Abstract
Objective
It remains unclear whether the CHADS2, CHA2DS2-VASc, or R2CHADS2 score is the most useful for the risk stratification of ischemic stroke/systemic thromboembolism (IS/SE) in Japanese patients with paroxysmal non-valvular atrial fibrillation (PNVAF).
Methods
We investigated the incidence of IS/SE on the basis of the CHADS2, CHA2DS2-VASc, and R2CHADS2 scores in 332 consecutive PNVAF patients (224 men, mean age: 65±13 years) who had not been administered anticoagulation therapy but who were administered antiarrhythmic drug therapy to maintain sinus rhythm between August 1995 and July 2008 before the 2008 Japanese Circulation Society guideline was issued (mean follow-up period: 53±35 months).
Results
The annual rates of IS/SE without underlying antiarrhythmic drug therapy are shown in the table included in this article. Higher CHADS2, CHA2DS2-VASc, and R2CHADS2 scores were associated with greater annual rates of IS/SE (p<0.001). In a multivariate logistic regression analysis adjusted for potentially confounding variables, the CHADS2 scores [odds ratio (OR): 4.74, 95% confidence interval (CI): 2.80-8.00, p<0.001], CHA2DS2-VASc scores (OR: 4.15, 95% CI: 2.57-6.71, p<0.001), and R2CHADS2 scores (OR: 1.94, 95% CI: 1.48-2.53, p<0.001) were significant independent predictors of IS/SE. The area under the receiver-operator characteristic curve for predicting IS/SE was 0.89 for CHA2DS2-VASc scores, 0.87 for CHADS2 scores, and 0.85 for R2CHADS2 scores (all, p<0.001), with no significant difference among the three scores.
Conclusion
In Japanese patients with PNVAF, the CHADS2, CHA2DS2-VASc, and R2CHADS2 scores are all useful for the risk stratification of IS/SE cases.
Keywords: CHADS2 score, CHA2DS2-VASc score, R2CHADS2 score, Japanese patients, non-valvular paroxysmal atrial fibrillation
Introduction
According to recent epidemiological studies in Europe and the United States, the prevalence of atrial fibrillation (AF) is about 4% in individuals in their 70s and about 10% in those over 80 years of age, showing a significant increase with age. In Japan, where the elderly population is increasing rapidly, the prevalence of AF in the elderly population is also high, occurring in about 2-3% of those in their 70s, and is expected to reach 1,000 per 100,000 population in 2010-2030 (1), with further increases in the future. AF is thus considered an important condition that will significantly affect the healthcare system in Japan.
AF is the most common sustained clinical arrhythmia in humans and not only impairs the quality of life but also causes serious complications, such as embolism and hemodynamic dysfunction. It also generates arrhythmia that worsens the cardiovascular prognosis in cases of left ventricular dysfunction (2).
The R2CHADS2 score has been newly proposed for stratifying patients with non-valvular atrial fibrillation (NVAF) according to the risk of stroke (3). We previously demonstrated that the CHADS2 and CHA2DS2-VASc scores were useful for risk stratification of cardiovascular events in Japanese patients with paroxysmal AF (4, 5). However, it remains unclear whether the CHADS2, CHA2DS2-VASc, or R2CHADS2 score is the most useful for the risk stratification of ischemic stroke/systemic thromboembolism (IS/SE) in Japanese patients with paroxysmal non-valvular atrial fibrillation (PNVAF).
We therefore investigated the incidence of IS/SE on the basis of the CHADS2, CHA2DS2-VASc, and R2CHADS2 scores in patients with PNVAF who did not receive anticoagulation therapy before the Japanese Circulation Society (JCS) guidelines were issued in 2008.
Materials and Methods
A total of 548 patients had paroxysmal AF confirmed based on symptoms and 12-lead surface electrocardiograms (ECG) and/or ambulatory 24-hour monitoring findings at Iwate Medical University School of Medicine between August 1995 and July 2008 before the publication of the Japan Circulation Society (JCS) guidelines in 2008. Our database, which was established in August 1995, contains data on all new patients admitted to Iwate Medical University School of Medicine in Morioka, Japan. The principle aim for establishing this hospital-based database is to monitor the prevalence and prognosis of cardiovascular diseases in a local area of Japan. The registry started in August 1995, and patients have been continually registered in the database annually. The study sample was drawn from this group and comprised 332 patients (224 men and 108 women; mean age: 65±13 years) who were not receiving anticoagulation therapy and in whom transthoracic echocardiography (TTE) had ruled out cardiac valvular disease. Valvular AF was defined as AF with mitral stenosis and/or a history of valvular surgery (both biological and mechanical valve). All subjects were treated on an outpatient basis every two to four weeks, underwent rhythm control therapy using antiarrhythmic drugs, and were followed for at least one year. All patients were screened for diabetes mellitus using fasting glucose and hemoglobin A1c levels. All patients also underwent a medical interview, chest X-ray, exercise tolerance test, and TTE or other appropriate noninvasive examinations for underlying cardiopulmonary diseases, and the investigators performed a pulmonary function test, chest computed tomography (CT), and cardiac catheterization whenever necessary.
Patients with congestive heart failure; serious bradyarrhythmia (e.g. sick sinus syndrome, atrioventricular block, bi-fascicular block or more); thyroid, hepatic, or renal dysfunction; child-bearing potential during the study period; a history of drug allergy; and receiving warfarin anticoagulation therapy were excluded from the study. In this study, the mean observation period was 53±35 months (range: 12 to 127 months).
