Abstract
Regulation of inflammation in intestinal mesothelial cells in the abdominal cavity is important for the pathogeny of clinical conditions, such as postoperative ileus, peritonitis and encapsulating peritoneal sclerosis. Here we have examined the inflammatory effect of lipopolysaccharide (LPS) and the anti-inflammatory effect of nicotinic acetylcholine receptor stimulation in rat intestinal mesothelial cells. LPS upregulated mRNA expression of interleukin-1β (IL-1β), tumor necrosis factor-α (TNF-α), monocyte chemotactic protein-1 (MCP-1) and inducible nitric oxide synthase (iNOS). The α7, α9 and α10 subunits of nicotinic acetylcholine receptor were detected in intestinal mesothelial cells. Nicotine (10 nM) significantly inhibited LPS-induced mRNA expression of IL-1β and iNOS, but not TNF-α and MCP-1. In addition, the α7 nicotinic acetylcholine receptor selective agonist, PNU-282987 (10 nM), significantly inhibited LPS-induced mRNA expression of IL-1β but not TNF-α, iNOS and MCP-1. Finally, we found that enteric nerves adhered to intestinal mesothelial cells located under the ileal serosa. In conclusion, intestinal mesothelial cells react to LPS to induce the production of nitric oxide from iNOS. The anti-inflammatory action of intestinal mesothelial cells expressing α7nAChR may be mediated via their connectivity with enteric nerves.
Keywords: inflammation, iNOS, mesothelial cells, nicotinic acetylcholine receptors
Mesothelial cells form a monolayer of valvate-like cells that cover organs in the body, including the abdominal, thoracic and pericardial cavities. Mesothelial cells in these organs are located on a thin basement membrane supported by connective tissue stroma. Large quantities of microvilli and pili are seen on the cell surface, where they protect the mesothelial surface from injury by friction. Microvilli contain water and serous effusions, while pili control the secretion of surfactant, together contributing to the physiological constancy of the serosa, which acts as the front-line barrier against bacteria, chemical substances and surgical stress. It is known that mesothelial cells secrete acid mucopolysaccharide, proteoglycan and phospholipids, which provide a lubricated body cavity wall to prevent damage to the wall cavity from adherence [27]. Recently, however, it has been reported that the mesothelium plays an important role in peritoneal homeostasis, such as immune surveillance, antigen presentation and wound healing [23].
Mesothelial cells produce various immune-regulatory factors, such as chemokines, cytokines, growth factors, reactive oxygen species (ROS), antioxidant enzymes and extracellular matrix molecules, which can regulate the initiation and subsequent recovery of serosal inflammation [23]. In regard to the innate immune system of the mesothelium, it has been reported that both human and mice mesothelial cells express toll-like receptors (TLRs) 1 to 6 at the mRNA level [8]. TLR-signaling can activate nuclear factor kappa-B (NF-κB) and mitogen-activated protein kinase (MAPK) to initiate inflammation during infectious stimuli [8]. Moreover, chemokines secreted from mesothelial cells cause the infiltration of neutrophils and monocytes, and regulate the emigration of macrophages from inflamed peritoneum.
In intestinal-resident macrophages, it has been found that lipopolysaccharide (LPS) can upregulate cyclooxygenase-2 (COX-2) expression, which in turn increases inducible nitric oxide synthase (iNOS) via prostaglandin E2 (PGE2)/EP2 and/or EP4 signaling [13, 21, 29]. Induced PGE2 and nitric oxide (NO) directly act upon intestinal smooth muscle cells to induce gastrointestinal motility disorders, such as postoperative ileus and peritonitis [14, 21, 28]. In addition, it is thought that peritoneal macrophages may also play an important role in the pathogeny of postoperative ileus [19]. All gut organs are covered with mesothelial cells of the abdominal cavity, suggesting the possibility that intestinal mesothelial cells (IMCs) may be involved in the supply of NO in both conditions, given that they may react to LPS to produce COX-2 and iNOS.
In recent years, accumulating evidence has shown that vagus nerve stimulation has anti-inflammatory effects via activation of macrophage α7 nicotinic acetylcholine receptors (α7nAChRs) in the spleen, named as a vagovagal cholinergic anti-inflammatory reflex [26, 30]. Furthermore, in the small intestine, a similar anti-inflammatory pathway regulated by α7nAChR exists in the myenteric plexus neural network [20, 32], and muscularis-resident macrophages expressing α7nAChR are candidates for the induction of this anti-inflammatory action [3, 20]. The peripheral vagus nerve preferentially interacts with the myenteric neural network, with nerve endings in close proximity to muscularis resident macrophages [3]. However, it is still unclear whether IMCs similarly react with the peripheral autonomic neuronal network. On the other hand, it has been reported that cell growth of human mesothelial cells is regulated through the expression of α7nAChR [31], lending support to the idea that IMCs, in addition to muscularis resident macrophages, can negatively regulate inflammation through an α7nAChR signaling pathway.
Peritoneal dialysis is one of the approaches for the treatment of kidney failure. Although this treatment is better than hemodialysis, because of odd-hour therapy and reduced cardiovascular invasiveness, long-term peritoneal dialysis causes encapsulating peritoneal sclerosis [37]. This is because mesothelial cells are repeatedly exposed to high glucose and low pH, resulting in a high osmotic pressure that promotes the secretion of various inflammatory mediators and induces peritoneal inflammation. Effective suppression of peritonitis in the clinic could reduce encapsulating peritoneal sclerosis and the risks associated with long-term peritoneal dialysis.
