Abstract
The efficacy of repeated lusutrombopag administration for thrombocytopenia in patients with chronic liver disease who undergo two or more planned invasive procedures is unknown. We herein report our findings regarding the effects of repeated lusutrombopag administration given to avoid platelet transfusion in a patient with chronic liver disease and thrombocytopenia. The platelet count showed a positive response to lusutrombopag treatment prior to the initial invasive procedure to treat a hepatoma, so platelet transfusion was not necessary. In conclusion, lusutrombopag may be a useful drug for patients with thrombocytopenia to avoid platelet transfusion in those undergoing two or more planned invasive procedures.
Keywords: lusutrombopag, thrombocytopenia, chronic liver disease, invasive procedure, hepatocellular carcinoma
Introduction
Treatment for hepatocellular carcinoma (HCC), the sixth-most frequent cancer in the world (1), is often invasive, with surgical resection, transcatheter arterial chemoembolization (TACE), and percutaneous radiofrequency ablation (RFA) often used, except for cases that receive systemic administration of a molecular-targeted agent (e.g. sorafenib). In the patients receiving these treatment, local tumor recurrence is frequently encountered (27% after 5 years and 36.9% after 10 years) (2), often necessitating repeated treatment for HCC.
Thrombocytopenia is the most common hematological abnormality encountered in patients with chronic liver disease (CLD) (3-5). Although mild to moderate thrombocytopenia, with a platelet count ranging from 50,000 to 150,000/μL, rarely causes spontaneous bleeding during an invasive procedure (e.g. a percutaneous liver biopsy, liver transplantation), severe thrombocytopenia (platelet count <50,000/μL) can significantly increase the risk of bleeding (3), thereby leading to a cancellation or delay of a scheduled invasive procedure (4).
Lusutrombopag (MulpletaⓇ) is an oral thrombopoietin receptor agonist (TPO-RA) developed by Shionogi & Company (Osaka, Japan), to reduce the need for platelet transfusion in patients with CLD who are scheduled to undergo an invasive procedure and was approved for use in Japan on September 28, 2015. In a phase 3 trial of lusutrombopag given to CLD patients scheduled for a single invasive procedure in Japan, 3 mg was administered once daily for 7 days prior to the procedure, and the findings were reported (6, 7). Eltrombopag, a thrombopoietin-receptor agonist similar to lusutrombopag, was also found to be effective in increasing the platelet count when administered over three cycles in patients with chronic immune thrombocytopenia (ITP) (8). However, the efficacy of repeated lusutrombopag administration for thrombocytopenia in patients with CLD who undergo two or more planned invasive procedures is unknown.
We herein report our findings regarding the efficacy of repeated lusutrombopag administration to avoid platelet transfusion in a patient with CLD and thrombocytopenia who showed a positive response prior to undergoing the initial invasive procedure.
Case Report
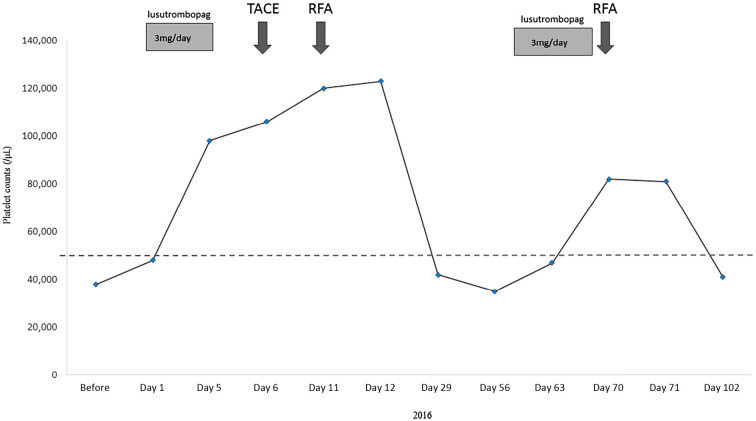
A 62-year-old Japanese woman with a history of HCC treatment and hepatitis C virus infection was referred to us for liver cirrhosis. One year earlier, her HCC had been initially treated by ultrasound-guided RFA after the patient underwent platelet transfusion, as she had chronic thrombocytopenia (platelets <50,000/μL) due to liver cirrhosis. The recurrence of an HCC nodule in segment 4 of the liver was diagnosed by dynamic computed tomography (CT) in January 2016 (Fig. 1A). At the time of the diagnosis of recurrence, the liver cirrhosis was staged as Child B, and the platelet count was 38,000/μL (Table). Therefore, we prescribed the daily oral administration of 3 mg of lusutrombopag beginning 1 week prior to recurrent HCC treatment. Her platelet count increased to 98,000/μL, well over the criterion for lusutrombopag discontinuation (50,000/μL), at 5 days after beginning lusutrombopag administration, so it was discontinued (Fig. 2). Treatment for HCC was performed using TACE and RFA without platelet transfusion (Fig. 1B), because the platelet count gradually increased to 123,000/μL by day 11 after beginning lusutrombopag administration (Fig. 2).
