Abstract
A 54-year-old man was treated with mycophenolate mofetil (MMF) after undergoing living donor renal transplantation. Two years later, he experienced repeated episodes of diarrhea, and his C-reactive protein (CRP) level was found to be 12.63 mg/dL. Ileocolonoscopy showed multiple deep, punched-out ulcers that were similar to Behçet's disease (BD) and cytomegalovirus (CMV) in the ileum. CMV infection was suspected. However, anti-cytomegalovirus agents were ineffective. The patient was subsequently diagnosed with gastrointestinal toxicity of MMF and MMF was switched to mizoribine. His symptoms improved immediately, and his CRP level normalized. Six months later, the patient's mucosa was healed.
Keywords: mycophenolate mofetil, gastrointestinal toxicity, ileal ulcer, renal transplantation
Introduction
Mycophenolate mofetil (MMF) is widely used for immunosuppression therapy in organ transplant recipients. Although its association with gastrointestinal (GI) toxicity (including diarrhea) is well known, there have been few reports of the associated endoscopic findings. We herein present the case of a patient who developed deep ulcers in the ileum and who improved after the withdrawal of MMF.
Case Report
A 54-year-old man underwent living donor renal transplantation in 20XX, after which he was treated with MMF (1 g/day), tacrolimus (3 mg/day), and methylprednisolone (4 mg/day). His relevant history included cholecystectomy at 44 years of age. His father was diagnosed with pulmonary tuberculosis. He had no history of non-steroidal anti-inflammatory drug use.
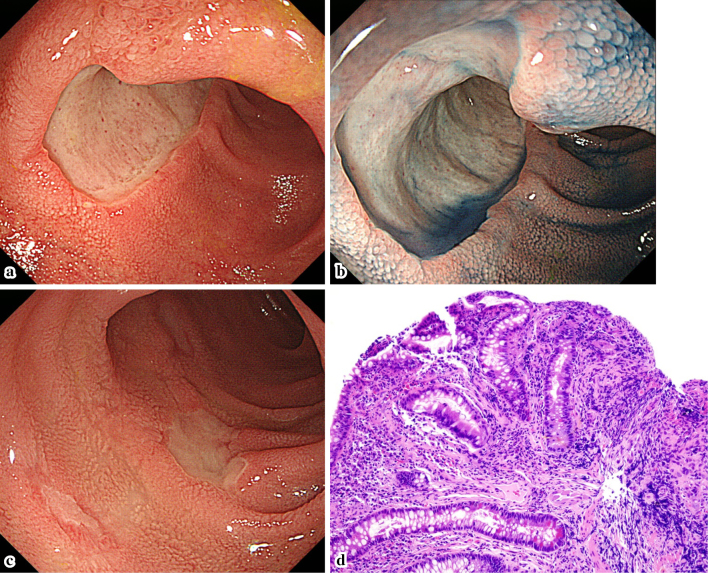
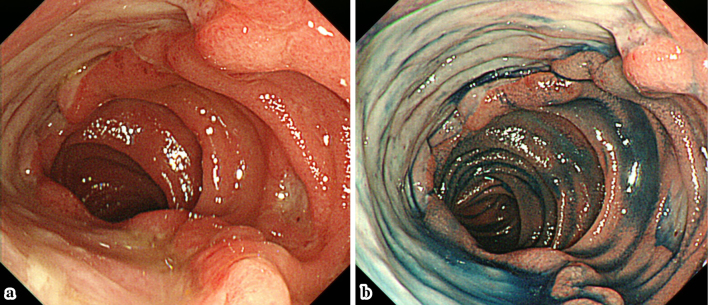
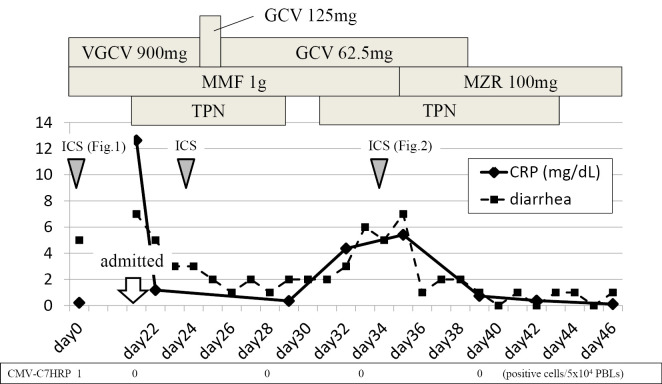
Two years after the transplant, the patient developed watery diarrhea, which occurred 5-6 times a day. Ileocolonoscopy showed multiple deep ulcers in the ileum (Fig. 1a-c). The pathological findings showed mild crypt distortion (Fig. 1d). Interferon gamma release assays (T-SPOTⓇ, Oxford Immunotec, Abingdon, UK) were positive, but a mucosal culture and a PCR for tuberculosis were both negative. Immunostaining for cytomegalovirus (CMV) was negative, and CMV-C7HRP-positive cells were present at only at one cell per 5×104 peripheral blood leukocytes in the patient's serum; however, CMV infection was