With this in mind, we examined the inflammatory effect of LPS and the anti-inflammatory effect of α7nAChR stimulation in rat IMCs. We found that IMCs react to LPS, in turn inducing the iNOS gene to produce NO. In addition, IMCs were found to express α7nAChR and produce an anti-inflammatory action that could potentially be mediated through the attachment of these cells to enteric nerves.
MATERIALS AND METHODS
Animals
Seven- to twelve-week-old male Sprague-Dawley rats were used in this study. Rats were housed under controlled conditions (25°C, 12 hr light-dark cycles). All animal experiments were performed according to the Guide for Animal Use and Care published by the University of Tokyo and were approved by the Institutional Review Board of The University of Tokyo (approval code P10-482).
Isolation and culture of IMCs
The IMCs were isolated according to recent reports [9, 18]. Briefly, the intestines of rats were isolated, washed with Hank’s balanced salt solution (HBSS) and incubated in 0.25% trypsin in HBSS for 30 min. The fluids were then centrifuged at 200 g for 5 min at 4°C before aspirating the supernatant and re-suspending the pellets in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% fetal bovine serum (FBS). The isolated IMCs were incubated in 60 mm dishes at 37°C in a 95% O2 / 5% CO2 incubator until 60–80% confluent. The culture solution was changed the day following isolation and every 3–4 days thereafter. For experiments, primary culture cells (P-0) were used. Cells were cultured with 10% FBS in the incubator. Serum starvation was performed 24 hr before the experiments.
Immunostaining
IMCs cultured on cover glasses were fixed in 5% neutral buffered formalin at 37°C for 5 min. After fixation, the cells were washed three times in Tris-buffered saline (TBS) for 30 min each, then with 0.1% Triton-X100 in TBS containing 1% bovine serum albumin (BSA) for 90 sec. After membrane permeabilization, the cells were incubated with 1% BSA in TBS at room temperature for 30 min to reduce non-specific binding. Subsequently, the cells were incubated with 1:100 diluted mouse vimentin antibody (Nichirei Co., Tokyo, Japan, H912) in TBS with 2% BSA at room temperature for 1 hr. After washing with TBS for 30 min at 4°C, the cells were incubated with 1:250 diluted Alexa Fluor® 488 goat anti-mouse IgG secondary antibody and 1:50 diluted Rhodamine-Phalloidin (Molecular Probes, Eugene, OR, U.S.A., R-415), which is an F-actin probe conjucted to red fluorescent dye and stabilizes actin filaments, in TBS at room temperature for 1 hr. After washing twice with TBS, the cells were incubated with DAPI (1 µg/ml) for 5 min. The cells were washed twice and mounted on glass slides. We used an Eclipse E800 (Nikon) for observation.
Semi-quantitative RT-PCR
Total RNA was extracted from IMCs using TRIzol reagent (Molecular Research Center, Inc., Cincinnati, OH, U.S.A.) according to the manufacturer’s instructions. Total RNA was reverse transcribed using ReverTra Ace in conjunction with random 9-mer oligonucleotide primers (Takara Bio, Otsu, Japan) at 30°C for 10 min, 42°C for 1 hr and 99°C for 5 min. RT-PCR was performed using Taq DNA polymerase (Takara Bio) and a Thermal Cycler TP600 (Takara Bio). The cDNA was amplified for 32 cycles, consisting of 98°C for 10 sec, 55–58°C for 30 sec and 72°C for 1 min. Expression values were normalized to GAPDH mRNA levels and expressed relative to the control sample. PCR products were resolved on 2% agarose gels containing 0.015 µl/ml ethidium bromide. Bands were visualized with a UV transilluminator (TOYOBO, Osaka, Japan), and their density was measured using NIH image software (Image J, ver 1.51). Primer sets and the expected sizes for RT-PCR are shown in Table 1.
Table 1. Primer sets and expected product sizes for semi-quantitative RT-PCR.
