ABSTRACT
Marek's disease virus (MDV) is a highly contagious alphaherpesvirus that infects chickens and causes a deadly neoplastic disease. We previously demonstrated that MDV infection arrests cells in S phase and that the tegument protein VP22 plays a major role in this process. In addition, expression of VP22 induces double-strand breaks (DSBs) in the cellular DNA, suggesting that DNA damage and the associated cellular response might be favorable for the MDV life cycle. Here, we addressed the role of DNA damage in MDV replication and pathogenesis. We demonstrated that MDV induces DSBs during lytic infection in vitro and in the peripheral blood mononuclear cells of infected animals. Intriguingly, we did not observe DNA damage in latently infected MDV-induced lymphoblastoid cells, while MDV reactivation resulted in the onset of DNA lesions, suggesting that DNA damage and/or the resulting DNA damage response might be required for efficient MDV replication and reactivation. In addition, reactivation was significantly enhanced by the induction of DNA damage using a number of chemicals. Finally, we used recombinant viruses to show that VP22 is required for the induction of DNA damage in vivo and that this likely contributes to viral oncogenesis.
IMPORTANCE Marek's disease virus is an oncogenic alphaherpesvirus that causes fatal T-cell lymphomas in chickens. MDV causes substantial losses in the poultry industry and is also used in small-animal models for virus-induced tumor formation. DNA damage not only is implicated in tumor development but also aids in the life cycle of several viruses; however, its role in MDV replication, latency, and reactivation remains elusive. Here, we demonstrate that MDV induces DNA lesions during lytic replication in vitro and in vivo. DNA damage was not observed in latently infected cells; however, it was reinitiated during reactivation. Reactivation was significantly enhanced by the induction of DNA damage. Recombinant viruses that lacked the ability to induce DNA damage were defective in their ability to induce tumors, suggesting that DNA damage might also contribute to cellular transformation processes leading to MDV lymphomagenesis.
KEYWORDS: herpesvirus, Marek's disease virus, DNA damage, oncogenesis, viral replication, VP22, cell cycle
INTRODUCTION
Marek's disease (MD) is an oncogenic lymphoproliferative disease caused by Marek's disease virus (MDV), also referred as to Gallid herpesvirus 2. MDV is a member of the Alphaherpesvirinae subfamily, mostly due to its genomic organization. However, MDV shows similarities with gammaherpesviruses, considering its lymphotropic nature and oncogenic properties (1). Infection of susceptible chickens with very virulent MDV strains induces a rapid onset of tumors within 3 to 4 weeks and a high rate of mortality. Intriguingly, MDV and human herpesvirus 6 (HHV-6) integrate their genomes into the telomeres of latently infected cells (2–5), allowing the long-life persistence of the virus in the host. Therefore, MDV is used to assess herpesvirus integration as well as virus-induced lymphomagenesis (6). The MDV life cycle is complex and can be broken down into four phases (7, 8): (i) an early cytolytic phase, corresponding to the replication of MDV in B and T lymphocytes during the first week of infection; (ii) the establishment of a latent infection in CD4+ T lymphocytes between 7 and 10 days postinfection (dpi), during which MDV is thought to integrate its genome into host telomeres; (iii) reactivation of the virus from latently infected cells, which is accompanied by its late replication and continuous shedding of the virus from the feather follicle epithelium; and (iv) the tumorigenic phase, characterized by the transformation of CD4+ T lymphocytes and the development of T-cell lymphoma.
Several viral oncogenes have been identified, such as the latent oncoprotein Meq and the viral telomerase RNA subunit vTR (9–15); however, the exact mechanism leading to lymphoma development remains poorly understood. We recently demonstrated that during lytic replication MDV triggers cell proliferation and subsequently delays the cell cycle in S phase (16). In addition, we showed that the tegument protein VP22 is able to induce S-phase arrest in the absence of other viral proteins. This blockade is associated with a massive onset of double-strand breaks (DSBs). The VP22 tegument protein is encoded by the UL49 viral gene and is part of the MDV virion. VP22 is involved in the cell-to-cell spread of MDV and is essential for MDV replication (17). Beyond its role in MDV replication, VP22 potentially contributes to the establishment of latency and/or transformation by its ability to interact with DNA/histones, to interfere with cell cycle progression, and to mediate DNA damage. Moreover, it was shown that a recombinant MDV expressing a VP22 with a C-terminal green fluorescent protein (GFP) tag is highly attenuated in vivo, suggesting that VP22 plays a role in MDV-induced tumorigenesis (18). In addition, we have previously observed that such a modification of the VP22 C terminus abolishes its ability to modulate the cell cycle and to induce DNA damage upon overexpression of the protein in proliferating cells (16). Of note, the fusion of GFP to the N terminus of VP22 did not affect these properties in vitro and only mildly attenuated the virus in vivo (16, 19).
Chromosomal aberrations and modulation of the DNA damage response (DDR) are commonly encountered during viral infections and are important for the viral life cycle, as reviewed previously (20–26). This has been particularly evidenced in herpesvirus infections, for which the ataxia telangiectasia-mutated (ATM) and ATM- and Rad3-related (ATR) DNA damage pathway proteins play a beneficial role for viral replication (27–31). Effectors of the DDR and DNA repair pathways also facilitate virus maintenance and the establishment of latency (31–33). Moreover, in the case of oncogenic viruses, such as Epstein-Barr virus (EBV) and Kaposi's sarcoma-associated herpesvirus (KSHV), the deregulation of these pathways and the induction of DNA damage are of particular importance since genomic instability promotes the establishment of neoplastic processes (34–40). DNA damage has been previously observed in the blood of chickens infected with uncharacterized field viral strains and diagnosed with MD (41); however, it remained unclear if this damage occurs in lymphocytes and if this is also the case during early infection.
In the present study, we aimed to elucidate the kinetics of DNA damage in MDV infection to determine the role of DNA damage in the MDV life cycle. We demonstrated that DNA breaks accumulate in lytically infected cells in vivo and in vitro but not in latently infected cells. We also showed that DNA damage and/or DDR is actively induced upon MDV lytic replication and reactivation from the latent stage. We demonstrated in vivo, using recombinant viruses, that VP22 is required for the induction of DNA damage. Also, we observed that a recombinant virus that lacked the ability to induce DNA damage is defective in the induction of tumors, suggesting that DNA damage induction might participate in the oncogenicity of MDV.
RESULTS
MDV replication induces double-strand breaks in the host genome.
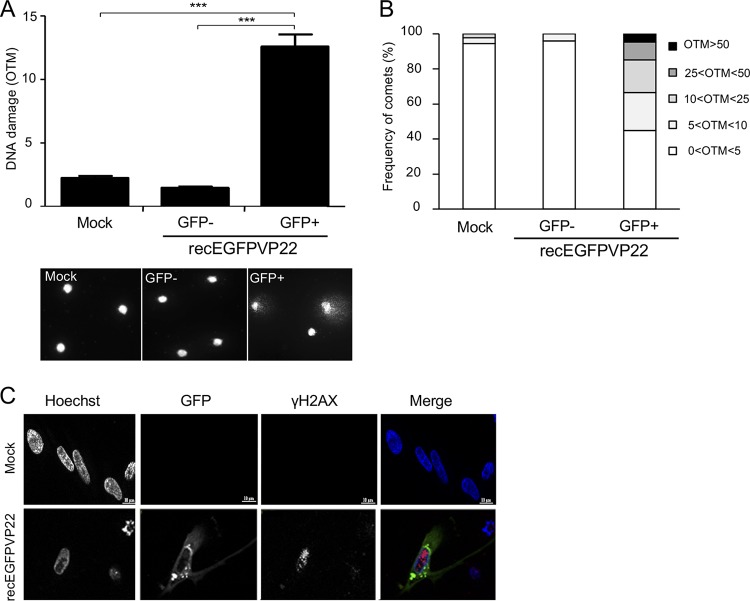
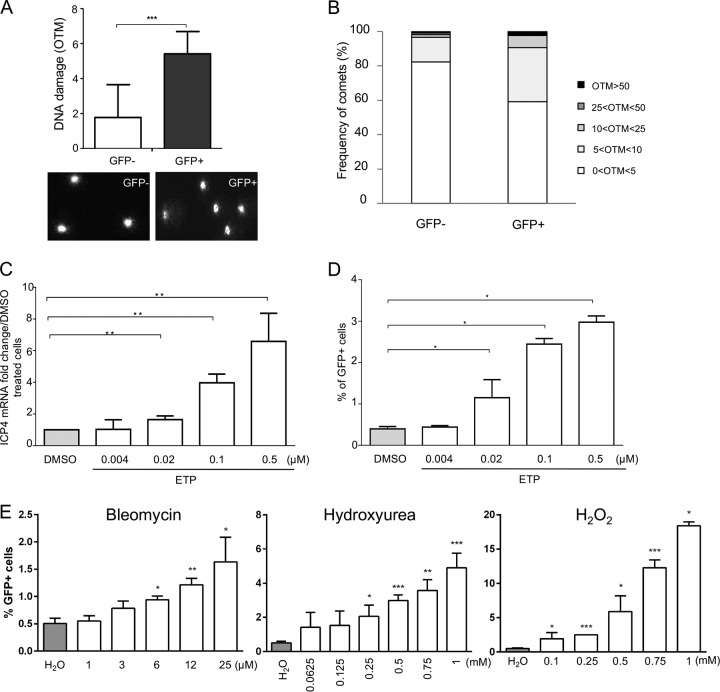
Until now, it remained unknown if MDV induces DNA damage during virus replication. Therefore, we infected primary chicken embryonic skin cells (CESCs) with a recombinant virus containing enhanced GFP (EGFP) fused to the N terminus of VP22 (recEGFPVP22). GFP-positive MDV-infected and GFP-negative cells were sorted by flow cytometry at 96 h postinfection (hpi), and DNA damage was assessed by an alkaline comet assay. MDV infection increased the rate of DNA damage by 8.5-fold and 6-fold compared to that in GFP-negative and mock-infected cells, respectively (Fig. 1A). In addition, up to 30% of the infected cells had an olive tail moment (OTM) score of greater than 10, indicative of highly damaged DNA (Fig. 1B). To identify the nature of the DNA damage in infected cells, we monitored the expression and localization of the phosphorylated form of H2AX (γ-H2AX), a marker classically used to detect double-strand breaks (DSBs). Immunofluorescence analyses revealed a significant increase in the intensity of γ-H2AX and a typical localization of the protein as foci in the nucleus of CESCs infected with recEGFPVP22 (Fig. 1C), indicative of the presence of DSBs.
