Abstract
A major role of the corpus luteum (CL) is to produce progesterone (P4). The CL has immature vasculature shortly after ovulation, suggesting it exists under hypoxic conditions. Hypoxia-inducible factor-1 (HIF1) induces the expression of glucose transporter 1 (GLUT1). To clarify the physiological roles of GLUT1 in bovine CL, we examined GLUT1 mRNA expression in the CL under hypoxic conditions by quantitative RT-PCR. We also measured the effects of glucose (0–25 mM) and GLUT1 inhibitors (cytochalasin B, STF-31) on P4 production in bovine luteal cells. GLUT1 mRNA expression in bovine CL was higher at the early luteal stage compared to the other later stages. Hypoxia (3% O2) increased GLUT1 mRNA expression in early luteal cells, but not in mid luteal cells. Glucose (0–25 mM) increased P4 production in early luteal cells, but not in mid luteal cells. Both GLUT1 inhibitors decreased P4 production in early and mid luteal cells. Overall, the results suggest that GLUT1 (possibly induced by hypoxic conditions in the early CL) plays a role in the establishment and development of bovine CL, especially in supporting luteal P4 synthesis at the early luteal stage.
Keywords: corpus luteum, glucose transporter-1, HIF1, hypoxia, progesterone
The corpus luteum (CL) is an organ that is temporarily formed during the female reproductive cycle. The CL is formed from a ruptured follicle after ovulation and is coupled with rapid angiogenesis [29, 30, 32, 33], which is stimulated by a variety of growth factors [30, 31, 33]. The strongest growth factor is vascular endothelial growth factor (VEGF) [8]. VEGF is strongly induced by hypoxia-inducible factor-1 (HIF1) [9], and plays a role in the angiogenesis of newly formed CLs in cattle [2, 11, 23]. Follicle rupture shortly after ovulation has been suggested to be induced under hypoxic conditions as a result of bleeding and due to its immature vasculature [1, 23]. In addition to the angiogenic action of VEGF, several systems are thought to be induced as a result of HIF1 activity during CL formation.
HIF1 is an obligatory heterodimeric protein composed of HIF1A and the aryl hydrocarbon receptor nuclear translocator (ARNT), both of which are members of the basic-helix-loop-helix (bHLH)-containing PER-ARNT-SIM (PAS) domain family [39]. ARNT expression is not affected by oxygen concentration, whereas HIF1A is rapidly ubiquitinated under hypoxia, which targets the protein for degradation by the proteasome [13, 15, 34]. In bovine CL, HIF1A expression is highest during luteal development and has a similar expression pattern to that of VEGF [23]. This suggests HIF1 is important for angiogenesis during bovine CL development. HIF1 induces the transcription of genes related to several physiological systems, such as angiogenesis, erythropoiesis, glycolysis, and apoptosis [40]. Glucose transporter 1 (GLUT1) is one of 13 facilitative sugar transporters that has tissue-specific distribution and is induced by HIF1 [40]. GLUT1 is expressed in the ovaries of rat [17, 18], mouse [44], dog [29], and sheep [42]. GLUT1 mRNA is also expressed in bovine CL [22]. However, the roles of GLUT1 and its relationship with hypoxia in bovine CL remain unclear.
In the present study, we investigated the physiological roles of GLUT1 in bovine CL by examining GLUT1 mRNA expression in the CL during the estrous cycle. The effects of hypoxia on GLUT1 mRNA expression in cultured bovine luteal cells was also examined. Furthermore, to examine whether glucose and GLUT1 regulate luteal progesterone (P4) synthesis, we evaluated the effects of glucose and GLUT1 inhibitors on P4 production in cultured luteal cells.
MATERIALS AND METHODS
Collection of CLs
Ovaries with CLs from Holstein cows were collected at a local abattoir 10–20 min after exsanguination. Luteal stages were classified as early, developing, mid, late, or regressed by macroscopic observation of the ovary and uterus, as previously described [21]. After stage determination, CLs (n=4/stage) were immediately separated from the ovaries, rapidly frozen in liquid nitrogen, and stored at −80°C until being processed for RNA isolation. For cell culture experiments, ovaries with CLs (1–3 ovaries per experiment) were submerged in ice-cold physiological saline and transported to the laboratory.
Cell isolation
Early and mid luteal tissues were enzymatically dissociated, and luteal cells were cultured as previously described [25, 27]. The luteal cells were suspended in a culture medium, consisting of DMEM and Ham’s F-12 medium (Life Technologies Corp., Grand Island, NY, U.S.A.; No. 12634-010), supplemented with 5% calf serum (Life Technologies Corporation; No. 16170-078) and 20 µg/ml gentamicin (Wako Pure Chemical Industries, Osaka, Japan; No. 078-06061). Cell viability was determined to be greater than 85% by trypan blue exclusion. Cells in the cell suspension consisted of approximately 70% small luteal cells, 20% large luteal cells, 10% endothelial cells or fibrocytes, and no erythrocytes [25].