| Target gene | Primer sequences |
Product sizes (bp) | |
|---|---|---|---|
| Forward | Reverse | ||
| GAPDH | TCCCTCAAGATTGTCAGCAA | AGATCCACAACGGATACATT | 308 |
| Mesothelin | TAGCCCCTGARGACATCCRSCAGTGG | CAACCGCCACATRACACTG | 251 |
| Keratin 5 | AAGGCCCAGTACGAGGACATT | GGTGTTCATGAGCTCCTGGTA | 351 |
| COX-2 | CTGTATCCCGCCCTCGTGGTG | ACTTGCGTTGATGGTGGCTGTCTT | 282 |
| iNOS | AAGRGAGTGYTGTTCCAGGT | CCACCAGCTTCTTCAAMGTG | 184 |
| IL-1β | TCCATGAGCTTTGTACAAGG | GGTGCTGATGACCAGTTGG | 246 |
| TNF-α | AAATGGGCTCCCTCTCATCA | AGCCTTGTCCCTTGAAGAGA | 248 |
| MCP-1 | CAACTCTCACTGAAGCCAGA | AAATGGATCTACATCTTGCA | 600 |
| α7nAChR | ATGGTGGCAAATGCCTAAG | CTCGGAAGCCAATGTAGAGC | 204 |
| α9nAChR | TCCTGGACCTACAATGGAAA | CTCCCAGAGAGACCTTCTCC | 295 |
| α10nAChR | AGATTGGAAGCGTCTGGCTA | TGCTCATCTGGCATTGAGTCTTA | 391 |
Electron microscopy
Intestines were placed in a fixative containing 3% glutaraldehyde and 4% paraformaldehyde in 0.1 M phosphate buffer, pH 7.4, for 2 hr at room temperature. Tissues were then post-fixed in 1% osmium tetroxide for 2 hr at 4°C, rinsed in distilled water, block-stained with saturated aqueous uranyl acetate solution for 3 hr, dehydrated in a graded series of ethyl alcohol and embedded in Epon 812. Ultrathin sections were cut with a Leica ultramicrotome, stained with uranyl acetate and lead citrate, and examined using a Hitachi H-7650 electron microscope (Hitachi, Tokyo, Japan).
Data analysis
Results are expressed as mean ± SEM. Data were evaluated using Student’s t-test for two groups and one-way ANOVA followed by Tukey’s test for comparisons between more than three groups. P values ˂0.05 were considered statistically significant.
RESULTS
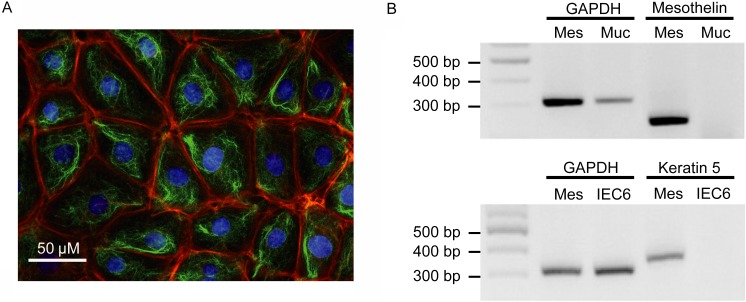
Figure 1A shows typical results of immunohistochemistry of cultured rat primary IMCs. IMCs grew with a pavement-like form and a mesh-like vimentin filamentous network inside the cytoplasm. Actin fiber networks formed peripherally to the cells. Total RNA was extracted from cultured rat IMCs, the IEC-6 rat epithelial cell line and rat intestinal mucosa, with RT-PCR performed for keratin 5 and mesothelin, marker proteins for IMCs. Keratin 5 was detected in primary cultured IMCs but not the IEC-6 cell line, while mesothelin was expressed in IMCs and IEC6 but not in intestinal mucosa (Fig. 1B).
Fig. 1.
Characterization of rat primary cultured IMCs. (A) Typical immunohistochemistry images of cultured rat IMCs. Red, green or blue signals indicate actin, vimentin or the cell nucleus, respectively. Scale bar represents 50 µm. (B) The mRNA expression of mesothelin and keratin 5 in rat mesothelial cells (MES). Intestinal mucosa (MUC) and the intestinal epithelial cell line, IEC6, were used for comparison. Product sizes were 308 bp for GAPDH, 351 bp for keratin 5 and 251 bp for mesothelin.
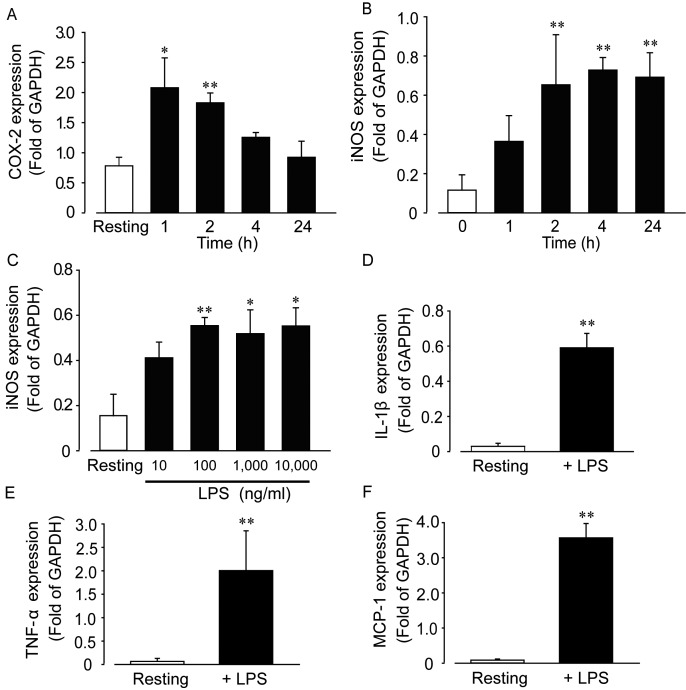
We next investigated time- and concentration-dependent changes in COX-2 and iNOS expression mediated by LPS (Fig. 2A and 2B). Results indicated that COX-2 and iNOS gene induction reached a maximum 2 and 4 hr after stimulation with LPS, respectively. COX-2 expression was down-regulated close to resting levels within 24 hr following LPS stimulation. In contrast, iNOS expression was maintained for more than 24 hr after stimulation. We also investigated the effect of LPS concentration on iNOS induction over a 4 hr stimulation period. Results indicated that 100 ng/ml LPS induced the maximum level of induction (Fig. 2C). We subsequently investigated the effect of LPS (100 ng/ml, 4 hr stimulation) on interleukin-1β (IL-1β), tumor necrosis factor-α (TNF-α) and monocyte chemotactic protein-1 (MCP-1) expression (Fig. 2D–F), with results showing that expression of all three genes was significantly increased by LPS stimulation.