Definition
Paroxysmal AF was defined as AF terminating spontaneously within seven days of onset (6). Permanent (chronic) AF was defined as AF refractory to antiarrthythimc drug therapy or electrical cardioversion and where a sinus rhythm could not be maintained for more than 12 months, as assessed by ECG. Ischemic stroke was confirmed based on typical neurological symptoms and the presence of a ≥3 mm infarct area, as obtained by brain CT or magnetic resonance imaging (MRI), which was performed in all patients. The diagnosis of hypertension followed the 2009 Japanese Society of Hypertension (JSH) guidelines (7). Dyslipidemia was defined as fasting serum cholesterol of ≥220 mg/dL and triglycerides of ≥150 mg/dL (8). AF was divided into three types depending on the time of onset: diurnal type (7:00 to 17:00), nocturnal type (17:00 to 7:00), and mixed type (any time) (9). Chronic obstructive pulmonary disease was defined as a forced expiratory volume in one second of <70%, as measured by a pulmonary function test. Systemic embolism was defined as an acute vascular occlusion of an extremity or organ, documented by means of imaging or surgery.
CHADS2 and CHA2DS2-VASc scores were defined according to 2006 the American Heart Association (AHA) guidelines (10) and 2010 the European Society of Cardiology (ESC) guidelines (11), respectively. The R2CHADS2 score (3) awards one point each for the presence of congestive heart failure, hypertension, age ≥75 years old, and diabetes mellitus and two points for prior stroke or transient ischemic attack and renal dysfunction (estimated glomerular filtration rate [eGFR] <60 mL/min/m2).
Protocol for antiarrhythmic drug therapy
In patients in whom TTE revealed a left ventricular ejection fraction ≥40% after spontaneous or pharmacological/electrical cardioversion, Class I or III antiarrhythmic drugs were administered based on the judgment of the outpatient attending physician. When TTE by contrast showed a left ventricular ejection fraction <40% after spontaneous or pharmacological/electrical cardioversion, aprindine, bepridil, or amiodarone was administered based on the judgment of the outpatient attending physician.
To confirm recurrence of AF, we performed a subjective assessment through history taking. We also obtained recordings from a standard 12-lead ECG and a portable monitor at the time of the medical examination after 2-4 weeks of administration or a change in antiarrhythmic drugs. Furthermore, ambulatory 24-hour ECG monitoring was performed every 3 months to detect recurrence of AF if considered necessary by the outpatient attending physician.
If AF became permanent despite antiarrhythmic therapy, β-blockers, Ca antagonists, or digitalis was administered orally to control the ventricular rate.
Protocol for antiplatelet therapy
Before publication of the Japanese Circulation Society guidelines (12) in November 2001, the attending physicians administered antiplatelet therapy at their own discretion. After November 2001, antiplatelet therapy was generally performed in accordance with the guidelines, but the decision to administer antiplatelet therapy was left to the physician. Doses of aspirin were 81 to 100 mg/day.
The subjects of the present study had not received anticoagulation therapy or had received only aspirin. We examined the patient distributions with regard to the CHADS2, CHA2DS2-VASc, and R2CHADS2 scores; patient background factors; ischemic stroke; and systemic embolism in patients with PNVAF in whom anticoagulant therapy was not administered before the JCS guideline issued in 2008.
Statistical analyses
The obtained values were expressed as the mean and standard deviation. The patient characteristics were compared between subgroups with the Mann-Whitney U test, and patient percentages were compared with the chi-squared test. The percentage of patients without IS/SE was evaluated using the Kaplan-Meier method, and differences between subgroups were tested for significance using the log-rank test. A multivariate logistic analysis was used to identify predictive factors for ischemic stroke and systemic embolism, and the Hosmer-Lemeshow goodness-of-fit test was used to validate the model. All statistical analyses were performed using the SPSS 13.0 statistical software package. A p value <0.05 was considered statistically significant.
Ethical issues
The ethical committee at Iwate Medical University School of Medicine granted approval for this study, and all of the patients gave their informed consent.
Results
Patient characteristics
All patient characteristics are shown in Tables 1-3.
Table 1.
Patient Characteristics.
| Number | 32 | ||
|---|---|---|---|
| Follow-up period (months) | 53±35 | LVDd (mm) | 46±5 |
| Age (years) | 65±13 | LAD (mm) | 34±6 |
| Male : female | 224:108 | LVEF (%) | 69±10 |
| Hypertension | 142 (43%) | RAAS inhibitors | 80 (24%) |
| Diabetes Mellitus | 42 (13%) | Statins | 45 (14%) |
| Dyslipidemia | 44 (13%) | Antithrombotic therapy ; | |
| Smoking habits | 89 (27%) | None | 223 (67%) |
| Alcohol habits | 134 (40%) | Aspirin | 109 (33%) |
| Hyperuricemia | 19 (6%) | ANP during SNR (pg/mL) | 38±37 |
| Underlying heart disease | 65 (20%) | Onset of AF | |
| Underlying pulmonary disease | 18 (5%) | diurnal : nocturnal : mixed | 65:129:138 |
| AF history (months) | 18±32 |
Table 2.
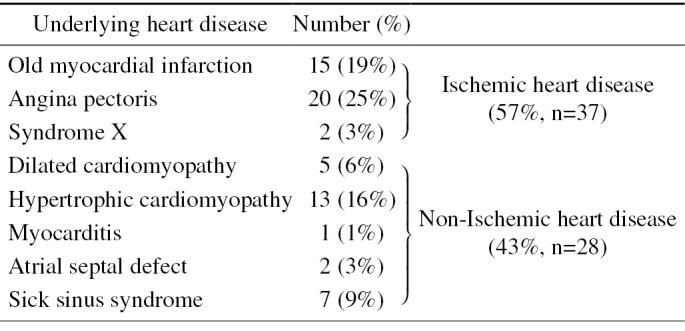
Details of Underlying Heart Disease.
Table 3.