Cell culture and experiments
For the determination of mRNA expression, dispersed luteal cells were seeded at 2.0 × 105 viable cells per ml in 24-well cluster dishes (Greiner Bio-One, Frickenhausen, Germany; No. 662160), while 48-well cluster dishes (Thermo Fischer Scientific, Rochester, NY, U.S.A.; No. 130187) were used for the determination of P4 production. In both instances cells were cultured in a N2-O2-CO2-regulated incubator with a humidified atmosphere of 5% CO2 at 37.5°C (ESPEC Corp., Osaka, Japan; No. BNP-110). After 12 hr of culture, the medium was replaced with fresh medium containing 0.1% BSA and 5 ng/ml sodium selenite. Thereafter the experiments described below were carried out. Glucose-free medium (Nakalai Tesque, Kyoto, Japan; No. 09893-05) was specifically used for the experiment examining the effects of glucose (0.25, 2.5 and 25 mM) on P4 production. Cell culture under conditions examining different levels of O2 (3 or 20%) was performed using N2-O2-CO2-regulated incubators (ASTEC, Fukuoka, Japan; No. APM30D), and cells were cultured for 24 hr. Following incubation, the cell culture supernatant was used for the determination of P4 concentrations, and total cellular RNA was extracted for the determination of GLUT1 mRNA. For GLUT1 inhibition, cytochalasin B (a non-specific GLUT inhibitor; Sigma-Aldrich, St. Louis, MO, U.S.A.; No. C6762; 10 µM) and STF-31 (a specific GLUT inhibitor; Sigma-Aldrich; No. SML1108; 10 µM) were added with or without human chorionic gonadotropin (hCG; ASKA Pharmaceutical Co., Ltd., Tokyo, Japan; 1 U/ml).
RNA isolation and cDNA synthesis
Total RNA was prepared from CL tissue and cultured luteal cells using RNeasy, according to the manufacturer’s directions (Qiagen GmbH, Hilden, Germany; No. 74106). Total RNA (1 µg) was reverse transcribed using a PrimeScript 1st strand cDNA synthesis kit (Takara Bio Inc., Otsu, Japan; No. 6110A).
Real-time polymerase chain reaction (PCR)
Gene expression was measured by real-time PCR using a 7500 FAST thermal cycler (Applied Biosystems, Tokyo, Japan) and the KAPA FAST ABI prism qPCR kit (KAPA, Boston, MA, U.S.A.; No. KK4604) starting with 1 ng of reverse-transcribed total RNA. The expression of 18S ribosomal RNA (18SrRNA) was used as an internal control. Specific primers with 50–60% GC-contents for GLUT1 and 18SrRNA were synthesized. Briefly, the primers for GLUT1 were 5′-AGACACCTGAGGAGCTGTTC-3′ (5′primer, 20 mer) and 5′-GACATCACTGCTGGCTGAAG-3′ (3′primer, 20 mer); and for 18S rRNA were 5′-TCGCGGAAGGATTTAAAGTG-3′ (5′primer, 20 mer) and 5′-AAACGGCTACCACATCCAAG-3′ (3′primer, 20 mer). The PCR conditions were 95°C for 10 min, followed by 40 cycles of 95°C for 15 sec and 60°C for 60 sec. Use of the KAPA FAST ABI prism qPCR mixture at elevated temperatures resulted in reliable and sensitive quantification of the RT-PCR products with high linearity (Pearson correlation coefficient r>0.99). To analyze the relative level of expression of each mRNA, the 2-ΔΔCT method was used [20].
P4 concentration determination
Concentrations of P4 were determined directly from the cell culture medium using an enzyme immunoassay (EIA), as previously described [24]. The standard curve ranged from 0.391 to 100 ng/ml, and the effective dose of the assay for 50% inhibition (ED50) was 4.5 ng/ml. The intra- and inter-assay coefficients of variation were 5.3% and 8.4%, respectively.
Statistical analysis
All experimental data are shown as the mean ± SEM. Data on the effects of glucose and GLUT1 inhibitors on P4 production are shown as a percentage of the control. The statistical significance of differences between the amounts of GLUT1 mRNA (Fig. 1) during the estrous cycle and on the effects of glucose and GLUT1 inhibitors on P4 production (Figs. 3 and 4) were assessed by analysis of variance (ANOVA) followed by a multiple comparison with Bonferroni correction. The statistical significance of the difference between various oxygen conditions on the amounts of GLUT1 mRNA (Fig. 2) were assessed by Student’s t-test.