Fig. 2.
Effect of LPS on the mRNA expression of inflammatory mediators in rat IMCs. Time-dependent changes in COX-2 (A) or iNOS (B) mRNA expression mediated by LPS (1 µg/ml, n=4–6 each). (C) Concentration-dependent change in iNOS expression mediated by LPS (10–10,000 ng/ml, n=4−6 each). Data are mean ± SEM; *P<0.05 and **P<0.01 vs. resting values. (D–F) Upregulation of inflammatory mediators (IL-1β, TNF-α and MCP-1) by LPS (100 ng/ml) after 4 hr. Each column shows mean ± SEM (n=4–6); *P<0.05 and **P<0.01 vs. resting or before stimulation with LPS.
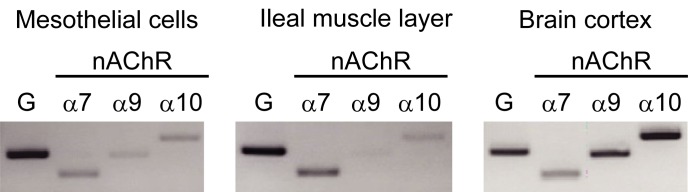
Because normal human mesothelial cells express α7nAChR [4, 31], we examined the subtypes of nAChRs expressed in rat IMCs, rat ileal smooth muscle and rat brain. Expression of nAChR subunits, α7, α9 and α10, were all detected in rat IMCs, as well as in ileal smooth muscle and brain (Fig. 3).
Fig. 3.
mRNA expression of α7, α9 and α10 subunits of nAChRs in IMCs, ileal muscle layer and brain cortex in rat. The expression of α7, α9 and α10 nAChR subunits was measured in rat IMCs, with rat ileal muscle and brain cortex used for comparison or as a positive control, respectively. The product sizes were 308 bp for GAPDH, 204 bp for α7nAChR, 295 bp for α9nAChR and 391 bp for α10nAChR.
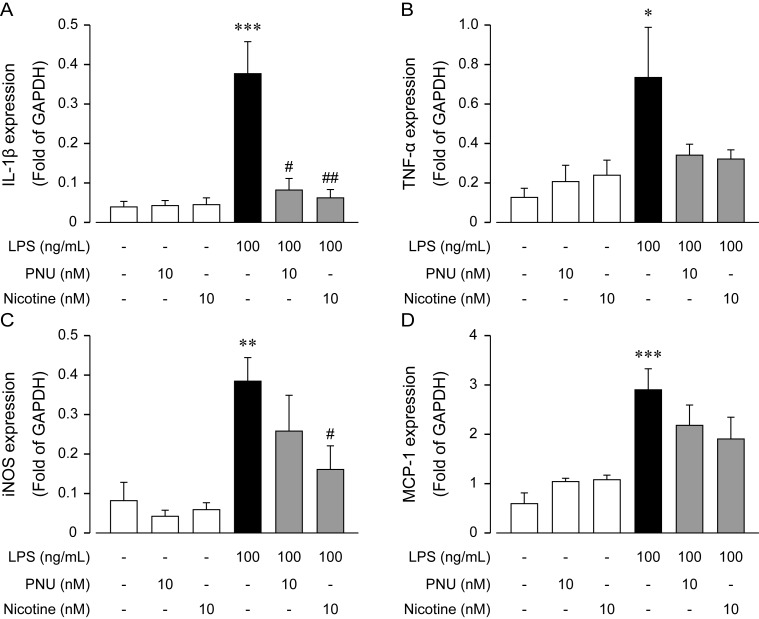
Subsequently, we compared the effect of nicotine (10 nM) on the LPS-induced mRNA expression of the inflammatory mediators, IL-1β, TNF-α, iNOS and MCP-1. Results indicated that nicotine significantly inhibited IL-1β and iNOS gene expression, but not TNF-α and MCP-1 (Fig. 4). We next examined the effect of PNU-282987 (PNU), one of α7nAChR selective agonist, on the LPS-induced mRNA expression of the inflammatory mediators, IL-1β, TNF-α, iNOS and MCP-1. Results showed that PNU significantly inhibited IL-1β gene expression, but not TNF-α, iNOS and MCP-1 (Fig. 4). However, in case of TNF-α, we considered that nicotine and PNU tended to inhibit the mRNA expression.
Fig. 4.
Anti-inflammatory effect of PNU-282987 and nicotine on LPS-induced mRNA expression of inflammatory mediators in IMCs. Rat mesothelial cells were treated with PNU-282987 (10 nM) and nicotine (10 nM) in the presence or absence of LPS (100 ng/ml) for 4 hr. PNU-282987 and nicotine were added 30 min before LPS application. (A–D) show results from IL-1β, TNF-α, iNOS and MCP-1, respectively. Each column shows mean ± SEM from four independent experiments (n=4−15); *P<0.05, **P<0.01 and ***P<0.001 vs. control values, and #P<0.05 and ##P<0.01 vs. LPS values.