FIG 1.
Induction of DNA damage in cells lytically infected with MDV. CESCs were infected with 104 PFU of recEGFPVP22. (A) Analysis of DNA damage in mock- or recEGFPVP22-infected CESCs. At 4 dpi, EGFP-positive and -negative cells were sorted by flow cytometry, and DNA damage in 2 × 105 cells was analyzed by alkaline comet assays. Two slides per comet assay were prepared for each condition and analyzed using CometScore software. Results are presented as the mean OTM score ± SD (***, P < 0,001) (top), and representative photographs of comets are shown (bottom). (B) Frequency distribution of the comets with respect to their OTM values. (C) Expression and localization of γ-H2AX in CESCs infected with recEGFPVP22. At 4 dpi, mock- and recEGFPVP22-infected CESCs were subjected to immunofluorescence using a mouse anti-γ-H2AX monoclonal antibody and an Alexa Fluor 594-conjugated secondary antibody (red). Nuclei were stained with Hoechst 33342 (blue), and infected cells expressing EGFP-tagged VP22 were directly visualized by fluorescence microscopy (green).
Induction of DNA damage enhances MDV replication.
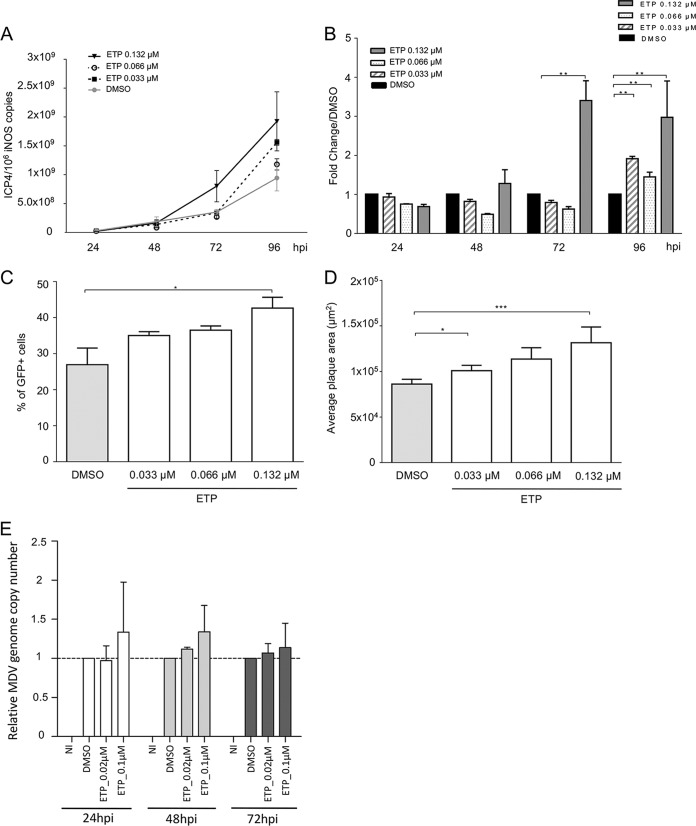
To determine if the induction of DNA damage and/or the subsequent DDR is beneficial for MDV replication, we infected CESCs with recEGFPVP22 in the presence or absence of the potent DSB inducer etoposide (ETP) (42) and monitored MDV replication by real-time quantitative PCR (qPCR) (Fig. 2A and B). At 96 hpi, the level of MDV replication in infected cells in the presence of ETP was significantly increased compared to that in dimethyl sulfoxide (DMSO)-treated control cells, and the greatest increase was observed at the highest ETP concentration (Fig. 2A and B). To confirm that the observed effect was indeed due to the induction of DNA damage, we also tested a number of pharmacological agents known to generate single-strand breaks, DSBs, replicative stress, and/or oxidative stress (bleomycin, hydroxyurea [HU], and H2O2). As for ETP treatment, CESCs were infected with recEGFPVP22 and treated with these drugs at different concentrations, and the MDV copy number was assayed by qPCR (Table 1). The overall effect of the DNA-damaging agents tested was more moderate than that of ETP on MDV-infected cells, although all reagents tended to increase slightly the level of viral replication, underlining that DNA damage enhances MDV replication. In addition, we could demonstrate that ETP increases the percentage of viable GFP-VP22-expressing cells compared to that of DMSO-treated control cells in a dose-dependent manner (Fig. 2C). This increase in infected cells was significant for the highest ETP concentrations. Furthermore, MDV plaques were also significantly larger upon induction of DNA damage (Fig. 2D), indicating that the virus spread more efficiently to surrounding cells. Beyond that, we also assessed the effect of ETP treatment on MDV replication in T cells, the natural target of MDV infection. RECC-CU91 T cells were infected with strain RB-1B_TK-GFP in the presence of ETP, and the MDV copy number was monitored by qPCR (Fig. 2E). The impact of ETP treatment on MDV replication in T cells was more mitigated than that in CESCs; however, MDV genome copy numbers slightly increased from 1 dpi when the cells were treated with the highest ETP concentration.
FIG 2.
DNA damage induction enhances MDV replication. (A to D) CESCs were infected with recEGFPVP22 and treated at 6 hpi with etoposide (ETP), bleomycin, hydroxyurea (HU), and H2O2 at the indicated concentrations or with DMSO or H2O (as negative controls). (A and B) At 24, 48, 72, and 96 hpi, DNA was extracted from cells treated with ETP and MDV replication was assessed using qPCR. For each group, the number of MDV genome copies (corresponding to the ICP4 copy number) was normalized to 106 cells (estimated by the iNOS copy number). (A) Representative growth curve from a total of 3 independent experiments. Means ± SDs from triplicate qPCRs are indicated. (B) Fold change in the number of MDV copies in ETP-treated cells relative to the value for DMSO-treated cells. **, P < 0.05. (C) Number of cells lytically infected with MDV upon ETP treatment. The percentage of viable GFP-positive infected cells was determined at 96 hpi by fluorescence-activated cell sorting. Viable cells were detected using the viability dye eFluor 780. Means ± SDs are represented as bars. *, P < 0.05. (D) Effect of ETP on MDV plaque size. Images of fluorescent MDV plaques were taken and plaque sizes were measured at 48 hpi. Means ± SDs are presented as histograms. *, P < 0.05; ***, P < 0.001. (E) Impact of ETP-induced DNA damage on MDV replication in RECC-CU91 T cells. RECC-CU91 cells were infected with strain RB-1B_TK-GFP and treated with 0.02 and 0.1 μM ETP or with DMSO (as a negative control). At 24, 48, and 72 hpi, the MDV genome copy number was quantified by qPCR, and the data are shown as the mean fold change ± SD relative to the value for DMSO-treated cells. NI, noninfected.
TABLE 1.
Effect of DNA damage inducers on MDV replication in CESCs
| Treatment | Concn (μM) | Mean level of ICP4 expression ± SD (108)/106 iNOS copies |
|---|---|---|
| H2O | 1.46 ± 0.22 | |
| Bleomycin | 0.125 | 1.33 ± 0.05 |
| 0.25 | 2.25 ± 0.04 | |
| 0.5 | 2.16 ± 0.14 | |
| 1 | 2.47 ± 0.33 | |
| Hydroxyurea | 10 | 2.55 ± 0.03 |
| 25 | 2.6 ± 0.005 | |
| 50 | 1.76 ± 0.03 | |
| 75 | 1.83 ± 0.25 | |
| H2O2 | 12.5 | 1.91 ± 0.009 |
| 25 | 1.6 ± 0.03 | |
| 50 | 1.38 ± 0.05 | |
| 100 | 2.12 ± 0.23 |
Taken together, our data demonstrate that additional induction of DNA damage and/or the subsequent DDRs are beneficial for MDV replication.
MDV replication induces ROS and NO production in CESCs.