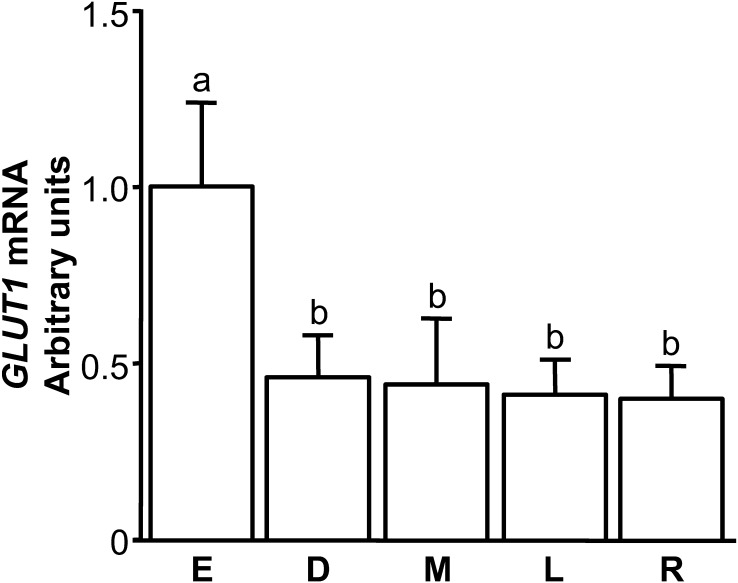
Fig. 1.
Changes in the relative amounts of GLUT1 mRNA in the bovine CL throughout the estrous cycle (E: early, Days 2–3; D: developing, Days 5–6; M: mid, Days 8–12; L: late, Days 15–17; R: regressed luteal stages, Days 19–21). Data are the means ± SEM for 4 samples/stage and are expressed as relative ratios of GLUT1 mRNA to 18S rRNA. Different letters indicate significant differences (P<0.05), as determined by ANOVA followed by a multiple comparison with Bonferroni correction.
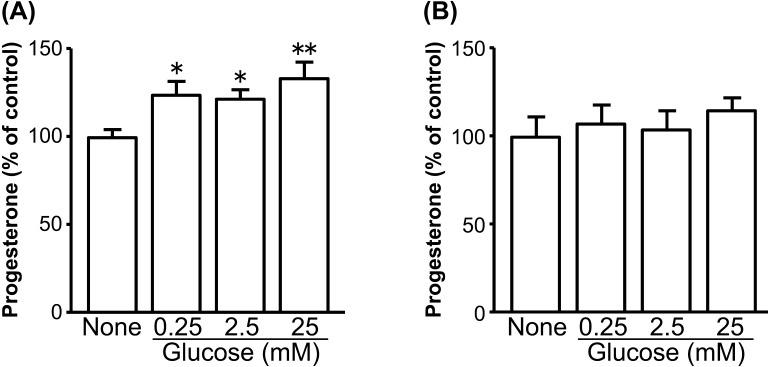
Fig. 3.
Dose-dependent effects of glucose on P4 production by cultured bovine early luteal cells (A) and mid luteal cells (B). The cells were cultured in glucose-free medium without or with glucose (0.25, 2.5 and 25 mM) for 24 hr (early: n=3, mid: n=3). All values are represented as a percentage of the control (none) value (mean ± SEM). The concentration of P4 in the early and mid luteal control cells was 193 ± 25.7 ng/ml and 613 ± 36.7 ng/ml, respectively. Asterisks indicate significant differences compared with none (*P<0.05, **P<0.01), as determined by ANOVA followed by a multiple comparison with Bonferroni correction.
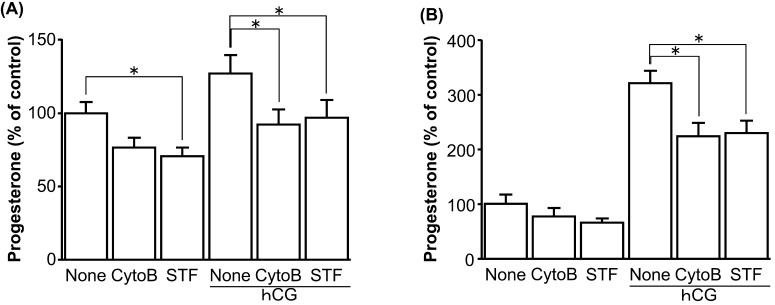
Fig. 4.