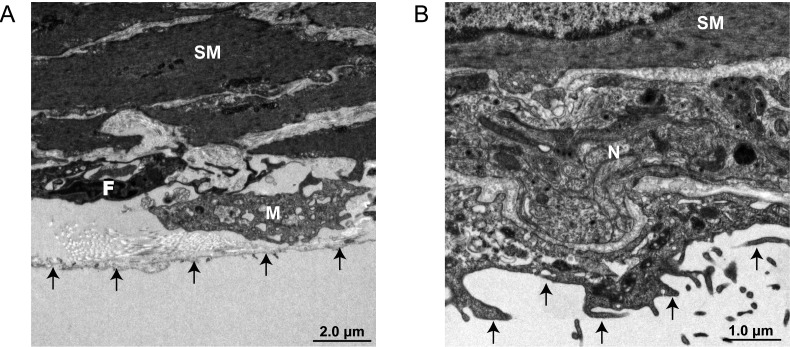
Finally, we performed electron microscopic analysis for evidence that an enteric neural network exists within the serosa of the small intestine. In this analysis, IMCs were found to be attached to fibroblast-like cells and subserosal resident macrophages (Fig. 5A). In addition, the results indicated the existence of an enteric neural network peripheral to the intestinal serosa that demonstrated connectivity with IMCs (Fig. 5B).
Fig. 5.
Electron microscopic images of ileal serosa. (A) IMCs attached with fibroblast-like cells and resident macrophages. (B) Peripheral enteric neural network which adhered to IMCs distributed in the intestinal serosa. SM, smooth muscle cell; F, fibroblast like cell; M, macrophage; arrows, mesothelial cells.
DISCUSSION
Mesothelin is a differentiation antigen that can be considered a good marker protein for IMCs, since its expression is confined to mesothelial cells derived from the pleural cavity, pericardium and peritoneum [5, 6]. In the present study, the primary cells that we isolated were shown to express mesothelin, supporting the concept that they represented IMCs. Further testing indicated that intact intestinal mucosa did not express mesothelin as expected; however, the cultured IEC-6 rat epithelial cell line did express mesothelin. There are reports that mesothelin is overexpressed in some cancer cells, such as pancreatic adenocarcinoma, ovarian cancer, pulmonary adenocarcinoma and mesothelioma [1, 12, 24, 25], which may explain the expression of mesothelin in the IEC-6 line, an immortalized cell strain. Further evidence that the primary cultured cells were IMCs was provided by the fact that they were positive for vimentin (typical of mesenchymal stromal cells) and also keratin 5 (another marker of the mesothelium).
TLRs (types 1 to 6) are known to be expressed in mesothelial cells and to participate in innate immune responses [8]. In macrophages, it is well known that LPS, the film component of the gram-negative bacillus, activates TLR4, which in turn induces expression of iNOS, COX-2 and inflammatory cytokines [17]. In the present study, rat IMCs stimulated with LPS induced the expression of the inflammatory mediators, COX-2, iNOS, IL-1β, TNF-α and MCP-1, supporting the notion that IMC-associated inflammatory immunoresponses following LPS stimulation depend on TLR4 activation, as previously reported [8].
In recent years, a vagus nerve-mediated cholinergic anti-inflammatory reflex in the spleen was identified, in addition to a neural anti-inflammatory mechanism regulated by glucocorticoid production and the hypothalamic–pituitary–adrenal (HPA) axis [11, 30]. In the gastrointestinal tract, another local anti-inflammatory pathway that does not require the involvement of the spleen has been demonstrated [3, 20, 32]. The target cells of the cholinergic anti-inflammatory reflex are macrophages expressing α7nAChR [10, 34, 35], a monomeric nAChR formed by α7 subunit pentamers [7]. α7nAChR is mainly expressed in central neural cells, but is also expressed in immunoreactive cells, such as macrophages [7]. Recent work has revealed that normal human mesothelial cells, and also human mesothelioma cell lines, express functional α7nAChRs [4, 30], indicating the possibility that α7nAChRs may also be expressed in rat IMCs. In this study, nicotine inhibited the mRNA expression of the inflammatory mediators, iNOS, IL-1β and TNF-α, suggesting that nAChRs may indeed be involved in the anti-inflammatory actions of IMCs.
We further examined the effect of nicotine and a selective α7nAChR agonist, PNU, in this experimental system. Not only PNU but also nicotine inhibited mRNA expression of IL-1β and tended to inhibit TNF-α in rat IMCs. However, PNU did not reduce mRNA expression iNOS, while nicotine significantly inhibited. These findings suggest that not only α7nAChR but also other types of nAChR participate in the anti-inflammatory action of nicotine in rat IMCs. In fact, in the current study, three types of nAChR subunits, α7, α9 and α10, were detected in IMCs. It has been reported that the α9 and α10 subunits might participate in the anti-inflammatory action mediated by nicotine, suggesting the existence of complexed nAChRs formed by α7/α9/α10 subunits [22]. Similarly, an anti-inflammatory role has been described for complex nAChR of the α4/β2 subtype in the gastrointestinal tract [33]. On the other hand, it is also possible to be considered that PNU tended to inhibit the LPS-induced iNOS induction as similar with the case of nicotine treatment. From the viewpoint of this idea, PNU and nicotine may activate the same nAChR, possibly α7nAChR, because PNU and nicotine caused similar reaction against the LPS-induced cytokines expression. Recent reports showed that JAK2-STAT3 pathway might function as pivotal bridge between α7nAChRs and NF-κB regulation [2]. Moreover, IκB, which was recognized as a key regulator of NF-κB, prevented the NF-κB activation in resting state [2], suggesting this regulation might happen in IMC. Further studies are required to increase our understanding around this point.