Figure 1.
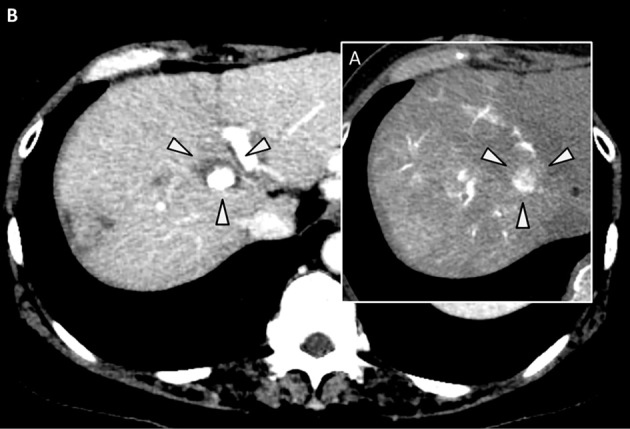
A: Arterial phase of dynamic CT imaging. The arrowheads show HCC recurrence in the fourth segment of the liver. B: Arterial phase of dynamic CT imaging. The arrowheads show the area of ablation by RFA. The central high- density area represents lipiodol retention by HCC after TACE. HCC: hepatocellular carcinoma, RFA: percutaneous radiofrequency ablation, TACE: transcatheter arterial chemoembolization
Table.
Laboratory Results on Admission.
| WBCs | 1,770 | /μL (3,800-8,600) | TP | 6.7 | g/dL (6.6-8.1) |
| RBCs | 3.97×106 | /μL (386-492) | Albumin | 3.2 | g/dL (4.1-5.1) |
| Hb | 13.3 | g/dL (11.6-14.8) | Total bilirubin | 2.4 | mg/dL (1.4-1.5) |
| Platelets | 3.8×104 | /μL (15.8-34.8) | Direct bilirubin | 0.9 | mg/dL (0.1-0.5) |
| %Prothrombin | 59.1 | % (70-130) | AST | 63 | U/L (13-30) |
| PT-INR | 1.35 | (0.90-1.10) | ALT | 57 | U/L (7-23) |
| LDH | 320 | U/L (124-222) | |||
| ALP | 357 | U/L (106-322) | |||
| GGT | 20 | U/L (9-32) | |||
| NH3 | 53 | μg/dL (19-54) | |||
| AFP | 5 | ng/mL (<20) | |||
| DCP | 21 | mAU/mL (<40) |
Normal ranges are shown in parentheses.
WBCs: white blood cells, RBCs: red blood cells, PT-INR: international normalized ratio of prothrombin time, TP: total protein, AST: aspartate aminotransferase, ALT: alanine aminotransferase, ALP: alkaline phosphatase, GGT: γ-glutamyltransferase, AFP: alpha-fetoprotein, DCP: des-gamma-carboxy prothrombin
Figure 2.
Clinical course. Initially, 3 mg of lusutrombopag was administered each day from Day 1 to Day 5. The platelet count increased to 98,000/μL on Day 5, so the administration was discontinued. As a second treatment, the administration of lusutrombopag was restarted from Day 60 to Day 66. TACE: transcatheter arterial chemoembolization, RFA: percutaneous radiofrequency ablation
In April 2016, a new hypervascular nodule in segment 5 of the liver was detected by dynamic computed tomography (CT) (Fig. 3A). The administration of lusutrombopag was restarted because the platelet count at the time of the diagnosis of HCC recurrence was 35,000/μL. The administration was continued for the full seven-day period, as the platelet count did not exceed the criterion for lusutrombopag discontinuation at five days after beginning the administration. However, at the end of the administration period, the count was 82,000/μL, and the serum FDP was 3.5 μg/mL, both within the respective normal ranges. Therefore, we treated the patient's recurrent HCC by RFA without platelet transfusion (Fig. 2, 3B). There were no adverse events, such as portal thrombus, during either of the treatment procedures. Portal thrombus was also not detected by dynamic CT after RFA.
