Abstract
The incidence of spontaneous intracerebral hematoma (SICH) is even now high worldwide, especially higher in Japan than in Western countries, despite the development of advances in blood pressure (BP) management and food/alcohol intake education. Although mortality and morbidity for SICH are high, some controversies remain regarding the appropriate acute phase of treatment. Recent studies have revealed that BP lowering treatment than 140 mmHg resulted in better outcomes. However the efficacy of surgical treatment for SICH has not been well established, with the exception of that for cerebellar SICH over 3 cm in diameter and life-saving procedures, although many randomized control studies and systematic reviews focused on surgical treatment have been reported. In this review, we summarize some issues and discuss strategies in development for the treatment of SICH.
Keywords: intracerebral hematoma, surgery, review
Introduction
Non-traumatic spontaneous (hypertensive) intracerebral hematoma (SICH) is a devastating disease with higher rates of mortality and morbidity than those of ischemic stroke, with an annual incidence of 10–30 per 100,000.1) Accounting for nearly 2 million (10–15%) of strokes each year in Western countries, SICH represents a major public health problem and frequently causes severe neurological deficits in survivors.1–3) However, the incidence of SICH is higher in Asian countries, particularly in Chinese and Japanese and these racial differences are mostly seen in the incidence of deep intracerebral hemorrhage and are most prominent in young and middle-aged people.1) Studies have revealed that both hypertension and high alcohol intake are strong risk factors for SICH, and that the incidence of SICH decreases in areas with improved medical management of blood pressure (BP).4–6) However, a recent Japanese cohort study reported that the rate of SICH was 1.14 per 1,000 age-adjusted person-years in Japan and revealed a discouraging result: Although the incidence of SICH drastically declined between the 1960s and 1970s in Japan due to nationwide improvements in BP control and decreases in alcohol intake, this decline has since leveled off, likely due to the increased incidence of thalamic hemorrhage in older adults in recent years.7) Furthermore, increases in the population of older adults as well as the use of anticoagulant/antiplatelet medication such as warfarin and DOAC or NOAC (direct oral anticoagulant or non-vitamin K oral anticoagulants), suggest that the incidence of SICH may increase in the future (Table 1).8–13) Therefore, new treatment options are necessary in order to improve neurological deficits and patient quality of life following SICH.
Table 1.
SICH bleeding rate and the influence of anti-palatelet/anti-coagulant medications
| Anti-platelet or Anti-coagulant SICH | |||
|---|---|---|---|
| Medications | Bleeding rate (% per year) | Clinical study | Reversal agent |
| None | |||
| 1961–1974 | 0.203 (Japanese) | Hisayama (1st cohort)7) | |
| 1988–2001 | 0.114 (Japanese) | Hisayama (3rd cohort)7) | |
| >80 years old 1988–2001 | About 0.4 (Japanese) | Hisayama (3rd cohort)7) | |
| Anti-platelet | |||
| Single | 0.2–0.3 | Systematic review8) | |
| 0.34 (Japanese) | BAT study9) | ||
| Double | 0.60 (Japanese) | BAT study9) | |
| Against warfarin | |||
| Warfarin | 0.3–1.2 | Systematic review10,11) | Vitamin K |
| 0.62 (Japanese) | BAT study9) | Non-specific agents | |
| PCC | |||
| rFVIIa | |||
| Warfarin + anti-platelet | 0.96 (Japanese) | BAT study9) | FFP |
| DOAC (NOAC) | Against all DOAC(NOAC)s13) | ||
| Dagigatoran | 0.32 (RR 0.41 vs warfarin) | RE-LY12) | Idarucizumb (only for Dagigatoran) |
| Andexanet alfa* | |||
| Ciraparantag (PER977)* | |||
| *Now not approved | |||
| Rivaroxaban | 0.50 (RR 0.67 vs warfarin) | ROCKET-AF12) | non-specific agents |
| 0.34 (RR 0.73 vs warfarin; Japanese) | J-ROCKET-AF12) | PCC | |
| rFVIIa | |||
| Apixaban | 0.24 (RR 0.51 vs warfarin) | ARISTOTLE12) | FFP |
| Edoxaban | 0.26 (RR 0.54 vs warfarin) | ENGAGE12) | |
FFP: fresh frozen plasma, PCC: prothrombin complex concentrate, rFVIIa: recombinant activated factor VII, RR: relative risk.
