Abstract
We report an 80-year-old woman with EGFR-mutant lung adenocarcinoma with multiple brain metastases (BMs). All lesions including BM showed a successful resolution after initiating daily 150 mg erlotinib. However, a grade 2 bilirubin-increase developed, and it was necessary to reduce the dose of erlotinib to 50 mg every other day, which aggravated BM. Switching erlotinib to afatinib led to the resolution of BM without an increase in the bilirubin level. Our results indicate that afatinib is an important treatment option when erlotinib-induced hepatotoxicity develops, regardless of the patients' age. Particularly in those patients with BM, switching to afatinib may be preferable to reducing the dose of erlotinib.
Keywords: afatinib, erlotinib, dose-reduction, hepatotoxicity, brain metastases, leptomeningeal metastases
Introduction
Despite the efficacy of epidermal growth factor receptor (EGFR)-tyrosine kinase inhibitors (TKIs) in the treatment of brain metastasis (BM) from non-small cell lung cancer (NSCLC) with EGFR mutation (1), a subset of NSCLC patients has been reported to suffer from isolated central nervous system (CNS) failure after obtaining a clinical benefit from EGFR-TKIs (2). The underlying mechanism of the isolated development of BM is attributable to the low concentrations of EGFR-TKIs in central spinal fluid due to the blood-brain barrier (3). Thus, an adequate dosage of EGFR-TKIs is important, especially in NSCLC patients with BM, and reducing the dose of an EGFR-TKI might result in CNS progression.
On the other hand, 33-70% of patients treated with 1st-generation EGFR-TKIs develop hepatotoxicity as an adverse event (4-6), which may necessitate dose reduction. We herein present a case of NSCLC with multiple BMs, which became aggravated after reducing the dose of erlotinib due to hepatotoxicity, but which was successfully managed after switching to afatinib.
Case Report
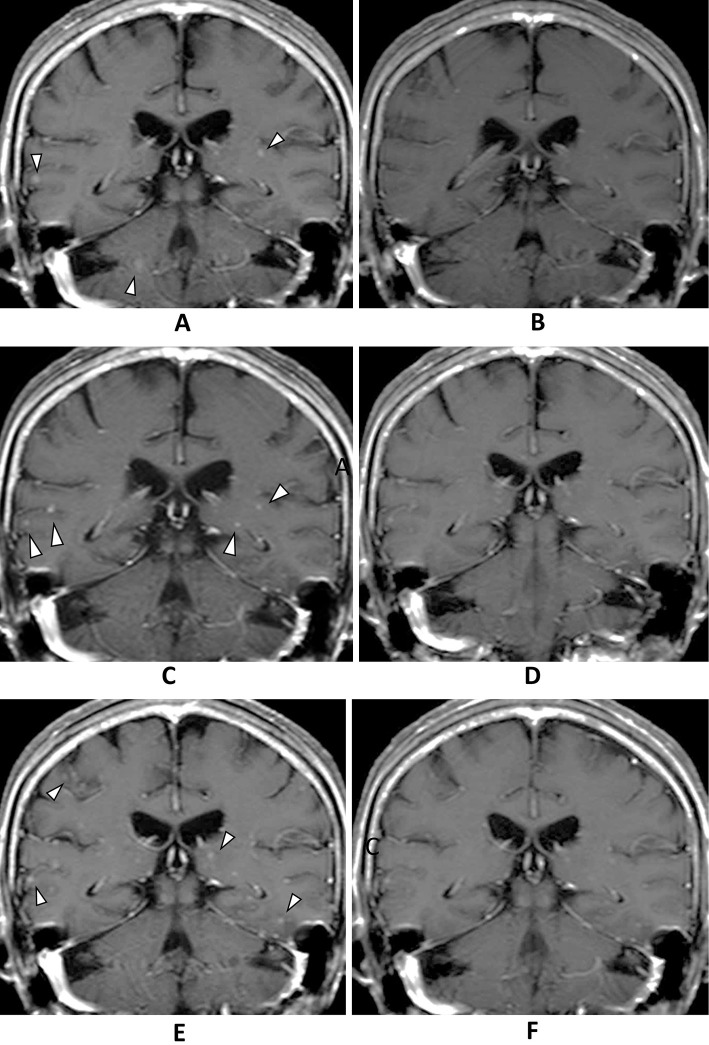
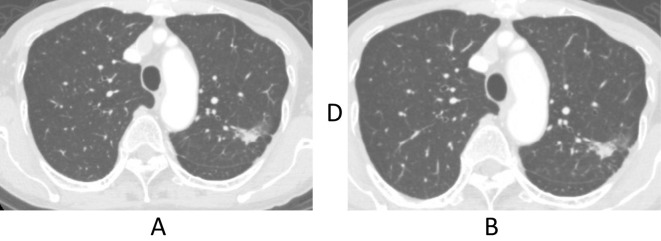
An 80-year-old woman, diagnosed with Stage IV adenocarcinoma (cT2N2M1b; exon 21 L858R mutation, PUL, OSS, and BM), was treated with EGFR-TKI in August 2015 as part of a clinical trial (FLAURA) that compared osimertinib and gefitinib (or erlotinib). Although all of the patient's lesions (including multiple BMs) showed a partial response, the trial drug was discontinued in November 2015 due to the development of interstitial pneumonia, which was successfully treated with prednisolone. A month after withholding the trial drug, the asymptomatic BM lesions showed an apparent deterioration, while the extracranial lesions remained stable (Fig. 1A). Because she refused whole brain radiotherapy, erlotinib was started as a second-line therapy at a daily dose of 150 mg in December 2015. However, on the 4th day after the initiation of erlotinib treatment, her serum total bilirubin level markedly increased to 2.5 mg/dL (Grade 2 toxicity according to the CTCAE version 4.0); her aspartate aminotransferase and alanine aminotransferase levels remained within the normal ranges. After temporal discontinuation, erlotinib was re-initiated in January 2016 and the dose of erlotinib was adjusted with carefully monitoring of the total bilirubin level. At the beginning of February 2016 (100 mg every other day), the BM lesions had apparently improved with stable extracranial lesions and no findings of biliary obstruction (Fig. 1B, 2A). However, her serum total bilirubin reached a peak of 2.7 mg/dL at a dose of 75 mg every other day. Thus, it was necessary to reduce the dose of erlotinib to 50 mg