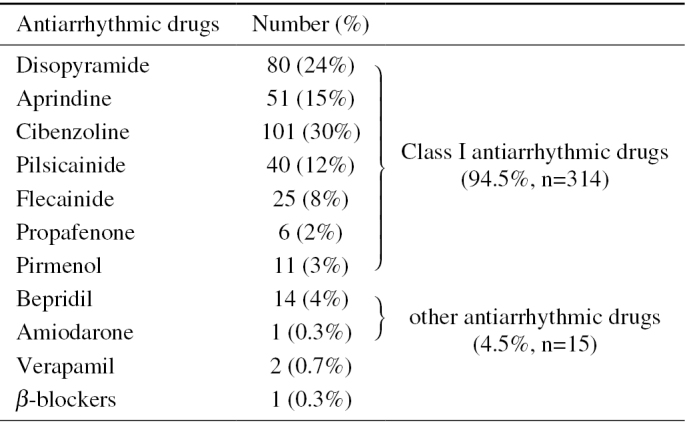
Selected Antiarrhythmic Drugs.
Distribution of CHADS2, CHA2DS2-VASc, and R2CHADS2 scores in patients with PNVAF
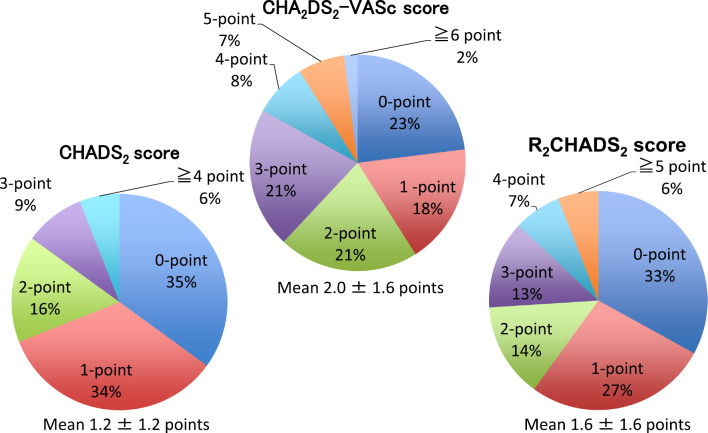
The CHADS2, CHA2DS2-VASc, and R2CHADS2 score distributions are shown in Fig. 1. The mean CHADS2 score was 1.2±1.2 points, the mean CHA2DS2-VASc score was 2.0±1.6 points, and the mean R2CHADS2 score was 1.6±1.6 points.
Figure 1.
Distribution of PNVAF patients on the basis of CHADS2, CHA2DS2-VASc, and R2CHADS2 scores.
The survival rate free from IS/SE among the CHADS2, CHA2DS2-VASc, and R2CHADS2 score groups
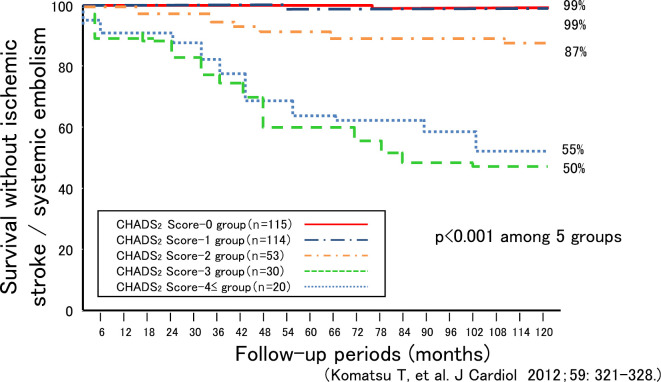
The respective survival rates free from IS/SE on the basis of the CHADS2 score at 12, 36, 60, 90, and 120 months of follow-up were as follows: score value 0: 100%, 100%, 100%, 99%, and 99%; score value 1: 100%, 100%, 99%, 99%, and 99%; score value 2: 98%, 94%, 89%, 87%, and 85%; score value 3: 93%, 77%, 60%, 50%, and 47%; and score value ≥4: 90%, 80%, 65%, 60%, and 50% (Fig. 2). There was a significant difference in the survival rate during the follow-up period among the 5 groups (p<0.001).
Figure 2.
Survival curve free from ischemic stroke/systemic embolism on the basis of the CHADS2 score.
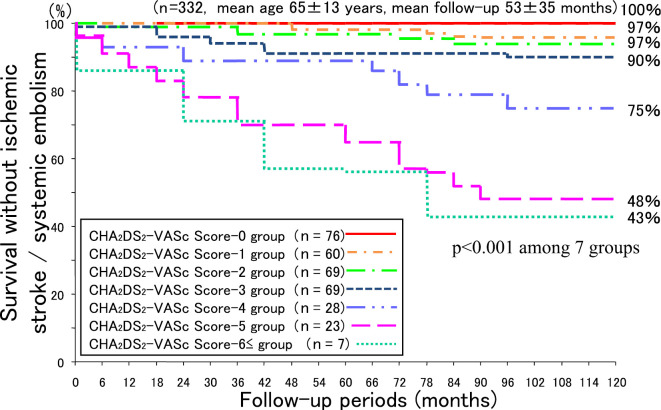
The respective survival rates free from IS/SE on the basis of the CHA2DS2-VASc score at 12, 36, 60, 90, and 120 months of follow-up were as follows: score value 0: 100%, 100%, 100%, 100%, and 100%; score value 1: 100%, 100%, 98%, 98%, and 97%; score value 2 group: 99%, 99%, 97%, 97%, and 97%; score value 3: 99%, 94%, 91%, 91%, and 90%; score value 4: 96%, 93%, 89%, 79%, and 75%; score value 5: 96%, 78%, 70%, 52%, and 48%; and score value ≥6: 86%, 71%, 57%, 43%, and 43% (Fig. 3). Here as well, there was a significant difference in the survival rate during the follow-up period among the 7 groups (p<0.001).
