Abstract
Natural infection with larval Echinococcus multilocularis was recognized in one of eight Norway rats, Rattus norvegicus, caught indoors in 2009 in Ebetsu, Hokkaido, northern Japan. Cystic lesions were found in the right median and lateral lobes of the liver, with numerous alveolar cysts in the periphery of the lesions. Protoscolices were formed within large cysts. The laminated layers of the cysts were positive for PAS staining. Nested PCR using the primers specific for Taenia mitochondrial 12S rDNA yielded a 250-bp product, and the sequence of the PCR product matched that of E. multilocularis isolates from Hokkaido and Germany. This is the third natural alveolar hydatidosis in R. norvegicus in Japan.
Keywords: alveolar hydatidosis, Echinococcus multilocularis, indoor infection, Norway rat, protoscolex
Natural infection with Echinococcus multilocularis in Norway rats, Rattus norvegicus, has been described in three reports so far in the literature [3, 5, 8]. The first report revealed infection in four Norway rats in forest-steppe areas, Novosibirsk, Republic of Kazakhstan [5]. The other two reported a total of two infected Norway rats captured outdoors in Hokuto, formerly Kamiiso-cho, Hokkaido, northern Japan [3, 8].
We report a case of natural alveolar hydatid infection in a Norway rat caught indoors in Ebetsu, Hokkaido, Japan. This is the third report of natural E. multilocularis infection in R. norvegicus in Japan.
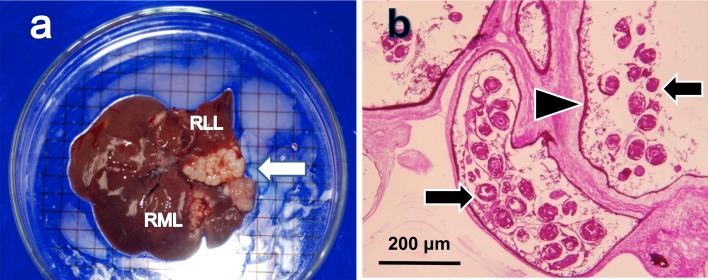
In May, 2009, eight rats were captured in a livestock feed storage house of Rakuno Gakuen University in Ebetsu, Hokkaido, northern Japan. These rats were identified as R. norvegicus because their head and body length was longer than tail length and their auricles did not reach the eyes when bent forward. Autopsy revealed cystic lesions in the liver of one rat. This rat was female, weighed 160 g, and was young because the uterus showed no signs of previous pregnancy. The cystic lesions were found in the right median and lateral lobes of the liver, with numerous small alveolar cysts present in the periphery of the lesions (Fig. 1a). Many protoscolices were formed within brood capsules in multilocular cysts (Fig. 1b). The laminated layers of the cysts were positive for PAS staining (Fig. 1b).
Fig. 1.
Pathological findings in the infected Norway rat. Cystic lesions (arrow) in the right median lobe (RML) and lateral lobe (RLL) of the liver (a). The grid size is 5 mm. Histopathological findings. Protoscolices (arrows) and laminated layers (arrowhead) (b). PAS staining, magnification ×100.