Effect of GLUT1 inhibitors (cytochalasin B [CytoB], STF-31 [STF]) on P4 production by cultured bovine early luteal cells (A) and mid luteal cells (B). Cells were cultured in medium containing 5% calf serum without or with GLUT1 inhibitor (cytochalasin B; 10 µM, STF-31; 10 µM) in combination without or with hCG stimulation (1 U/ml) for 24 hr (early: n=6, mid: n=5). All values are represented as a percentage of the control (none without hCG) value (mean ± SEM). The concentration of P4 in the controls for early and mid luteal cells was 238 ± 19.5 ng/ml and 674 ± 58.0 ng/ml, respectively. Asterisks indicate significant differences (P<0.05), as determined by ANOVA followed by a multiple comparison with Bonferroni correction.
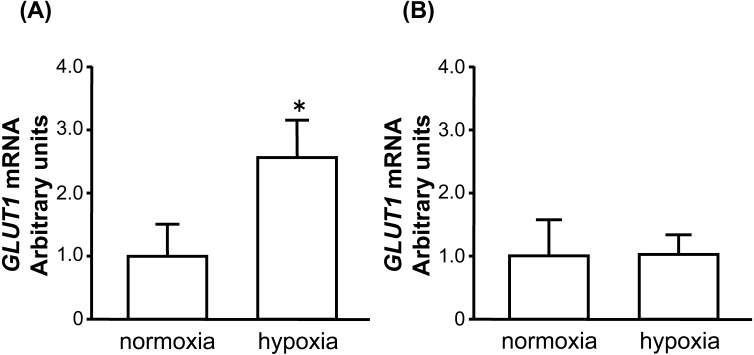
Fig. 2.
Effect of hypoxia on GLUT1 mRNA expression in cultured bovine early luteal cells (A) and mid luteal cells (B). Cells were cultured under 20% O2 (normoxia) or 3% O2 (hypoxia) for 24 hr. The amount of GLUT1 mRNA is expressed relative to the amount of 18S rRNA (early: n=5, mid: n=5). An asterisk indicates a significant difference between oxygen tension (P<0.05), as determined by the Student’s t-test.
RESULTS
GLUT1 mRNA expression
GLUT1 mRNA was expressed in bovine CL throughout the estrous cycle and was highest at the early luteal stage (Fig. 1; P<0.05). GLUT1 mRNA expression in early luteal cells increased significantly after 24 hr of hypoxia (Fig. 2A; P<0.05). Mid luteal cells did not show the same response (Fig. 2B).
Effects of glucose and GLUT1 inhibitors on P4 production in early and mid luteal cells
Glucose increased P4 production in early luteal cells in a dose-dependent manner (Fig. 3A; none vs. 0.25, 2.5 mM glucose: P<0.05; none vs. 25 mM glucose: P<0.01), whereas it did not affect P4 production by mid luteal cells (Fig. 3B). The nonselective GLUT inhibitor cytochalasin B only decreased P4 production in early luteal cells stimulated by hCG (Fig. 4A; P<0.05), while the GLUT1-specific inhibitor STF-31 decreased P4 production in early luteal cells without and with hCG stimulation (Fig. 4A; P<0.05). Both GLUT1 inhibitors decreased P4 production in mid luteal cells stimulated by hCG (Fig. 4B; P<0.05). Minor inhibitory effects were also observed in mid luteal cells without hCG stimulation (Fig. 4B).
DISCUSSION
The present study reconfirmed the previous finding that GLUT1 mRNA was expressed throughout the estrous cycle in bovine CL [22], and revealed that GLUT1 mRNA expression levels in the CL were greatest at the early luteal stage. Compared to normal culture conditions (20% O2), GLUT1 mRNA expression increased in bovine early luteal cells, but not in mid luteal cells, under hypoxic conditions (3% O2). Development of the bovine early CL is suggested to occur under hypoxic conditions, since HIF1A protein expression is highest at early luteal stage CL than at the other CL stages [23]. The present study also found that glucose is necessary for luteal P4 production, since GLUT1 inhibition resulted in decreased P4 production. These findings suggest that, in early CL tissue, hypoxic conditions induce HIF1A expression, which induces GLUT1 expression [40], which in turn increases P4 production, the primary role of the CL. GLUT1 is also suggested to function in the bovine mid CL, since GLUT1 mRNA is expressed in bovine CL throughout the estrous cycle. However, GLUT1 mRNA expression did not increase under hypoxic conditions in mid luteal cells, whereas GLUT1 inhibition was still found to decrease P4 production in mid luteal cells. These results suggest GLUT1 in bovine mid CL is induced by a pathway other than hypoxia, and produces a large amount of P4 needed in the mid CL.
