Abstract
Objective
To assess the correlation between the angiographic appearance of cerebral collateral pathways or the degree of internal carotid artery stenosis (ICAS) and reduced cerebrovascular reactivity (CVR) estimated by single-photon emission computed tomography (SPECT) image analysis in patients with unilateral ICAS.
Methods
A retrospective analysis was performed in 42 patients with unilateral ICAS who underwent cerebral angiography and acetazolamide-challenged SPECT of the brain. Cerebral blood flow quantitation was performed using the quantitative SPECT/dual-table autoradiography method. The CVR in the middle cerebral artery (MCA) territory was evaluated using the stereotactic extraction estimation based on the Japanese extracranial-intracranial bypass trial (SEE-JET) program and classified as reduced (<18.4%) or non-reduced (≥18.4%). Angiographic collateralization was classified as circle of Willis (type 1), extracranial-intracranial (type 2), and leptomeningeal (type 3). The degree of ICAS was defined as severe (≥70% stenosis) or non-severe (<70%).
Results
Eight patients showed reduced CVR, including 6 (46%) of 13 with type 3 collaterals and 2 (7%) of 29 without type 3 collaterals (p=0.006). In contrast, type 1 and type 2 collaterals and severe ICAS were not significantly associated with reduced CVR.
Conclusion
In patients with unilateral ICAS, leptomeningeal collaterals are strongly correlated with reduced CVR in the MCA territory, which presumably increases the risk of cerebral hyperperfusion after carotid artery stenting (CAS). Therefore, these findings may be clinically applicable to the perioperative management of CAS.
Keywords: cerebral angiography, cerebral blood flow, QSPECT/DTARG, SEE-JET, vasomotor reactivity
Introduction
The utility of acetazolamide (ACZ)-challenged single-photon emission computed tomography (SPECT) of the brain for evaluating the cerebrovascular reactivity (CVR) has been established in many studies (1-11). One recent study suggested that the evaluation of CVR using SPECT is useful for predicting the development of post-carotid endarterectomy (CEA) hyperperfusion (11). In addition, a reduced CVR as measured by SPECT is increasingly being reported as a predictor of cerebral hyperperfusion after carotid artery stenting (CAS) (8, 9). Cerebral hyperperfusion after CAS is associated with increased morbidity and mortality; therefore, the prediction of its occurrence is of clinical value. Several reports have described a correlation between a reduction in the CVR and the angiographic appearance of cerebral collateral pathways or the degree of carotid steno-occlusive lesions (4, 5, 10, 12-17). Determining the strength of this correlation may be useful for identifying patients with reduced CVR based on cerebral and carotid angiograms and may aid in the perioperative management of CAS, as patients with reduced CVR can be promptly managed more strictly to prevent cerebral hyperperfusion after CAS.
Most studies of reduced CVR have included patients with carotid artery occlusion who are not eligible for CAS (4, 5, 10, 12-17) and have used various modalities, such as positron emission tomography (PET) (12, 13), SPECT (4, 5, 10), xenon-enhanced computed tomography (Xe-CT) (14), perfusion computed tomography (CT) (17), and transcranial Doppler ultrasonography (TCD) (15, 16), for the evaluation of cerebral hemodynamics. In studies using SPECT (4, 5, 10), the methods of cerebral blood flow (CBF) measurement and the analytical techniques for processing SPECT images differed among studies, which is an inherent drawback. Therefore, in this study, we evaluated the CVR using an accurate SPECT image analysis technique, in which the CBF quantitation was performed with quantitative SPECT/dual-table autoradiography (QSPECT/DTARG), thereby enabling the accurate determination of the CBF (18-21). Image data obtained from QSPECT/DTARG were then examined using the stereotactic extraction estimation based on the Japanese extracranial-intracranial bypass trial (SEE-JET) program. This approach can automatically analyze data for the whole brain in the stereotactic space and calculate the CVR for each cerebral artery territory objectively and reproducibly (22).
To our knowledge, no studies have assessed the correlation between the angiographic appearances of cerebral collateral pathways or the degree of carotid artery stenosis and the CVR estimated by an accurate SPECT image analysis exclusively in patients with carotid artery stenosis. The aim of this study was to evaluate the correlation between cerebral collateral patterns or the degree of internal carotid artery stenosis (ICAS) and reduced CVR in patients with unilateral ICAS.