The morphological finding of an association of the autonomic nervous system with the gastrointestinal serosa is unprecedented. In this study, we have shown for the first time clear morphological evidence for such an association by electron microscopy. It remains uncertain whether the nerve fascicle distributed under the serosa in fact represents the efferent vagus nerve, but the data suggest the possibility of an anti-inflammatory response capacity via peripheral cholinergic innervation to the serosa. Alternatively, since the acetylcholine-synthesizing enzyme choline acetyltransferase has been reported in a wide range of cell types, including endothelial, epithelial, muscle, immune and mesothelial cells, it may be that mechanisms involving non-neuronal acetylcholine are available to react with intestinal target cells, such as IMCs [15, 16, 36].
In summary, we have found that IMCs have an immunoresponse capability in response to LPS-stimulation that results in the induction of inflammatory mediators, such as COX-2, iNOS, IL-1β, TNF-α and MCP-1. In addition, we have provided evidence that α7AChRs, and potentially other type of nAChRs, play an anti-inflammatory role in IMCs, which may in turn be regulated by peripheral autonomic innervation under the serosa. As such, IMCs represent an important target for therapeutic strategies to reduce the risk of postoperative ileus, peritonitis and encapsulating peritoneal sclerosis.
Acknowledgments
This work was supported by Grants-in-Aid for Scientific Research from the Ministry of Education, Japan (MH; 24248050 and HO; 25221205) and in part by the Smoking Research Foundation (MH). No conflicts of interest, financial or otherwise, are declared by the authors. HO and MH designed the research; WO, TM and KH performed experiments; SM, NK, SI, HO and MH discussed the research study; TM and MH wrote the paper.
REFERENCES
- 1.Argani P., Iacobuzio-Donahue C., Ryu B., Rosty C., Goggins M., Wilentz R. E., Murugesan S. R., Leach S. D., Jaffee E., Yeo C. J., Cameron J. L., Kern S. E., Hruban R. H.2001. Mesothelin is overexpressed in the vast majority of ductal adenocarcinomas of the pancreas: identification of a new pancreatic cancer marker by serial analysis of gene expression (SAGE). Clin. Cancer Res. 7: 3862–3868. [PubMed] [Google Scholar]
- 2.Báez-Pagán C. A., Delgado-Vélez M., Lasalde-Dominicci J. A.2015. Activation of the Macrophage α7 Nicotinic Acetylcholine Receptor and Control of Inflammation. J. Neuroimmune Pharmacol. 10: 468–476. doi: 10.1007/s11481-015-9601-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cailotto C., Gomez-Pinilla P. J., Costes L. M., van der Vliet J., Di Giovangiulio M., Némethova A., Matteoli G., Boeckxstaens G. E.2014. Neuro-anatomical evidence indicating indirect modulation of macrophages by vagal efferents in the intestine but not in the spleen. PLOS ONE 9: e87785. doi: 10.1371/journal.pone.0087785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Catassi A., Paleari L., Servent D., Sessa F., Dominioni L., Ognio E., Cilli M., Vacca P., Mingari M., Gaudino G., Bertino P., Paolucci M., Calcaterra A., Cesario A., Granone P., Costa R., Ciarlo M., Alama A., Russo P.2008. Targeting alpha7-nicotinic receptor for the treatment of pleural mesothelioma. Eur. J. Cancer 44: 2296–2311. doi: 10.1016/j.ejca.2008.06.045 [DOI] [PubMed] [Google Scholar]
- 5.Chang K., Pastan I.1996. Molecular cloning of mesothelin, a differentiation antigen present on mesothelium, mesotheliomas, and ovarian cancers. Proc. Natl. Acad. Sci. U.S.A. 93: 136–140. doi: 10.1073/pnas.93.1.136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang K., Pastan I., Willingham M. C.1992. Isolation and characterization of a monoclonal antibody, K1, reactive with ovarian cancers and normal mesothelium. Int. J. Cancer 50: 373–381. doi: 10.1002/ijc.2910500308 [DOI] [PubMed] [Google Scholar]
- 7.Cloëz-Tayarani I., Changeux J. P.2007. Nicotine and serotonin in immune regulation and inflammatory processes: a perspective. J. Leukoc. Biol. 81: 599–606. doi: 10.1189/jlb.0906544 [DOI] [PubMed] [Google Scholar]