Consistent with the present results, Nishimoto et al. [22] reported high GLUT1 mRNA expression in bovine CL stage I (Days 1–4 after ovulation). However, they also reported high expression in stage II (Days 5–10) and stage III (Days 11–17). The discrepancies may be due to differences in stage classification between the two studies. We classified Days 2–3, as early stage CLs, which could result in excessive expression in the early CL. Our finding of high GLUT1 expression in the early CL is also consistent with the dynamics of HIF1, whose expression is highest at the early luteal stage in bovine CL [23]. However, further analyses of the timings of expression of GLUT1, HIF1 and other proteins, are needed to clarify the sequence of these events.
The dynamics of GLUT1 mRNA expression in CL during the estrous cycle observed in the present study are similar to those of HIF1 as well as VEGF in bovine CL [23]. These results are in agreement with the recent report for canine CL [29]. Similar expression level dynamics of SCL2A1 (GLUT1 gene), HIF1A, and VEGF were demonstrated in canine CL during diestrus, and suggest a functional inter-relationship that results in the formation of the GLUT1 protein [29]. Rather than bovine luteal cells, GLUT1 mRNA has been demonstrated to be induced by hypoxic conditions in several types of cells in several species, including bovine retinal endothelial cells [37], rat fibroblasts [4], mouse mammary epithelial cells [36], rat and ovine placental cells [5], porcine proximal tubule cells [43], and human glioma cells [19]. These results, together with the present findings, suggest that hypoxic conditions in the CL induce HIF1, resulting in the transcription of GLUT1 and VEGF to support the physiological function of CL across species.
Cyclic mono-phosphate (cAMP) was found to stimulate GLUT1 transcription in 3T3-L1 adipocytes following 2–16 hr of culture [14]. This suggests cAMP directly modulates GLUT1 levels in 3T3-L1 adipocytes. cAMP was also found to stimulate the transcriptional activity of the GLUT1 promoter in rat myoblasts and a 33 bp sequence lying 5′ upstream of the transcriptional start site was important for the effects of 8-bromo-cAMP [38]. Ogura et al. [26] also demonstrated by Northern and immunoblot analyses that 8-bromo-cAMP stimulates GLUT1 mRNA and protein expression in a human choriocarcinoma cell line. In the present study, GLUT1 inhibitors, cytochalasin B and STF-31, significantly inhibited P4 production in hCG-stimulated cells, whereas their inhibitory effects were weak in cells without hCG stimulation. hCG binds to the LH/CG receptor which connects adenylate cyclase inside the cells to induce cAMP accumulation for downstream signal transduction [12, 41]. Results from the present study therefore suggest that following LH/CG receptor stimulation GLUT1 expression increases and cAMP accumulates, thereby resulting in increased P4 production by bovine luteal cells.
In bovine CL, P4 is thought to be a luteotropic factor since it exerts a positive feedback loop on its receptor [35] and protects luteal cells from apoptosis [28]. In endometrial cells, P4 has been suggested to directly regulate GLUT1 expression via its own receptor [10, 16], thereby enhancing GLUT1 expression and translocation to the plasma membrane [16]. In the bovine ovary, hypoxic conditions have been suggested to support P4 synthesis during luteinization [6, 7]. Our finding that hypoxic conditions increased GLUT1 mRNA expression suggests that GLUT1 supports P4 synthesis during luteinization in the bovine ovary. It is also possible that P4 enhances GLUT1 mRNA expression via its own receptor, thereby increasing luteal glucose uptake [3] and corresponding with higher steroidogenic outputs during CL establishment.
In conclusion, our results suggest that the hypoxic conditions during early bovine CL development enhance GLUT1 expression via the transcriptional activity of HIF1. GLUT1, in turn, contributes to CL function by supporting P4 production.
Acknowledgments
This research was supported by Grant-in-Aid for Young Scientists (B) (Grant Number JP16K18803) of the Japan Society for the Promotion of Science (JSPS). We are grateful to Dr. Tsuyoshi Yamaguchi and Dr. Tatsufumi Usui of Tottori University for the technical assistance in measuring P4 concentrations. We are also grateful to Dr. Naoki Kitamura of Tottori University for the technical assistance in isolating bovine luteal cells.