Materials and Methods
Patient population
Between April 2010 and March 2014, 76 patients with ICAS were evaluated with cerebral angiography, and 50 of these patients were assessed using ACZ-challenged SPECT of the brain. Eight patients with occlusion or stenosis (≥30%) of the contralateral ICA or stenosis (≥30%) of the distal segment of the ipsilateral ICA were excluded from the study. The remaining 42 patients (39 men, 3 women; age 62 to 90 years, mean 74 years) were retrospectively reviewed. The intervals between cerebral angiography and SPECT were 1, 2, 4, and 6 months in 30, 9, 2, and 1 patients, respectively. Sixteen patients presented with minor stroke and four with transient ischemic attacks relevant to the ICAS. Twenty-two patients exhibited asymptomatic ICAS. On magnetic resonance imaging (MRI), none of the patients showed evidence of severe stenosis, occlusion of the basilar artery, or large cortical infarction. This study was approved by the ethics committee of our hospital.
CBF studies
Quantitative CBF and CVR in response to an ACZ challenge were assessed by SPECT using iodine[123]-N-isopropyl-p-iodoamphetamine (IMP) following the DTARG protocol (18). A rotating dual-headed gamma camera (E.CAM) and an LMEGP parallel collimator (both from Toshiba Medical Systems, Tokyo, Japan) were used. The energy range centered on 158 keV with a width of 20%, and 2-min data collection per 180° was performed 14 times in continuous mode. The matrix size was 64×64 pixels. Dynamic SPECT was performed for 28 minutes with 1 rotation of 2 minutes performed twice: the first scan as the resting state was acquired from 0 to 28 minutes, and the second scan with ACZ challenge was acquired from 30 to 58 minutes. At 4 minutes per frame, each of the 2 dynamic scan periods required 7 frames.
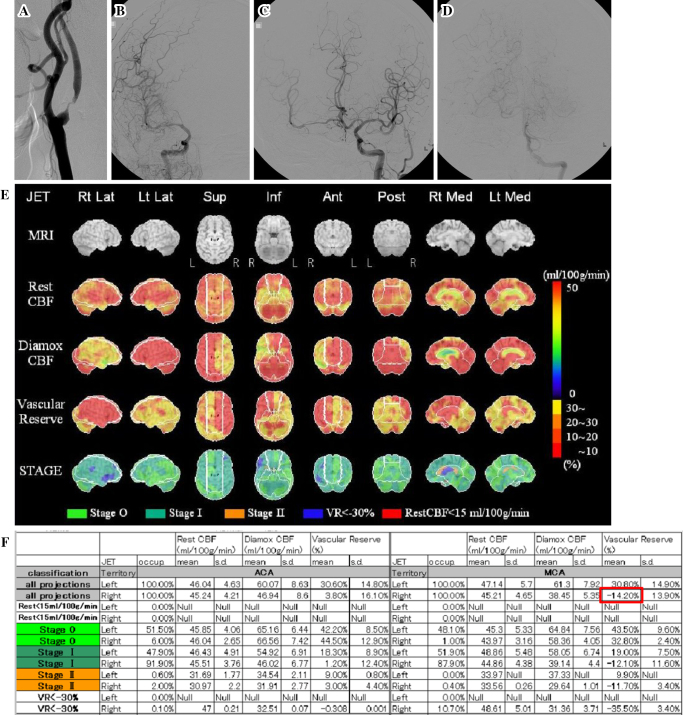
A dose of IMP (111 MBq) was intravenously infused twice over a 1-min period at 0 and 30 minutes. ACZ (15 mg/kg) was administered intravenously 20 minutes after the first IMP injection. At 10 minutes after the first IMP injection, 2.5 mL of arterial blood was obtained from the femoral artery, and the standard input function was calibrated. Whole-blood radioactivity was measured using a well counter cross-calibrated to the SPECT apparatus. CBF quantitation was performed with the QSPECT/DTARG method, which automatically and accurately corrects attenuated absorption and scattered radiation (19-21). SPECT image processing was performed with the SEE-JET software program (Figure E) (22) using quantitative image data obtained via the QSPECT/DTARG method at rest and after ACZ challenge with three-dimensional stereotactic surface projection (3D-SSP) (23). In 3D-SSP, the image tilt of individual subjects is adjusted and linearly converted into 3D stereotaxic coordinates (Talairach standard brain) through the anterior commissure-posterior commissure line. After anatomic standardization, brain surface extraction is performed with the maximum pixel value shown in the vertical direction in the cerebral cortex from a prescribed brain surface on the stereotaxic coordinate system in the cortex of a standard brain (24). The SEE-JET program can automatically measure the CBF values at rest and after an ACZ challenge for the territories of the anterior cerebral artery (ACA), middle cerebral artery (MCA), and posterior cerebral artery (PCA), and can also automatically calculate the CVR for all cerebral coordinate systems. The CVR was defined as (CBF after ACZ challenge - CBF at rest) / CBF at rest ×100 (%).
