Abstract
Mucosa-associated lymphoid tissue lymphoma is a common type of primary pulmonary carcinoma, but the presence of polypoid nodules is extremely rare. We herein report two cases with multiple nodules in the trachea. One case involved polypoid nodules and airway stenosis mimicking asthma; the other case had concurrent nontuberculous mycobacterial infection. The diagnosis of both cases was confirmed by bronchoscopy. The two cases were sensitive to radiotherapy and chemotherapy, respectively.
Keywords: mucosa-associated lymphoid tissue (MALT) lymphoma, airway stenosis, asthma, nontuberculous mycobacterial infection
Introduction
Mucosa-associated lymphoid tissue (MALT) lymphoma is a distinct subgroup of non Hodgkin's lymphoma (NHL) that accounts for approximately 5% of all NHLs (1, 2). Pulmonary extranodal malignant lymphoma is a rare entity that accounts for <0.5% of primary pulmonary malignancies and <1% of all lymphomas (3, 4). MALT lymphoma features including multiple tiny nodules or diffuse wall thickening along the trachea and main stem bronchi have been reported previously, but such cases of MALT are very rare. In addition, MALT lymphomas have been associated with Sjögren's syndrome, dysgammaglobulinemia, amyloid deposits, collagen vascular diseases, Helicobacter pylori infection, and AIDS (1, 5). Chronic inflammatory diseases can evoke oligo- or monoclonal cell proliferation in mucosal lymphoid tissue, resulting in the development of lymphoma (6). In this report, we present two cases with multiple nodules in the trachea. One case involved polypoid nodules and airway stenosis mimicking asthma; the other case involved a concurrent nontuberculous mycobacterial infection.
Case Reports
Case 1
A 47-year-old woman had suffered from dry cough and exertional dyspnea for 4 years. She was treated with inhaled corticosteroids, followed by systemic corticosteroids for one month, as bronchial asthma. However, these treatments were not effective, and the patient was referred to our hospital.
On a physical examination, her peripheral arterial blood oxygen saturation (SpO2) was 93% on room air, but chest auscultation revealed stridor. Computed tomography (CT) showed severe and widespread tracheobronchial stenosis (Fig. 1A, B). Laboratory findings showed moderate leukocytosis with a left shift and an increase in neutrophilic granulocytes, with relatively normal levels of lymphocytes, monocytes, and acidophilic granulocytes. Her liver function and serum albumin level were moderately abnormal, but other laboratory values were normal. The soluble interleukin-2 receptor (s-IL2R) level was 131.0 U/mL (Table). The forced expiratory volume 1.0 second (FEV1) was 420 mL (FEV1%-G; 17.94%) and vital capacity (VC) 3,410 mL (%VC; 120%) on a pulmonary function test.
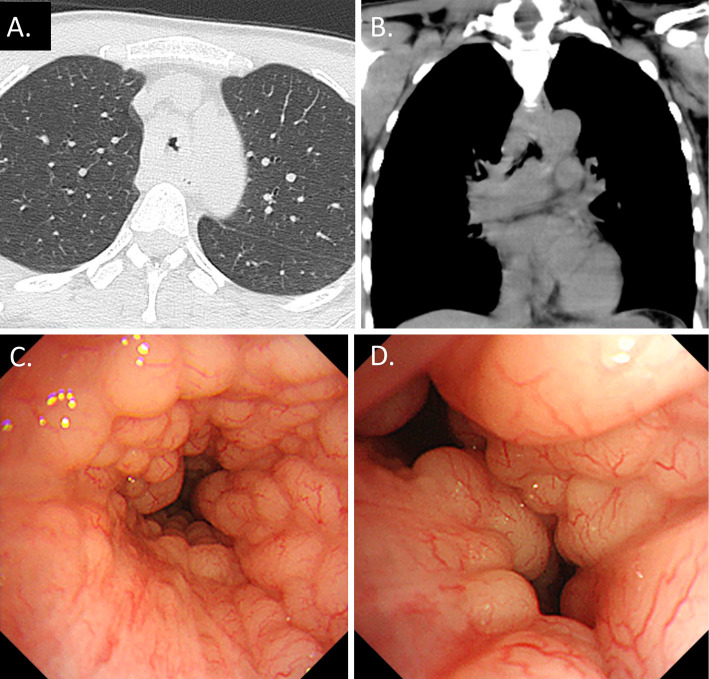
Figure 1.