- 8.Colmont C. S., Raby A. C., Dioszeghy V., Lebouder E., Foster T. L., Jones S. A., Labéta M. O., Fielding C. A., Topley N.2011. Human peritoneal mesothelial cells respond to bacterial ligands through a specific subset of Toll-like receptors. Nephrol. Dial. Transplant. 26: 4079–4090. doi: 10.1093/ndt/gfr217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Debray-García Y., Sánchez E. I., Rodríguez-Muñoz R., Venegas M. A., Velazquez J., Reyes J. L.2016. Diabetes and exposure to peritoneal dialysis solutions alter tight junction proteins and glucose transporters of rat peritoneal mesothelial cells. Life Sci. 161: 78–89. doi: 10.1016/j.lfs.2016.07.018 [DOI] [PubMed] [Google Scholar]
- 10.de Jonge W. J., van der Zanden E. P., The F. O., Bijlsma M. F., van Westerloo D. J., Bennink R. J., Berthoud H. R., Uematsu S., Akira S., van den Wijngaard R. M., Boeckxstaens G. E.2005. Stimulation of the vagus nerve attenuates macrophage activation by activating the Jak2-STAT3 signaling pathway. Nat. Immunol. 6: 844–851. doi: 10.1038/ni1229 [DOI] [PubMed] [Google Scholar]
- 11.Floto R. A., Smith K. G.2003. The vagus nerve, macrophages, and nicotine. Lancet 361: 1069–1070. doi: 10.1016/S0140-6736(03)12902-9 [DOI] [PubMed] [Google Scholar]
- 12.Hassan R., Kreitman R. J., Pastan I., Willingham M. C.2005. Localization of mesothelin in epithelial ovarian cancer. Appl. Immunohistochem. Mol. Morphol. 13: 243–247. doi: 10.1097/01.pai.00000141545.36485.d6 [DOI] [PubMed] [Google Scholar]
- 13.Hori M., Kita M., Torihashi S., Miyamoto S., Won K. J., Sato K., Ozaki H., Karaki H.2001. Upregulation of iNOS by COX-2 in muscularis resident macrophage of rat intestine stimulated with LPS. Am. J. Physiol. Gastrointest. Liver Physiol. 280: G930–G938. [DOI] [PubMed] [Google Scholar]
- 14.Kalff J. C., Schraut W. H., Billiar T. R., Simmons R. L., Bauer A. J.2000. Role of inducible nitric oxide synthase in postoperative intestinal smooth muscle dysfunction in rodents. Gastroenterology 118: 316–327. doi: 10.1016/S0016-5085(00)70214-9 [DOI] [PubMed] [Google Scholar]
- 15.Kawashima K., Fujii T.2003. The lymphocytic cholinergic system and its contribution to the regulation of immune activity. Life Sci. 74: 675–696. doi: 10.1016/j.lfs.2003.09.037 [DOI] [PubMed] [Google Scholar]
- 16.Kawashima K., Fujii T., Moriwaki Y., Misawa H.2012. Critical roles of acetylcholine and the muscarinic and nicotinic acetylcholine receptors in the regulation of immune function. Life Sci. 91: 1027–1032. doi: 10.1016/j.lfs.2012.05.006 [DOI] [PubMed] [Google Scholar]
- 17.Kim T. W., Joh E. H., Kim B., Kim D. H.2012. Ginsenoside Rg5 ameliorates lung inflammation in mice by inhibiting the binding of LPS to toll-like receptor-4 on macrophages. Int. Immunopharmacol. 12: 110–116. doi: 10.1016/j.intimp.2011.10.023 [DOI] [PubMed] [Google Scholar]
- 18.Kim T. H., Lee K. B., Kang M. J., Park J. H.2012. Critical role of Toll-like receptor 2 in Bacteroides fragilis-mediated immune responses in murine peritoneal mesothelial cells. Microbiol. Immunol. 56: 782–788. doi: 10.1111/j.1348-0421.2012.00505.x [DOI] [PubMed] [Google Scholar]
- 19.Maehara T., Matsumoto K., Horiguchi K., Kondo M., Iino S., Horie S., Murata T., Tsubone H., Shimada S., Ozaki H., Hori M.2015. Therapeutic action of 5-HT3 receptor antagonists targeting peritoneal macrophages in post-operative ileus. Br. J. Pharmacol. 172: 1136–1147. doi: 10.1111/bph.13006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matteoli G., Gomez-Pinilla P. J., Nemethova A., Di Giovangiulio M., Cailotto C., van Bree S. H., Michel K., Tracey K. J., Schemann M., Boesmans W., Vanden Berghe P., Boeckxstaens G. E.2014. A distinct vagal anti-inflammatory pathway modulates intestinal muscularis resident macrophages independent of the spleen. Gut 63: 938–948. doi: 10.1136/gutjnl-2013-304676 [DOI] [PubMed] [Google Scholar]
- 21.Mikawa S., Ohta Y., Kaji N., Islam M. S., Murata T., Ozaki H., Hori M.2015. Time-dependent changes in inhibitory action of lipopolysaccharide on intestinal motility in rat. J. Vet. Med. Sci. 77: 1443–1449. doi: 10.1292/jvms.15-0198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mishra N. C., Rir-sima-ah J., Boyd R. T., Singh S. P., Gundavarapu S., Langley R. J., Razani-Boroujerdi S., Sopori M. L.2010. Nicotine inhibits Fc epsilon RI-induced cysteinyl leukotrienes and cytokine production without affecting mast cell degranulation through alpha 7/alpha 9/alpha 10-nicotinic receptors. J. Immunol. 185: 588–596. doi: 10.4049/jimmunol.0902227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mutsaers S. E.2002. Mesothelial cells: their structure, function and role in serosal repair. Respirology 7: 171–191. doi: 10.1046/j.1440-1843.2002.00404.x [DOI] [PubMed] [Google Scholar]