Figure.
A 74-year-old man presented with transient left hemiparesis caused by severe right internal carotid artery (ICA) stenosis. The patient displayed two types of collateral flow: circle of Willis collaterals and leptomeningeal collaterals. The cerebrovascular reactivity (CVR) of the whole ipsilateral middle cerebral artery (MCA) territory was classified as “reduced” status (CVR <18.4%). A: Lateral view of the right carotid angiogram showing severe stenosis at the origin of the ICA. B: Anteroposterior (AP) view of the late arterial phase of the right carotid angiogram showing insufficient opacification of the right MCA territory. C: AP view of the left carotid angiogram demonstrating opacification of the right MCA territory via the anterior communicating artery. D: AP view of the left vertebral angiogram showing partial opacification of the right MCA territory via leptomeningeal anastomosis from the posterior cerebral artery. E: Stereotactic extraction estimation based on Japanese extracranial-intracranial bypass trial (SEE-JET) images. The top row shows the surface anatomy of the brain on magnetic resonance imaging, and the lower four columns show three-dimensional stereotactic surface projection format views of the cerebral blood flow (CBF) at rest (Rest CBF), CBF after acetazolamide challenge (Diamox CBF), CVR (Vascular Reserve, VR), and severity of hemodynamic cerebral ischemia (STAGE), from left to right. The CVR is defined as (Diamox CBF-Rest CBF)/Rest CBF ×100 (%). In this case, the CVR was noticeably reduced in the right MCA territory. F: Physiological data analyzed by the SEE-JET program for the anterior cerebral artery and MCA territories. The VR in the right MCA territory was -14.20% (red frame).
In this study, the mean CVR in the whole MCA territory was evaluated (Figure F) because the CVR in the territory of the ACA on the affected side was strongly influenced by collateral flow via the anterior communicating artery from the contralateral side, and the CVR in the territory of the PCA was supplied mainly by the vertebrobasilar artery. A reduced CVR was defined as <18.4%, because defining the mean CVR in the whole MCA territory using a cut-off value of 18.4% was shown to have high sensitivity and specificity for predicting the development of post-CEA hyperperfusion in 500 patients with ipsilateral ICAS (11), based on a 3D stereotactic region of interest (ROI) template (3DSRT) analysis (25) of SPECT image data obtained using the ARG method (26). A 3DSRT analysis allows for the fully automated designation of ROIs in the entire brain and the calculation of the regional CBF and can estimate the CVR in the MCA territory objectively and reproducibly, similarly to the SEE-JET analysis. We therefore used the cut-off value of 18.4% in this study in a large number of patients with ICAS.
Cerebral angiography
Digital subtraction angiography (DSA) was performed on a biplane Integris Allura (Philips Healthcare, Best, The Netherlands). Utilizing a 4F radial and/or femoral artery sheath (Radifocus Introducer II; Terumo, Tokyo, Japan), selective injections were made using a 4F catheter (MS-K; Medikit, Tokyo, Japan and/or OK-2M; Gadelius Medical, Tokyo, Japan) into the bilateral common carotid arteries and at least the unilateral vertebral arteries in all 42 patients. Images were acquired after automated injection (Mark V ProVis auto-injector; Medrad, Indianola, USA) of the following doses of iopamidol (Iopamiron 300; Bayer Healthcare, Berlin, Germany), a nonionic contrast agent: 8 mL into the common carotid artery at 6 mL/s, and 5 mL into the vertebral artery at 3 mL/s. The frame rates were 3 frames/s in the arterial and capillary phases and 2 frames/s in the venous phase (Figure A-D). Collateral flow patterns supplying the MCA territory were classified as type 1, circle of Willis collaterals (via the circle of Willis from the anterior and/or posterior communicating arteries; Figure C); type 2, extracranial-intracranial (EC-IC) collaterals (via the ipsilateral ophthalmic artery from the external carotid artery, the only EC-IC pathway in the study); or type 3, leptomeningeal collaterals (via anastomotic channels across the surface of the brain from adjacent vascular territories; Figure D). A patient with more than one collateral flow pattern was counted as one in a univariate analysis of the factors associated with reduced CVR (Table 1).
