Abstract
Objective
An acute exacerbation (AE) of idiopathic pulmonary disease (IPF) represents a life threatening condition. The activities of daily living (ADL) and quality of life of patients who survive an AE of IPF (AE-IPF) are often diminished. However, the association between AE-IPF and the ADL has yet to be evaluated.
To evaluate the effect of AE-IPF on the ADL.
Methods, Patients
Patients treated for AE-IPF from 2010 to 2014 were identified. We retrospectively evaluated their ADL before and after AE-IPF using a modified Barthel index (BI) composed of 6 items.
Results
Twenty-eight of the 47 AE-IPF patients remained alive at 3 months after the onset of AE-IPF. The BI values of 22 survivors (79%) showed a full score (70 points) before the onset of AE-IPF. The evaluation of the BI scores at four weeks after the onset of AE-IPF revealed that the scores of 12 patients had decreased by >15 points and more than half of the survivors showed scores of <55. Logistic regression analyses showed that persistent hypeoxemia at 28 days after an AE, both at exertion (odds ratio, 24.20; 95% confidence interval, 2.42-242.31; p=0.009) and at rest (odds ratio, 21.00; 95% confidence interval, 2.05-215.18; p=0.010), was associated with a >15-point decrease in the BI score at 4 weeks after AE-IPF.
Conclusion
AE-IPF survivors with persistent hypoxemia showed diminished ADL after treatment.
Keywords: acute exacerbation, idiopathic pulmonary fibrosis, activities of daily living, hypoxemia, quality of life
Introduction
Idiopathic pulmonary fibrosis (IPF) is chronic, progressive fibrotic lung disease of unknown cause (1). IPF is fatal, with death typically resulting from a slow decline in the lung function, lung cancer, or an acute exacerbation (AE) (2,3). AE-IPF is defined by the presence of idiopathic diffuse alveolar damage causing the acceleration of fibroproliferative changes. The lack of consensus regarding prompt treatment can be life threatening and is the principal cause of death in IPF patients (4). AE-IPF survivors often have a diminished pulmonary function, activities of daily living (ADL), and quality of life (QOL).
Maintaining the QOL of IPF patients requires an accurate assessment of the extent of ADL impairment after AE-IPF. Although numerous studies have investigated the disturbance and recovery of ADL in people with stable and exacerbated chronic obstructive pulmonary disease (COPD) (5-10), few studies have examined the relationship between IPF and ADL (11,12). Moreover, the optimal approach to assessing the ADL of IPF patients remains a matter of debate. In this retrospective study, we used the Barthel index (BI) (13), an easily calculated and widely accepted measurement, to evaluate the time course of ADL impairment after AE-IPF. The severity of ADL impairment and the factors associated with ADL impairment in AE-IPF patients were also determined.
Materials and Methods
Study design
The changes in the ADL of AE-IPF patients over time (1, 8, 15, and 29 days after AE) were measured using the BI, as described below. Information on the ADL before AE-IPF was obtained from ADL evaluations that were conducted at our hospital within 3 months before the AE. If these evaluations had not been conducted at our hospital, the patients were questioned directly. The clinical findings, laboratory data, and information on the evaluation of the patients' ADL were collected retrospectively from the patients' medical records. Hypoxemia was defined by an SpO2 value of <90%. The presence of hypoxemia at 4 weeks after AE-IPF was assessed using a 6-minute walk test. These data were then used to assess the factors associated with a decreased BI score at 29 days after the onset of AE-IPF.
Patients
We reviewed the records of all patients who received a diagnosis of AE-IPF at Toho University Omori Medical Center during the period from 2010 to 2014. The definitions of IPF and AE are detailed below. There were a total of 47 AE-IPF patients. The 28 patients who were alive at 3 months after the AE were defined as survivors in the analysis.
The diagnosis of IPF
IPF was diagnosed retrospectively based on high-resolution computed tomography (HRCT) images and the histopathological findings from a lung biopsy, according to the American Thoracic Society/European Respiratory Society/Japanese Respiratory Society/Latin American Thoracic Association guidelines (1). Seven patients (15%) underwent surgical lung biopsy before the onset of AE-IPF, and all specimens showed a usual pattern of interstitial pneumonia (UIP). The HRCT of all of the patients were evaluated by 2 pulmonologists (T.I. and S.S.) and 1 chest radiologist (A.K.).
