Abstract
We investigated the antidepressant-like effect of lactoferrin (Lf) in a repeated forced-swim test (FST) stress mouse model. FST was performed on days 1, 2, 7 and 14. Bovine Lf (bLf) or bovine serum albumin (BSA) was supplemented at 1% to the commercial diet after the first FST throughout the experimental period. The FST-control and FST+BSA group showed a marked increase in immobility time on day 2, which remained increased up to the 14th day, while the FST+bLf group showed a significant lower immobility time. Brain-derived neurotrophic factor (BDNF) content in the hippocampus significantly decreased in all of FST treated groups. These results suggest that bLf may improve the depressive-like symptoms induced by repeated FST.
Keywords: antidepressant, BDNF, corticosterone, lactoferrin, repeated forced-swim test
Lactoferrin (Lf) is an iron-binding glycoprotein present in body fluids, such as tears, saliva, milk, pancreatic juice and bile. Lf has many bioactive effects as an antiviral [1], antibacterial [12], anti-tumor [17] and anti-inflammatory agent [19]. To clarify the physiological effects of Lf, we reported the characteristic transporting system for Lf [14]. Intraduodenally administered bovine Lf (bLf) is transported into the blood circulation via thoracic duct lymph fluid in adult rats. This finding indicates that the bLf transported to epithelial cells from the lumen reaches the blood circulation via the lymphatic pathway, suggesting that it could be distributed to the whole body, if an effective dose was administered. Kamemori et al. [6] have reported the transportation of intravenously administered bLf into the cerebrospinal fluid (CSF). Administered bLf was immunohistochemically detected in the vesicular membranes of endothelial cells in cerebral blood vessels 10 min after the infusion. Numerous immunoreactive small vesicles were also detected in the ependymal cells of the choroid plexus. Moreover, the bLf concentration in the CSF was significantly increased at 1–2 hr after the intravenous infusion of bLf (10 or 30 mg/kg). These findings clearly demonstrate that Lf is possibly transported into the brain matter even in adult animals.
Depressive disorders are a major psychological burden in humans and are treated with selective serotonin reuptake inhibitors (SSRI) and other anti-depressants. Repeated forced-swim stress is an animal model of chronic depression [2]. It shows increasing immobilized behavior over time and sensitizes to the effects of SSRI or other anti-depressant drugs.
In our previous studies, we have reported that bLf suppresses the distress induced by electric-shock [5] or psychological stress in rats [13]. Lf also has a suppressive effect on the increment of plasma corticosterone in maternal separated rat pups [13]. Increased hypothalamus-pituitary-adrenal (HPA) axis activity has been reported to highly relate with depressaive disorder [3]. We have also reported an anti-nociceptive effect of Lf in a mice tail-flick model [16], suggesting Lf is effective in the central nervous system in both rats and mice. Within these contexts, we hypothesized that Lf has an anti-depressant-like effect. The major purpose of this study was to assess the antidepressant-like effect of Lf using the repeated forced-swim stress mouse model. We also investigated glucocorticoid release and brain-derived neurotrophic factor (BDNF) content in the hippocampus in relation to the antidepressant-like effect of Lf.
Male ICR strain mice aged 5 weeks were purchased from the Tokyo Experimental Animal Co., Ltd. (Tokyo, Japan). The mice were acclimated for 1 week, and then used for experiments at 6 weeks of age. The animals were housed in a temperature-controlled (22 ± 1°C) room under a constant day/night cycle (lights on 07:00–19:00). Mice were housed 3 per cage for all experiments. Food (standard chow, CE-2, Nihon-Crea, Tokyo, Japan) and water were available ad libitum. The experimental procedure used in the present study and care of animals were approved by the Experimental Animal Committee of Tottori University.
Bovine Lf was purchased from Wako Pure Chemical Co., Ltd. (Osaka, Japan), and bovine serum albumin (BSA) was purchased from Sigma Aldrich Japan (Tokyo, Japan).
The forced-swim test (FST) was conducted using a plexiglass cylinder with a 15 cm diameter and 25 cm height, filled with warm water (25°C) to a depth of 10 cm. Mice were divided into 6 groups: groups 1 and 4 received a normal diet (control), groups 2 and 5 received a 1% BSA-supplemented diet, and groups 3 and 6 received a 1% bLf-supplemented diet. FST was performed only in groups 4 (FST-control), 5 (FST+BSA) and 6 (FST+bLf). Repeated FST was performed according to a previous report [8] with slight modifications. Before the FST, the mice were left quiet for at least 1 hr to adapt to the experimental room. Each mouse was placed individually in the cylinder for 15 min on day 1 and for 5 min on days 2, 7 and 14. All experiments were carried out between 13:00 and 17:00. The behavior of each mouse was recorded during the FST using a video camera (Handycam, Sony, Tokyo, Japan), and immobility time was measured using SMART 3.0 software (Panlab Harvard Apparatus, Barcelona, Spain). Each mouse was judged to be immobile when it ceased struggling and remained floating motionless in the water. Immobile criteria were determined as less than 0.6 cm2/sec from preliminary experiments (data are not shown) and judged every 0.5 sec by the software. Following the first FST trial, we started to feed BSA or bLf to the animal.