Table 1.
Factors with a Potential Association with Reduction of CVR in the MCA Territory.
| Reduced CVR (n=8) | Non-reduced CVR (n=34) | p value | |
|---|---|---|---|
| Age (year), mean±SD | 73.4±8.6 | 74.1±5.2 | 0.688 |
| Male sex, n (%) | 8 (100) | 31 (91) | NS |
| Hypertension, n (%) | 6 (75) | 30 (88) | 0.319 |
| Diabetes mellitus, n (%) | 5 (63) | 10 (29) | 0.110 |
| Dyslipidemia, n (%) | 4 (50) | 19 (56) | NS |
| Degree of ICA stenosis (%), mean±SD | 85.8±11.4 | 71.6±14.8 | 0.019 |
| Collateral flow, n (%) | 6 (75) | 12 (35) | 0.056 |
CVR: cerebrovascular reactivity, ICA: internal carotid artery, MCA: middle cerebral artery, NS: not significant
The degree of ICAS was calculated using cerebral DSA with the method described in the North American Symptomatic Carotid Endarterectomy Trial (NASCET) (27, 28). Patients were divided into two groups based on the degree of ICAS according to the NASCET criteria: severe (≥70% stenosis) and non-severe (<70% stenosis). All angiograms were reviewed independently by a neurointerventional radiologist (K. M.) and neurologist (H. S.) who were blinded to information from the SPECT studies.
Statistical analysis
Descriptive statistics are expressed as the mean±standard deviation (SD). Fisher's exact test was used for categorical variables, and a Mann-Whitney U test was used for continuous variables. A p value of <0.05 was considered significant. All analyses were performed using SPSS ver. 18.0 (IBM, Somers, USA).
Results
The study population consisted of 42 patients with unilateral ICAS. The mean degree of ICAS was 74.3±15.1%, with a range of 38% to 95%. Cerebral collateral pathways were observed in 18 (43%) of 42 patients, including 5 with only type 1 collaterals, 5 with only type 3 collaterals, 5 with both type 1 and 3 collaterals, and 3 with all three collateral types. The mean CVR in the whole MCA territory was 42.4±27.1%, with a range of -26.1% to 89.7%. The CVR was reduced in eight patients.
The results of a univariate analysis of the factors related to a reduction in the CVR are shown in Table 1. There were no significant differences in the age, sex, collateral flow, or rates of hypertension, diabetes mellitus, or dyslipidemia among the patients with and without reduced CVR. However, the degree of ICAS was more severe in patients with reduced CVR than in those with a non-reduced CVR (85.8% vs. 71.6%, p=0.019).
Correlations between the CVR and collateral flow patterns are shown in Table 2. Only type 3 (leptomeningeal) collaterals were significantly associated with reduced CVR. The CVR was reduced in 6 of 13 patients with type 3 collaterals and in 2 of 29 patients without type 3 collaterals (46% vs. 7%, p=0.006). The correlation between the CVR and the degree of ICAS is shown in Table 3. The CVR was reduced in 7 of 27 patients with angiographic evidence of ≥70% ICAS and in 1 of 15 patients with angiographic evidence of <70% ICAS, but the difference between the groups was not significant (26% vs. 7%, p=0.222).
Table 2.