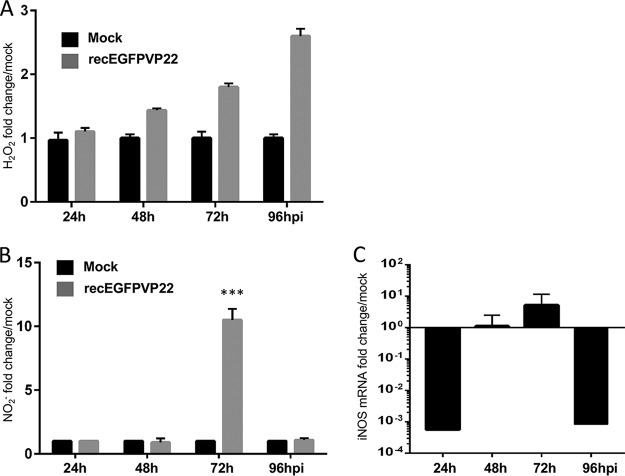
Next we assessed if MDV-induced DNA damage is mediated by oxidative stress, a common cause of DSBs (43). We first monitored the level of reactive oxygen species (ROS) by measuring the production of hydrogen peroxide (H2O2) in the supernatant of MDV- or mock-infected cells (Fig. 3A). A significant accumulation of H2O2 was detected in the supernatant of infected cells from 48 to 96 hpi. Besides ROS, reactive nitrogen species, such as nitric oxide (NO), also play a role in metabolic stress and oxidative DNA damage in cells (44). We measured the level of NO production in the supernatant of infected and mock-infected cells and observed a significant increase in the amount of NO at 72 hpi (Fig. 3B). Intriguingly, this increase in the level of NO production coincided with an increase in the level of expression of iNOS at 72 hpi (Fig. 3C). Our data suggest that MDV infection is associated with an increased level of reactive oxygen and nitrogen species that could contribute to the DNA damage in infected cells.
FIG 3.
MDV replication induces production of ROS and NO. CESCs were mock infected or infected with recEGFPVP22. (A) ROS accumulation in the supernatants of mock- and recEGFPVP22-infected cells. At 24, 48, 72, and 96 hpi, the supernatants of mock- and recEGFPVP22-infected cells were collected, and the level of H2O2 accumulation was quantified using an ROS-Glo kit (Promega). Results were normalized to the RLU values obtained from mock-infected cells and are expressed as means ± SDs. (B) NO production in the supernatants of mock- and recEGFPVP22-infected cells. At the indicated time points, the supernatants of mock- and recEGFPVP22-infected cells were collected, and nitrite accumulation was quantified using the Griess reaction. Results are presented as the mean fold change in the level of NO2− in the supernatants of infected cells relative to that in the supernatants of mock-infected cells ± SDs. ***, P < 0.001. (C) Expression of inducible nitric oxide synthase (iNOS) in MDV-infected cells. Total mRNA was isolated from mock- and MDV infected CESCs at the indicated time points, and qRT-PCRs were performed with iNOS-specific primers. Results were normalized to the level of GAPDH expression and are expressed as the mean fold change in iNOS mRNA expression compared to that in mock-infected cells ± SD.
DNA damage induction in chicken PBMCs upon MDV infection.
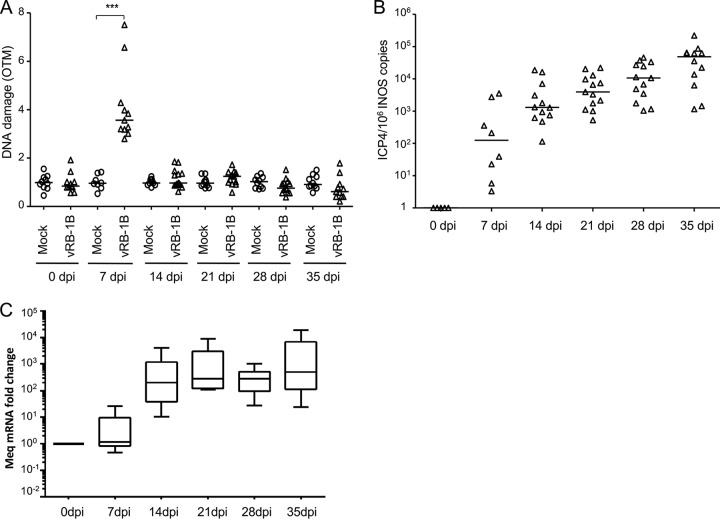
To determine if MDV induces DNA lesions in vivo, we infected chickens with the very virulent MDV strain vRB-1B. Blood was collected from all animals at various time points. The viral load was assessed by qPCR on whole blood, and DNA damage was determined using the alkaline comet assay on peripheral blood mononuclear cells (PBMCs). In addition, the establishment of viral latency was followed by analysis of the expression of Meq mRNA in noninfected and vRB-1B-infected PBMCs by quantitative reverse transcription-PCR (qRT-PCR). Intriguingly, DNA damage was significantly increased (about 3-fold higher) in the PBMCs of infected chickens during the lytic phase of the MDV life cycle at 7 dpi (Fig. 4A). In contrast, no increase in the number of DNA lesions was observed in the latent phase of infection after 14 dpi. The increased amount of DNA damage was associated with a 100-fold increase in the viral load in the blood during the lytic phase of infection (Fig. 4B). After day 14, high levels of the major oncogene Meq, corresponding to latently infected or MDV-transformed cells, were detected by qRT-PCR (Fig. 4C), a finding in agreement with previous reports on the establishment of latency (45, 46). Thus, our data show that MDV early lytic infection is associated with transient DNA damage in PBMCs, while no DNA damage was detected at later stages of infection.
FIG 4.
Induction of DNA damage in PBMCs of chickens infected with MDV. Specific-pathogen-free (SPF) susceptible White Leghorn chicks (B13/B13 haplotype) were inoculated intramuscularly with 1,000 PFU of the very virulent MDV strain vRB-1B. DNA damage onset in PBMCs from 10 noninfected chickens (circles) and 13 birds infected with vRB-1B (triangles) was assessed. Blood was collected from all birds at the indicated time points. (A) Analysis of DNA damage in PBMCs isolated from mock- and vRB-1B-infected chickens by alkaline comet assays. Two slides per comet assay were prepared for each animal at each time point. A minimum of 50 comets were observed and further analyzed on each replicate slide using CometScore software. Results are presented as a dot plot, with each dot representing an animal and the mean OTM for each group being indicated as a bar. ***, P < 0.001. (B) Viral load estimated after extraction of DNA from whole blood and quantification of MDV genome copies using qPCR. For each group, the number of ICP4 copies in the MDV genome was normalized to 106 copies of the cellular genome, estimated by the detection of iNOS copies. The median copy numbers are indicated as bars. (C) Meq mRNA expression upon MDV infection. Total RNA was extracted from PBMCs isolated from the blood of birds infected with vRB-1B. Quantitative RT-PCRs were performed in order to detect the expression of Meq mRNA. The level of gene expression was normalized to that of GAPDH expression, and fold changes are presented as box plots (minimum and maximum).
VP22 contributes to DNA damage upon MDV infection in vivo.
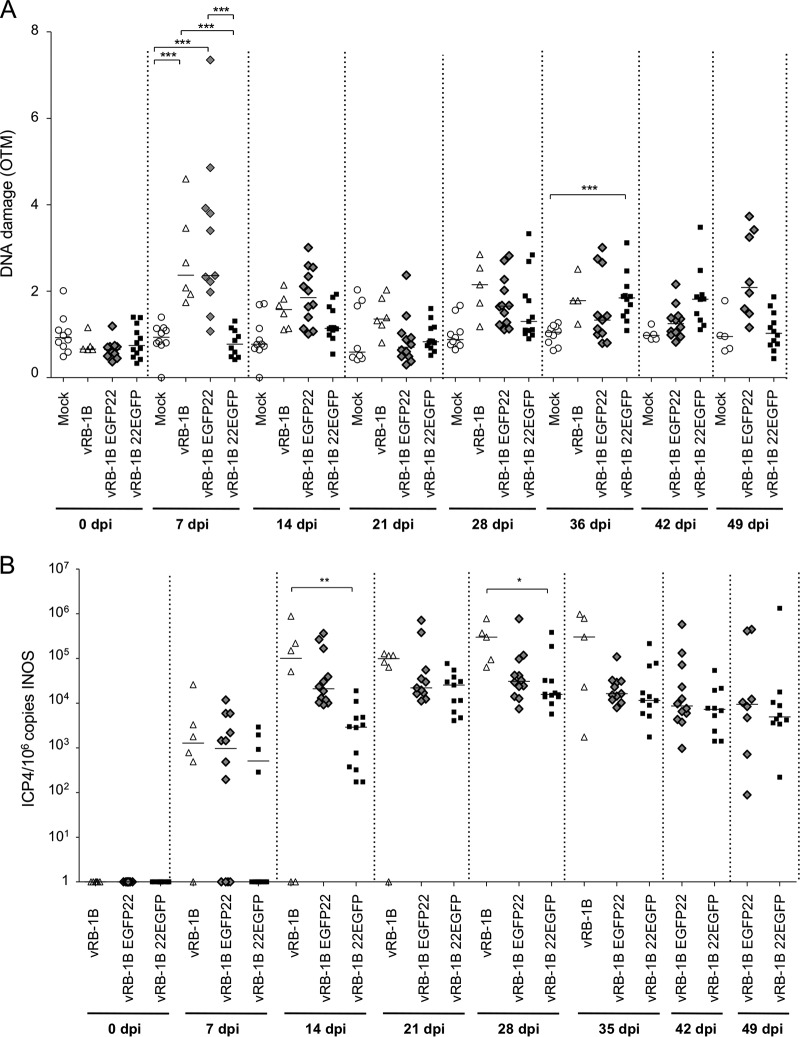
We previously demonstrated that overexpression of VP22 arrests the cell cycle and induces DNA damage in vitro (16). In addition, we showed that MDV harboring EGFP fused to the C terminus of VP22 (vRB-1B 22EGFP) is severely attenuated, while fusion to the N terminus of VP22 (vRB-1B EGFP22) induces only a mild decrease in oncogenicity (18, 19), suggesting that the C-terminal fusion affects VP22 function. Based on this observation, we assessed the induction of DNA damage mediated by these recombinant viruses in vivo. We infected specific-pathogen-free (SPF) chickens with either wild-type vRB-1B, vRB-1B 22EGFP, or vRB-1B EGFP22 and monitored DNA damage, virus replication, and tumor development. Intriguingly, DNA damage at day 7 was observed only in the PBMCs of birds infected with wild-type vRB-1B and vRB-1B EGFP22, suggesting that the fusion of GFP to the N terminus of VP22 does not affect DNA damage induction (Fig. 5A). In contrast, the levels of DNA damage in the PBMCs of vRB-1B 22EGFP-infected animals was comparable to those in the PBMCs of mock-infected chickens, indicating that fusion of GFP to the C terminus of VP22 disrupts its ability to mediate DNA lesions. To ensure that this effect was not just due to reduced virus replication, we monitored the virus load in PBMCs by qPCR and could demonstrate that virus replication was only mildly reduced at day 7. In contrast, a significant decrease in the vRB-1B 22EGFP load compared with the parental virus load was observed at 14 and 28 dpi (Fig. 5B). Since DNA damage also plays an important role in cancer development, we also monitored the tumor incidence in the infected chickens. As observed previously, tumor formation was severely impaired in chickens infected with vRB-1B 22EGFP (31%), which cannot induce DNA damage, while tumors were efficiently induced by wild-type vRB-1B (100%) and vRB-1B EGFP22 (66%). Our data show that DNA damages are dependent on a functional VP22 and that tumor formation is severely impaired for a virus that cannot induce DNA damage.