Blood samples (0.1 ml) were collected at 30 min after the FST on days 2, 7 and 14 from the tail vein. Blood samples were also collected at15:00–16:00 on day 15 to measure basal plasma corticosterone levels. The blood was transferred into an EDTA-containing tube and centrifuged (5,000 × g, 5 min, 4°C). All samples were stored at −80°C until measurement for levels of corticosterone. Plasma corticosterone was determined by an ELISA kit (Assaypro, St. Charles, MO, U.S.A.).
After all behavioral experiments and blood collections were completed, mice were sacrificed by decapitation. The hippocampus was rapidly removed and stored at −80°C until assays were performed. For the measurement of BDNF content, protein samples were obtained from the hippocampus. Protein concentration was determined by a protein assay reagent (Bio-Rad, Hercules, CA, U.S.A.). BDNF content was determined by an ELISA kit (Promega, Madison, WI, U.S.A.). Normalized BDNF concentration was shown as BDNF/protein concentration.
All data are expressed as mean ± SD. Differences between the groups were statistically investigated using two-way repeated analysis of variance (ANOVA) for the immobility time in the FST and plasma corticosterone levels immediately after the FST. We also used two-way ANOVA for the basal plasma corticosterone level and hippocampal BDNF content. If there is significant difference by the factor, we used Bonferroni’s post hoc test. In all cases, a P value of <0.05 was considered to indicate statistical significance.
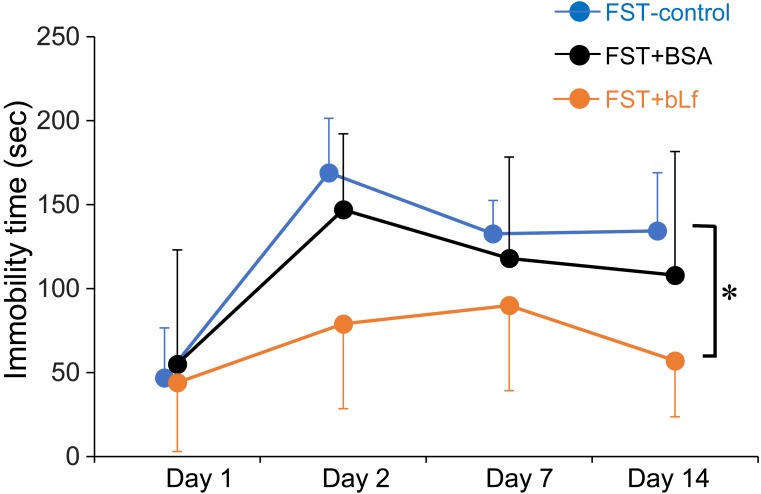
Immobility time in the FST-control and FST+BSA groups showed a marked increase after the day 2, while the FST-bLf showed a slight increase. Two-way repeated ANOVA showed a significant difference among the tree groups (F(2,50)=10.49, P<0.001), and the Bonferroni’s post hoc test showed a significant difference (P<0.05) between the FST-control and FST+bLf groups (Fig. 1).
Fig. 1.
Suppressive effects of bovine lactoferrin on the immobility time in the forced-swim test. Data show an immobility time within the 5 min FST. In the FST on day 1, the immobility time was measured during the first 5 min period. Each value represents the mean ± SD (n=5–6). Statistical analysis was performed by two-way repeated ANOVA. *P<0.05 comparison between FST-control and FST+bLf group.