Correlation between CVR and Collateral Flow Patterns in the MCA Territory.
| Collateral type | Reduced CVR (n=8) | Non-reduced CVR (n=34) | p value |
|---|---|---|---|
| Circle of Willis collaterals | |||
| Visible (n=13) | 3 | 10 | 0.686 |
| Invisible (n=29) | 5 | 24 | |
| EC-IC collaterals | |||
| Visible (n=3) | 0 | 3 | NS |
| Invisible (n=39) | 8 | 31 | |
| Leptomeningeal collaterals | |||
| Visible (n=13) | 6 | 7 | 0.006 |
| Invisible (n=29) | 2 | 27 |
CVR: cerebrovascular reactivity, EC-IC: extracranial-intracranial, MCA: middle cerebral artery, NS: not significant
Table 3.
Correlation between CVR of the MCA Territory and ICA Stenosis.
| Degree of stenosis at the origin of the ICA | Reduced CVR (n=8) | Non-reduced CVR (n=34) | p value |
|---|---|---|---|
| <70% (n=15) | 1 | 14 | 0.222 |
| ≥70% (n=27) | 7 | 20 |
CVR: cerebrovascular reactivity, ICA: internal carotid artery, MCA: middle cerebral artery
Discussion
Our results show that the presence of leptomeningeal collaterals on cerebral angiography was robustly correlated with a reduction of the CVR of the ipsilateral MCA territory in patients with unilateral ICAS, based on a SEE-JET analysis of SPECT image data obtained using the QSPECT/DTARG method. These results may be clinically informative for the perioperative management of CAS, since if leptomeningeal collaterals are confirmed by cerebral angiography, preventive measures such as strict control of the blood pressure for cerebral hyperperfusion after CAS can be implemented perioperatively.
The evaluation of cerebral hemodynamic status is important in patients with carotid steno-occlusive diseases and has been performed using modalities such as PET (12, 13), SPECT (1-11), Xe-CT (14), perfusion CT (17), perfusion MRI (29), and TCD (15, 16, 30-33). However, the techniques used for the image data analysis differ among these studies, and each technique has drawbacks. For example, in many studies (1, 3, 4, 6-9, 12, 14, 17), the image data analysis was performed using the ROI method, which is commonly used in conventional image data analyses but has poor reproducibility because of the operator-dependent selection of the ROI. In other studies (2, 4, 5), the CVR was evaluated qualitatively using a color scale, but the results lacked accuracy and could easily be influenced by personal bias.
In this study, we analyzed the CVR using SPECT. Several studies (1-11) have shown that SPECT can assess the CVR and cerebral perfusion with good sensitivity, and recent studies (8, 9) have suggested that a reduced CVR may be a predictor of cerebral hyperperfusion after CAS. One difference from these studies is that we used the QSPECT/DTARG method for the accurate evaluation of the quantitative CBF and analyzed the image data using the SEE-JET program. This program can reproducibly perform a CVR assessment of the whole cerebral artery territory with an automatic analysis that is independent of the operator (18-22).
Cerebral collateral flow patterns are generally classified as primary and secondary, depending on the autoregulatory capacity. Circle of Willis collaterals are primary and can rapidly compensate for decreased cerebral perfusion pressure, whereas collateral flow via the ophthalmic artery and flow via leptomeningeal vessels is secondary (15, 17, 30). The presence of secondary collaterals on TCD is correlated with a compromised hemodynamic condition, as indicated by relatively low CO2 reactivity (15) and a lower percentage increase of the MCA mean blood velocity after ACZ challenge (30). Leptomeningeal collaterals on cerebral angiography have also been associated with cerebral hemodynamic impairment measured by PET (12), SPECT (5), and Xe-CT (14). In addition, a recent study (17) found that secondary collaterals on cerebral angiography were significantly correlated with severe hemodynamic impairment, as indicated by an increased cerebral blood volume and a delayed time to peak in the ipsilateral hemisphere on perfusion CT. These findings suggest that leptomeningeal collaterals are associated with a severely compromised flow status of the brain. However, these studies (5, 12, 14, 15, 17, 30) were performed in patients with carotid artery diseases, including occlusion.
To date, only a few reports have assessed the correlation between cerebral collateral patterns and cerebral hemodynamics in patients with carotid artery stenosis (4, 31, 33). Those studies showed a poor correlation between the collateral pathways and CVR, which is inconsistent with our results. However, the evaluation of leptomeningeal collaterals was limited in these studies. In particular, two of the studies used TCD (31, 33), which has a limited ability to detect distal branches of intracranial vessels, despite being a useful tool for detecting cerebral collateral patterns. In contrast, we evaluated three types of cerebral collateral pathways, including leptomeningeal collaterals, using cerebral angiography. Although invasive, this method is considered to be the gold standard for evaluating the anatomy of the collateral circulation, particularly for leptomeningeal collaterals (16, 17, 34). This may account for our finding of a significant correlation between leptomeningeal collaterals and the reduced CVR in patients with ICAS.