A: Lung window. B: Mediastinal window. C, D: Bronchoscopic findings. Chest CT showing aggressive tracheobronchial stenosis. Bronchoscopy revealing multiple small nodular mucosal lesions with a cobblestone appearance along the trachea and main carina.
Table.
Symptoms, Physical Examination Findings, and Laboratory Findings of the Two Cases.
| Case 1 | Case 2 | |
|---|---|---|
| Symptoms | Dry cough, exertional dyspnea | Hemoptysis |
| Physical examination | ||
| SpO2(%) | 93 | 96 |
| Chest auscultation | stridor | none |
| Laboratory findings | ||
| Soluble interleukin-2 receptor (U/mL) | 131 | 476 |
| C-reactive protein (mg/dL) | 0.02 | 0.17 |
| Lactate dehydrogenase (IU/L) | 150 | 186 |
| IgG (mg/dL) | 828 | 1,828 |
| IgM (mg/dL) | 55 | 92 |
| IgA (mg/dL) | 148 | 326 |
SpO2: arterial blood oxygen saturation measured by pulse oximetry in room air
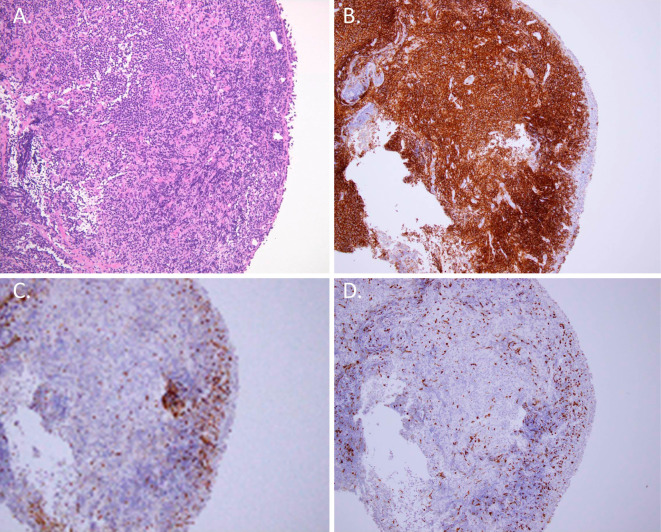
An examination using a flexible bronchoscope revealed multiple nodular mucosal lesions mimicking a cobblestone appearance along the trachea and main carina (Fig. 1C, D). A pathological examination of a biopsied specimen showed diffuse infiltrates of lymphoid cells within the mucosa (Fig. 2A). In addition, the cells showed positive immunohistochemical staining for cluster of differentiation (CD) 20 (Fig. 2B) but were negative for CD3 (Fig. 2C), CD 10, cyclin D1, and k-light chain expression. The cells demonstrated weak Ki-67 staining (Fig. 2D). Therefore, we made a diagnosis of extranodal marginal zone MALT lymphoma. Radiation and steroid therapy was performed, and the severe and widespread tracheobronchial stenosis recovered rapidly.
Figure 2.
A: Light microscopic image showing infiltration of lymphoid cells into the epithelium (Hematoxylin and Eosin staining; 40× magnification). B, C, and D: The cells demonstrated a B-cell origin according to positive CD20 staining (B), negative CD3 staining (C), and weak Ki-67 staining (D) (immunohistochemical staining; 40× magnification).
Case 2
A 61-year-old woman who had been treated for depression consulted our hospital for hemoptysis.