- 24.Ordóñez N. G.2003. Application of mesothelin immunostaining in tumor diagnosis. Am. J. Surg. Pathol. 27: 1418–1428. doi: 10.1097/00000478-200311000-00003 [DOI] [PubMed] [Google Scholar]
- 25.Ordóñez N. G.2003. Value of mesothelin immunostaining in the diagnosis of mesothelioma. Mod. Pathol. 16: 192–197. doi: 10.1097/01.MP.0000056981.16578.C3 [DOI] [PubMed] [Google Scholar]
- 26.Rosas-Ballina M., Olofsson P. S., Ochani M., Valdés-Ferrer S. I., Levine Y. A., Reardon C., Tusche M. W., Pavlov V. A., Andersson U., Chavan S., Mak T. W., Tracey K. J.2011. Acetylcholine-synthesizing T cells relay neural signals in a vagus nerve circuit. Science 334: 98–101. doi: 10.1126/science.1209985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roth J.1973. Ultrahistochemical demonstration of saccharide components of complex carbohydrates at the alveolar cell surface and at the mesothelial cell surface of the pleura visceralis of mice by means of concanavalin A. Exp. Pathol. (Jena) 8: 157–167. [PubMed] [Google Scholar]
- 28.Schwarz N. T., Kalff J. C., Türler A., Engel B. M., Watkins S. C., Billiar T. R., Bauer A. J.2001. Prostanoid production via COX-2 as a causative mechanism of rodent postoperative ileus. Gastroenterology 121: 1354–1371. doi: 10.1053/gast.2001.29605 [DOI] [PubMed] [Google Scholar]
- 29.Tajima T., Murata T., Aritake K., Urade Y., Michishita M., Matsuoka T., Narumiya S., Ozaki H., Hori M.2012. EP2 and EP4 receptors on muscularis resident macrophages mediate LPS-induced intestinal dysmotility via iNOS upregulation through cAMP/ERK signals. Am. J. Physiol. Gastrointest. Liver Physiol. 302: G524–G534. doi: 10.1152/ajpgi.00264.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tracey K. J.2009. Reflex control of immunity. Nat. Rev. Immunol. 9: 418–428. doi: 10.1038/nri2566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Trombino S., Cesario A., Margaritora S., Granone P., Motta G., Falugi C., Russo P.2004. Alpha7-nicotinic acetylcholine receptors affect growth regulation of human mesothelioma cells: role of mitogen-activated protein kinase pathway. Cancer Res. 64: 135–145. doi: 10.1158/0008-5472.CAN-03-1672 [DOI] [PubMed] [Google Scholar]
- 32.Tsuchida Y., Hatao F., Fujisawa M., Murata T., Kaminishi M., Seto Y., Hori M., Ozaki H.2011. Neuronal stimulation with 5-hydroxytryptamine 4 receptor induces anti-inflammatory actions via α7nACh receptors on muscularis macrophages associated with postoperative ileus. Gut 60: 638–647. doi: 10.1136/gut.2010.227546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van der Zanden E. P., Snoek S. A., Heinsbroek S. E., Stanisor O. I., Verseijden C., Boeckxstaens G. E., Peppelenbosch M. P., Greaves D. R., Gordon S., De Jonge W. J.2009. Vagus nerve activity augments intestinal macrophage phagocytosis via nicotinic acetylcholine receptor alpha4beta2. Gastroenterology 137: 1029–1039, 1039.e1–1039.e4. doi: 10.1053/j.gastro.2009.04.057 [DOI] [PubMed] [Google Scholar]
- 34.Wang H., Yu M., Ochani M., Amella C. A., Tanovic M., Susarla S., Li J. H., Wang H., Yang H., Ulloa L., Al-Abed Y., Czura C. J., Tracey K. J.2003. Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature 421: 384–388. doi: 10.1038/nature01339 [DOI] [PubMed] [Google Scholar]
- 35.Wehner S., Behrendt F. F., Lyutenski B. N., Lysson M., Bauer A. J., Hirner A., Kalff J. C.2007. Inhibition of macrophage function prevents intestinal inflammation and postoperative ileus in rodents. Gut 56: 176–185. doi: 10.1136/gut.2005.089615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wessler I., Kilbinger H., Bittinger F., Unger R., Kirkpatrick C. J.2003. The non-neuronal cholinergic system in humans: expression, function and pathophysiology. Life Sci. 72: 2055–2061. doi: 10.1016/S0024-3205(03)00083-3 [DOI] [PubMed] [Google Scholar]
- 37.Williams J. D., Craig K. J., Topley N., Von Ruhland C., Fallon M., Newman G. R., Mackenzie R. K., Williams G. T., Peritoneal Biopsy Study Group. 2002. Morphologic changes in the peritoneal membrane of patients with renal disease. J. Am. Soc. Nephrol. 13: 470–479. [DOI] [PubMed] [Google Scholar]