Regarding the correlation between the cerebral hemodynamics and the degree of carotid stenosis, we defined severe stenosis as ≥70% according to the NASCET criteria, which is similar to the definitions in other studies (5, 16, 32). Using this approach, we noted no significant correlation between severe carotid stenosis and a reduction in the CVR (<18.4%). Powers et al. (12, 13) also found a poor correlation between the degree of carotid stenosis and cerebral hemodynamic status in a PET study, but other reports have suggested a good correlation between the CVR measured by SPECT and the degree of carotid steno-occlusive lesions estimated by cerebral angiography (4, 5). One recent study (10) found that the CVR estimated by SPECT imaging, with a SEE-JET analysis of image data obtained with the ARG method, was moderately correlated with the degree of ICAS. Some studies using TCD for evaluation of cerebral hemodynamics have found that severe (≥70%) carotid stenosis is significantly associated with cerebral vasomotor reactivity impairment, indicated by a lower percentage increase in the mean MCA velocity after ACZ injection (16) and a low breath-holding index (32). The differences in the results of these studies may be due to differences in factors such as the study design, techniques, and imaging modalities.
There are several limitations associated with the current study, including its retrospective design and the inclusion of only a small number of patients. Given the risk of selective cerebral arteriography, six-vessel cerebral angiography was not used for the evaluation of the collateral circulation. In addition, our results cannot be applied to patients with severe stenosis of the bilateral ICAs or occlusion of the contralateral ICA because the development of collateral flow in those patients would differ from that in patients with only unilateral ICAS. Finally, although reduced CVR was defined as <18.4%, the true threshold of CVR as a predictor of cerebral hyperperfusion after CAS has yet to be established. Further studies are needed to clarify these issues.
Conclusion
This is the first study to assess the correlation between the angiographic appearance of cerebral collateral pathways or the degree of ICAS and the CVR estimated by SPECT imaging in patients with unilateral ICAS, using an analysis with the SEE-JET program and image data obtained via QSPECT/DTARG. The results showed that the presence of leptomeningeal collaterals on cerebral angiography is strongly correlated with reduced CVR of the ipsilateral MCA territory in these patients. These findings may aid in the perioperative management of CAS.
The authors state that they have no Conflict of Interest (COI).
References
- 1. Russell D, Dybevold S, Kjartansson O, Nyberg-Hansen R, Rootwelt K, Wiberg J. Cerebral vasoreactivity and blood flow before and 3 months after carotid endarterectomy. Stroke 21: 1029-1032, 1990. [DOI] [PubMed] [Google Scholar]
- 2. Cikrit DF, Burt RW, Dalsing MC, et al. Acetazolamide enhanced single photon emission computed tomography (SPECT) evaluation of cerebral perfusion before and after carotid endarterectomy. J Vasc Surg 15: 747-753, 1992. [PubMed] [Google Scholar]
- 3. Kuroda S, Kamiyama H, Abe H, Houkin K, Isobe M, Mitsumori K. Acetazolamide test in detecting reduced cerebral perfusion reserve and predicting long-term prognosis in patients with internal carotid artery occlusion. Neurosurgery 32: 912-918, 1993. [DOI] [PubMed] [Google Scholar]
- 4. Hosoda K, Fujita S, Kawaguchi T, Shose Y, Shibata Y, Tamaki N. Influence of degree of carotid artery stenosis and collateral pathways and effect of carotid endarterectomy on cerebral vasoreactivity. Neurosurgery 42: 988-994, 1998. [DOI] [PubMed] [Google Scholar]