every other day. In April 2016, multiple small BMs developed; however, the extracranial lesions remained stable (Fig. 1C, 2B). Moreover, cerebrospinal fluid puncture was performed and leptomeningeal carcinomatosis (LMC) was diagnosed. In May 2016, we switched from erlotinib to afatinib (40 mg daily). The BM lesions and LMC showed radiological resolution at one month after the initiation of afatinib, without any concomitant increase in the patient's bilirubin level (Fig. 1D). However, grade 2 diarrhea and grade 2 anorexia developed and the dose was reduced to 20 mg every other day. Follow-up MRI in August 2016 showed the apparent aggravation of the BM lesions and LMC (Fig. 1E), although the extracranial lesions remained stable. She was subsequently diagnosed with depression in July 2016, which suggested that the anorexia might have been caused by depression and not by afatinib. We therefore increased the afatinib dose to 20 mg daily with close monitoring. A month after increasing the afatinib dose, the obvious resolution of the BM lesions and LMC was observed (Fig. 1F). Afatinib therapy was continued until a deterioration of her LMC-related symptoms in December 2016.
Figure 1.
Multiple small nodules (arrowhead) were present in the cerebrum (A). Although no brain metastasis was identified after the initiation of erlotinib (B) in February 2016, multiple small BMs (arrowhead) was found to have developed in the cerebrum (C). In June 2016, after the initiation of afatinib, no brain metastasis was detected (D); however, multiple tiny nodules were found to have developed in the cerebrum and abnormal leptomeningeal enhancement was observed in the sulci in August 2016 (E). These lesions disappeared at one month after increasing the dose of afatinib (F).
Figure 2.
The primary lesion remained stable during the clinical course (A: February 2016, B: April 2016).
Discussion
In the present case, although the patient's extracranial lesions remained stable, multiple BMs and LMC developed after the dose of erlotinib was reduced due to an increase in the serum total bilirubin level. Importantly, after switching from erlotinib to afatinib, BM and LMC quickly resolved without an increase in the serum bilirubin level. Our results have two clinical implications.
First, the findings suggest the utility of afatinib as an important alternative drug when erlotinib-induced hepatotoxicity develops, even in elderly NSCLC patients. To our knowledge, there are five documented cases in which afatinib treatment was successful following the development of severe hepatotoxicity due to the administration of a 1st-generation EGFR-TKI (7-9). Generally, 1st-generation EGFR-TKIs are metabolized in the liver by cytochrome P450 (CYP)-dependent enzymes, such as CYP3A4 (10). Afatinib exhibits high plasma protein-binding (95% in healthy volunteers); thus, only a small fraction of the administered drug is directly subjected to hepatic metabolism (11). This characteristic is primarily responsible for the low incidence of afatinib-induced hepatotoxicity (12, 13). However, all five of these previously documented patients were younger than our patient and under 75 years of age. Although it remains unclear whether afatinib is safe in elderly patients, Kashiwabara reported on five elderly patients with poor PS, who were mostly safely treated with afatinib - similar to our case (14). These findings suggest that afatinib may be an important alternative drug for cases in which erlotinib-induced hepatotoxicity develops - even in elderly NSCLC patients - however, careful monitoring would be needed.