REFERENCES
- 1.Amselgruber W. M., Schäfer M., Sinowatz F.1999. Angiogenesis in the bovine corpus luteum: an immunocytochemical and ultrastructural study. Anat. Histol. Embryol. 28: 157–166. doi: 10.1046/j.1439-0264.1999.00195.x [DOI] [PubMed] [Google Scholar]
- 2.Berisha B., Schams D., Kosmann M., Amselgruber W., Einspanier R.2000. Expression and tissue concentration of vascular endothelial growth factor, its receptors, and localization in the bovine corpus luteum during estrous cycle and pregnancy. Biol. Reprod. 63: 1106–1114. doi: 10.1095/biolreprod63.4.1106 [DOI] [PubMed] [Google Scholar]
- 3.Chase C. C., Jr., Del Vecchio R. P., Smith S. B., Randel R. D.1992. In vitro metabolism of glucose by bovine reproductive tissues obtained during the estrous cycle and after calving. J. Anim. Sci. 70: 1496–1508. doi: 10.2527/1992.7051496x [DOI] [PubMed] [Google Scholar]
- 4.Chen C., Pore N., Behrooz A., Ismail-Beigi F., Maity A.2001. Regulation of glut1 mRNA by hypoxia-inducible factor-1. Interaction between H-ras and hypoxia. J. Biol. Chem. 276: 9519–9525. doi: 10.1074/jbc.M010144200 [DOI] [PubMed] [Google Scholar]
- 5.Das U. G., Sadiq H. F., Soares M. J., Hay W. W., Jr., Devaskar S. U.1998. Time-dependent physiological regulation of rodent and ovine placental glucose transporter (GLUT-1) protein. Am. J. Physiol. 274: R339–R347. [DOI] [PubMed] [Google Scholar]
- 6.Fadhillah Y., Yoshioka S., Nishimura R., Okuda K.2014. Hypoxia promotes progesterone synthesis during luteinization in bovine granulosa cells. J. Reprod. Dev. 60: 194–201. doi: 10.1262/jrd.2014-014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fadhillah Y., Yoshioka S., Nishimura R., Yamamoto Y., Kimura K., Okuda K.2017. Hypoxia-inducible factor 1 mediates hypoxia-enhanced synthesis of progesterone during luteinization of granulosa cells. J. Reprod. Dev. 63: 75–85. doi: 10.1262/jrd.2016-068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferrara N., Davis-Smyth T.1997. The biology of vascular endothelial growth factor. Endocr. Rev. 18: 4–25. doi: 10.1210/edrv.18.1.0287 [DOI] [PubMed] [Google Scholar]
- 9.Forsythe J. A., Jiang B. H., Iyer N. V., Agani F., Leung S. W., Koos R. D., Semenza G. L.1996. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol. Cell. Biol. 16: 4604–4613. doi: 10.1128/MCB.16.9.4604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frolova A., Flessner L., Chi M., Kim S. T., Foyouzi-Yousefi N., Moley K. H.2009. Facilitative glucose transporter type 1 is differentially regulated by progesterone and estrogen in murine and human endometrial stromal cells. Endocrinology 150: 1512–1520. doi: 10.1210/en.2008-1081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gabler C., Plath-Gabler A., Killian G. J., Berisha B., Schams D.2004. Expression pattern of fibroblast growth factor (FGF) and vascular endothelial growth factor (VEGF) system members in bovine corpus luteum endothelial cells during treatment with FGF-2, VEGF or oestradiol. Reprod. Domest. Anim. 39: 321–327. doi: 10.1111/j.1439-0531.2004.00517.x [DOI] [PubMed] [Google Scholar]
- 12.Garverick H. A., Smith M. F., Elmore R. G., Morehouse G. L., Agudo L. S., Zahler W. L.1985. Changes and interrelationships among luteal LH receptors, adenylate cyclase activity and phosphodiesterase activity during the bovine estrous cycle. J. Anim. Sci. 61: 216–223. doi: 10.2527/jas1985.611216x [DOI] [PubMed] [Google Scholar]
- 13.Huang L. E., Gu J., Schau M., Bunn H. F.1998. Regulation of hypoxia-inducible factor 1α is mediated by an O2-dependent degradation domain via the ubiquitin-proteasome pathway. Proc. Natl. Acad. Sci. U.S.A. 95: 7987–7992. doi: 10.1073/pnas.95.14.7987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaestner K. H., Flores-Riveros J. R., McLenithan J. C., Janicot M., Lane M. D.1991. Transcriptional repression of the mouse insulin-responsive glucose transporter (GLUT4) gene by cAMP. Proc. Natl. Acad. Sci. U.S.A. 88: 1933–1937. doi: 10.1073/pnas.88.5.1933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kallio P. J., Wilson W. J., O’Brien S., Makino Y., Poellinger L.1999. Regulation of the hypoxia-inducible transcription factor 1α by the ubiquitin-proteasome pathway. J. Biol. Chem. 274: 6519–6525. doi: 10.1074/jbc.274.10.6519 [DOI] [PubMed] [Google Scholar]