- 5. Ozgur HT, Kent Walsh T, Masaryk A, et al. Correlation of cerebrovascular reserve as measured by acetazolamide-challenged SPECT with angiographic flow patterns and intra- or extracranial arterial stenosis. AJNR Am J Neuroradiol 22: 928-936, 2001. [PMC free article] [PubMed] [Google Scholar]
- 6. Hosoda K, Kawaguchi T, Shibata Y, et al. Cerebral vasoreactivity and internal carotid artery flow help to identify patients at risk for hyperperfusion after carotid endarterectomy. Stroke 32: 1567-1573, 2001. [DOI] [PubMed] [Google Scholar]
- 7. Ogasawara K, Yukawa H, Kobayashi M, et al. Prediction and monitoring of cerebral hyperperfusion after carotid endarterectomy by using single-photon emission computerized tomography scanning. J Neurosurg 99: 504-510, 2003. [DOI] [PubMed] [Google Scholar]
- 8. Kaku Y, Yoshimura S, Kokuzawa J. Factors predictive of cerebral hyperperfusion after carotid angioplasty and stent placement. AJNR Am J Neuroradiol 25: 1403-1408, 2004. [PMC free article] [PubMed] [Google Scholar]
- 9. Iwata T, Mori T, Tajiri H, Nakazaki M. Predictors of hyperperfusion syndrome before and immediately after carotid artery stenting in single-photon emission computed tomography and transcranial color-coded real-time sonography studies. Neurosurgery 68: 649-655, 2011. [DOI] [PubMed] [Google Scholar]
- 10. Tomura N, Otani T, Koga M, Ishiyama K. Correlation between severity of carotid stenosis and vascular reserve measured by acetazolamide brain perfusion single photon emission computed tomography. J Stroke Cerebrovasc Dis 22: 166-170, 2013. [DOI] [PubMed] [Google Scholar]
- 11. Oshida S, Ogasawara K, Saura H, et al. Does preoperative measurement of cerebral blood flow with acetazolamide challenge in addition to preoperative measurement of cerebral blood flow at the resting state increase the predictive accuracy of development of cerebral hyperperfusion after carotid endarterectomy? Results from 500 cases with brain perfusion single-photon emission computed tomography study. Neurol Med Chir (Tokyo) 55: 141-148, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Powers WJ, Press GA, Grubb RL Jr, Gado M, Raichle ME. The effect of hemodynamically significant carotid artery disease on the hemodynamic status of the cerebral circulation. Ann Intern Med 106: 27-34, 1987. [DOI] [PubMed] [Google Scholar]
- 13. Powers WJ. Cerebral hemodynamics in ischemic cerebrovascular disease. Ann Neurol 29: 231-240, 1991. [DOI] [PubMed] [Google Scholar]
- 14. Smith HA, Thompson-Dobkin J, Yonas H, Flint E. Correlation of xenon-enhanced computed tomography-defined cerebral blood flow reactivity and collateral flow patterns. Stroke 25: 1784-1787, 1994. [DOI] [PubMed] [Google Scholar]
- 15. Hofmeijer J, Klijn CJ, Kappelle LJ, Van Huffelen AC, Van Gijn J. Collateral circulation via the ophthalmic artery or leptomeningeal vessels is associated with impaired cerebral vasoreactivity in patients with symptomatic carotid artery occlusion. Cerebrovasc Dis 14: 22-26, 2002. [DOI] [PubMed] [Google Scholar]
- 16. Russell SM, Woo HH, Siller K, Panasci D, Leroux PD. Evaluating middle cerebral artery collateral blood flow reserve using acetazolamide transcranial Doppler ultrasound in patients with carotid occlusive disease. SurgNeurol 70: 466-470, 2008. [DOI] [PubMed] [Google Scholar]
- 17. Cheng XQ, Tian JM, Zuo CJ, Liu J, Zhang Q, Lu GM. Quantitative perfusion computed tomographymeasurements of cerebral hemodynamics: correlation with digital subtraction angiography identified primary and secondary cerebral collaterals in internal carotid artery occlusive disease. Eur J Radiol 81: 1224-1230, 2012. [DOI] [PubMed] [Google Scholar]
- 18. Kim KM, Watabe H, Hayashi T, Ishiyama K. Quantitative mapping of basal and vasareactive cerebral blood flow using split-dose 123I-iodoamphetamine and single photon emission computed tomography. Neuroimage 33: 1126-1135, 2006. [DOI] [PubMed] [Google Scholar]