Figure 3.
Survival curve free from ischemic stroke/systemic embolism on the basis of the CHA2DS2-VASc score.
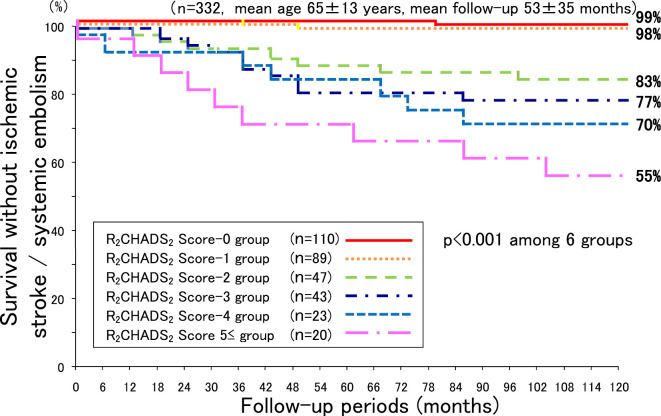
The respective survival rates free from IS/SE on the basis of the R2CHADS2 score at 12, 36, 60, 90, and 120 months of follow-up were as follows: score value 0: 100%, 100%, 100%, 99%, and 99%; score value 1: 100%, 100%, 98%, 98%, and 98%; score value 2: 98%, 92%, 87%, 85%, and 83%; score value 3: 98%, 91%, 79%, 77%, and 77%; score value 4: 91%, 91%, 83%, 70%, and 70%; and score value ≥5: 95%, 75%, 70%, 60%, and 55% (Fig. 4). Here as well, there was a significant difference in the survival rate during the follow-up period among the 6 groups (p<0.001).
Figure 4.
Survival curve free from ischemic stroke/systemic embolism on the basis of the R2CHADS2 score.
Annual incidence of IS/SE on the basis of the CHADS2, CHA2DS2-VASc, and R2CHADS2 scores
Table 4 shows the annual rates of IS/SE by CHADS2, CHA2DS2-VASc, and R2CHADS2 score. In each category, the higher value groups had higher annual rates of IS/SE. In total patients, the annual rates of IS/SE in the no anticoagulant therapy group and in the aspirin group were 2.6%/year and 3.1%/year, respectively.
Table 4.
Incidences and Annual Rates of Ischemic Stroke/Systemic Embolism on the Basis of the CHADS2, CHA2DS2-VASc, and R2CHADS2 Scores.
| CHADS2score | Number | Follow up period (months) | Annual rate (%/year; 95%CI) |
|---|---|---|---|
| Score 0 | (n=115) | 46±32 | 0.21 (0.10-0.33) |
| Score 1 | (n=114) | 51±35 | 0.93 (0.79-1.07) |
| Score 2 | (n=53) | 65±39 | 2.78 (2.61-2.96) |
| Score 3 | (n=30) | 68±39 | 9.41 (8.98-9.85) |
| Score 4 ≤ | (n=20) | 55±30 | 10.90 (10.18-11.67) |
| CHA2DS2-VASc score | Number | ollow up period (months) | Annual rate (%/year; 95%CI) |
| Score 0 | (n=76) | 45±33 | 0 |
| Score 1 | (n=60) | 42±31 | 0.60 (0.45-0.76) |
| Score 2 | (n=69) | 58±34 | 0.95 (0.73-1.18) |
| Score 3 | (n=69) | 62±39 | 1.96 (1.65-2.28) |
| Score 4 | (n=28) | 55±32 | 5.45 (5.06-5.85) |
| Score 5 | (n=23) | 69±33 | 9.06 (8.41-9.72) |
| Score 6≤ | (n=7) | 50±29 | 13.70 (11.79-15.62) |
| R2CHADS2score | Number | Follow up period (months) | Annual rate (%/year; 95%CI) |
| Score 0 | (n=110) | 47±33 | 0.23 (0.12-0.35) |
| Score 1 | (n=89) | 49±35 | 0.56 (0.36-0.77) |
| Score 2 | (n=47) | 62±39 | 3.29 (3.00-3.58) |
| Score 3 | (n=43) | 56±32 | 4.98 (4.57-5.40) |
| Score 4 | (n=23) | 53±37 | 5.80 (5.13-6.47) |
| Score 5 ≤ | (n=20) | 70±32 | 7.71 (5.81-9.61) |
Non-valvular paroxysmal AF (n=332, mean age 65±13 years, mean follow-up 53±35 months)
Predictors of IS/SE in patients with PNVAF
In a multivariate logistic regression analysis adjusted for other potentially confounding variables, the CHADS2 score [odds ratio (OR): 4.735, 95% confidence interval (CI): 2.803-7.998, p<0.001], CHA2DS2-VASc score (OR: 4.152, 95% CI: 2.570-6.709, p<0.001), R2CHADS2 score (OR: 1.937, 95% CI: 1.481-2.533, p<0.001), and mixed type onset (OR: 3.380, 95% CI: 1.133-10.08, p=0.003 in Table 5A; OR: 3.120, 95% CI: 1.018-9.565, p=0.046 in Table 5B; and OR: 2.782, 95% CI: 1.021-7.584, p=0.045 in Table 5C) were significant independent predictors of IS/SE.
Table 5A.