- 16.Kim S. T., Moley K. H.2009. Regulation of facilitative glucose transporters and AKT/MAPK/PRKAA signaling via estradiol and progesterone in the mouse uterine epithelium. Biol. Reprod. 81: 188–198. doi: 10.1095/biolreprod.108.072629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kodaman P. H., Behrman H. R.1999. Hormone-regulated and glucose-sensitive transport of dehydroascorbic acid in immature rat granulosa cells. Endocrinology 140: 3659–3665. doi: 10.1210/endo.140.8.6938 [DOI] [PubMed] [Google Scholar]
- 18.Kol S., Ben-Shlomo I., Ruutiainen K., Ando M., Davies-Hill T. M., Rohan R. M., Simpson I. A., Adashi E. Y.1997. The midcycle increase in ovarian glucose uptake is associated with enhanced expression of glucose transporter 3. Possible role for interleukin-1, a putative intermediary in the ovulatory process. J. Clin. Invest. 99: 2274–2283. doi: 10.1172/JCI119403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li S., Wang J., Wei Y., Liu Y., Ding X., Dong B., Xu Y., Wang Y.2015. Crucial role of TRPC6 in maintaining the stability of HIF-1α in glioma cells under hypoxia. J. Cell Sci. 128: 3317–3329. doi: 10.1242/jcs.173161 [DOI] [PubMed] [Google Scholar]
- 20.Livak K. J., Schmittgen T. D.2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔC(T) Method. Methods 25: 402–408. doi: 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- 21.Miyamoto Y., Skarzynski D. J., Okuda K.2000. Is tumor necrosis factor α a trigger for the initiation of endometrial prostaglandin F(2α) release at luteolysis in cattle? Biol. Reprod. 62: 1109–1115. doi: 10.1095/biolreprod62.5.1109 [DOI] [PubMed] [Google Scholar]
- 22.Nishimoto H., Matsutani R., Yamamoto S., Takahashi T., Hayashi K. G., Miyamoto A., Hamano S., Tetsuka M.2006. Gene expression of glucose transporter (GLUT) 1, 3 and 4 in bovine follicle and corpus luteum. J. Endocrinol. 188: 111–119. doi: 10.1677/joe.1.06210 [DOI] [PubMed] [Google Scholar]
- 23.Nishimura R., Okuda K.2010. Hypoxia is important for establishing vascularization during corpus luteum formation in cattle. J. Reprod. Dev. 56: 110–116. doi: 10.1262/jrd.09-162E [DOI] [PubMed] [Google Scholar]
- 24.Nishimura R., Sakumoto R., Tatsukawa Y., Acosta T. J., Okuda K.2006. Oxygen concentration is an important factor for modulating progesterone synthesis in bovine corpus luteum. Endocrinology 147: 4273–4280. doi: 10.1210/en.2005-1611 [DOI] [PubMed] [Google Scholar]
- 25.Nishimura R., Bowolaksono A., Acosta T. J., Murakami S., Piotrowska K., Skarzynski D. J., Okuda K.2004. Possible role of interleukin-1 in the regulation of bovine corpus luteum throughout the luteal phase. Biol. Reprod. 71: 1688–1693. doi: 10.1095/biolreprod.104.032151 [DOI] [PubMed] [Google Scholar]
- 26.Ogura K., Sakata M., Okamoto Y., Yasui Y., Tadokoro C., Yoshimoto Y., Yamaguchi M., Kurachi H., Maeda T., Murata Y.2000. 8-bromo-cyclicAMP stimulates glucose transporter-1 expression in a human choriocarcinoma cell line. J. Endocrinol. 164: 171–178. doi: 10.1677/joe.0.1640171 [DOI] [PubMed] [Google Scholar]
- 27.Okuda K., Miyamoto A., Sauerwein H., Schweigert F. J., Schams D.1992. Evidence for oxytocin receptors in cultured bovine luteal cells. Biol. Reprod. 46: 1001–1006. doi: 10.1095/biolreprod46.6.1001 [DOI] [PubMed] [Google Scholar]
- 28.Okuda K., Korzekwa A., Shibaya M., Murakami S., Nishimura R., Tsubouchi M., Woclawek-Potocka I., Skarzynski D. J.2004. Progesterone is a suppressor of apoptosis in bovine luteal cells. Biol. Reprod. 71: 2065–2071. doi: 10.1095/biolreprod.104.028076 [DOI] [PubMed] [Google Scholar]