FIG 5.
Role of VP22 and DNA damage in MDV-mediated oncogenicity in chickens. SPF White Leghorn chicks were inoculated with 1,500 PFU of vRB-1B, vRB-1B EGFP22, or vRB-1B 22EGFP. Blood was collected from all birds at the indicated time points, and PBMCs were isolated. (A) DNA damage was quantified from 2 × 105 PBMCs using the alkaline comet assay. Results are presented as dot plots, with each dot representing an animal. For each group, the median OTM is indicated as a bar, ***, P < 0.001. (B) The MDV viral load was evaluated by qPCR on DNA extracted from PBMCs. The number of MDV copies per 106 cells is presented as a dot plot, with each dot representing an animal. For each group, the median is indicated as a bar. *, P < 0.05; **, P < 0.005.
MDV reactivation is accompanied and enhanced by DNA damage.
Next, we set out to determine if DNA damage is induced upon MDV reactivation. We used a lymphoblastoid cell line that expresses GFP fused to the tegument protein UL47 upon reactivation (3867K cells), as described previously (47). We sorted EGFP-positive and -negative cells and assessed DNA damage by comet assays. DNA damage was significantly increased in reactivating (GFP-positive) cells compared to the latent, GFP-negative cells (Fig. 6A), indicating that MDV reactivation in T cells is associated with DNA damage. An increased proportion of reactivating cells (8%) also showed high levels of DNA damage (OTM > 10), while only minimal damage was seen in latently infected cells (Fig. 6B). Next, we evaluated if DNA damage could also increase reactivation. We induced DNA damage in 3867K cells with increasing concentrations of ETP for 48 h. Both ICP4 expression and the number of GFP-expressing cells were significantly increased in a dose-dependent manner (Fig. 6C and D). To confirm the effect of DNA damage on MDV reactivation, we treated 3867K cells with bleomycin, HU, and H2O2. MDV reactivation was significantly increased in a dose-dependent manner for all three DNA damage-inducing drugs (Fig. 6E). Taken together, our data demonstrate that DNA damage is induced upon MDV reactivation and that induction of DNA damages seems to be favorable for MDV reactivation.
FIG 6.
DNA damage during MDV reactivation. 3867K cells undergoing MDV lytic replication were sorted by cytometry on the basis of the expression of the UL47 gene tagged with EGFP. (A) DNA damage analysis in lytically infected (GFP-positive) and latently infected (GFP-negative) cells. The alkaline comet assay was performed on EGFP-positive and -negative sorted cells. Results are presented as the mean OTM ± SD (***, P < 0.001) (top), and representative comet images are shown (bottom). (B) Frequency distribution of the comets with respect to their OTM values. (C to E) Effect of DNA-damaging pharmacological agents on MDV reactivation. 3867K cells were treated with etoposide (ETP), bleomycin, hydroxyurea (HU), or H2O2 at the indicated concentrations for 48 h. DMSO and H2O were added to the culture media as negative controls. (C) MDV replication was evaluated by quantifying the expression of mRNA for the immediate early gene ICP4 by qRT-PCR. The level of ICP4 expression was normalized to the level of expression of GAPDH, and results are presented as means ± SDs. **, P < 0.005. (D and E) Number of 3867K cells in which MDV was reactivated. The percentage of viable GFP-positive cells (expressing the EGFP-tagged UL47 protein) was determined by cytometry 48 h posttreatment. Viable cells were labeled using the viability dye eFluor 780. Means ± SDs are represented as bars. *, P < 0.05. Results are representative of those from 3 independent experiments realized in triplicate.
DISCUSSION
The hallmark of the present study is the observation of an onset of DNA lesions in cells sustaining MDV replication in vitro and in vivo. This was initially shown in vitro in MDV-infected CESCs, in which we detected DNA DSBs at 96 hpi. In vivo, we demonstrated that MDV early cytolytic replication is associated with an increase in DNA damages in the PBMCs of infected chickens early after infection (7 dpi). Moreover, we showed in vitro that lymphoblastoid cells (3867K cells) undergoing MDV replication induced from the spontaneous reactivation of the virus are also affected by DNA damage. Of note, the DNA damage sustained during MDV reactivation in the PBMCs of birds infected with the highly virulent RB-1B strain was not statistically significantly different from that in the PBMCs of mock-infected birds at 21 dpi, the time point at which a peak of viral reactivation is expected. This might be due to the fact that only a small number of CD4+ T cells reactivate in the blood and due to the low sensitivity of the comet assay.
Intriguingly, DNA lesions were detected at 7 dpi in the PBMCs of chickens infected with the vRB-1B or vRB-1B EGFP22 virus but not in the PBMCs of chickens infected with the attenuated vRB-1B 22EGFP virus, even though all 3 viruses showed similar robust viral DNA replication, as was assessed from the qPCR results. This observation may indicate that MDV replication is not sufficient to induce DNA breaks. However, at 14 and 28 dpi the attenuated vRB-1B 22EGFP virus displayed a replication rate lower than that of the wild-type vRB-1B virus. We could assume that this growth defect might be associated with the low rate of DNA lesions occurring during the early replication of the virus (at 7 dpi) and, thus, that DNA damage might be favorable for MDV replication.
These observations also confirmed that VP22 is a major viral determinant associated with DNA damages in vivo. The VP22 tegument protein is abundantly expressed during viral lytic infection and essential for MDV replication (17, 48). In a previous study, we also reported that the overexpression of VP22 leads to DSB induction in proliferating cells and that this activity of VP22 depends on an unmodified C-terminal extremity (16). Herein, we show in vivo, in an infectious context, that VP22 is involved in the induction of DNA lesions observed during MDV early cytolytic infection and that the modification of the C-terminal extremity of the protein subverted the ability of MDV to trigger DNA damage in PBMCs. It should also be noted that the level of DNA lesions detected at 7 dpi in PBMCs from infected birds was somewhat surprising, given the low number of circulating infected cells, and seems to indicate that noninfected cells might also be subjected to DNA damage. The lesions observed in the noninfected population could be attributed to the inflammatory immune response and/or paracrine signaling molecules that are emitted from infected cells and responsible for a bystander effect (49–51). Also, despite conflicting reports about the intercellular trafficking property of the VP22 protein, we cannot exclude the possibility that VP22 could spread to noninfected surrounding cells and contribute to the generation of DNA lesions in these cells (52–54).
The mechanism by which VP22 is involved in the onset of DNA lesions is still unclear. MDV VP22 could have direct genotoxic activity on DNA, since VP22 can interact with DNA and histones (16, 48), or could activate cellular metabolism pathways leading to DNA damage. In support of the latter hypothesis, we showed that MDV infection triggers oxidative stress in CESCs. We detected an increase in the level of hydrogen peroxide production from 48 hpi in CESCs infected with MDV. In addition, a higher level of nitrites associated with an increase in the level of iNOS mRNA expression was detected at 72 hpi in the supernatant of MDV-infected CESCs than in the supernatant of mock-infected cells. Previous studies reported that MDV infection influences the production of NO (41, 55). A correlation between the virulence of MDV strains and their ability to induce NO was notably established, with the most virulent strains inducing the highest level of NO (55). It is believed that NO plays a role in MDV pathogenesis through its involvement in the immune suppression observed early after infection (55). Nevertheless, the role of NO on MDV replication is still not clearly elucidated. NO production was identified as an antiviral process by inhibiting MDV replication in vitro and in vivo (56, 57). However, Jarosinski et al. demonstrated that despite a strong NO response, chickens infected with very virulent MDV strains showed an enhanced cytolytic infection (55).
Oxidative stress is a major generator of DNA breaks, including DSBs (58). We could thus hypothesize that the oxidative stress generated during MDV replication could participate in the generation of DNA lesions, which in turn would facilitate MDV replication and, consequently, potentiate the virulence of MDV. We have indeed demonstrated that DNA damage seems to favor the replication of the virus, since we have shown that DNA-damaging pharmacological agents can promote MDV replication and enhance MDV reactivation from latent infection. Of note, the impact on MDV replication seemed to depend on the drug used and thus probably on the nature of the damages generated and/or the associated DNA damage response (DDR).