The diagnosis of AE-IPF
The diagnostic criteria for AE-IPF were based on the definition proposed by Collard et al. (4), namely 1) a previous or current diagnosis of IPF, 2) the unexplained worsening or development of dyspnea in the past 30 days, 3) an HRCT scan showing new bilateral ground-glass opacities and/or consolidation superimposed on a background reticular or honeycomb pattern, 4) no evidence of pulmonary infection in the cultures of the patient's bronchoalveolar lavage fluid, endotracheal aspirate, or sputum and negative results on blood tests for other potentially infectious pathogens, and 5) the exclusion of left heart failure, pulmonary embolism, and other possible causes of acute lung injury.
The evaluation of ADL
The ADL was assessed before and after the AE (days 1, 8, 15, and 29) using the BI (13). Although a relationship between the BI and a diminished ADL has not been demonstrated in patients with respiratory disease, we selected this index based on its convenience and wide acceptance, and because it is routinely used to evaluate the ADL of inpatients in our hospital. The BI evaluates the ADL in relation to feeding, transferring, grooming, toilet use, bathing, dressing, walking, bowel/bladder control, and ascending/descending stairs. For the purpose of this study, the categories bowel/bladder control and ascending/descending stairs were removed because they are strongly affected by the severity of respiratory failure and the type of care that is provided-including the extent of bed rest and urethral catheterization. Thus, the modified BI in our study consisted of 7 items (Table 1). The total scores ranged from 0-70, with a higher score indicating better ADL. The changes in the BI score at day 29 in comparison to before the onset of the AE were arbitrarily classified as mild (a decrease of <10 points), moderate (10-15 points), and severe (>15 points).
Table 1.
Barthel Index.
| Feeding | Bathing | |||
| 0 | unable | 0 | dependent | |
| 5 | needs help cutting, spreading butter, etc | 5 | independent (or in shower) | |
| 10 | independent | |||
| Mobility | ||||
| Transfer | 0 | immobile or <50 yards | ||
| 0 | unable, no sitting balance | 5 | wheelchair independent. Including corners, >50 yards | |
| 5 | major help (one or two people, physical), can sit | 10 | walks with help of one person (verbal or physical) >50 yards | |
| 10 | minor help (verbal or physical) | 15 | independent (but may use any aid; for example, stick) >50 yards | |
| Grooming | Dressing | |||
| 0 | needs to help with personal care | 0 | dependent | |
| 5 | independent face/hair/teeth/shaving (implements provided) | 5 | needs help but can do about half unaided | |
| 10 | independent (including buttons, zips, laces, etc) | |||
| Toilet use | ||||
| 0 | dependent | |||
| 5 | needs some help, but can do something alone | |||
| 10 | independent (on and off, dressing, wiping) | |||
The original evaluation criteria for the BI comprise nine items: feeding, transferring, grooming, toilet use, bathing, dressing, walking, bowel/bladder control, and ascending/descending stairs. For this analysis, bowel/bladder control and ascending/descending stairs were excluded.
Statistical analysis
Fisher's exact test or the χ2 test and Mann-Whitney U test were used to compare the characteristics of survivors and non-survivors. The factors associated with a diminished BI score were identified by a logistic analysis. The p values of < 0.05 were considered to indicate statistical significance.
Ethical considerations
This study was reviewed and approved by the Institutional Review Board of Toho University Omori Medical Center (project approval No. 27-166) and was conducted according to the principles expressed in the Declaration of Helsinki.
Results
Patient characteristics
The clinical characteristics, laboratory findings, and treatments of the patients are shown in Table 2. The median age at the AE was 74 years (range, 58-86). Twelve patients received no medical treatment for the chronic progression of IPF before the AE. Diagnostic bronchoalveolar lavage and endotracheal aspiration were not performed in any of the patients because of the severity of their respiratory failure. Recombinant thrombomodulin was used to treat AE-IPF, as described previously (14). There was no significant difference in the treatments administered to the survivors and non-survivors. The survivors showed a higher PaO2/FiO2 ratio at the onset of AE-IPF in comparison to the non-survivors (p=0.008).
Table 2.