On a physical examination, her vital signs were stable. Her peripheral SpO2 was 96% on room air. A systemic examination of her chest and abdomen did not reveal any significant abnormalities. Chest X-ray and CT revealed small pulmonary nodules and bronchiectasis in the middle lobe and lingular segment (Fig. 3A). The laboratory data showed moderate thrombocytopenia and an abnormal C-reactive protein level. Her serum glycopeptidolipid core IgA antibody level was positive (2.44 U/mL). All other data were normal. The s-IL2R level was 476.0 U/mL (Table).
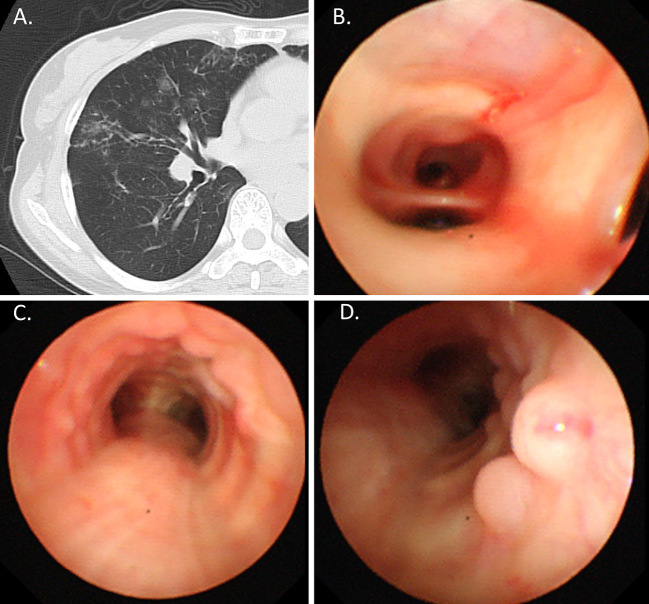
Figure 3.
A: Lung window. B, C, and D: Bronchoscopic findings. Chest CT showing small pulmonary nodules and bronchiectasis in the middle lobe (A). Bronchoalveolar lavage from the middle lobe (B). Bronchoscopic findings revealing multiple small nodular mucosal lesions with a cobblestone appearance along the trachea (C and D).
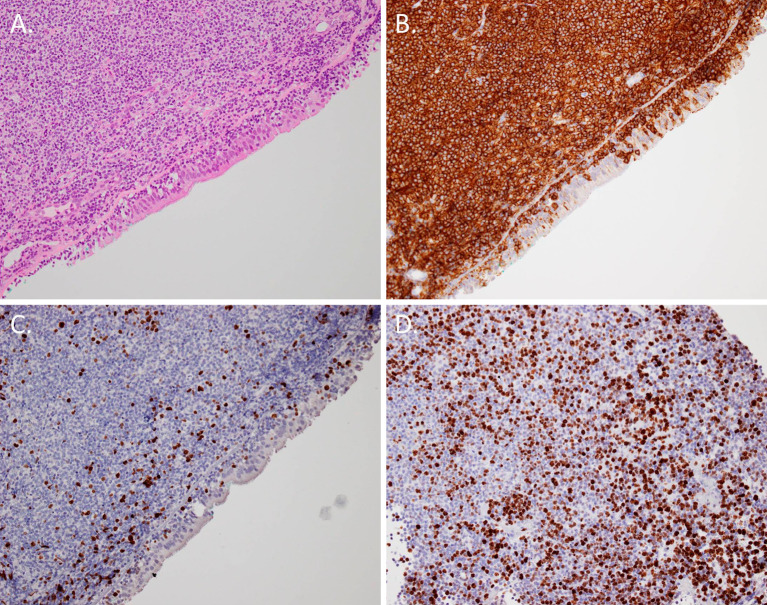
Bronchoscopy yielded a diagnosis of Mycobacterium intracellulare in the bronchial lavage fluid collected from the middle lobe (Fig. 3B). In addition, multiple nodular mucosal lesions in the lower and middle trachea were observed (Fig. 3C, D). Microscopic examination of biopsy specimens from the tracheal mass showed infiltration of lymphoid cells into the epithelium (Fig. 4A). The cells demonstrated a B-cell origin according to positive CD20 staining (Fig. 4B). The cells showed negative staining for CD3, CD56, chromogranin A, synaptophysin, CD5, CD10, and cyclin D1. The monoclonality of the tumor cells was demonstrated immunohistochemically. Areas of both weak and moderate Ki-67 staining were observed (Fig. 4C, D). Therefore, we diagnosed the patient with MALT lymphoma with coexisting diffuse large B cell lymphoma. The multiple nodular mucosal lesions in the trachea and small pulmonary nodules were successfully treated with R-CHOP chemotherapy (rituximab, cyclophosphamide, vincristine, doxorubicin, etoposide, and prednisolone).