- 29.Papa P. C., Sousa L. M., Silva R. S., de Fátima L. A., da Fonseca V. U., do Amaral V. C., Hoffmann B., Alves-Wagner A. B., Machado U. F., Kowalewski M. P.2013. Glucose transporter 1 expression accompanies hypoxia sensing in the cyclic canine corpus luteum. Reproduction 147: 81–89. doi: 10.1530/REP-13-0398 [DOI] [PubMed] [Google Scholar]
- 30.Redmer D. A., Reynolds L. P.1996. Angiogenesis in the ovary. Rev. Reprod. 1: 182–192. doi: 10.1530/ror.0.0010182 [DOI] [PubMed] [Google Scholar]
- 31.Reynolds L. P., Killilea S. D., Redmer D. A.1992. Angiogenesis in the female reproductive system. FASEB J. 6: 886–892. [PubMed] [Google Scholar]
- 32.Reynolds L. P., Grazul-Bilska A. T., Redmer D. A.2000. Angiogenesis in the corpus luteum. Endocrine 12: 1–9. doi: 10.1385/ENDO:12:1:1 [DOI] [PubMed] [Google Scholar]
- 33.Reynolds L. P., Grazul-Bilska A. T., Killilea S. D., Redmer D. A.1994. Mitogenic factors of corpora lutea. Prog. Growth Factor Res. 5: 159–175. doi: 10.1016/0955-2235(94)90003-5 [DOI] [PubMed] [Google Scholar]
- 34.Salceda S., Caro J.1997. Hypoxia-inducible factor 1α (HIF-1α) protein is rapidly degraded by the ubiquitin-proteasome system under normoxic conditions. Its stabilization by hypoxia depends on redox-induced changes. J. Biol. Chem. 272: 22642–22647. doi: 10.1074/jbc.272.36.22642 [DOI] [PubMed] [Google Scholar]
- 35.Schams D., Berisha B.2004. Regulation of corpus luteum function in cattle--an overview. Reprod. Domest. Anim. 39: 241–251. doi: 10.1111/j.1439-0531.2004.00509.x [DOI] [PubMed] [Google Scholar]
- 36.Shao Y., Wellman T. L., Lounsbury K. M., Zhao F. Q.2014. Differential regulation of GLUT1 and GLUT8 expression by hypoxia in mammary epithelial cells. Am. J. Physiol. Regul. Integr. Comp. Physiol. 307: R237–R247. doi: 10.1152/ajpregu.00093.2014 [DOI] [PubMed] [Google Scholar]
- 37.Takagi H., King G. L., Aiello L. P.1998. Hypoxia upregulates glucose transport activity through an adenosine-mediated increase of GLUT1 expression in retinal capillary endothelial cells. Diabetes 47: 1480–1488. doi: 10.2337/diabetes.47.9.1480 [DOI] [PubMed] [Google Scholar]
- 38.Viñals F., Ferré J., Fandos C., Santalucia T., Testar X., Palacín M., Zorzano A.1997. Cyclic adenosine 3′,5′-monophosphate regulates GLUT4 and GLUT1 glucose transporter expression and stimulates transcriptional activity of the GLUT1 promoter in muscle cells. Endocrinology 138: 2521–2529. doi: 10.1210/endo.138.6.5217 [DOI] [PubMed] [Google Scholar]
- 39.Wang G. L., Jiang B. H., Rue E. A., Semenza G. L.1995. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc. Natl. Acad. Sci. U.S.A. 92: 5510–5514. doi: 10.1073/pnas.92.12.5510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wenger R. H.2002. Cellular adaptation to hypoxia: O2-sensing protein hydroxylases, hypoxia-inducible transcription factors, and O2-regulated gene expression. FASEB J. 16: 1151–1162. doi: 10.1096/fj.01-0944rev [DOI] [PubMed] [Google Scholar]
- 41.Williams M. T., Clark M. R., Ling W. Y., LeMaire W. J., Caron M. G., Marsh J. M.1978. Role of cyclic AMP in the actions of luteinizing hormone on steroidogenesis in the corpus luteum. Adv. Cyclic Nucleotide Res. 9: 573–582. [PubMed] [Google Scholar]
- 42.Williams S. A., Blache D., Martin G. B., Foot R., Blackberry M. A., Scaramuzzi R. J.2001. Effect of nutritional supplementation on quantities of glucose transporters 1 and 4 in sheep granulosa and theca cells. Reproduction 122: 947–956. doi: 10.1530/rep.0.1220947 [DOI] [PubMed] [Google Scholar]
- 43.Zapata-Morales J. R., Galicia-Cruz O. G., Franco M., Martinez Y Morales F.2014. Hypoxia-inducible factor-1α (HIF-1α) protein diminishes sodium glucose transport 1 (SGLT1) and SGLT2 protein expression in renal epithelial tubular cells (LLC-PK1) under hypoxia. J. Biol. Chem. 289: 346–357. doi: 10.1074/jbc.M113.526814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhou J., Bievre M., Bondy C. A.2000. Reduced GLUT1 expression in Igf1-/- null oocytes and follicles. Growth Horm. IGF Res. 10: 111–117. doi: 10.1054/ghir.2000.0147 [DOI] [PubMed] [Google Scholar]