The response to different treatments might also vary between cells according to their lineage (lymphoid, fibroblastic) and their proliferative potential (primary versus cell lines). Hence, ETP treatment (inducing mainly DSBs) resulted in an increase in the level of MDV replication in CESCs, while bleomycin, hydroxyurea, and H2O2 treatments had a weaker effect on MDV replication in CESCs. One explanation might be that the low proliferative rate of CESCs may counteract the activity of drugs inducing damage during S phase. ETP also had a mild effect on MDV replication in the RECC-CU91 T-cell line. Also, RECC-CU91 cells were initially transformed with reticuloendotheliosis virus (REV); we could thus hypothesize that the presence of a replicative retrovirus might disturb the DNA damage responses in these cells and/or have an impact on MDV replication. On the other hand, all treatments induced a significant increase in the level of MDV reactivation in a lymphoid cell line transformed by MDV. We cannot currently specify whether DNA damages only or the induction of the DDR associated with the onset of damage promotes MDV replication.
As previously demonstrated for a number of viruses and especially herpesviruses, the DDR plays a major role in viral replication (for reviews, see references 20, 22 to 24, 26, and 59). Unfortunately, we were not able to characterize more precisely the DDR pathways induced during MDV infection in the present study due to a lack of specific tools cross-reacting with chicken proteins. Nevertheless, we hypothesized that MDV infection induces the activation of a DDR as it was demonstrated for other herpesviruses. We identified at least two processes that could contribute to DDR pathway activation: (i) the generation of DNA lesions in cellular DNA triggered upon MDV replication and (ii) the increase in oxidative stress in MDV-infected CESCs. Elevated levels of ROS are known to activate DDR pathways, as demonstrated, notably, upon EBV infection, in which the latent protein EBNA1 promotes ROS accumulation and, consequently, ATM-dependent DDR activation (34). Moreover, our previous study demonstrated that MDV induces S-phase arrest in fibroblasts (16). This constitutive S-phase induction may generate a favorable environment for viral replication but could also lead to replicative stress, a potent mechanism responsible of DDR induction. Although we currently cannot determine the DDR pathways activated by MDV, we can speculate that ATM signaling may be induced in response to the DSBs and to the oxidative stress generated during MDV infection (60, 61). Of note, ATM pathway activation seems to be a common characteristics of herpesvirus infections (as previously reported notably for human herpes simplex virus 1 [HSV-1], cytomegalovirus [CMV], EBV, and KSHV), and in most cases, ATM has been demonstrated to be beneficial for viral replication (28, 62–64).
Finally, a major point of interest of the present study is the potential involvement of the onset of DNA lesions in MDV-induced lymphomagenesis. Many reports have shown that DNA damage and the DDR can contribute to genomic instability in cells and, in turn, to the development of tumors. Moreover, DSBs are among the most deleterious lesions occurring in cells, and if they are not repaired, the accumulation of DSBs can promote cell death or the loss of genome integrity, possibly leading to carcinogenesis (65). Several studies demonstrated the interplay between oncogenic viruses and DNA damage/DDR signaling and its crucial implication in virus-mediated tumorigenesis (for reviews, see references 35, 39, and 40). Our data support these findings, since we observed a higher rate of DNA damage in PBMCs from chickens infected with virulent MD viruses (vRB-1B and vRB-1B EGFP22) and, to a significantly lesser extent, in PBMCs from birds infected with an attenuated recombinant virus (vRB-1B 22EGFP). The low oncogenicity of the vRB-1B 22EGFP virus might well be associated with its diminished replication capacity (as observed at day 14 pi). However, in the light of our results, one can also speculate that the viral phenotype is due to the absence of an early onset of DNA lesions in infected leukocytes that otherwise might contribute to MDV-induced tumorigenesis. One hypothesis would be that the enhanced MDV replication could result in an increased number of latently infected cells from which transformed T cells originate. DNA damage could also play a direct role in the establishment of viral latency and, more precisely, in the process of integration of the viral genome. We indeed cannot exclude the possibility that the DNA lesions observed at an early time point of MDV infection could arise before or concomitantly with MDV latent infection in CD4+ T lymphocytes and thus could facilitate MDV genome integration either directly or indirectly by triggering DDR and DNA repair pathways, especially homologous recombination, as it was previously suggested for hepatitis B virus, human papillomaviruses, Merkel cell polyomavirus, and EBV (2, 3, 66, 67). However, one should not underestimate the impact of a potential genomic instability originating from the DNA damage generated during MDV replication. It is indeed conceivable that, in a particular sequence of events, including cell cycle deregulation, reprogramming of gene expression by viral oncogenes (notably Meq), and telomerase activation, the accumulation of DNA lesions upon MDV infection may also contribute to the transformation process, ultimately leading to MD lymphoma formation.
MATERIALS AND METHODS
Cells and viruses.
Primary chicken embryonic skin cells (CESCs) were prepared from 12-day-old specific-pathogen-free (SPF) White Leghorn (LD1) chicken embryos and maintained in culture as previously described (48). The MDCC-3867K cell line was derived from a renal lymphoma induced upon infection of a chicken with the highly pathogenic recombinant vRB-1B 47EGFP virus encoding the UL47 gene fused to the enhanced green fluorescent protein (EGFP) (47). 3867K cells were cultured in RPMI 1640 supplemented with 2 mM glutamine, 1% pyruvate, 1% nonessential amino acids, 1% glucose, 10% tryptose phosphate broth, and 10% fetal bovine serum (FBS) and maintained at 41°C in a 5% CO2 atmosphere. RECC-CU91 T cells, a reticuloendotheliosis virus (REV)-transformed chicken T-cell line, were cultured in RPMI 1640 supplemented with 1% pyruvate, 1% nonessential amino acids, 10% FBS, and penicillin-streptomycin and maintained at 41°C in a 5% CO2 atmosphere (68).
To visualized virus-infected cells, EGFP was fused to the 5′ end of the UL49 gene in the avirulent BAC20 strain, resulting in recEGFPVP22 (69). Very virulent, spread-competent vRB-1B virus was reconstituted from the infectious bacterial artificial chromosome (BAC) of the RB-1B strain as described previously (70). In addition, recombinant vRB-1B viruses that had previously been generated with EGFP fused to the 5′ and 3′ ends of VP22, termed vRB-1B EGFP22 and vRB-1B 22EGFP, respectively, were used (18, 19). All recombinant viruses were reconstituted, propagated, and titrated as described previously (69).
Infections of RECC-CU91 T cells were performed by cocultivation with infected CESCs (71). One million CESCs were infected with 3 × 104 PFU of RB-1B_TK-GFP, which expresses GFP under the control of the early HSV-1 thymidine kinase (TK) promoter for 3 to 4 days in 6-well plates. Subsequently, 106 RECC-CU91 T cells were added to the highly infected CESC monolayer for 16 h at 41°C. The RECC-CU91 cells were carefully removed at days 1, 2, and 3 postinfection for further analysis.
Pharmacological induction of DNA damages.
DNA damage was induced in cells by etoposide (ETP; Sigma-Aldrich), bleomycin (Calbiochem), hydroxyurea (HU; Sigma-Aldrich), and hydrogen peroxide (H2O2; Sigma-Aldrich) treatments. At 6 h postinfection, CESCs infected with recEGFPVP22 were treated by addition to the culture medium of the pharmacological agents at the appropriate concentration (0.033, 0.066, or 0.132 μM ETP; 0.125 to 1 μM bleomycin; 10 to 75 μM HU, and 12.5 to 100 μM H2O2). ETP-treated infected cells were analyzed at 24, 48, 72, and 96 h postinfection, and bleomycin-, hydroxyurea-, and hydrogen peroxide-treated cells were analyzed at 72 h postinfection. RECC-CU91 cells were treated at the time of infection with 0.02 μM or 0.1 μM ETP and treated for 24, 48, and 72 hpi. Treatments of 3867K cells were performed for 48 h with 0.004 to 0.5 μM ETP, 1 to 25 μM bleomycin, 0.0625 to 1 mM HU, and 0.1 to 1 mM H2O2. In all experiments, DMSO (for ETP) or H2O (for bleomycin, HU, and H2O2) was used as a negative control and was added to the culture medium at a volume equivalent to that used for the highest concentration of the drug treatments.
Animal experiments.
Two in vivo experiments were carried out in strict compliance with the French legislation for animal experiments and ethics and approved by the local ethics committee (Comité d'Ethique pour l'Expérimentation Animale de Val de Loire [CEEA VdL] protocol number 2012-09-3). In the first experiment (Fig. 4), 24-day-old SPF White Leghorn chicks (B13/B13 haplotype) were infected intramuscularly (pectoral muscles) with 1,000 PFU of vRB-1B (n = 13) or mock infected (n = 10) and housed in isolation units. In the second experiment (Fig. 5), chickens were infected with either 1,500 PFU of vRB-1B (n = 6), vRB-1B EGFP22 (n = 12), or vRB-1B 22EGFP (n = 13) or mock infected (n = 10) as described above. Of note, in order to inoculate an equal amount of virus into the chickens, the vRB-1B EGFP22 and vRB-1B 22EGFP viral inocula were exclusively constituted of EGFP-positive sorted CESCs (i.e., infected cells). Birds were evaluated daily for symptoms of MD. In the case of clinical evidence of MD, the chickens were euthanized and examined postmortem for the presence of gross MD lesions. At the end of the experiments (35 dpi in experiment 1 and 49 dpi in experiment 2), all surviving birds were euthanized and necropsied. To assess the DNA damage in peripheral blood mononuclear cells and to follow the viral load, blood was collected from all birds at 0, 7, 14, 21, 28, and 35 dpi for experiment 1 and 0, 7, 14, 21, 28, 35 42, and 49 dpi for experiment 2 and placed in tubes with 3% sodium citrate. PBMCs were isolated from 1 ml of whole blood using lymphocyte separation medium (LSM; Eurobio, France) as previously described (19).