Predictors of Ischemic Stroke/Systemic Embolism in Patients with PNVAF Not Receiving Antithrombotic Therapy.
| Variables | Odds ratio (95%CI) | p value |
|---|---|---|
| CHADS2 score | 4.735 (2.803 - 7.998) | <0.001 |
| Mixed type (time of AF onset) | 3.380 (1.133 - 10.08) | 0.003 |
| Statins | 3.185 (0.978 - 13.72) | 0.068 |
| Age (years) | 0.956 (0.908 - 1.007) | 0.091 |
| RAAS inhibitors | 2.106 (0.667 - 6.648) | 0.145 |
| Chronic AF | 1.420 (0.490 - 4.116) | 0.204 |
| Underlying heart disease | 1.662 (0.244 - 11.30) | 0.518 |
| Underlying pulmonary disease | 0.801 (0.231 - 2.771) | 0.604 |
| AF recurrence | 0.984 (0.899 - 1.077) | 0.726 |
| LVDd (mm) | 1.005 (0.974 - 1.038) | 0.726 |
| AF history (months) | 0.988 (0.908 - 1.074) | 0.739 |
| LAD (mm) | 1.007 (0.960 - 1.056) | 0.770 |
| LVEF (%) | 0.988 (0.908 - 1.074) | 0.788 |
| Male | 1.150 (0.394 - 3.357) | 0.799 |
- A multivariate logistic regression analysis -
AF: atrial fibrillation, RAAS: renin-angiotensin-aldosterone system, LVDd: left ventricular end-diastolic dimension, LAD: left atrial dimension, LVEF: left ventricular ejection fraction
Table 5B.
Predictors of Ischemic Stroke/Systemic Embolism in Patients with PNVAF Not Receiving Antithrombotic Therapy.
| (N=332, mean age 65±13 years, mean follow-up 53±35 months) | ||
|---|---|---|
| Variables | Odds ratio (95%CI) | p value |
| CHA2DS2-VASc score | 4.152 (2.570 - 6.709) | <0.001 |
| Mixed type (time of AF onset) | 3.120 (1.018 - 9.565) | 0.046 |
| Male | 2.907 (0.991 - 8.530) | 0.052 |
| Age (years) | 0.965 (0.901 - 1.012) | 0.098 |
| Statins | 2.743 (0.892 - 11.41) | 0.113 |
| RAAS inhibitors | 2.095 (0.752 - 5.837) | 0.157 |
| Chronic AF | 1.871 (0.586 - 5.972) | 0.290 |
| Underlying pulmonary disease | 0.537 (0.279 - 11.61) | 0.537 |
| AF history (months) | 1.003 (0.989 - 1.016) | 0.702 |
| AF recurrence | 1.256 (0.379 - 4.161) | 0.709 |
| LAD (mm) | 0.985 (0.904 - 1.075) | 0.739 |
| LVEF (%) | 0.993 (0.949 - 1.039) | 0.765 |
| LVDd (mm) | 0.988 (0.903 - 1.082) | 0.795 |
| Underlying heart disease | 1.119 (0.388 - 3.225) | 0.835 |
- A multivariate logistic regression analysis -
AF: atrial fibrillation, RAAS: renin-angiotensin-aldosterone system, LAD: left atrial dimension, LVEF: left ventricular ejection fraction, LVDd: left ventricular end-diastolic dimension
Table 5C.
Predictors of Ischemic Stroke/Systemic Embolism in Patients with PNVAF Not Receiving Antithrombotic Therapy.
| (N=332, mean age 65±13 years, mean follow-up 53±35 months) | ||
|---|---|---|
| Variables | Odds ratio (95%CI) | p value |
| R2CHADS2 score | 1.937 (1.481 - 2.533) | <0.001 |
| Mixed type (time of AF onset) | 2.782 (1.021 - 7.584) | 0.045 |
| Statins | 2.920 (0.922 - 9.246) | 0.068 |
| Chronic AF | 1.778 (0.643 - 4.918) | 0.268 |
| RAAS inhibitors | 1.610 (0.659 - 3.933) | 0.296 |
| LAD (mm) | 1.031 (0.962 - 1.105) | 0.385 |
| Symptomatic AF | 0.634 (0.225 - 1.738) | 0.388 |
| AF history (months) | 1.003 (0.991 - 1.015) | 0.651 |
| Male | 1.199 (0.471 - 3.051) | 0.704 |
| Underlying pulmonary disease | 1.336 (0.252 - 7.081) | 0.734 |
| LVDd (mm) | 1.011 (0.934 - 1.094) | 0.789 |
| LVEF (%) | 0.934 (0.957 - 1.037) | 0.846 |
| Underlying heart disease | 1.015 (0.406 - 2.539) | 0.974 |
| AF recurrence | 1.005 (0.335 - 3.019) | 0.993 |
- A multivariate logistic regression analysis -
AF: atrial fibrillation, RAAS: renin-angiotensin-aldosterone system, LAD: left atrial dimension, LVDd: left ventricular end-diastolic dimension, LVEF: left ventricular ejection fraction
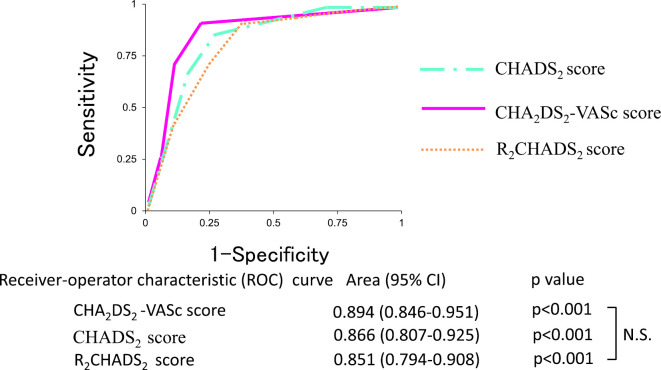
Predictability of IS/SE by CHADS2, CHA2DS2-VASc, and R2CHADS2 score using a receiver operating characteristic (ROC) curve
When the predictability of IS/SE was compared based on the area under the ROC curve, the CHADS2 score was 0.865 (p<0.001), the CHA2DS2-VASc score was 0.899 (p<0.001), and the R2CHADS2 score was 0.851 (p<0.001). All of the parameters were useful for predicting the occurrence of IS/SE. The area under the ROC curve for the CHA2DS2-VASc score was higher than that for the CHADS2 and R2CHADS2 scores, but not to a significant degree (Fig. 5).