Characteristics of the AE-IPF Patients.
| Survived patients (n=28) | Fetal patients (n=19) | p value | |
|---|---|---|---|
| Median age (years old) (range) | 75 (58-86) | 74 (68-85) | 0.914 |
| Never smoker (%) | 4 (15%) | 1 (5%) | 0.635 |
| Male (%) | 24 (86%) | 18 (95%) | 0.635 |
| Pulmonary function before AE-IPF | |||
| %FVC | 77±19 | 73±18 | 0.575 |
| %FEV1.0 | 102±22 | 91±22 | 0.162 |
| %DLCO | 52±17 | 55±14 | 0.571 |
| Treatment before AE-IPF | |||
| CS | 3 (11%) | 5 (26%) | 0.240 |
| Cyclosporine A | 1 (4%) | 2 (11%) | 0.557 |
| Pirfenidone | 5 (18%) | 3 (16%) | 1.000 |
| N-acetylcysteine | 12 (43%) | 6 (32%) | 0.546 |
| No medication | 14 (50%) | 8 (42%) | 0.767 |
| Respiratory failure before AE-IPF | 4 (14%) | 5 (26%) | 0.453 |
| Clinical parameters at AE-IPF onset | |||
| White blood cell (/μL) | 9,800±3,200 | 11,000±2,900 | 0.590 |
| C-reactive protein (mg/dL) | 7.3±5.9 | 8.2±4.6 | 0.174 |
| LDH (IU/L) | 336±70 | 378±145 | 0.198 |
| KL-6 (U/mL) | 1,509±1,135 | 1,418±1,026 | 0.832 |
| SP-D (ng/mL) | 321±201 | 437±348 | 0.144 |
| SP-A (ng/mL) | 91±39 | 87±46 | 0.074 |
| PaO2/FiO2 ratio | 277±88 | 194±111 | 0.008* |
| Medications for AE-IPF | |||
| CS pulse therapy | 27 (100%) | 19 (100%) | 1.000 |
| CS maintenance therapy | 27 (100%) | 19 (100%) | 1.000 |
| Cyclosporine A | 23 (85%) | 15 (79%) | 1.000 |
| Recombinant thrombomodulin | 17 (63%) | 7 (37%) | 0.143 |
| Hypoxemia after AE-IPF | |||
| At rest | 8 (29%) | - | - |
| On exertion | 9 (32%) | - | - |
| Degree of lowering of BI after AE-IPF | |||
| 0 | 6 (21%) | - | - |
| mild (5-15) | 10 (36%) | - | - |
| severe (20-) | 12 (43%) | - | - |
| Median Survival duration after AE-IPF onset (days) (range) | 13 (2-82) | - |
*p<0.01.
AE: acute exacerbation, IPF: idiopathic pulmonary fibrosis, FVC: forced vital capacity, FEV1.0: forced expiratory volume in one second, DLCO: diffusing capacity of the lung for carbon monoxide, CS: corticosteroids, LDH: lactate dehydrogenase
Evaluation of the ADL
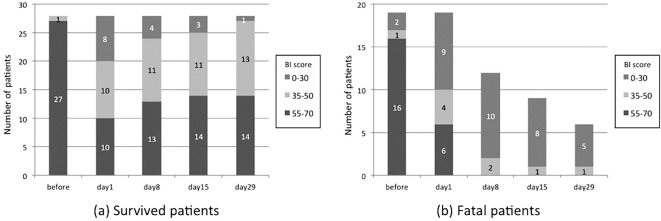
The BI scores before and after AE-IPF are shown in Figs. 1 and 2. The BI scores before the AE were higher among survivors than among non-survivors (p=0.023). At 4 weeks after the onset of AE-IPF, the BI scores of half of the survivors were lower than 55. The BI values of 22 survivors (79%) were highest before the onset of AE-IPF. In comparison to the survivors, a larger proportion of non-survivors had BI values of <35 on the first day of AE-IPF.
Figure 1.
The time course of the total Barthel index (BI) score. The numbers on the bar indicate the number of patients in each BI score range.
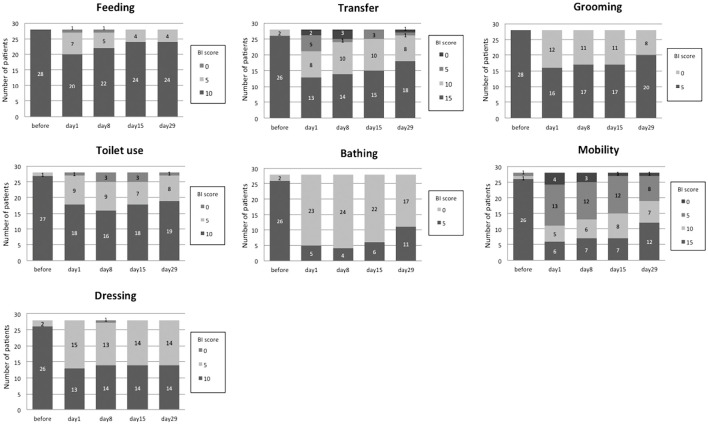
Figure 2.