Second, when erlotinib-induced hepatotoxicity develops, a switch to afatinib may be preferable to reducing the dose of erlotinib, especially in NSCLC patients with BM. To date, it is unclear whether an EGFR-TKI should be replaced by another EGFR-TKI in the event of a toxicity that requires a dose-reduction. In cases in which the primary lesion is stable, it is a common practice to reduce the dose of the causative drug. On the other hand, high serum concentrations of EGFR-TKIs are considered important in NSCLC patients with BM to cross the blood-brain barrier (3). Erloitnib was recently reported to be superior to gefitinib in the management of BM and LMC (15, 16). The superiority of erlotinib over gefitinib is attributable to the lower maximum tolerable dose (MTD) of gefitinib, which is one-third of that of erlotinib (17, 18). However, reduced-dose of erlotinib has been reported to exacerbate BM (19). In fact, in the present case, CNS progression was observed one month after we reduced the dosage to 50 mg every other day, despite the fact that the extracranial disease remained stable. Importantly, the erlotinib dose was reduced due to the development of hepatotoxicity, which could be managed by switching afatinib. Besides, afatinib has been reported to be effective in NSCLC patients, even patients heavily pretreated with 1st-generation EGFR-TKIs (20). Considering the importance of maintaining high serum concentrations of EGFR-TKIs for NSCLC patients with BM, switching to afatinib - as opposed to reducing the dose of erlotinib - represents an important therapeutic option. In addition, in the present case, the temporary aggravation of BM occurred after the dose of afatinib was reduced to 20 mg every other day. However, the patient improved after the dose was increased to 20 mg daily. Although the development of adverse events requiring afatinib dose-reduction correlates with high serum concentrations (21), the optimal dose of afatinib should be cautiously determined, particularly for NSCLC patients with BM.
In conclusion, we reported the case of patient with BM that was aggravated after the dosage of erlotinib was reduced due to the development of hepatotoxicity; however, the case was successfully managed after switching to afatinib. The present case suggests that afatinib may be an important treatment option for cases in which erlotinib-induced hepatotoxicity develops, regardless of the patients' age. Switching to afatinib would be preferable to reducing the dose of erlotinib, particularly in patients with BM.
Author's disclosure of potential Conflicts of Interest (COI).
Akimasa Sekine: Honoraria, Boehringer Ingelheim and Chugai Pharmaceuticals. Terufumi Kato: Honoraria, AstraZeneca, Boehringer Ingelheim and Chugai Pharmaceuticals; Research funding, Boehringer Ingelheim and Chugai Pharmaceuticals. Takashi Ogura: Honoraria, Boehringer Ingelheim.