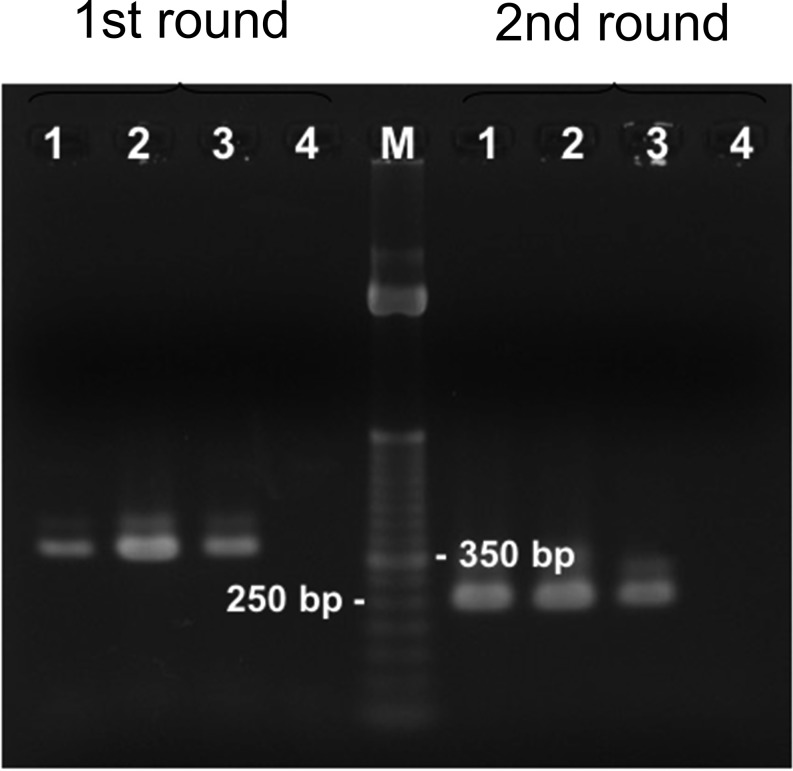
Total DNA was extracted from the cysts and protoscolices that were isolated separately from ethanol-fixed specimens and subjected to nested PCR amplification using mitochondrial 12S rDNA primers specific for the genus Taenia according to von Nickisch-Rosenegk et al. [10]. PCR yielded a band of about 350 bp on the first round and a band of about 250 bp on the second round, each corresponding to those from an E. multilocularis isolated in Hokkaido (Fig. 2). Nucleotide sequence of the PCR product was essentially identical to those of E. multilocularis isolates from Hokkaido and Germany (Fig. 3).
Fig. 2.
Nested PCR amplification of DNA from protoscolices and cysts of the infected Norway rat using mitochondrial 12S rDNA primers specific for Taenia spp. Lane 1, protoscolices; lane 2, cysts: lane 3, a Hokkaido isolate; and lane 4, negative control. M, 100-bp marker.
Fig. 3.
Sequence alignment of the PCR product. RNO, protoscolices from the infected Norway rat; HOK, Hokkaido isolate (GenBank accession no. AB024424); GER, German isolate (GenBank accession no. L49455).
The source of the present infection most likely is fox feces and the site of infection inside the feed storage house. The storage house was infested with numerous Norway rats throughout the year. Its floor was contaminated with fox feces. Red foxes, Vulpes vulpes schrencki, were seen wandering within the school premises and invading the storage house through a broken part of the exterior wall, most actively during winter. The storage house stood close to the Nopporo Forest Park which is heavily contaminated by E. multilocularis. Lagapa et al. [4] collected fox feces in the park and revealed a high prevalence of E. multilocularis eggs or coproantigen throughout the year, with almost 30 and 57% of the feces positive for eggs and coproantigen, respectively, from November to December. These pieces of evidence indicate that the present infection occurred indoors through the feces of E. multilocularis-infected red foxes.
Natural alveolar hydatidosis is not common in Norway rats. Its occurrences have been described in three reports in the literature [3, 5, 8]. The first report appeared in 1961 and described E. multilocularis infection in four of 50 Norway rats captured in forest-steppe areas, near Novosibirsk, Republic of Kazakhstan; the infected rats had no protoscolices [5]. The other two reported E. multilocularis infection in Norway rats captured in two consecutive years at the same garbage dump in Hokuto, southern Hokkaido, Japan [3, 8]. The 1992 report identified infection in one of 42 rats [8], and the 1993 report found one of 78 rats infected [3]. These two rats were infected with larval E. multilocularis and had protoscolices in the liver cysts [3, 8]. Thus, the present report is the third describing natural E. multilocularis infection in Norway rats in Japan and probably the forth in the world.