The time course of the total BI score of the survivors according to the severity of their condition. The numbers on the bars indicate the number of patients for each BI score.
Fig. 2 shows the time course of the BI scores of the survivors. The maximum impairment was observed on days 1 or 8 for all of the ADL items. A majority of patients required some assistance in bathing or mobility at 4 weeks after AE-IPF; however, most required no assistance while eating.
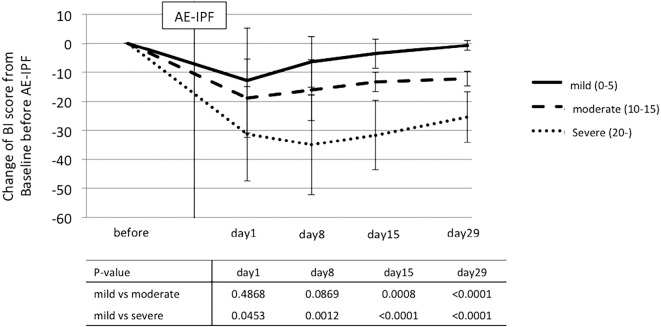
Among the survivors, the severity of ADL impairment on day 29 after the AE was classified as mild, moderate, or severe. The difference in the BI change on days 1, 8, 15, and 29 after AE-IPF in the 3 groups (classified according to severity) are shown in Fig. 3. The BI value of the severe group, but not the other groups, was decreased on day 8. The BI score of the severe group on day 8 and the moderate group on day 15 showed significant differences in comparison to the mild group.
Figure 3.
The difference in the time courses of the BI scores of the survivors according to the BI score change on day 29. The p values for each date indicate the significance of the difference in the BI change between the 2 groups.
The factors associated with severe ADL impairment after AE-IPF
The BI scores of 12 patients changed by >15 points, indicating the severe impairment of their ADL. A previous study of stroke patients reported that a 20-point increase in the BI value reflects the need for daily assistance (15). We evaluated age, the PaO2/FiO2 ratio, the serum LDH titer, and the presence of hypoxemia (before and 28 days after the AE) as risk factors for severe ADL impairment. The results are shown in Table 3. A logistic regression analysis showed that severe ADL impairment was associated with hypoxemia on exertion [odds ratio (OR), 24.20; 95% confidence interval (CI), 2.42-242.31; p=0.009] and at rest (OR, 21.00; 95% CI, 2.05-215.18; p=0.010) at 28 days after AE. The presence of hypoxemia before AE-IPF was not associated with severe ADL impairment (OR, 5.00; 95% CI, 0.44-55.63; p=0.190).
Table 3.
Univariate Logistic Analysis of the Risk for Lowering BI Score More than 15 Points 4 Weeks after AE-IPF.
| OR (95%CI) | p value | |
|---|---|---|
| Age | 1.04 (0.92-1.18) | 1.040 |
| Minimum PaO2/FiO2 ratio | 1.01 (0.99-1.03) | 0.079 |
| Respiratory failure | ||
| On exertion | 24.20 (2.42-242.31) | 0.009** |
| At rest | 21.00 (2.05-215.18) | 0.010* |
| Before AE-IPF | 5.00 (0.45-55.63) | 0.190 |
| LDH | 0.99 (0.98-1.00) | 0.139 |
*p<0.05, **p<0.01.
Hypoxemia was defined as an SpO2 value <90%. Hypoxemia at exertion and at rest was assessed using a 6-minute walk test on day 29 after AE-IPF. All patients with hypoxemia before AE-IPF had an SpO2 value<90% at rest.
AE: acute exacerbation, IPF: idiopathic pulmonary fibrosis, LDH: lactate dehydrogenase
Discussion
Chronic lung diseases resulting in respiratory failure, dyspnea, and cough have a considerable adverse effect on a patient's QOL and ADL, through the slow progression of respiratory impairment and the exacerbation of disease. Although data have accumulated on the ADL of COPD patients in recent years, few studies have evaluated the ADL of IPF patients, and most such studies have focused on chronic impairment following an AE (11,12). This is the first report on the relationship between AE-IPF and the ADL.