DNA extraction and qPCR.
To quantify the viral load, DNAs were extracted from 30 μl whole blood (animal experiment 1) or from 2 × 106 isolated cells (PBMCs in animal experiment 2 and CESC and RECC-CU91 cells) using a QIAamp DNA minikit according to the manufacturer's instructions (Qiagen). MDV genome copies were quantified by real-time quantitative PCR (qPCR) as previously described (19, 55). The MDV genome was detected using primers and probes against the ICP4 gene, and the amount was normalized to 106 copies of the cellular genome, quantified by detection of the iNOS gene.
RNA extraction and qRT-PCR.
RNAs were extracted from 106 noninfected and recEGFPVP22-infected CESCs, PBMCs isolated from vRB-1B-infected chickens, and 3867K cells, using an RNeasy minikit following the manufacturer's instructions (Qiagen). RNAs were treated with RNase-free RQ1 DNase (Promega, France), and the RNA concentration was measured with a NanoDrop spectrophotometer. One microgram of total RNA was reverse transcribed using 100 μg/ml oligo(dT) primers (Promega) and Moloney murine leukemia virus reverse transcriptase (Promega). The expression of the genes of interest was then assessed by quantitative reverse transcription-PCR (qRT-PCR) using Supermix SYBR green (Bio-Rad) as previously described (16). The sequences of the specific primer pairs used for the amplification of the viral and cellular genes are depicted in Table 2. Expression of the chicken glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene was used for normalization, and the relative changes in gene expression were determined by the 2−ΔΔCT threshold cycle (CT) method.
TABLE 2.
Primer pairs used for qRT-PCR and qPCR
| Target | Orientationa | Sequence | GenBank accession no. |
|---|---|---|---|
| ICP4 | For | 5′-TTTCTAGCAAGGAGCGACGC-3′ | NC_002229.3 |
| Rev | 5′-CTGACTTGCGCTTACGGGAA-3′ | ||
| Meq | For | 5′-GTCCCCCCTCGATCTTTCTC-3′ | AY571783.1 |
| Rev | 5′-CGTCTGCTTCCTGCGTCTTC-3′ | ||
| iNOS | For | 5′-TACTGCGTGTCCTTTCAACG-3′ | U46504 |
| Rev | 5′-CCCATTCTTCTTCCAACCTC-3′ | ||
| GAPDH | For | 5′-TGATGATATCAAGAGGGTAGTGAAG-3′ | K01458 |
| Rev | 5′-TCCTTGGATGCCATGTGGACCAT-3′ |
For, forward; Rev, reverse.
Cell sorting and flow cytometry analysis.
Sorting of CESCs infected with recombinant viruses expressing fluorescent VP22 proteins was performed at 4 dpi. The 3867K cells exhibiting MDV lytic replication were sorted on the basis of the expression of the UL47 protein tagged with EGFP. Mock-infected CESCs (negative control) were also sorted to avoid an experimental bias linked to sorting. Damaged cells and debris were eliminated on the basis of morphological criteria. EGFP-positive and -negative cells were sorted using a MoFlo high-speed cell sorter (Beckman Coulter, Fort Collins, CO, USA).
The percentage of lytically infected 3867K and RECC-CU91 T cells was determined by cytometry on the basis of the expression of GFP (associated with the expression of UL47 and the TK promoter, respectively). Cell viability was estimated using the fixable viability dye eFluor 780 (eBioscience) at a dilution of 1:1,000. Staining was performed for 15 min on ice in the dark. Cells were washed twice in phosphate-buffered saline (PBS) before being fixed with 1% paraformaldehyde (PFA). Cell viability was analyzed with a 780/40 nm band-pass filter.
Alkaline comet assay.
Alkaline comet assays were performed with 2 × 105 cells as previously described (16, 72). For the comet assays, two slides were prepared for each condition. Comets were observed using an Axiovert 200 M inverted epifluorescence microscope (Zeiss), and images were taken with an Axiocam MRm camera (Zeiss). A minimum of 50 comets was analyzed for each replicate using CometScore software (version 1.5; TriTek). The olive tail moment (OTM) parameter was calculated on the basis of the tail length and the relative proportion of DNA contained in the tail. Results are presented as the mean OTM ± standard deviation (SD) calculated for each condition or as a distribution of the comets with respect to their respective OTM value (i.e., the percentage of cells presenting a defined OTM).
ROS assay.
Reactive oxygen species (ROS) production was assayed from the supernatants (80 μl) of mock- and recEGFPVP22-infected CESCs at 24, 48, 72, and 96 hpi using an ROS-Glo H2O2 assay following the manufacturer's instructions (Promega). Luminescence quantification was performed using a Glomax multidetection system luminometer (Promega). Results were recorded as relative luminescent units (RLU). Assays were done in triplicates at each time point. Results obtained from infected cells were normalized to those from mock-infected cells and are expressed as means ± SDs.
NO assay.
The amount of nitric oxide (NO) produced from infected and mock-infected CESCs was measured at 24, 48, 72, and 96 hpi by detecting the accumulation of nitrite (NO2−) in the culture media using the Griess reaction (73). Fifty microliters of cell culture supernatant was collected at each time point in a 96-well plate (in triplicates) and incubated for 10 min in the dark with 100 μl of the Griess reagent mixture (1:1), consisting of 1% sulfanilamide (Sigma-Aldrich) in 1.2 N hydrochloric acid and 0.3% N-1-naphthylethylenediamine dihydrochloride (Sigma-Aldrich). The absorbance at 540 nm was then measured. Nitrite concentrations were calculated with reference to a calibration curve established using standard solutions of sodium nitrite (Sigma-Aldrich) diluted in culture medium to concentrations ranging from 0 to 200 μM. A positive control consisting of the supernatant of Escherichia coli-infected cells was included in the assay, as was cell-free medium as a negative control.
Fluorescence microscopy.
CESCs were grown on glass coverslips and infected with recEGFPVP22. At 4 dpi, infected and noninfected cells were fixed with 4% PFA for 20 min at room temperature and permeabilized with 0.5% Triton X-100 for 5 min at room temperature. After blocking with PBS, 0.1% Triton X-100, and 2% bovine serum albumin (BSA), the cells were incubated with mouse monoclonal IgG antibody directed against phospho-histone H2AX (Ser139) (clone JBW301; Millipore) at a dilution of 1:500. Alexa Fluor 594-conjugated goat anti-mouse IgG secondary antibody (Invitrogen) was used at 1:2,000. Nuclei were counterstained with Hoechst 33342 (Invitrogen). EGFP fluorescence was directly observed from cells expressing the viral EGFP-tagged VP22 protein. Cells were observed under an Axiovert 200 M inverted epifluorescence microscope equipped with a 40× Plan Neofluar oil/differential interference contrast (DIC) objective or a 63× Plan Apochromat oil/DIC and an Apotome imaging system (Zeiss). Images were captured with a AxioCam MRm charge-coupled-device camera (Zeiss) by using AxioVision software.
MDV plaque size measurement assay.
At 48 hpi, CESC monolayers infected with recEGFPVP22 and treated with ETP were fixed with 4% PFA. The fluorescence emitted from the viral EGFP-tagged VP22 protein was detected using an Axiovert 200 M inverted epifluorescence microscope equipped with a 5× Fluar objective. Viral plaques were measured and analyzed as previously described (69).
Statistical analysis.
All graphs were prepared and statistics were performed using GraphPad Prism software (version 5.02; San Diego, CA, USA). Data are presented as the means ± SDs or medians. The one-way analysis of variance test was used to compare the differences among multiple groups, and the Mann-Whitney test (two-tailed) was used to compare nonparametric variables between two groups. Significant differences were determined using Student's t test. P values of <0.05 were considered statistically significant, as indicated in the figure legends.
ACKNOWLEDGMENTS
We thank F. Paillard for editing the manuscript and D. Pasdeloup and S. Trapp for their constructive comments on and corrections of the manuscript, as well as C. Berthault and K. Courvoisier for their technical help.