Figure 3.
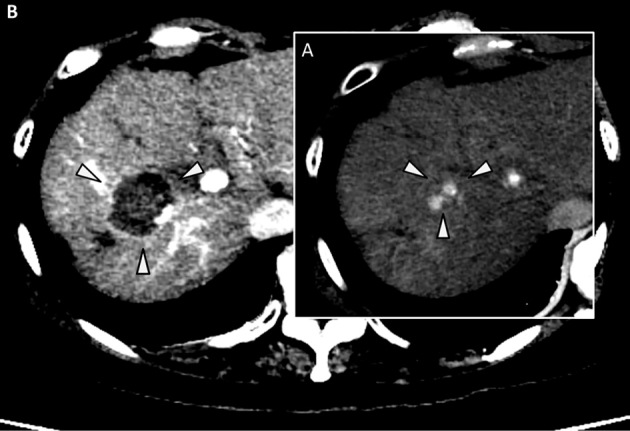
A: Arterial phase of dynamic CT imaging. The arrowheads show HCC recurrence in the fifth segment of the liver. B: Arterial phase of dynamic CT imaging. The arrowheads show the area of ablation by RFA. HCC: hepatocellular carcinoma, RFA: percutaneous radiofrequency ablation
Discussion
To our knowledge, this is the first report to show the efficacy of repeated administration of lusutrombopag in a patient with CLD who underwent two planned invasive procedures (RFA and TACE) for HCC. Lusutrombopag is a TPO-RA that acts selectively on the human TPO receptor and activates signal transduction pathways that promote the proliferation and differentiation of bone marrow progenitor cells into megakaryocytes, thereby increasing the platelet count (6, 7). When given at 3 mg once a day for 7 days in patients with thrombocytopenia scheduled to undergo invasive procedures, lusutrombopag was found to significantly reduce the need for platelet transfusions compared to a placebo in a randomized, double blind, placebo-controlled multicenter phase 3 trial (IapicCTI-132323) conducted in Japan, in which the proportion of patients not requiring platelet transfusion was 79.2% with lusutrombopag (p<0.0001) versus 12.5% with the placebo (6, 7). The proportion of responders (platelet count >50,000/μL and >20,000/μL compared to baseline) was greater with lusutrombopag than with the placebo (77.1% vs. 6.3%; p<0.0001), and a platelet count >50,000/μL was also maintained for a significantly longer period of time with lusutrombopag than with the placebo (22.1 days without platelet transfusion vs. 3.3 days with platelet transfusion; p<0.0001).
Genetically-modified mice with a human form of the mouse TPO-receptor that received repeated administration of oral lusutrombopag demonstrated a dose-dependent increase in the platelet count (9). However, no study has investigated the efficacy of repeated lusutrombopag administration for thrombocytopenia in patients with CLD undergoing two or more planned invasive procedures.
Other treatment options include splenic artery embolization, splenectomy, and a transjugular intrahepatic portosystemic shunt, each of which can be costly, invasive, and possibly unsuitable for patients with advanced liver disease. In contrast, platelet transfusion is an effective non-invasive method of increasing the platelet count. However, the duration of a platelet count >50,000/μL with platelet transfusion is too short for repeated invasive treatments for HCC. Furthermore, repeated platelet transfusion can lead to a platelet-refractory condition, a significant clinical problem that complicates the provision of platelet transfusion and is associated with adverse clinical outcomes and increased health care costs (10).
A critical adverse event of lusutrombopag administration is portal thrombus. In our case, the serum values of FDP, a biomarker of deep vein thrombosis, were within the normal range after retreatment with lusutrombopag, and portal thrombus was not detected by dynamic CT after RFA. In the previously noted phase 3 trial, the frequency of portal thrombus did not differ between the lusutrombopag at 3 mg a day group and the placebo group (2.1% in both). However, in a phase 2 trial, the frequency of portal thrombus was 8.7% in the cohort given lusutrombopag at 4 mg once a day. Therefore, lusutrombopag must be discontinued in order to prevent portal thrombus when the platelet count exceeds the criterion for discontinuation (50,000/μL) within 5 days after beginning administration.