Figure 4.
A: Microscopy of the corresponding pathologic samples showing infiltration of lymphoid cells (Hematoxylin and Eosin staining; 40× magnification). B: The small lymphoid cells were B cells based on the CD20 staining (immunohistochemical staining; 40× magnification). C, D: The cells demonstrated weak and moderate Ki-67 staining (immunohistochemical staining; 40× magnification).
Discussion
Although the stomach is the most frequently involved organ in MALT lymphoma, the lung is one of the most frequently involved non gastrointestinal sites (7). MALT is a lymphoid aggregate located in the submucosal area of the bronchioles that plays a central role in the mucosal immunity of the airways. Long-lasting antigen stimuli promote hyperplasia in MALT (8, 9). Chronic inflammatory diseases can evoke oligo- or monoclonal cell proliferation in mucosal lymphoid tissue, resulting in the development of lymphoma (6). Our patients included one case with nontuberculous mycobacterial infection in Case 2. Such an infection has been mentioned in another report (10), so this case might be of interest.
In Case 1, multiple and small nodular mucosal lesions coexisted in the trachea and main-stem bronchi. Such widespread tracheobronchial stenosis is extremely rare (11, 12). The patient had suffered from dry cough and exertional dyspnea for four years. She was treated with inhaled corticosteroids and sometimes systemic corticosteroids for bronchial asthma. This long period might have led to severe and widespread tracheobronchial stenosis from MALT lymphoma. The laboratory data were normal, including the s-IL2R level, but the bronchoscopic examination supported a diagnosis of MALT lymphoma.
An active search is underway for the most efficient bronchoscopic imaging tool for the detection of cancer (13, 14). Using bronchoscopic imaging to detect and investigate precancerous lesions in the bronchial mucosa may represent a turning point in the elucidation of neoplastic transformation (15-17). In the present study, there were no cases suspected of being MALT lymphoma prior to a bronchoscopic examination. One case was treated for bronchial asthma with severe and widespread tracheobronchial stenosis of unknown origin. The other case had hemoptysis from a nontuberculous mycobacterial infection. Endoscopic technology has advanced markedly and become widespread over the past 10 years (13, 14). Consultation of pulmonary specialists might be necessary for the rapid diagnosis of diseases of unknown origin in the trachea, bronchus, and pulmonary regions. A bronchoscopic examination appears to be a useful tool for diagnosing nontubercular mycobacterial infections, but diagnosing infections using sputum samples from patients with hemosputum or small pulmonary nodules and bronchiectasis can be difficult. Active adaptation of a bronchoscopic examination and careful observation in the trachea and bronchus are certainly effective for the diagnosis of unexpected diseases, including MALT lymphoma.
The authors state that they have no Conflict of Interest (COI).
Acknowledgement
We thank Drs. Yoko Shinnou and Keina Nagakita for their helpful discussions on the pathological diagnosis.