Figure 5.
Predictive ability of the CHADS2, CHA2DS2-VASc, and R2CHADS2 scores for ischemic stroke/systemic embolism based on the receiver operating characteristic curve.
Discussion
Major findings of the present study
During antiarrhythmic drug therapy to maintain sinus rhythm in patients with PNVAF, higher CHADS2, CHA2DS2-VASc, and R2CHADS2 scores were associated with higher annual rates of IS/SE in patients with PNVAF not receiving anticoagulant therapy. All three parameters were independent predictors for IS/SE. In addition, the discriminative ability for the incidence of IS/SE in patients with PNVAF was compared by ROC. No significant differences were observed among the parameters, indicating that all score schemes were useful for risk stratification.
A comparison of the CHADS2, CHA2DS2-VASc, and R2CHADS2 scores in NVAF patients
NVAF is a risk factor for IS/SE. The annual rate of ischemic stroke among patients with NVAF (approximately 5%) has been shown to be 2- to 7-fold higher than in subjects without AF (13-15). In general, the CHADS2 score is recommended for the risk stratification of IS/SE or determining whether or not to introduce anticoagulant therapy in patients with NVAF (16-18). The CHADS2 score was estimated as the sum of points obtained after assigning one point each for age ≥75 years, hypertension, diabetes mellitus, and heart failure and two points for previous IS/SE.
The CHA2DS2-VASc score was established in the 2010 ESC guidelines and incorporated other risk factors in addition to those mentioned in the CHADS2 score, such as cardiomyopathy, age 65 to 74 years, a history of myocardial infarction, aortic plaque, vascular disease, and gender (female). The CHA2DS2-VASc score was estimated as the sum of points obtained after assigning one point each for age 65 to 74 years, hypertension, diabetes mellitus, heart failure, vascular disease, and gender (female) and two points each for previous IS/SE and age ≥75 years (19).
The R2CHADS2 score, which accounts for renal dysfunction (Crr <60 mL/min/m2), has also been proposed for the risk stratification of IS/SE (3). Piccini et al. found that the R2CHADS2 score enhanced the stroke risk assessment on the basis of the net reclassification index by 8.2% compared with the CHADS2 score and by 6.2% compared with the CHA2DS2-VASc score (3).
To our knowledge, there have been no studies comparing the CHADS2, CHA2DS2-VASc and R2CHADS2 scores in Japanese patients with PNVAF not receiving anticoagulation therapy. However, our study showed that the CHADS2, CHA2DS2-VASc, and R2CHADS2 scores were all useful for the risk stratification of IS/SE in Japanese patients with PNVAF with no significant differences among the three scores. These results may reflect ethnic differences in stroke risk assessment in patients with NVAF.
Chronic renal failure and cardiovascular complications
Chronic kidney disease (CKD) is usually considered a risk factor for cardiovascular complications. In patients with chronic renal failure, arteriosclerosis is enhanced (20, 21) by vascular inflammation and protein catabolism as well as by poor nutrition. In addition, patients with chronic renal failure have high oxidative stress. It has been reported that oxidative stress can activate several complements, increase vascular endothelial adhesion molecules, deteriorate endothelial NO production via reactive oxygen species, and induce the development of vascular endothelial dysfunction (22, 23). Serum concentrations of asymmetric dimethylarginine [asymmetric dimethyl arginine (ADMA)], an endogenous NOS inhibitor of the release of NO from arginine, have been shown to be increased in patients with not only renal dysfunction but also cerebral infarction (24-26). However, the structure of the brain blood vessels is similar to that of the renal ones from an anatomical perspective (27), which may help analyze the mechanisms underlying the onset of stroke in patients with renal dysfunction. We therefore investigated the relationship between the R2CHADS2 score and IS/SE in patients with NVAF, as the above findings suggest that the R2CHADS2 score, which accounts for renal dysfunction, may be more useful than the CHADS2 score for the risk stratification of IS/SE in patients with NVAF.
Association of chronic renal failure with AF
The 2013 Japanese guideline of chronic renal failure recommend renal dysfunction be considered a risk factor for cardiovascular disease, and a number of previous reports have described the close relationship between AF and chronic renal failure (28-32). Soliman et al. reported that the incidence of AF increased in patients ≥70 years of age with moderate renal dysfunction (average eGFR 43.6 mL/min/m2) when their eGFR was ≤45 mL/min/m2 (33). Furthermore, Watanabe et al. reported that the incidence of AF was newly found in 2,947 of 235,818 patients with chronic renal failure during 4.5 years of follow-up. They further found that the hazard ratio of patients with eGFR 30-59 mL/min/m2 was 1.32 (95% CI: 1.08-1.62), and the hazard ratio of patients with eGFR <30 mL/min/m2 was 1.52 (95% CI: 0.89-2.77) (34). However, the Atherosclerosis Risk in Communities (ARIC) study, which compared the cardiovascular prognosis between patients with and without a history of AF during 10.1 years of follow-up, found that an impaired renal function was not an independent predictor in patients with AF (35), and Roldán et al. reported that the addition of renal dysfunction to the CHADS2 and CHA2DS2-VASc score as a risk factor of cardiovascular events did not improve the predictive probability in patients with AF (36). In the present study, no significant difference was observed among the CHADS2, CHA2DS2-VASc, and R2CHADS2 scores for discriminative ability of IS/SE in Japanese patients with PNVAF, and our results were consistent with those of previous studies.
However, it constitutes the R2 CHADS 2 score is moderate to severe renal dysfunction, so further examination is particularly important in patients with mild renal dysfunction.