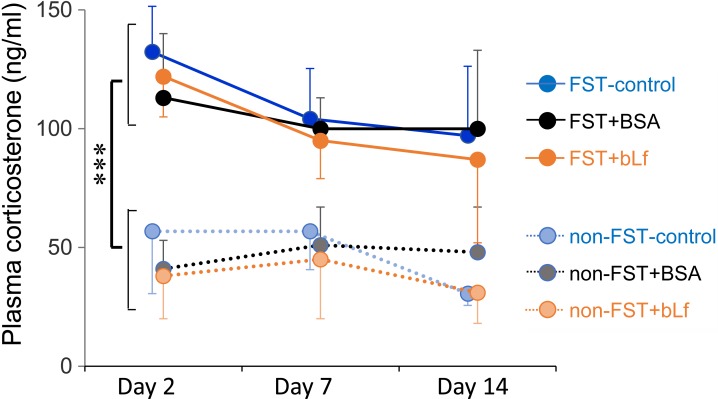
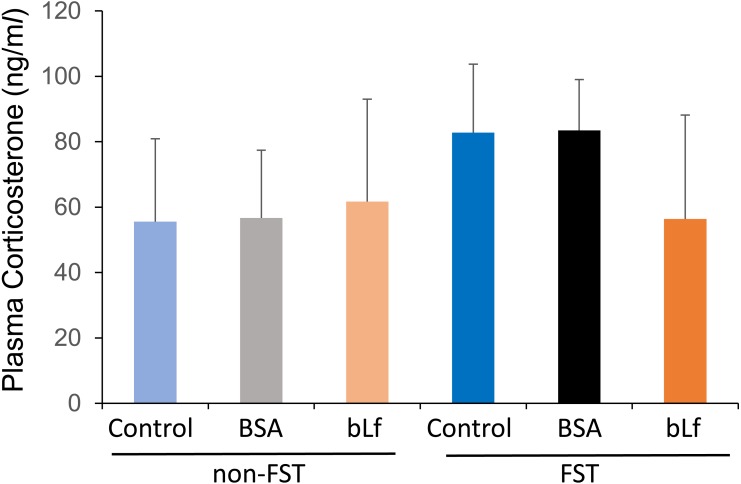
Plasma corticosterone levels in all non-FST groups didn’t show any changes during the experimental period. Two-way repeated ANOVA showed a significant higher plasma corticosterone level in the FST treated groups (F(5,104)=57.72, P<0.001) (Fig. 2). However, there were no significant differences by bLf supplementation. Although the plasma corticosterone level on day 15 (24 hr after the last FST) tended to increase in the FST-control and FST+BSA groups, two-way ANOVA didn’t show any significant changes (F(5,34)=3.808, P=0.0604) by FST treatment (Fig. 3).
Fig. 2.
Changes in plasma corticosterone levels immediately after the FST in each experimental day. Data are presented as mean ± SD (n=5−6). Statistical analysis was performed by two-way repeated ANOVA. ***P<0.001 comparison between non-FST and FST groups.
Fig. 3.
Changes in basal plasma corticosterone levels on day 15. Data are represented as mean ± SD (n=5–6). Statistical analysis was performed by two-way ANOVA.
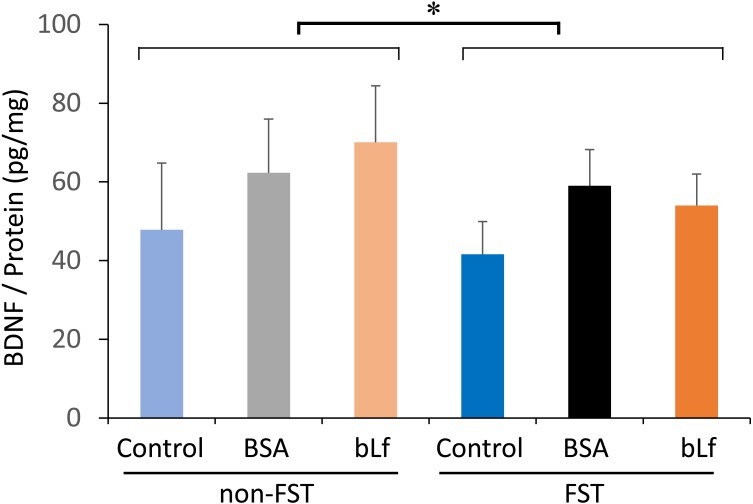
BDNF content in the hippocampus showed a significant decrease (F(5,34)=4.326, P<0.05) by FST treatment. However, there were no significant differences by bLf supplementation (Fig. 4).
Fig. 4.
Changes in BDNF content in the hippocampus on day 15. Data are represented as mean ± SD (n=5–6). Statistical analysis was performed by two-way ANOVA. *P<0.05 comparison between non-FST and FST groups.
In the present study, the repeated FST mouse model satisfactorily mimicked depressive behavior, measured as the increase in immobility time. We demonstrated that bLf has antidepressant-like effects in this model.