The efficacy and safety of repeated administration of lusutrombopag have not been clearly elucidated. However, another TPO-receptor antagonist, eltrombopag, has been reported to be generally well-tolerated in patients with chronic ITP, and the safety was consistent (11-13). In addition, Bussel et al. showed that repeated administration of eltrombopag maintained its efficacy in increasing the platelet count in patients with ITP, and adverse events such as headache were minimal (14). However, findings concerning the repeated administration of TPO receptor agonists in patients with CLD have not been reported. In the present patient, the rate of platelet increase after the first administration of lusutrombopag seemed to differ from that after the second administration. This difference might have been due to the liver function and a decrease in the baseline platelet count induced by the initial treatment for HCC, rather than a difference in the platelet response to the administration of lusutrombopag. Alternatively, the platelet count response might have occurred later than expected after the second administration of the drug. Additional studies of the repeated administration of lusutrombopag are needed to ensure the efficacy and safety in patients with CLD and thrombocytopenia.
In conclusion, lusutrombopag may be a useful drug for CLD patients with thrombocytopenia to avoid platelet transfusion in those undergoing two or more planned invasive procedures.
The authors state that they have no Conflict of Interest (COI).
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin 55: 74-108, 2005. [DOI] [PubMed] [Google Scholar]
- 2.Kim YS, Lim HK, Rhim H, Lee MW, Choi D, Lee WJ, Paik SW, Koh KC, Lee JH, Choi MS, Gwak GY, Yoo BC. Ten-year outcomes of percutaneous radiofrequency ablation as first-line therapy of early hepatocellular carcinoma: analysis of prognostic factors. J Hepatol 58: 89-97, 2013. [DOI] [PubMed] [Google Scholar]
- 3.Afdhal N, McHutchinson J, Brown R, et al. Thrombocytopenia associated with chronic liver disease. J Hepatol 48: 1000-1007, 2008. [DOI] [PubMed] [Google Scholar]
- 4.Maan R, de Knegt RJ, Veldt BJ. Management of thrombocytopenia in chronic liver disease: focus on pharmacotherapeutic strategies. Drugs 75: 1981-1992, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Qamar AA, Grace ND, Groszmann RJ, et al. ; Portal Hypertension Collaborative Group Incidence, prevalence, and clinical significance of abnormal hematologic indices in compensated cirrhosis. Clin Gastroenterol Hepatol 7: 689-695, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pharmaceuticals and Medical Devices Agency MulpletaⓇ (lusutrombopag) tablets: Japanese prescribing information (in Japanese) [Internet]. [cited 2016 Nov. 19]. Available from: http://www.pmda.go.jp/
- 7. Shionogi Shionogi receives marketing and manufacturing approval in Japan for MULPLETAⓇ tablets 3 mg for improvement of thrombocytopenia [media release] [Internet]. [cited 2016 Nov. 19]. Available from: http://www.shionogi.co.jp/en/
- 8.Bussel JB, Saleh MN, Vasey SY, Mayer B, Arning M, Stone NL. Repeated short-term use of eltrombopag in patients with chronic immune thrombocytopenia (ITP). Br J Haematol 160: 538-546, 2013. [DOI] [PubMed] [Google Scholar]
- 9.Kim ES. Lusutrombopag: First global approval. Drugs 76: 155-158, 2016. [DOI] [PubMed] [Google Scholar]
- 10.Stanworth SJ, Navarrete C, Estcourt L, Marsh J. Platelet refractoriness--practical approaches and ongoing dilemmas in patient management. Br J Haematol 171: 297-305, 2015. [DOI] [PubMed] [Google Scholar]
- 11.Bussel JB, Cheng G, Saleh MN, et al. Eltrombopag for the treatment of chronic idiopathic thrombocytopenic purpura. N Engl J Med 357: 2237-2247, 2007. [DOI] [PubMed] [Google Scholar]
- 12.Bussel JB, Provan D, Shamsi T, et al. Effect of eltrombopag on platelet counts and bleeding during treatment of chronic idiopathic thrombocytopenic purpura: a randomised, double-blind, placebo-controlled trial. Lancet 373: 641-648, 2009. [DOI] [PubMed] [Google Scholar]
- 13.Cheng G, Saleh MN, Marcher C, et al. Eltrombopag for management of chronic immune thrombocytopenia (RAISE): a 6-month, randomised, phase 3 study. Lancet 377: 393-402, 2011. [DOI] [PubMed] [Google Scholar]
- 14.Bussel JB, Saleh MN, Vasey SY, Mayer B, Arning M, Stone NL. Repeated short-term use of eltrombopag in patients with chronic immune thrombocytopenia (ITP). Br J Haematol 160: 538-546, 2013. [DOI] [PubMed] [Google Scholar]