References
- 1.Iuchi T, Shingyoji M, Sakaida T, et al. Phase II trial of gefitinib alone without radiation therapy for Japanese patients with brain metastases from EGFR-mutant lung adenocarcinoma. Lung Cancer 82: 282-287, 2013. [DOI] [PubMed] [Google Scholar]
- 2.Lee YJ, Choi HJ, Kim SK, et al. Frequent central nervous system failure after clinical benefit with epidermal growth factor receptor tyrosine kinase inhibitors in Korean patients with nonsmall-cell lung cancer. Cancer 116: 1336-1343, 2010. [DOI] [PubMed] [Google Scholar]
- 3.Ricciardi S, de Marinis F. Multimodality management of non-small cell lung cancer patients with brain metastases. Curr Opin Oncol 22: 86-93, 2010. [DOI] [PubMed] [Google Scholar]
- 4.Mitsudomi T, Morita S, Yatabe Y, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol 11: 121-128, 2010. [DOI] [PubMed] [Google Scholar]
- 5.Goto K, Nishio M, Yamamoto N, et al. A prospective, phase II, open-label study (JO22903) of first-line erlotinib in Japanese patients with epidermal growth factor receptor (EGFR) mutation-positive advanced non-small-cell lung cancer (NSCLC). Lung Cancer 82: 109-114, 2013. [DOI] [PubMed] [Google Scholar]
- 6.Inoue A, Kobayashi K, Usui K, et al. First-line gefitinib for patients with advanced non-small-cell lung cancer harboring epidermal growth factor receptor mutations without indication for chemotherapy. J Clin Oncol 27: 1394-1400, 2009. [DOI] [PubMed] [Google Scholar]
- 7.Toba H, Sakiyama S, Takizawa H, Tangoku A. Safe and successful treatment with afatinib in three postoperative non-small cell lung cancer patients with recurrences following gefitinib/erlotinib-induced hepatotoxicity. J Med Invest 63: 149-151, 2016. [DOI] [PubMed] [Google Scholar]
- 8.Ueda H, Hayashi H, Kudo K, Takeda M, Nakagawa K. Successful treatment with afatinib after gefitinib- and erlotinib-induced hepatotoxicity. Invest New Drugs 34: 797-799, 2016. [DOI] [PubMed] [Google Scholar]
- 9.Zenke Y, Umemura S, Sugiyama E, et al. Successful treatment with afatinib after grade 3 hepatotoxicity induced by both gefitinib and erlotinib in EGFR mutation-positive non-small cell lung cancer. Lung Cancer 99: 1-3, 2016. [DOI] [PubMed] [Google Scholar]
- 10.Ling J, Johnson KA, Miao Z, et al. Metabolism and excretion of erlotinib, a small molecule inhibitor of epidermal growth factor receptor tyrosine kinase, in healthy male volunteers. Drug Metab Dispos 34: 420-426, 2006. [DOI] [PubMed] [Google Scholar]
- 11.Stopfer P, Marzin K, Narjes H, et al. Afatinib pharmacokinetics and metabolism after oral administration to healthy male volunteers. Cancer Chemother Pharmacol 69: 1051-1061, 2012. [DOI] [PubMed] [Google Scholar]
- 12.Sequist LV, Yang JC, Yamamoto N, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol 31: 3327-3334, 2013. [DOI] [PubMed] [Google Scholar]
- 13.Takeda M, Okamoto I, Nakagawa K. Pooled safety analysis of EGFR-TKI treatment for EGFR mutation-positive non-small cell lung cancer. Lung Cancer 88: 74-79, 2015. [DOI] [PubMed] [Google Scholar]
- 14.Kashiwabara K, Semba H, Fujii S, Tsumura S. Tolerability and efficacy of afatinib at a low starting dosage in 10 elderly or low performance status patients with advanced refractory non-small-cell lung cancer. Respiratory investigation 54: 468-472, 2016. [DOI] [PubMed] [Google Scholar]
- 15.Bai H, Han B. The effectiveness of erlotinib against brain metastases in non-small cell lung cancer patients. Am J Clin Oncol 36: 110-115, 2013. [DOI] [PubMed] [Google Scholar]
- 16.Deng Y, Feng W, Wu J, et al. The concentration of erlotinib in the cerebrospinal fluid of patients with brain metastasis from non-small-cell lung cancer. Mol Clin Oncol 2: 116-120, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fukuoka M, Yano S, Giaccone G, et al. Multi-institutional randomized phase II trial of gefitinib for previously treated patients with advanced non-small-cell lung cancer (The IDEAL 1 Trial) [corrected]. J Clin Oncol 21: 2237-2246, 2003. [DOI] [PubMed] [Google Scholar]
- 18.Kris MG, Natale RB, Herbst RS, et al. Efficacy of gefitinib, an inhibitor of the epidermal growth factor receptor tyrosine kinase, in symptomatic patients with non-small cell lung cancer: a randomized trial. JAMA 290: 2149-2158, 2003. [DOI] [PubMed] [Google Scholar]
- 19.Lampson BL, Nishino M, Dahlberg SE, et al. Activity of erlotinib when dosed below the maximum tolerated dose for EGFR-mutant lung cancer: Implications for targeted therapy development. Cancer 2016. (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoffknecht P, Tufman A, Wehler T, et al. Efficacy of the irreversible ErbB family blocker afatinib in epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor (TKI)-pretreated non-small-cell lung cancer patients with brain metastases or leptomeningeal disease. J Thorac Oncol 10: 156-163, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang JC, Sequist LV, Zhou C, et al. Effect of dose adjustment on the safety and efficacy of afatinib for EGFR mutation-positive lung adenocarcinoma: post hoc analyses of the randomized LUX-Lung 3 and 6 trials. Ann Oncol 27: 2103-2110, 2016. [DOI] [PubMed] [Google Scholar]