The FST is a behavioral test useful for screening drugs with potential antidepressant activity [11]. Animals re-exposed to the FST exhibit higher scores for immobility over the course of retesting [8], suggesting that the repeated FST, a severe stress model, causes chronic depressive-like behavior. Though it is well established that the exposure to repeated severe stress can evoke depressive-like behavior in an animal, Van den Berg et al. [18] have reported that an emotional stimulus giving a forced perception of another rat receiving footshock (FS) has the opposite effect to that of a FS stimulus in rats. In their study, FS for 5 consecutive days decreased motor activities of rats for at least 15 days, whereas an emotional stimulus increased these activities in the period from 30 min up to at least 15 days. The findings of the study suggest that mechanisms regulating the responses to stress use different pathways according to the stress condition. It appears that bLf suppresses one of the pathways related to stress responses, because bLf is more effective under certain stress conditions [5]. In our study, bLf suppressed the increased immobility time during the FST for at least on day 2. This suggests bLf may be more effective in the early period.
In the present study, plasma corticosterone levels were increased in all of FST treated groups, while bLf had no effects on the plasma corticosterone immediately after the FST. In contrast, basal plasma corticosterone level tended to decrease in FST-bLf group. The acute stress-induced glucocorticoid increase is usually a beneficial response that helps the body avoid injury; however, in conditions of over-exposure to stress, such as chronic stress, glucocorticoid hypersecretion reduces glucocorticoid receptor (GR) levels and impairs HPA axis negative feedback [15]. It should be clarified whether bLf can modulate glucocorticoid receptor expression in a future study.
In the present study, BDNF content in the hippocampus was significantly decreased by FST treatment. Chronic stress application markedly reduces BDNF mRNA levels in hippocampal tissue [10], and antidepressant drugs can protect against depression-induced BDNF down-regulation in the hippocampus [15]. Some reports have shown that glucocorticoids or GR may influence BDNF expression in the hippocampus [7]. In our study, hippocampal BDNF content tended to be higher by supplementation of BSA or bLf. Some amino acids, including taurine [9], L-tryptophan [20] and L-arginine [4], have antidepressants like effect in FST. Beta-alanine has an anxiolytic-like effect and increases BDNF concentration in the hippocampus [9]. BSA or bLf supplementation may cause some changes in the amino acid content in the brain, while we did not measure the plasma amino acid concentration in mice. Thus, future studies should clarify the influence of BSA and bLf on the BDNF expression.
In conclusion, bLf may improve the depressive-like symptoms induced by repeated FST, which may be partially related to the suppression of increased basal plasma corticosterone.
Acknowledgments
This work was partly supported by a Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (KAKENHI grant number 15K14878).
REFERENCES
- 1.Andersen J. H., Osbakk S. A., Vorland L. H., Traavik T., Gutteberg T. J.2001. Lactoferrin and cyclic lactoferricin inhibit the entry of human cytomegalovirus into human fibroblasts. Antiviral Res. 51: 141–149. doi: 10.1016/S0166-3542(01)00146-2 [DOI] [PubMed] [Google Scholar]
- 2.Dal-Zotto S., Martí O., Armario A.2000. Influence of single or repeated experience of rats with forced swimming on behavioural and physiological responses to the stressor. Behav. Brain Res. 114: 175–181. doi: 10.1016/S0166-4328(00)00220-5 [DOI] [PubMed] [Google Scholar]
- 3.Givalois L., Li S., Pelletier G.2002. Central nitric oxide regulation of the hypothalamic-pituitary-adrenocortical axis in adult male rats. Brain Res. Mol. Brain Res. 102: 1–8. doi: 10.1016/S0169-328X(02)00218-8 [DOI] [PubMed] [Google Scholar]
- 4.Inan S. Y., Yalcin I., Aksu F.2004. Dual effects of nitric oxide in the mouse forced swimming test: possible contribution of nitric oxide-mediated serotonin release and potassium channel modulation. Pharmacol. Biochem. Behav. 77: 457–464. doi: 10.1016/j.pbb.2003.12.024 [DOI] [PubMed] [Google Scholar]