- 19. Iida H, Narita Y, Kado H, et al. Effects of scatter and attenuation correction on quantitative assessment of regional cerebral blood flow with SPECT. J Nucl Med 39: 181-189, 1998. [PubMed] [Google Scholar]
- 20. Iida H, Nakagawara J, Hayashida K, et al. Multicenter evaluation of a standardized protocol for rest and acetazolamide cerebral blood flow assessment using a quantitative SPECT reconstruction program and split-dose 123I-iodoamphetamine. J Nucl Med 51: 1624-1631, 2010. [DOI] [PubMed] [Google Scholar]
- 21. Yoneda H, Shirao S, Koizumi H, et al. Reproducibility of cerebral blood flow assessment using a quantitative SPECT reconstruction program and split-dose 123I-iodoamphetamine in institutions with different γ-cameras and collimators. J Cereb Blood Flow Metab 32: 1757-1764, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mizumura S, Nakagawara J, Takahashi M, et al. Three-dimensional display in staging hemodynamic brain ischemia for JET study: objective evaluation using SEE analysis and 3D-SSP display. Ann Nucl Med 18: 13-21, 2004. [DOI] [PubMed] [Google Scholar]
- 23. Minoshima S, Frey KA, Koeppe RA, Foster NL, Kuhl DE. A diagnostic approach in Alzheimer's disease using three-dimensional stereotactic surface projections of fluorine-18-FDG PET. J Nucl Med 36: 1238-1248, 1995. [PubMed] [Google Scholar]
- 24. Minoshima S, Koeppe RA, Frey KA, Kuhl DE. Anatomic standardization: linear scaling and nonlinear warping of functional brain images. J Nucl Med 35: 1528-1537, 1994. [PubMed] [Google Scholar]
- 25. Takeuchi R, Yonekura Y, Matsuda H, Konishi J. Usefulness of a three-dimensional stereotaxic ROI template on anatomically standardised 99mTc-ECD SPET. Eur J Nucl Med Mol Imaging 29: 331-341, 2002. [DOI] [PubMed] [Google Scholar]
- 26. Iida H, Itoh H, Nakazawa M, et al. Quantitative mapping of regional cerebral blood flow using iodine-123-IMP and SPECT. J Nucl Med 35: 2019-2030, 1994. [PubMed] [Google Scholar]
- 27. North American, Symptomatic Carotid, Endarterectomy Trial, Steering Committee. North American Symptomatic Carotid Endarterectomy Trial. Methods, patient characteristics, and progress. Stroke 22: 711-720, 1991. [DOI] [PubMed] [Google Scholar]
- 28. North American Symptomatic Carotid Endarterectomy Trial Collaborators Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N Engl J Med 325: 445-453, 1991. [DOI] [PubMed] [Google Scholar]
- 29. Mandell DM, Han JS, Poublanc J, et al. Mapping cerebrovascular reactivity using blood oxygen level-dependent MRI in Patients with arterial steno-occlusive disease: comparison with arterial spin labeling MRI. Stroke 39: 2021-2028, 2008. [DOI] [PubMed] [Google Scholar]
- 30. Müller M, Schimrigk K. Vasomotor reactivity and pattern of collateral blood flow in severe occlusive carotid artery disease. Stroke 27: 296-299, 1996. [DOI] [PubMed] [Google Scholar]
- 31. Telman G, Kouperberg E, Sprecher E, Hoffman A, Yarnitsky D. Assessment of ophthalmic artery collateral pathway in the hemispheric cerebral hemodynamics in patients with severe unilateral carotid stenosis. Neurol Res 25: 309-311, 2003. [DOI] [PubMed] [Google Scholar]
- 32. Krdžić I, Čovičković-Šternić N, Katsiki N, Isenović ER, Radak Ð. Correlation of carotid artery disease severity and vasomotor response of cerebral blood vessels. Angiology 66: 481-487, 2015. [DOI] [PubMed] [Google Scholar]
- 33. Prokin AL, Slankamenac P, Kovačević P, Kaloci SR, Živanović ŽD. Cerebral vasomotor reactivity and apnea test in symptomatic and asymptomatic high-grade carotid stenosis. Srp Arh Celok Lek 143: 520-524, 2015. [PubMed] [Google Scholar]
- 34. Romero JR, Pikula A, Nguyen TN, Nien YL, Norbash A, Babikian VL. Cerebral collateral circulation in carotid artery disease. Curr Cardiol Rev 5: 279-288, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]