suspected based on the form of the ulcer. However, treatment with valganciclovir (VGCV 900 mg/day) for 22 days was ineffective, and his symptoms worsened. He was then admitted to our hospital on day 22. At the time, his vital signs were normal and skin, eyes, and genitals were not involved; however, the frequency of his diarrhea episodes had increased to >10 times/day and the laboratory data showed a highly elevated C-reactive protein (CRP) level (12.63 mg/dL). On the other hand, the patient was negative for CMV-C7HRP. The other laboratory data are shown in Table. Total parenteral nutrition (TPN) improved his symptoms and CRP levels. However, bloody stools were seen and the ulcer grew larger on day 24. The antiviral agents were then switched to ganciclovir (GCV, 125 mg on the first day, and 62.5 mg daily thereafter). Oral feeding was started on day 29, and soon his symptoms and CRP level worsened. On day 32, the ulcer in the ileum became even larger and deeper (Fig. 2). Because the clinical course was considered to be unusual for a CMV infection, GI toxicity of MMF was suspected, and the patient was switched from MMF to mizoribine (MZR, 100 mg/day). His symptoms improved immediately, and his CRP level normalized. Six months later, the ileal mucosa was healed (Fig. 3). The patient's clinical course is shown in Fig. 4.
Figure 1.
The endoscopic and pathological findings on admission. a-c: The endoscopic findings on admission. Multiple punched-out ulcers were seen in the ileum. d: The pathological findings. Mild crypt distortion was seen.
Table.
Laboratory Data on Admission.
| WBC | 6,370/μL | TP | 6.11 g/dL | CRP | 12.63 mg/dL |
| STAB | 12.0% | ALB | 3.55 g/dL | ||
| SEG | 77.0% | T-bil | 0.53 mg/dL | MMF | 2.3 μg/mL(1.0-3.0) |
| LYMP | 3.0% | AST | 22.4 IU/L | Tacrolimus | 8.6 ng/mL |
| MONO | 5.0% | ALT | 12.9 IU/L | ||
| EOS | 3.0% | ALP | 436 IU/L | CMV-C7HRP : negative | |
| RBC | 466×104/μL | γ-GTP | 34.5 IU/L | IGRAs : positive | |
| Hb | 13.8 g/dL | LDH | 268 IU/L | Blood culture : negative | |
| HCT | 39.5% | BUN | 15.6 mg/dL | Stool culture : normal flora | |
| MCV | 84.8 fL | Cr | 1.43 mg/dL | ||
| MCH | 29.6 pg | ||||
| MCHC | 34.9% | ||||
| PLT | 27.0×104/μL | ||||
MMF: mycophenolate mofetil, CMV: cytomegalovirus, IGRAs: interferon-gamma release assays
Figure 2.