- 5.Kamemori N., Takeuchi T., Hayashida K., Harada E.2004. Suppressive effects of milk-derived lactoferrin on psychological stress in adult rats. Brain Res. 1029: 34–40. doi: 10.1016/j.brainres.2004.09.015 [DOI] [PubMed] [Google Scholar]
- 6.Kamemori N., Takeuchi T., Sugiyama A., Miyabayashi M., Kitagawa H., Shimizu H., Ando K., Harada E.2008. Trans-endothelial and trans-epithelial transfer of lactoferrin into the brain through BBB and BCSFB in adult rats. J. Vet. Med. Sci. 70: 313–315. doi: 10.1292/jvms.70.313 [DOI] [PubMed] [Google Scholar]
- 7.Kumamaru E., Numakawa T., Adachi N., Kunugi H.2011. Glucocorticoid suppresses BDNF-stimulated MAPK/ERK pathway via inhibiting interaction of Shp2 with TrkB. FEBS Lett. 585: 3224–3228. doi: 10.1016/j.febslet.2011.09.010 [DOI] [PubMed] [Google Scholar]
- 8.Mezadri T. J., Batista G. M., Portes A. C., Marino-Neto J., Lino-de-Oliveira C.2011. Repeated rat-forced swim test: reducing the number of animals to evaluate gradual effects of antidepressants. J. Neurosci. Methods 195: 200–205. doi: 10.1016/j.jneumeth.2010.12.015 [DOI] [PubMed] [Google Scholar]
- 9.Murakami T., Furuse M.2010. The impact of taurine- and beta-alanine-supplemented diets on behavioral and neurochemical parameters in mice: antidepressant versus anxiolytic-like effects. Amino Acids 39: 427–434. doi: 10.1007/s00726-009-0458-x [DOI] [PubMed] [Google Scholar]
- 10.Numakawa T., Adachi N., Richards M., Chiba S., Kunugi H.2013. Brain-derived neurotrophic factor and glucocorticoids: reciprocal influence on the central nervous system. Neuroscience 239: 157–172. doi: 10.1016/j.neuroscience.2012.09.073 [DOI] [PubMed] [Google Scholar]
- 11.Porsolt R. D., Le Pichon M., Jalfre M.1977. Depression: a new animal model sensitive to antidepressant treatments. Nature 266: 730–732. doi: 10.1038/266730a0 [DOI] [PubMed] [Google Scholar]
- 12.Strøm M. B., Haug B. E., Rekdal O., Skar M. L., Stensen W., Svendsen J. S.2002. Important structural features of 15-residue lactoferricin derivatives and methods for improvement of antimicrobial activity. Biochem. Cell Biol. 80: 65–74. doi: 10.1139/o01-236 [DOI] [PubMed] [Google Scholar]
- 13.Takeuchi T., Kitagawa H., Harada E.2004. Evidence of lactoferrin transportation into blood circulation from intestine via lymphatic pathway in adult rats. Exp. Physiol. 89: 263–270. doi: 10.1113/expphysiol.2003.026633 [DOI] [PubMed] [Google Scholar]
- 14.Takeuchi T., Hayashida K., Inagaki H., Kuwahara M., Tsubone H., Harada E.2003. Opioid mediated suppressive effect of milk-derived lactoferrin on distress induced by maternal separation in rat pups. Brain Res. 979: 216–224. doi: 10.1016/S0006-8993(03)02941-X [DOI] [PubMed] [Google Scholar]
- 15.Thompson Ray M., Weickert C. S., Wyatt E., Webster M. J.2011. Decreased BDNF, trkB-TK+ and GAD67 mRNA expression in the hippocampus of individuals with schizophrenia and mood disorders. J. Psychiatry Neurosci. 36: 195–203. doi: 10.1503/jpn.100048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsuchiya T., Takeuchi T., Hayashida K., Shimizu H., Ando K., Harada E.2006. Milk-derived lactoferrin may block tolerance to morphine analgesia. Brain Res. 1068: 102–108. doi: 10.1016/j.brainres.2005.11.002 [DOI] [PubMed] [Google Scholar]
- 17.Tsuda H., Sekine K., Takasuka N., Toriyama-Baba H., Iigo M.2000. Prevention of colon carcinogenesis and carcinoma metastasis by orally administered bovine lactoferrin in animals. Biofactors 12: 83–88. doi: 10.1002/biof.5520120113 [DOI] [PubMed] [Google Scholar]
- 18.Van den Berg C. L., Lamberts R. R., Wolterink G., Wiegant V. M., Van Ree J. M.1998. Emotional and footshock stimuli induce differential long-lasting behavioural effects in rats; involvement of opioids. Brain Res. 799: 6–15. doi: 10.1016/S0006-8993(98)00397-7 [DOI] [PubMed] [Google Scholar]
- 19.Ward P. P., Uribe-Luna S., Conneely O. M.2002. Lactoferrin and host defense. Biochem. Cell Biol. 80: 95–102. doi: 10.1139/o01-214 [DOI] [PubMed] [Google Scholar]
- 20.Wong P. T., Ong Y. P.2001. Acute antidepressant-like and antianxiety-like effects of tryptophan in mice. Pharmacology 62: 151–156. doi: 10.1159/000056088 [DOI] [PubMed] [Google Scholar]