References
- 1. Harris NL, Jaffe ES, Stein H, et al. A revised European American classification of lymphoid neoplasms: a proposal from the International Lymphoma Study Group. Blood 84: 1361-1392, 1994. [PubMed] [Google Scholar]
- 2. Armitage JO, Weisenburger DD. New approach to classifying non Hodgkin's lymphomas: clinical features of the major histologic subtypes. Non Hodgkin's Lymphoma Classification Project. J Clin Oncol 16: 2780-2795, 1998. [DOI] [PubMed] [Google Scholar]
- 3. Ahmed S, Siddiqui AK, Rai KR. Low grade B cell bronchial associated lymphoid tissue (BALT) lymphoma. Cancer Invest 20: 1059-1068, 2002. [DOI] [PubMed] [Google Scholar]
- 4. Koss MN. Malignant and benign lymphoid lesions of the lung. Ann Diagn Pathol 8: 167-187, 2004. [DOI] [PubMed] [Google Scholar]
- 5. McGuinness G, Scholes JV, Jagirdar JS, et al. Unusual lymphoproliferative disorders in nine adults with HIV or AIDS: CT and pathologic findings. Radiology 197: 59-65, 1995. [DOI] [PubMed] [Google Scholar]
- 6. Anaya JM, McGuff HS, Banks PM, Talal N. Clinicopathological factors relating malignant lymphoma with Sjögren's syndrome. Semin Arthritis Rheum 25: 337-346, 1996. [DOI] [PubMed] [Google Scholar]
- 7. Zucca E, Conconi A, Pedrinis E, et al. Nongastric marginal zone B cell lymphoma of mucosa associated lymphoid tissue. Blood 101: 2489-2495, 2003. [DOI] [PubMed] [Google Scholar]
- 8. Brandtzaeg P, Jahnsen FL, Farstad IN. Immune functions and immunopathology of the mucosa of the upper respiratory pathways. Acta Otolaryngol 116: 149-159, 1996. [DOI] [PubMed] [Google Scholar]
- 9. Tirouvanziam R, Khazaal I, N'Sondé V, et al. Ex vivo development of functional human lymph node and bronchus-associated lymphoid tissue. Blood 99: 2483-2489, 2002. [DOI] [PubMed] [Google Scholar]
- 10. Otsuki T, Nishino R, Akita S, et al. A case of mucosa-associated lymphoid tissue (MALT) lymphoma combined with nontuberculous mycobacteriosis and lung cancer. J Jpn Soc Respir Endosc 34: 113-119, 2012(in Japanese, Abstract in English). [Google Scholar]
- 11. Li H, Wang T, Wei X, et al. Marginal zone B-cell lymphoma of the pulmonary mucosa-associated lymphoid tissue: a case report. Oncol Lett 10: 1731-1734, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yoon RG, Kim MY, Song JW, et al. Primary endobronchial marginal zone B-cell lymphoma of bronchus-associated lymphoid tissue: CT findings in 7 patients. Korean J Radiol 14: 366-374, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pahlevaninezhad H, Lee AM, Ritchie A, et al. Endoscopic Doppler optical coherence tomography and autofluorescence imaging of peripheral pulmonary nodules and vasculature. Biomed Opt Express 6: 4191-4199, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zaric B, Perin B, Stojsic V, et al. Detection of premalignant bronchial lesions can be significantly improved by combination of advanced bronchoscopic imaging techniques. Ann Thorac Med 8: 93-98, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Stojsić J, Adzić T, Marić D, et al. Histological types and age distribution of lung cancer operated patients over a 20-year period: a pathohistological based study. Srp Arh Celok Lek 139: 619-624, 2011. [PubMed] [Google Scholar]
- 16. Vrdoljak E, Wojtukiewicz MZ, Pienkowski T, et al. ; South Eastern European Research Oncology Group Cancer epidemiology in Central, South and Eastern European countries. Croat Med J 52: 478-487, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Stojsic J, Radojicic J, Markovic J, et al. Gender and age trends of histological types of lung cancer in a 20-year period: pathological perspective. J BUON 15: 136-140, 2010. [PubMed] [Google Scholar]