The endoscopic findings one month after the administration of antiviral agents. a, b: One month after the initiation of antiviral therapy. The patient’s ulcers were observed to have become bigger and deeper.
Figure 3.
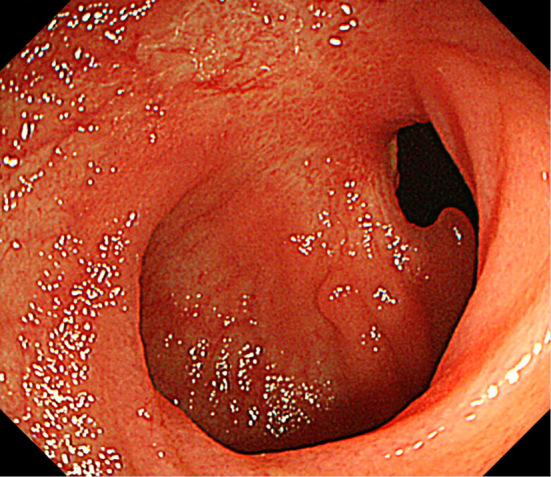
Endoscopic findings at six months after the withdrawal of MMF. The ileal mucosa was observed to have healed. MMF: mycophenolate mofetil
Figure 4.
The clinical course of the present case. The patient’s CMV-C7HRP level became negative after the administration of antiviral therapy; however, his diarrhea and CRP levels worsened with the start of oral feeding. The symptoms improved and a negative CRP level was achieved by switching MMF to MZR. VGCV: valganciclovir, GCV: ganciclovir, MMF: mycophenolate mofetil, MZR: mizoribine, TPN: total parenteral nutrition, ICS: ileo-colonoscopy, PBLs: peripheral blood leukocytes
Discussion
Combination therapy with prednisolone, tacrolimus, and MMF is recommended as the first-line immunosuppressive treatment to prevent rejection in patients after organ transplantation (1). GI toxicity is a well-known side effect of MMF. Diarrhea, which occurs in 8.3% of cases, is the most frequent symptom (2). The mechanism of this side effect is thought to involve an acyl glucuronide of mycophenolic acid (MPA), one of the MMF metabolites, which was found to induce the production of pro-inflammatory cytokines such as IL-6 and TNF-alpha (3).
The pathological examination of the intestinal ulcers associated with MMF reveals various findings, including neutrophil infiltration, crypt abscess, crypt distortion, crypt loss, and epithelial apoptosis (4-8). These findings are non-specific, and the condition cannot be diagnosed based on the pathological findings. In comparison, far fewer reports have described the endoscopic findings. To the best our knowledge, there have only been two case reports. One showed multiple shallow ulcers in the colon (8); the other showed a longitudinal ulcer similar to Crohn's disease in the colon (9). In the present case, endoscopy revealed deep and punched-out ulcers that were similar to Behçet's disease (BD), simple ulcer (SU), or CMV infection. In fact - despite the patient's low CMV-C7HRP level - CMV infection was initially suspected based on the form of the ulcer. The patient's CMV C7-HRP level became negative after the administration of antiviral agents, but the ulcer worsened. Furthermore, immunostaining for CMV was negative. This series of events made CMV infection unlikely. GCV and MZR are known to show anti-CMV activity and synergism, and it is difficult to rule out CMV infection completely; however, we think that it is reasonable to suggest that the patient's CMV infection was subclinical and that GI toxicity of MMF was the highly likely cause of the patient's symptoms (10).
In Japan, MZR is most often used for post-transplant patients who cannot tolerate MMF due to its side effects; thus, MMF was discontinued and MZR was started (11). In the present case, we did not observe any signs that were suggestive of BD, such as skin, oral, or genital lesions. There have been some reports of the development of oral ulcers as an adverse effect of MMF (12, 13). If oral ulcers had been seen in the present patient, it might have been difficult to distinguish his condition from BD.