The prevalence of infection in the present study is higher than that in the above field studies. The prevalence was 8% in the Novosibirsk survey [5], 2.4% in the 1992 report [8] and 1.3% in the 1993 report [3] of the surveys conducted in Hokkaido, Japan. In contrast, the prevalence in the present study was 13%, although the sample size was admittedly small. The low prevalence in these field studies suggests either a poor susceptibility of Norway rats to E. multilocularis or a low contamination of these fields with E. multilocularis. As for the susceptibility, an earlier attempt with intraperitoneal injection of E. multilocularis protoscolices failed to establish infection in white rats although the nature of white rats was not specified [11]. Inoculation by gavage of eggs from a Hokkaido isolate of E. multilocularis caused no infection in Wistar rats [2]. Only recently, a novel approach of implanting single vesicles of E. multilocularis in the liver succeeded in establishing hepatic lesions in Sprague-Dawley rats [13, 14]. With regard to environmental contamination levels, the Novosibirsk survey found 89 of 166 (54%) foxes infected with E. multilocularis [5]. The prevalence in foxes was not known in the area that Okamoto et al. [8] and Iwaki et al. [3] surveyed. However, E. multilocularis is endemic in Hokkaido, and its prevalence in foxes is generally high. For example, the prevalence of E. multilocularis coproantigen was 21% and over 50% in feces collected around fox dens in urban fringes of Sapporo, western Hokkaido [9] and of Koshimizu, eastern Hokkaido [6], respectively. These findings suggest that although Norway rats are poorly susceptible to E. multilocularis, they can be infected when the environment is heavily contaminated. We speculate that the high prevalence in the present study is a result of a high dose of infection caused by heavy contamination of the storage house with feces of red foxes inhabiting the near-by Nopporo Forest Park, a high-prevalence reservoir of E. multilocularis [4].
Our present findings indicate that Norway rats have a potential to be an intermediate host of E. multilocularis, suggesting that they can spread E. multilocularis and establish a life cycle of E. multilocularis in urban areas with domestic carnivores. Canine E. multilocularis infection outside Hokkaido was reported for the first time in 2006 in Saitama Prefecture, Honshu, Japan [12]. The second canine infection in Honshu was reported in 2016 in Aichi Prefecture [7]. An estimate describes that at least 30 pet dogs infected with E. multilocularis are annually brought into Honshu from Hokkaido [1]. Once the prevalence of E. multilocularis infection in dogs reaches the level where environmental contamination with E. multilocularis eggs is sufficiently heavy to infect Norway rats, these house rats can establish a life cycle of E. multilocularis in human residential areas with dogs.
Acknowledgments
We thank Dr. Yuzaburo Oku, Tottori University, and Dr. Kimpei Yagi, Hokkaido Institute of Public Health, for their advices during the course of investigation. We also thank Dr. Toshio Homma, Meiji University, for his critical reading of the manuscript and editorial assistance. This work was supported in part by grants for Research on Emerging and Re-emerging Infectious Diseases from the Ministry of Health, Labor and Welfare, Japan.