REFERENCES
- 1.Jha HC, Banerjee S, Robertson ES. 2016. The role of gammaherpesviruses in cancer pathogenesis. Pathogens 5:E18. doi: 10.3390/pathogens5010018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Delecluse HJ, Hammerschmidt W. 1993. Status of Marek's disease virus in established lymphoma cell lines: herpesvirus integration is common. J Virol 67:82–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaufer BB, Jarosinski KW, Osterrieder N. 2011. Herpesvirus telomeric repeats facilitate genomic integration into host telomeres and mobilization of viral DNA during reactivation. J Exp Med 208:605–615. doi: 10.1084/jem.20101402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arbuckle JH, Medveczky MM, Luka J, Hadley SH, Luegmayr A, Ablashi D, Lund TC, Tolar J, De Meirleir K, Montoya JG, Komaroff AL, Ambros PF, Medveczky PG. 2010. The latent human herpesvirus-6A genome specifically integrates in telomeres of human chromosomes in vivo and in vitro. Proc Natl Acad Sci U S A 107:5563–5568. doi: 10.1073/pnas.0913586107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wallaschek N, Sanyal A, Pirzer F, Gravel A, Mori Y, Flamand L, Kaufer BB. 2016. The telomeric repeats of human herpesvirus 6A (HHV-6A) are required for efficient virus integration. PLoS Pathog 12:e1005666. doi: 10.1371/journal.ppat.1005666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Osterrieder N, Kamil JP, Schumacher D, Tischer BK, Trapp S. 2006. Marek's disease virus: from miasma to model. Nat Rev Microbiol 4:283–294. doi: 10.1038/nrmicro1382. [DOI] [PubMed] [Google Scholar]
- 7.Calnek BW. 2001. Pathogenesis of Marek's disease virus infection. Curr Top Microbiol Immunol 255:25–55. [DOI] [PubMed] [Google Scholar]
- 8.McPherson MC, Delany ME. 2016. Virus and host genomic, molecular, and cellular interactions during Marek's disease pathogenesis and oncogenesis. Poult Sci 95:412–429. doi: 10.3382/ps/pev369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burnside J, Bernberg E, Anderson A, Lu C, Meyers BC, Green PJ, Jain N, Isaacs G, Morgan RW. 2006. Marek's disease virus encodes microRNAs that map to Meq and the latency-associated transcript. J Virol 80:8778–8786. doi: 10.1128/JVI.00831-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Engel AT, Selvaraj RK, Kamil JP, Osterrieder N, Kaufer BB. 2012. Marek's disease viral interleukin-8 promotes lymphoma formation through targeted recruitment of B cells and CD4+ CD25+ T cells. J Virol 86:8536–8545. doi: 10.1128/JVI.00556-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nair V. 2013. Latency and tumorigenesis in Marek's disease. Avian Dis 57:360–365. doi: 10.1637/10470-121712-Reg.1. [DOI] [PubMed] [Google Scholar]
- 12.Trapp S, Parcells MS, Kamil JP, Schumacher D, Tischer BK, Kumar PM, Nair VK, Osterrieder N. 2006. A virus-encoded telomerase RNA promotes malignant T cell lymphomagenesis. J Exp Med 203:1307–1317. doi: 10.1084/jem.20052240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kung HJ, Xia L, Brunovskis P, Li D, Liu JL, Lee LF. 2001. Meq: an MDV-specific bZIP transactivator with transforming properties. Curr Top Microbiol Immunol 255:245–260. [DOI] [PubMed] [Google Scholar]
- 14.Lupiani B, Lee LF, Cui X, Gimeno I, Anderson A, Morgan RW, Silva RF, Witter RL, Kung HJ, Reddy SM. 2004. Marek's disease virus-encoded Meq gene is involved in transformation of lymphocytes but is dispensable for replication. Proc Natl Acad Sci U S A 101:11815–11820. doi: 10.1073/pnas.0404508101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gimeno IM, Witter RL, Hunt HD, Reddy SM, Lee LF, Silva RF. 2005. The pp38 gene of Marek's disease virus (MDV) is necessary for cytolytic infection of B cells and maintenance of the transformed state but not for cytolytic infection of the feather follicle epithelium and horizontal spread of MDV. J Virol 79:4545–4549. doi: 10.1128/JVI.79.7.4545-4549.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Trapp-Fragnet L, Bencherit D, Chabanne-Vautherot D, Le Vern Y, Remy S, Boutet-Robinet E, Mirey G, Vautherot JF, Denesvre C. 2014. Cell cycle modulation by Marek's disease virus: the tegument protein VP22 triggers S-phase arrest and DNA damage in proliferating cells. PLoS One 9:e100004. doi: 10.1371/journal.pone.0100004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dorange F, Tischer BK, Vautherot JF, Osterrieder N. 2002. Characterization of Marek's disease virus serotype 1 (MDV-1) deletion mutants that lack UL46 to UL49 genes: MDV-1 UL49, encoding VP22, is indispensable for virus growth. J Virol 76:1959–1970. doi: 10.1128/JVI.76.4.1959-1970.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jarosinski KW, Arndt S, Kaufer BB, Osterrieder N. 2012. Fluorescently tagged pUL47 of Marek's disease virus reveals differential tissue expression of the tegument protein in vivo. J Virol 86:2428–2436. doi: 10.1128/JVI.06719-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Remy S, Blondeau C, Le Vern Y, Lemesle M, Vautherot JF, Denesvre C. 2013. Fluorescent tagging of VP22 in N-terminus reveals that VP22 favors Marek's disease virus (MDV) virulence in chickens and allows morphogenesis study in MD tumor cells. Vet Res 44:125. doi: 10.1186/1297-9716-44-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith S, Weller SK. 2015. HSV-I and the cellular DNA damage response. Future Virol 10:383–397. doi: 10.2217/fvl.15.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fortunato EA, Spector DH. 2003. Viral induction of site-specific chromosome damage. Rev Med Virol 13:21–37. doi: 10.1002/rmv.368. [DOI] [PubMed] [Google Scholar]
- 22.Lilley CE, Schwartz RA, Weitzman MD. 2007. Using or abusing: viruses and the cellular DNA damage response. Trends Microbiol 15:119–126. doi: 10.1016/j.tim.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 23.Sinclair A, Yarranton S, Schelcher C. 2006. DNA-damage response pathways triggered by viral replication. Expert Rev Mol Med 8:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Turnell AS, Grand RJ. 2012. DNA viruses and the cellular DNA-damage response. J Gen Virol 93:2076–2097. doi: 10.1099/vir.0.044412-0. [DOI] [PubMed] [Google Scholar]
- 25.Weitzman MD, Carson CT, Schwartz RA, Lilley CE. 2004. Interactions of viruses with the cellular DNA repair machinery. DNA Repair (Amst) 3:1165–1173. doi: 10.1016/j.dnarep.2004.03.018. [DOI] [PubMed] [Google Scholar]
- 26.Xiaofei E, Kowalik TF. 2014. The DNA damage response induced by infection with human cytomegalovirus and other viruses. Viruses 6:2155–2185. doi: 10.3390/v6052155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hau PM, Deng W, Jia L, Yang J, Tsurumi T, Chiang AK, Huen MS, Tsao SW. 2015. Role of ATM in the formation of the replication compartment during lytic replication of Epstein-Barr virus in nasopharyngeal epithelial cells. J Virol 89:652–668. doi: 10.1128/JVI.01437-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hollingworth R, Skalka GL, Stewart GS, Hislop AD, Blackbourn DJ, Grand RJ. 2015. Activation of DNA damage response pathways during lytic replication of KSHV. Viruses 7:2908–2927. doi: 10.3390/v7062752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mohni KN, Dee AR, Smith S, Schumacher AJ, Weller SK. 2013. Efficient herpes simplex virus 1 replication requires cellular ATR pathway proteins. J Virol 87:531–542. doi: 10.1128/JVI.02504-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mounce BC, Tsan FC, Droit L, Kohler S, Reitsma JM, Cirillo LA, Tarakanova VL. 2011. Gammaherpesvirus gene expression and DNA synthesis are facilitated by viral protein kinase and histone variant H2AX. Virology 420:73–81. doi: 10.1016/j.virol.2011.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lilley CE, Carson CT, Muotri AR, Gage FH, Weitzman MD. 2005. DNA repair proteins affect the lifecycle of herpes simplex virus 1. Proc Natl Acad Sci U S A 102:5844–5849. doi: 10.1073/pnas.0501916102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jha HC, Upadhyay SK, Prasad AJ M, Lu J, Cai Q, Saha A, Robertson ES. 2013. H2AX phosphorylation is important for LANA-mediated Kaposi's sarcoma-associated herpesvirus episome persistence. J Virol 87:5255–5269. doi: 10.1128/JVI.03575-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tarakanova VL, Stanitsa E, Leonardo SM, Bigley TM, Gauld SB. 2010. Conserved gammaherpesvirus kinase and histone variant H2AX facilitate gammaherpesvirus latency in vivo. Virology 405:50–61. doi: 10.1016/j.virol.2010.05.027. [DOI] [PubMed] [Google Scholar]
- 34.Gruhne B, Sompallae R, Masucci MG. 2009. Three Epstein-Barr virus latency proteins independently promote genomic instability by inducing DNA damage, inhibiting DNA repair and inactivating cell cycle checkpoints. Oncogene 28:3997–4008. doi: 10.1038/onc.2009.258. [DOI] [PubMed] [Google Scholar]
- 35.Hollingworth R, Grand RJ. 2015. Modulation of DNA damage and repair pathways by human tumour viruses. Viruses 7:2542–2591. doi: 10.3390/v7052542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koopal S, Furuhjelm JH, Jarviluoma A, Jaamaa S, Pyakurel P, Pussinen C, Wirzenius M, Biberfeld P, Alitalo K, Laiho M, Ojala PM. 2007. Viral oncogene-induced DNA damage response is activated in Kaposi sarcoma tumorigenesis. PLoS Pathog 3:1348–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu CC, Liu MT, Chang YT, Fang CY, Chou SP, Liao HW, Kuo KL, Hsu SL, Chen YR, Wang PW, Chen YL, Chuang HY, Lee CH, Chen M, Wayne Chang WS, Chen JY. 2010. Epstein-Barr virus DNase (BGLF5) induces genomic instability in human epithelial cells. Nucleic Acids Res 38:1932–1949. doi: 10.1093/nar/gkp1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xiao Y, Chen J, Liao Q, Wu Y, Peng C, Chen X. 2013. Lytic infection of Kaposi's sarcoma-associated herpesvirus induces DNA double-strand breaks and impairs non-homologous end joining. J Gen Virol 94:1870–1875. doi: 10.1099/vir.0.053033-0. [DOI] [PubMed] [Google Scholar]