The endoscopic appearance of an SU is quite similar to the appearance of an intestinal ulcer of BD. A MEDLINE search of the literature up to February 2017, which was performed using the search terms “simple ulcer” and “mycophenolate mofetil or mizoribine”, yielded no reports. Thus, there seems to be no relationship between SU and MMF or MZR.
Some intestinal ulcers associated with MMF may be difficult to distinguish from BD, SU or CMV infection. It is not possible to make an accurate diagnosis based on the pathological findings and it is important to be aware that the form of ulcers associated with MMF might vary.
The authors state that they have no Conflict of Interest (COI).
Acknowledgement
The authors would like to thank all of the staff of Oita University Hospital who took care of the patient.
References
- 1. Chadban SJ, Barraclough KA, Campbell SB, et al. KHA-CARI guideline: KHA-CARI adaptation of the KDIGO Clinical Practice Guideline for the Care of Kidney Transplant Recipients. Nephrology (Carlton) 17: 204-214, 2012. [DOI] [PubMed] [Google Scholar]
- 2. Iida M, Fukuda T, Ikegame K, et al. Use of mycophenolate mofetil in patients received allogeneic hematopoietic stem cell transplantation in Japan. Int J Hematol 93: 523-531, 2011. [DOI] [PubMed] [Google Scholar]
- 3. Wieland E, Shipkova M, Schellhaas U, et al. Induction of cytokine release by the acyl glucuronide of mycophenolic acid: a link to side effects? Clin Biochem 33: 107-113, 2000. [DOI] [PubMed] [Google Scholar]
- 4. Maes BD, Dalle I, Geboes K, et al. Erosive enterocolitis in mycophenolate mofetil-treated renal-transplant recipients with persistent afebrile diarrhea. Transplantation 75: 665-672, 2003. [DOI] [PubMed] [Google Scholar]
- 5. Parfitt JR, Jayakumar S, Driman DK. Mycophenolate mofetil-related gastrointestinal mucosal injury: variable injury patterns, including Graft-Versus-Host Disease-Like Changes. Am J Surg Pathol 33: 1355-1363, 2009. [DOI] [PubMed] [Google Scholar]
- 6. Al-Absi AI, Cooke CR, Wall BM, et al. Patterns of Injury in Mycophenolate mofetil-Related Colitis. Transplant Proc 42: 3591-3593, 2010. [DOI] [PubMed] [Google Scholar]
- 7. Liapis G, Boletis J, Skalioti C, et al. Histological spectrum of mycophenolate mofetil-related colitis: association with apoptosis. Histopathology 63: 649-658, 2013. [DOI] [PubMed] [Google Scholar]
- 8. Liu TC, Amorosino MS, Cerda S, Farraye FA. Mycophenolate mofetil-associated enterocolitis. Gastrointest Endosc 63: 707-708, 2006. [DOI] [PubMed] [Google Scholar]
- 9. Dost D, van Leerdam ME, van Dekken H, et al. Crohn's-like enterocolitis associated with mycophenolic acid treatment. Gut 57: 1330, 2008. [DOI] [PubMed] [Google Scholar]
- 10. Kuramoto T, Daikoku T, Yoshida Y, et al. Novel anticytomegalovirus activity of immunosuppressant mizoribine and its synergism with ganciclovir. J Pharmacol Exp Ther 333: 816-821, 2010. [DOI] [PubMed] [Google Scholar]
- 11. Kalluri HV, Hardinger KL. Current state of renal transplant immunosuppression: present and future. World J Transplant 2: 51-68, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Naranjo J, Poniachik J, Cisco D, et al. Oral ulcers produced by mycophenolate mofetil in two liver transplant Patients. Transplant Proc 39: 612-614, 2007. [DOI] [PubMed] [Google Scholar]
- 13. Savas N, Sevmis S, Karakayali H, Yilmaz U, Haberal M. Orogenital ulcers in a liver transplant recipient: discerning between mycophenolate-mofetil-induced complication and Behcet's disease. Clin Transplant 23: 147-149, 2009. [DOI] [PubMed] [Google Scholar]