We found that the ADL of IPF patients was affected by the persistence of hypoxemia after AE-IPF. The ADL scores of IPF patients are related to their Medical Research Council dyspnea grade and lung function (12), which indicates that the chronic progression of IPF impairs the ADL and suggests that an acute decline in the pulmonary function after AE-IPF reduces the ADL in proportion to the severity of impairment. Our present findings suggest that AE-IPF patients who show hypoxemia - which reflects severe disease progression after treatment - require additional medical and social support, to maintain or improve their ADL.
The health-related consequences of an AE of COPD (AE-COPD) have been more extensively investigated than the health-related consequences of AE-IPF. Because of its adverse effects on the cognitive function and ADL, AE-COPD can reduce a patient's QOL (5,9,16). Disturbances in a patient's physical activities after AE-COPD tend to resolve within 3 weeks of the onset of the exacerbation (9). In addition to this spontaneous remission of the adverse impact on the patient's ADL, pulmonary rehabilitation, which is a cornerstone in the management of stable disease (17), has been shown to improve patients' activity limitations and QOL in clinical trials (18,19) and a meta-analysis (20). However, the decrease in the ADL of AE-IPF patients is usually irreversible, as indicated by the persistent decrease in the ADL scores of the patients in the present study, particularly those with respiratory failure. In addition, Kozu et al. reported that pulmonary rehabilitation was less effective for IPF patients (11). This difference in the recovery after AE-IPF may be due to pathological fibrotic changes that affect the respiratory function. Greater control of the medical and social environment may be necessary after AE-IPF in patients with respiratory failure, due to the persistent decrease in their performance of ADL.
The present study is associated with several limitations. First, it was a retrospective study of a small number of patients. Because a patient's ADL can be affected by many factors, such as the care environment as well as their cognitive function and physical condition, a larger sample size is needed to increase the accuracy of the analysis. A second limitation is that it is unclear whether the BI is an appropriate instrument for evaluating the ADL in IPF patients. In patients with COPD, the ADL is evaluated by disease-specific indexes such as the University of California, San Diego Shortness of Breath Questionnaire (SOBQ) (21), a modified version of the Pulmonary Functional Status and Dyspnea Questionnaire (PFSDQ-M) (22,23), and the London Chest ADL Scale (LCADL) (24). However, no such index has been created or modified to evaluate the ADL of IPF patients. A final limitation is that a longer observational period might be necessary to clarify the trends in the impairment of physical activities. A study of COPD cases revealed that the performance of ADL improved after a patient was discharged from hospital (10); however, the survivors in the present study all remained hospitalized from days 1 to 29 after the onset of AE-IPF.
AE-IPF was associated with ADL impairment. Thus, to maintain the QOL of AE-IPF survivors with persistent hypoxemia, it may be necessary to pay more attention to the factors that disturb their ADL.
The authors state that they have no Conflict of Interest (COI).
Financial Support
This research was partially supported by the Practical Research Project for Rare Intractable Diseases from the Japan Agency for Medical Research and Development, AMED and by a grant from the Ministry of Health, Labour and Welfare of Japan awarded to the Study Group on Diffuse Pulmonary Disorders, Scientific Research/Research on intractable diseases.
References
- 1. Raghu G, Collard HR, Egan JJ, et al. . An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med 183: 788-824, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ley B, Collard HR, King TE Jr. Clinical course and prediction of survival in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 183: 431-440, 2011. [DOI] [PubMed] [Google Scholar]
- 3. Natsuizaka M, Chiba H, Kuronuma K, et al. . Epidemiologic survey of Japanese patients with idiopathic pulmonary fibrosis and investigation of ethnic differences. Am J Respir Crit Care Med 190: 773-779, 2014. [DOI] [PubMed] [Google Scholar]