Study limitations
Several limitations associated with the present study warrant mention. First, the patient background likely did not conform to the current AF status because the follow-up period in this study was from August 1995 to July 2008. According to a large-scale epidemiological study conducted from 2000 to 2010, the incidence of AF in the Japanese population increased by 1.4, and it is suspected that the incidence of female AF patients may be rapidly increasing in Japan (1). Second, the present study was conducted only in patients with paroxysmal AF, and it is unclear whether or not the same findings would be obtained in patients with persistent and permanent AF. Third, there are a number of methodological limitations hindering physicians from determining when AF actually recurred, and none of the currently available monitoring methods can make a correct evaluation, except for dedicated devices. Finally, the number of patients was relatively small. A large-scale multicenter study should be performed in Japanese patients with NVAF to further evaluate the utility of the CHADS2, CHA2DS2-VASc, and R2CHADS2 scores in risk stratification.
Conclusion
In Japanese patients with PNVAF, the CHADS2, CHA2DS2-VASc, and R2CHADS2 scores were all found to be useful for risk stratification of IS/SE.
The authors state that they have no Conflict of Interest (COI).
Acknowledgement
We thank all of the study participants and the physicians and co-medical staff of Iwate Medical University School. We are also deeply grateful for the direction provided by Professor Ken Okumura (Hirosaki University School of Medicine, Hirosaki, Japan).
References
- 1. Ohsawa M, Okayama A, Sakata K, et al. Rapid increase in estimated number of persons with atrial fibrillation in Japan: an analysis from national surveys on cardiovascular disease in 1980, 1990 and 2000. J Epidemiol 15: 194-196, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dries DL, Exner DV, Gersh BJ, Domanski MJ, Waclawiw MA, Stevenson LW. Atrial fibrillation is associated with an increased risk for mortality and heart failure progression in patients with asymptomatic and symptomatic left ventricular dysfunction A retrospective analysis of the SOLVD trials. J Am Coll Cardiol 32: 695-703, 1998. [DOI] [PubMed] [Google Scholar]
- 3. Piccini JP, Stevens SR, Chang YC, et al. Renal dysfunction as a predictor of stroke and systemic embolism in patient with nonvalvular atrial fibrillation. Circulation 127: 224-232, 2013. [DOI] [PubMed] [Google Scholar]
- 4. Komatsu T, Sato Y, Ozawa M, et al. Relationship between CHADS2 score and efficacy of antiarrhythmic drug therapy in patients with paroxysmal atrial fibrillation. Circ J 77: 639-645, 2013. [DOI] [PubMed] [Google Scholar]
- 5. Komatsu T, Tachibana H, Sato Y, et al. Relationship between CHA(2)DS(2)-VASc scores and ischemic stroke/cardiovascular events in Japanese patients with paroxysmal atrial fibrillation not receiving anticoagulant therapy. J Cardiol 59: 321-328, 2012. [DOI] [PubMed] [Google Scholar]
- 6. Sopher SM, Camm AJ. Atrial fibrillation: maintenance of sinus rhythm versus rate control. Am J Cardiol 77: 24A-37A, 1996. [DOI] [PubMed] [Google Scholar]
- 7. Ogihara T, Kikuchi K, Matsuoka H, et al. The Japanese Society of Hypertension guidelines for the management of hypertension (JSH2009). Hypertens Res 32: 3-107, 2009. [PubMed] [Google Scholar]
- 8. Investigating Committee of Guideline for Diagnosis and Treatment of Hyperlipidemias: Guidelines for diagnosis and treatment of hyperlipidemia in adults. J Jpn Atheroscler 25: 1-34, 1997. [Google Scholar]
- 9. Komatsu T, Tachibana H, Sato Y, et al. Efficacy of amiodarone for preventing the recurrence of symptomatic paroxysmal and persistent atrial fibrillation after cardioversion. Circ J 71: 46-51, 2007. [DOI] [PubMed] [Google Scholar]
- 10. Fuster V, Ryden LE, Cannom DS, et al. ACC/AHA/ESC guidelines for the management of patients with atrial fibrillation: executive summary: A report of the American College/American Heart Association Task Force on Practice guidelines and the European Society of Cardiology Committee for Practice guidelines and policy conferences developed in collaboration with the European heart Rhythm Association and the Heart Rhythm Society. Circulation 114: 700-752, 2006. [Google Scholar]
- 11. Camm AJ, Lip GY, De Caterina R, et al. 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation-developed with the special contribution of the European Heart Rhythm Association. Eur Heart J 33: 2719-2747, 2012. [DOI] [PubMed] [Google Scholar]
- 12. Kodama H, Aisawa Y, Inoue H, et al. (Report of Joint Research Group, Japanese Circulation Society): Guideline for drug treatment of arrhythmia. Circ J 68(Suppl IV): 981-1053, 2004. [Google Scholar]
- 13. Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke 22: 983-988, 1991. [DOI] [PubMed] [Google Scholar]
- 14. Krahn AD, Manfreda J, Tate RB, Mathewson FA, Cuddy TE. The natural history of atrial fibrillation: incidence, risk factors, and prognosis in the Manitoba Follow-Up Study. Am J Med 98: 476-484, 1995. [DOI] [PubMed] [Google Scholar]
- 15. Lévy S, Maarek M, Coumel P, et al. Characterization of different subsets of atrial fibrillation in general practice in France: the ALFA study. The College of French Cardiologists. Circulation 99: 3028-3035, 1999. [DOI] [PubMed] [Google Scholar]