REFERENCES
- 1.Doi R., Matsuda H., Uchida A., Kanda E., Kamiya H., Konno K., Tamashiro H., Nonaka N., Oku Y., Kamiya M.2003. [Possibility of invasion of Echinococcus into Honshu with pet dogs from Hokkaido and overseas]. Nippon Koshu Eisei Zasshi 50: 639–649 [In Japanese]. [PubMed] [Google Scholar]
- 2.Iwaki T., Inohara J., Oku Y., Shibahara T., Kamiya M.1995. Infectivity to rats with eggs of the Echinococcus multilocularis isolate from a Norway rat in Hokkaido, Japan. Kisechugaku Zasshi 44: 32–33. [Google Scholar]
- 3.Iwaki T., Hatakeyama S., Nonaka N., Miyaji S., Yokohata Y., Okamoto M., Ooi H., Oku Y., Kamiya M.1993. Surveys on larval Echinococcus multilocularis and other hepatic helminths in rodents and insectivores in Hokkaido, Japan, from 1985 to 1992. Kisechugaku Zasshi 42: 502–506. [Google Scholar]
- 4.Lagapa J. T. G., Oku Y., Kaneko M., Ganzorig S., Ono T., Nonaka N., Kobayashi F., Kamiya M.2009. Monitoring of environmental contamination by Echinococcus multilocularis in an urban fringe forest park in Hokkaido, Japan. Environ. Health Prev. Med. 14: 299–303. doi: 10.1007/s12199-009-0083-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lukashenko N. P., Zaorikhina V. I.1961. [Epidemiology of alveolar echinococcosis in central zone of the Baraba Forest Steppe in the Novosibirsk region]. Med. Prom. SSSR 30: 159–168 [In Russian]. [PubMed] [Google Scholar]
- 6.Morishima Y., Tsukada H., Nonaka N., Oku Y., Kamiya M.1999. Coproantigen survey for Echinococcus multilocularis prevalence of red foxes in Hokkaido, Japan. Parasitol. Int. 48: 121–134. doi: 10.1016/S1383-5769(99)00009-4 [DOI] [PubMed] [Google Scholar]
- 7.Morishima Y., Tomaru Y., Fukumoto S., Sugiyama H., Yamasaki H., Hashimoto C., Harada K.2016. Canine echinococcosis due to Echinococcus multilocularis: a second notifiable case from mainland Japan. Jpn. J. Infect. Dis. 69: 448–449. doi: 10.7883/yoken.JJID.2015.573 [DOI] [PubMed] [Google Scholar]
- 8.Okamoto M., Fujita O., Arikawa J., Kurosawa T., Oku Y., Kamiya M.1992. Natural Echinococcus multilocularis infection in a Norway rat, Rattus norvegicus, in southern Hokkaido, Japan. Int. J. Parasitol. 22: 681–684. doi: 10.1016/0020-7519(92)90020-L [DOI] [PubMed] [Google Scholar]
- 9.Tsukada H., Morishima Y., Nonaka N., Oku Y., Kamiya M.2000. Preliminary study of the role of red foxes in Echinococcus multilocularis transmission in the urban area of Sapporo, Japan. Parasitology 120: 423–428. doi: 10.1017/S0031182099005582 [DOI] [PubMed] [Google Scholar]
- 10.von Nickisch-Rosenegk M., Silva-Gonzalez R., Lucius R.1999. Modification of universal 12S rDNA primers for specific amplification of contaminated Taenia spp. (Cestoda) gDNA enabling phylogenetic studies. Parasitol. Res. 85: 819–825. doi: 10.1007/s004360050638 [DOI] [PubMed] [Google Scholar]
- 11.Webster G. A., Cameron T. W. M.1961. Observations on experimental infections with Echinococcus in rodents. Can. J. Zool. 39: 877–891. doi: 10.1139/z61-082 [DOI] [Google Scholar]
- 12.Yamamoto N., Morishima Y., Kon M., Yamaguchi M., Tanno S., Koyama M., Maeno N., Azuma H., Mizusawa H., Kimura H., Sugiyama H., Arakawa K., Kawanaka M.2006. The first reported case of a dog infected with Echinococcus multilocularis in Saitama prefecture, Japan. Jpn. J. Infect. Dis. 59: 351–352. [PubMed] [Google Scholar]
- 13.Yamashita M., Imagawa T., Nakaya K., Sako Y., Okamoto Y., Tsuka T., Osaki T., Okamoto M., Ito A.2013. Echinococcus multilocularis: Single hepatic lesion experimentally established without metastasis in rats. Exp. Parasitol. 135: 320–324. doi: 10.1016/j.exppara.2013.07.015 [DOI] [PubMed] [Google Scholar]
- 14.Yamashita M., Imagawa T., Sako Y., Okamoto M., Yanagida T., Okamoto Y., Tsuka T., Osaki T., Ito A.2017. Serological validation of an alveolar echinococcosis rat model with a single hepatic lesion. J. Vet. Med. Sci. 79: 308–313. doi: 10.1292/jvms.16-0513 [DOI] [PMC free article] [PubMed] [Google Scholar]