- 39.Nikitin PA, Luftig MA. 2011. At a crossroads: human DNA tumor viruses and the host DNA damage response. Future Virol 6:813–830. doi: 10.2217/fvl.11.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nikitin PA, Luftig MA. 2012. The DNA damage response in viral-induced cellular transformation. Br J Cancer 106:429–435. doi: 10.1038/bjc.2011.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Keles H, Fidan AF, Cigerci IH, Kucukkurt I, Karadas E, Dundar Y. 2010. Increased DNA damage and oxidative stress in chickens with natural Marek's disease. Vet Immunol Immunopathol 133:51–58. doi: 10.1016/j.vetimm.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 42.Walker JV, Nitiss JL. 2002. DNA topoisomerase II as a target for cancer chemotherapy. Cancer Invest 20:570–589. doi: 10.1081/CNV-120002156. [DOI] [PubMed] [Google Scholar]
- 43.Barzilai A, Yamamoto K. 2004. DNA damage responses to oxidative stress. DNA Repair (Amst) 3:1109–1115. doi: 10.1016/j.dnarep.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 44.Phoa N, Epe B. 2002. Influence of nitric oxide on the generation and repair of oxidative DNA damage in mammalian cells. Carcinogenesis 23:469–475. doi: 10.1093/carcin/23.3.469. [DOI] [PubMed] [Google Scholar]
- 45.Calnek BW, Schat KA, Ross LJ, Chen CL. 1984. Further characterization of Marek's disease virus-infected lymphocytes. II. In vitro infection. Int J Cancer 33:399–406. [DOI] [PubMed] [Google Scholar]
- 46.Shek WR, Calnek BW, Schat KA, Chen CH. 1983. Characterization of Marek's disease virus-infected lymphocytes: discrimination between cytolytically and latently infected cells. J Natl Cancer Inst 70:485–491. [PubMed] [Google Scholar]
- 47.Denesvre C, Remy S, Trapp-Fragnet L, Smith LP, Georgeault S, Vautherot JF, Nair V. 2015. Marek's disease virus undergoes complete morphogenesis after reactivation in T-lymphoblastoid cell line transformed by recombinant fluorescent marker virus. J Gen Virol 97:480–486. doi: 10.1099/jgv.0.000354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dorange F, El Mehdaoui S, Pichon C, Coursaget P, Vautherot JF. 2000. Marek's disease virus (MDV) homologues of herpes simplex virus type 1 UL49 (VP22) and UL48 (VP16) genes: high-level expression and characterization of MDV-1 VP22 and VP16. J Gen Virol 81:2219–2230. doi: 10.1099/0022-1317-81-9-2219. [DOI] [PubMed] [Google Scholar]
- 49.Palmai-Pallag T, Bachrati CZ. 2014. Inflammation-induced DNA damage and damage-induced inflammation: a vicious cycle. Microbes Infect 16:822–832. doi: 10.1016/j.micinf.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 50.Jaiswal M, LaRusso NF, Burgart LJ, Gores GJ. 2000. Inflammatory cytokines induce DNA damage and inhibit DNA repair in cholangiocarcinoma cells by a nitric oxide-dependent mechanism. Cancer Res 60:184–190. [PubMed] [Google Scholar]
- 51.Jaiswal H, Lindqvist A. 2015. Bystander communication and cell cycle decisions after DNA damage. Front Genet 6:63. doi: 10.3389/fgene.2015.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Elliott G, O'Hare P. 1997. Intercellular trafficking and protein delivery by a herpesvirus structural protein. Cell 88:223–233. doi: 10.1016/S0092-8674(00)81843-7. [DOI] [PubMed] [Google Scholar]
- 53.Wu F, Long J, Wang S, Xing J, Li M, Zheng C. 2012. Live cell imaging fails to support viral-protein-mediated intercellular trafficking. Arch Virol 157:1383–1386. doi: 10.1007/s00705-012-1308-9. [DOI] [PubMed] [Google Scholar]
- 54.Xue X, Huang J, Wang H. 2014. The study of the intercellular trafficking of the fusion proteins of herpes simplex virus protein VP22. PLoS One 9:e100840. doi: 10.1371/journal.pone.0100840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jarosinski KW, Yunis R, O'Connell PH, Markowski-Grimsrud CJ, Schat KA. 2002. Influence of genetic resistance of the chicken and virulence of Marek's disease virus (MDV) on nitric oxide responses after MDV infection. Avian Dis 46:636–649. doi: 10.1637/0005-2086(2002)046[0636:IOGROT]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 56.Djeraba A, Bernardet N, Dambrine G, Quere P. 2000. Nitric oxide inhibits Marek's disease virus replication but is not the single decisive factor in interferon-gamma-mediated viral inhibition. Virology 277:58–65. doi: 10.1006/viro.2000.0576. [DOI] [PubMed] [Google Scholar]
- 57.Xing Z, Schat KA. 2000. Inhibitory effects of nitric oxide and gamma interferon on in vitro and in vivo replication of Marek's disease virus. J Virol 74:3605–3612. doi: 10.1128/JVI.74.8.3605-3612.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Karanjawala ZE, Murphy N, Hinton DR, Hsieh CL, Lieber MR. 2002. Oxygen metabolism causes chromosome breaks and is associated with the neuronal apoptosis observed in DNA double-strand break repair mutants. Curr Biol 12:397–402. doi: 10.1016/S0960-9822(02)00684-X. [DOI] [PubMed] [Google Scholar]
- 59.Weitzman MD, Lilley CE, Chaurushiya MS. 2010. Genomes in conflict: maintaining genome integrity during virus infection. Annu Rev Microbiol 64:61–81. doi: 10.1146/annurev.micro.112408.134016. [DOI] [PubMed] [Google Scholar]
- 60.Guo Z, Deshpande R, Paull TT. 2010. ATM activation in the presence of oxidative stress. Cell Cycle 9:4805–4811. doi: 10.4161/cc.9.24.14323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Guo Z, Kozlov S, Lavin MF, Person MD, Paull TT. 2010. ATM activation by oxidative stress. Science 330:517–521. doi: 10.1126/science.1192912. [DOI] [PubMed] [Google Scholar]
- 62.E X, Pickering MT, Debatis M, Castillo J, Lagadinos A, Wang S, Lu S, Kowalik TF. 2011. An E2F1-mediated DNA damage response contributes to the replication of human cytomegalovirus. PLoS Pathog 7:e1001342. doi: 10.1371/journal.ppat.1001342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kudoh A, Fujita M, Zhang L, Shirata N, Daikoku T, Sugaya Y, Isomura H, Nishiyama Y, Tsurumi T. 2005. Epstein-Barr virus lytic replication elicits ATM checkpoint signal transduction while providing an S-phase-like cellular environment. J Biol Chem 280:8156–8163. doi: 10.1074/jbc.M411405200. [DOI] [PubMed] [Google Scholar]
- 64.Shirata N, Kudoh A, Daikoku T, Tatsumi Y, Fujita M, Kiyono T, Sugaya Y, Isomura H, Ishizaki K, Tsurumi T. 2005. Activation of ataxia telangiectasia-mutated DNA damage checkpoint signal transduction elicited by herpes simplex virus infection. J Biol Chem 280:30336–30341. doi: 10.1074/jbc.M500976200. [DOI] [PubMed] [Google Scholar]
- 65.Bohgaki T, Bohgaki M, Hakem R. 2010. DNA double-strand break signaling and human disorders. Genome Integr 1:15. doi: 10.1186/2041-9414-1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bill CA, Summers J. 2004. Genomic DNA double-strand breaks are targets for hepadnaviral DNA integration. Proc Natl Acad Sci U S A 101:11135–11140. doi: 10.1073/pnas.0403925101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chen Y, Williams V, Filippova M, Filippov V, Duerksen-Hughes P. 2014. Viral carcinogenesis: factors inducing DNA damage and virus integration. Cancers (Basel) 6:2155–2186. doi: 10.3390/cancers6042155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pratt WD, Morgan RW, Schat KA. 1992. Characterization of reticuloendotheliosis virus-transformed avian T-lymphoblastoid cell lines infected with Marek's disease virus. J Virol 66:7239–7244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Blondeau C, Marc D, Courvoisier K, Vautherot JF, Denesvre C. 2008. Functional homologies between avian and human alphaherpesvirus VP22 proteins in cell-to-cell spreading as revealed by a new cis-complementation assay. J Virol 82:9278–9282. doi: 10.1128/JVI.00598-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jarosinski KW, Margulis NG, Kamil JP, Spatz SJ, Nair VK, Osterrieder N. 2007. Horizontal transmission of Marek's disease virus requires US2, the UL13 protein kinase, and gC. J Virol 81:10575–10587. doi: 10.1128/JVI.01065-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Arumugaswami V, Kumar PM, Konjufca V, Dienglewicz RL, Reddy SM, Parcells MS. 2009. Latency of Marek's disease virus (MDV) in a reticuloendotheliosis virus-transformed T-cell line. II. Expression of the latent MDV genome. Avian Dis 53:156–165. [DOI] [PubMed] [Google Scholar]
- 72.Lebailly P, Devaux A, Pottier D, De Meo M, Andre V, Baldi I, Severin F, Bernaud J, Durand B, Henry-Amar M, Gauduchon P. 2003. Urine mutagenicity and lymphocyte DNA damage in fruit growers occupationally exposed to the fungicide captan. Occup Environ Med 60:910–917. doi: 10.1136/oem.60.12.910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ding AH, Nathan CF, Stuehr DJ. 1988. Release of reactive nitrogen intermediates and reactive oxygen intermediates from mouse peritoneal macrophages. Comparison of activating cytokines and evidence for independent production. J Immunol 141:2407–2412. [PubMed] [Google Scholar]