- 16. Go AS, Hylek EM, Chang Y, et al. Anticoagulation therapy for stroke prevention in atrial fibrillation: how well do randomized trials translate into clinical practice? JAMA 290: 2685-2692, 2003. [DOI] [PubMed] [Google Scholar]
- 17. Goldstein LB, Adams R, Alberts MJ, et al. Primary prevention of ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council: cosponsored by the Atherosclerotic Peripheral Vascular Disease Interdisciplinary Working Group; Cardiovascular Nursing Council; Clinical Cardiology Council; Nutrition, Physical Activity, and Metabolism Council; and the Quality of Care and Outcomes Research Interdisciplinary Working Group: the American Academy of Neurology affirms the value of this guideline. Stroke 37: 1583-1633, 2006. [DOI] [PubMed] [Google Scholar]
- 18. Braunwald E, Antman EM, Beasley JW, et al. Guidelines for the management of patients with an increased risk for mortality. Circulation 102: 1193, 2000. [DOI] [PubMed] [Google Scholar]
- 19. Phillippart R, Brunet-Bernard A, Clementy N, et al. Prognostic value of CHA2DS2-VASc score in patients with ‘non-valvular atrial fibrillation' and valvular heart disease: the Loire Valley Atrial Fibrillation Project. Eur Heart J 36: 1822-1830, 2015. [DOI] [PubMed] [Google Scholar]
- 20. Locatelli F, Pozzoni P, Tentori F, Del Vecchio L. Epidemiology of cardiovascular risk in patients with chronic kidney disease. Nephrol Dial Transplant 18(Suppl 7): vii2-vii9, 2003. [DOI] [PubMed] [Google Scholar]
- 21. Pecotis-Filho R, Lindholm B, Stenvinkel P. The malnutrition, inflammation, and atherosclerosis (MIA) syndrome: the heart of the matter. Nephrol Dial Transplant 17(Suppl 11): 28-31, 2002. [DOI] [PubMed] [Google Scholar]
- 22. Gusbeth-Tatomir P, Covic A. Causes and consequences of increased arterial stiffness in chronic kidney disease patients. Kidney Blood Press Res 30: 97-107, 2007. [DOI] [PubMed] [Google Scholar]
- 23. Satoh M, Fujimoto S, Haruna Y, et al. NAD(P)H oxidase and uncoupled nitric oxide synthase are major sources of glomerular superoxide in rats with experimental diabetic nephropathy. Am J Physiol Renal Physiol 288: F1144-F1152, 2005. [DOI] [PubMed] [Google Scholar]
- 24. Ravani P, Tripepi G, Malberti F, Testa S, Mallamaci F, Zoccali C. Asymmetrical dimethylarginine predicts progression to dialysis and death in patients with chronic kidney disease:a competing risks modeling approach. J Am Soc Nephrol 16: 2449-2455, 2005. [DOI] [PubMed] [Google Scholar]
- 25. Yilmaz MI, Saglam M, Caglar K, et al. The determinants of endothelial dysfunction in CKD: oxidative stress and asymmetric dimethylarginine. Am J Kidney Dis 47: 42-50, 2006. [DOI] [PubMed] [Google Scholar]
- 26. Nishiyama Y, Ueda M, Katsura K, et al. Asymmetric dimethylarginine (ADMA) as a possible risk marker for ischemic stroke. J Neurol Sci 290: 12-15, 2010. [DOI] [PubMed] [Google Scholar]
- 27. Kuwabara A, Satoh M, Tomita N, Sasaki T, Kashihara N. Deterioration of glomerular endothelial surface layer induced by oxidative stress is implicated in altered permeability of macromolecules in Zucker fatty rats. Diabetologia 53: 2056-2065, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ito S, Nagasawa T, Abe M, Mori T. Strain vessel hypothesis : a viewpoint for linkage of albuminuria and cerebrocardiovascular risk. Hypertens Res 32: 115-121, 2009. [DOI] [PubMed] [Google Scholar]
- 29. Ansari N, Manis T, Feinfeld DA. Symptomatic atrial arrhythmias in hemodialysis patients. Ren Fail 23: 71-76, 2001. [DOI] [PubMed] [Google Scholar]
- 30. Fabbian F, Catalano C, Lambertini D, et al. Clinical characteristics associated to atrial fibrillation in chronic hemodialysis patients. Clin Nephrol 54: 234-239, 2000. [PubMed] [Google Scholar]
- 31. Genovesi S, Pogliani D, Faini A, et al. Prevalence of atrial fibrillation and associated factors in a population of long-term hemodialysis patients. Am J Kidney Dis 46: 897-902, 2005. [DOI] [PubMed] [Google Scholar]
- 32. Horio T, Iwashima Y, Kamide K, et al. Chronic kidney disease as an independent risk factor for new-onset atrial fibrillation in hypertensive patients. J Hypertens 28: 1738-1744, 2010. [DOI] [PubMed] [Google Scholar]
- 33. Soliman EZ, Prineas RJ, Go AS, et al. Chronic kidney disease and prevalent atrial fibrillation. The Chronic Renal Insufficiency Cohort (CRIC). Am Heart J 159: 1102-1107, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Watanabe H, Watanabe T, Sasaki S, Nagai K, Roden DM, Aizawa Y. Close bidirectional relationship between chronic kidney disease and atrial fibrillation: the Niigata preventive medicine study. Am Heart J 158: 629-636, 2009. [DOI] [PubMed] [Google Scholar]
- 35. Banerjee A, Fauchier L, Vourc'h P, et al. Renal impairment and ischemic stroke risk assessment in patients with atrial fibrillation: the Loire Valley Atrial Fibrillation Project. J Am Coll Cardiol 61: 2079-2087, 2013. [DOI] [PubMed] [Google Scholar]
- 36. Roldán V, Marin F, Manzano-Fernandez S, et al. Does chronic kidney disease improve the predictive value of the CHADS2 and CHA2DS2-VASc stroke stratification risk scores for atrial fibrillation? Thromb Haemost 109: 956-960, 2013. [DOI] [PubMed] [Google Scholar]