- 4. Collard HR, Moore BB, Flaherty KR, et al. . Acute exacerbations of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 176: 636-643, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Clini EM, Crisafulli E, Costi S, et al. . Effects of early inpatient rehabilitation after acute exacerbation of COPD. Respir Med 103: 1526-1531, 2009. [DOI] [PubMed] [Google Scholar]
- 6. Bendstrup KE, Ingemann Jensen J, Holm S, Bengtsson B. Out-patient rehabilitation improves activities of daily living, quality of life and exercise tolerance in chronic obstructive pulmonary disease. Eur Respir J 10: 2801-2806, 1997. [DOI] [PubMed] [Google Scholar]
- 7. Skumlien S, Hagelund T, Bjortuft O, Ryg MS. A field test of functional status as performance of activities of daily living in COPD patients. Respir Med 100: 316-323, 2006. [DOI] [PubMed] [Google Scholar]
- 8. Bourbeau J, Ford G, Zackon H, Pinsky N, Lee J, Ruberto G. Impact on patients' health status following early identification of a COPD exacerbation. Eur Respir J 30: 907-913, 2007. [DOI] [PubMed] [Google Scholar]
- 9. Ehsan M, Khan R, Wakefield D, et al. . A longitudinal study evaluating the effect of exacerbations on physical activity in patients with chronic obstructive pulmonary disease. Ann Am Thorac Soc 10: 559-564, 2013. [DOI] [PubMed] [Google Scholar]
- 10. Tsai LL, Alison JA, McKenzie DK, McKeough ZJ. Physical activity levels improve following discharge in people admitted to hospital with an acute exacerbation of chronic obstructive pulmonary disease. Chron Respir Dis 13: 23-32, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kozu R, Senjyu H, Jenkins SC, Mukae H, Sakamoto N, Kohno S. Differences in response to pulmonary rehabilitation in idiopathic pulmonary fibrosis and chronic obstructive pulmonary disease. Respiration 81: 196-205, 2011. [DOI] [PubMed] [Google Scholar]
- 12. Kozu R, Jenkins S, Senjyu H. Evaluation of activity limitation in patients with idiopathic pulmonary fibrosis grouped according to Medical Research Council dyspnea grade. Arch Phys Med Rehabil 95: 950-955, 2014. [DOI] [PubMed] [Google Scholar]
- 13. Mahoney FI, Barthel DW. Functional evaluation: the Barthel index. Md State Med J 14: 61-65, 1965. [PubMed] [Google Scholar]
- 14. Isshiki T, Sakamoto S, Kinoshita A, Sugino K, Kurosaki A, Homma S. Recombinant human soluble thrombomodulin treatment for acute exacerbation of idiopathic pulmonary fibrosis: a retrospective study. Respiration 89: 201-207, 2015. [DOI] [PubMed] [Google Scholar]
- 15. Granger CV, Sherwood CC, Greer DS. Functional status measures in a comprehensive stroke care program. Arch Phys Med Rehabil 58: 555-561, 1977. [PubMed] [Google Scholar]
- 16. Dodd JW, Charlton RA, van den Broek MD, Jones PW. Cognitive dysfunction in patients hospitalized with acute exacerbation of COPD. Chest 144: 119-127, 2013. [DOI] [PubMed] [Google Scholar]
- 17. Casaburi R, ZuWallack R. Pulmonary rehabilitation for management of chronic obstructive pulmonary disease. N Engl J Med 360: 1329-1335, 2009. [DOI] [PubMed] [Google Scholar]
- 18. Murphy N, Bell C, Costello RW. Extending a home from hospital care programme for COPD exacerbations to include pulmonary rehabilitation. Respir Med 99: 1297-1302, 2005. [DOI] [PubMed] [Google Scholar]
- 19. Seymour JM, Moore L, Jolley CJ, et al. . Outpatient pulmonary rehabilitation following acute exacerbations of COPD. Thorax 65: 423-428, 2010. [DOI] [PubMed] [Google Scholar]
- 20. Puhan MA, Gimeno-Santos E, Scharplatz M, Troosters T, Walters EH, Steurer J. Pulmonary rehabilitation following exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev CD005305, 2011. [DOI] [PubMed] [Google Scholar]
- 21. Eakin EG, Resnikoff PM, Prewitt LM, Ries AL, Kaplan RM. Validation of a new dyspnea measure: the UCSD Shortness of Breath Questionnaire. University of California, San Diego. Chest 113: 619-624, 1998. [DOI] [PubMed] [Google Scholar]
- 22. Lareau SC, Meek PM, Roos PJ. Development and testing of the modified version of the pulmonary functional status and dyspnea questionnaire (PFSDQ-M). Heart Lung 27: 159-168, 1998. [DOI] [PubMed] [Google Scholar]
- 23. Lareau SC, Meek PM, Press D, Anholm JD, Roos PJ. Dyspnea in patients with chronic obstructive pulmonary disease: does dyspnea worsen longitudinally in the presence of declining lung function? Heart Lung 28: 65-73, 1999. [DOI] [PubMed] [Google Scholar]
- 24. Miravitlles M, Llor C, de Castellar R, Izquierdo I, Baro E, Donado E. Validation of the COPD severity score for use in primary care: the NEREA study. Eur Respir J 33: 519-527, 2009. [DOI] [PubMed] [Google Scholar]