Pathology and controversy of treatment
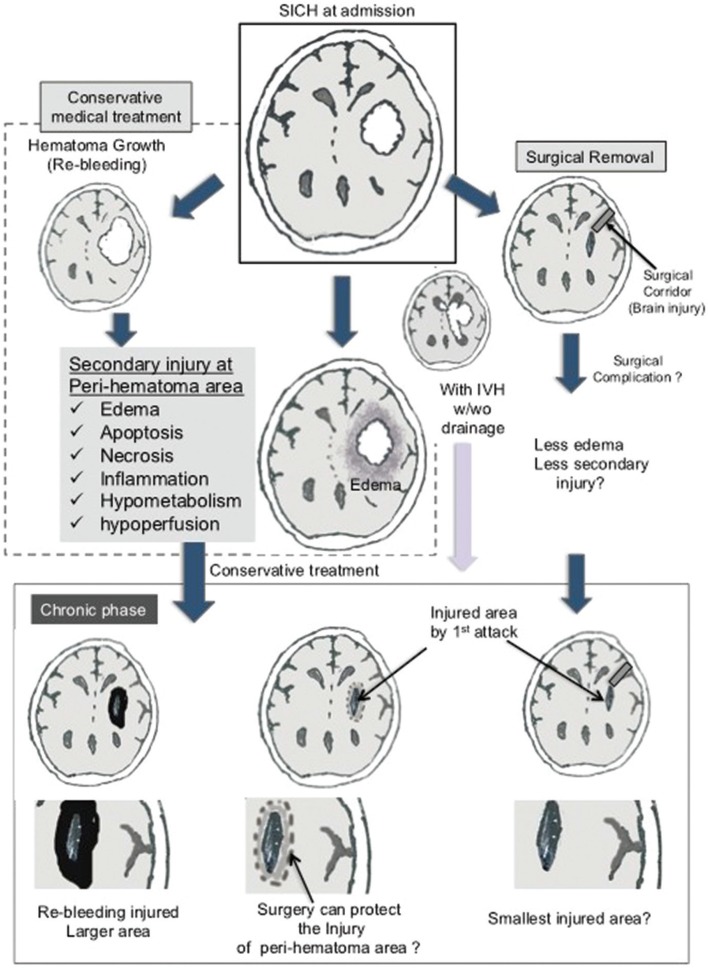
SICH occurs suddenly due to the rupture of vessels affected by hypertension-related degenerative changes in the cerebral lobes, basal ganglia, thalamus, brainstem, and cerebellum.2) Hematoma irreversibly impairs neuroglial structures at the site of bleeding, while hematoma regrowth (re-bleeding) and intraventricular hemorrhage are associated with poor outcomes.14–17) In the natural course, secondary injury in the peri-hematoma area may occur within several weeks (Fig. 1), resulting in the breakdown of the blood-brain barrier, tissue edema, protease activation, apoptosis, necrosis, inflammatory reactions, hypometabolism, and hypoperfusion.1,18–23) Peri-hematoma brain edema may last for 2 weeks, peaking 5–6 days after onset,24,25) although the aforementioned impairments may persist for several works and induce permanent injury to secondary areas. Furthermore, patients with higher intracranial pressure (ICP) undergoing conservative treatment may experience injury even in remote areas. Although hematoma evacuation may result in some level of injury (for example surgical corridor area), most neurosurgeons consider that early removal of hematoma may result in significantly greater improvements in brain function than conservative treatment.
Fig. 1.
Pathology and controversy of SICH treatment. Hematoma regrowth (re-bleeding) and intraventricular hemorrhage are important pathological lesions related to poor outcome. Conservative treatment without surgical evacuation may induce damage in the peri-hematoma area, although the effectiveness of surgery for SICH has not been established. IVH, intraventricular hemorrhage; SICH, spontaneous intracerebral hematoma.
Diagnosis and management principles
Computed tomography (CT) scan is convenient and generally used for emergent diagnosis of intracerebral hematoma and magnetic resonance image (MRI), especially gradient echo, is very useful with high sensitivity,26) although MRI is better for detection of acute ischemia and chronic hemorrhage.27) To confirm the diagnosis of SICH, we must exclude other obvious vascular lesions such as cerebral aneurysm, vascular malformation, arterio-venous fistula, moyamoya disease. Although cerebral angiography was a gold standard to rule out these vascular lesions, MRI, MR-angiography (MRA), CT-angiography (CTA) has been recently beneficial for emergent diagnosis of vascular lesions.28) One more usefulness of MRI for SICH is cerebral microbleeds (MBs), which are represented on T2*-weighted MRI images as spotty, low-intensity lesions, frequently detected in patients with stroke and can be used to predict SICH even in healthy elderly indivisuals.29)
The mortality of in-hospital SICH is significantly lower when treated in intensive-care neurological unit.30) After admission to emergecy department, airway control, BP control, ICP control treatment, and anticoagulation reversal must be started as soon as possible.2) The possible treatments to reduce ICP are the following: 1) 30 degrees of head elevation,31) 2) intravenous mannitol infusion,2) 3) mild hypothermia32,33) 4) surgical evacuation, 5) Ventricular drainage, However there are some issues and controversies in these treatments.
Prevention of ICH regrowth: control of BP
Hematoma regrowth (re-bleeding) has been recognized as an important risk factor for early neurological deterioration and poor outcomes (Fig. 1). Approximately 73% of patients assessed within 3 hours of onset experience some degree of hematoma regrowth, with nearly 40% exhibiting clinically prominent enlargement.8) Although BP management is recommended for the prevention of SICH, excessive decreases in BP may induce hypoperfusion/ischemic complications in patients with increased ICP. Several studies have therefore aimed to determine the appropriate BP level in patients with SICH.
Reducing systolic BP (SBP) to 160 mmHg or below with nicardipine appears to be well tolerated and feasible for acute ICH.34) Previous studies have further revealed that low-grade SBP reduction is independently associated with poor clinical outcomes,35) and that greater reductions in SBP (>20 mmHg) over 7 days were associated with a lower risk of poor outcomes.36) Furthermore, SBP variability during the initial 24 hours of acute intracerebral hemorrhage is independently associated with neurological deterioration and unfavorable outcomes.37)
The INTERACT2 study group randomly assigned 2,839 patients into either an intensive SBP lowering group (<140 mmHg) or a guideline-recommended SBP lowering group (<180 mmHg). However, they reported no significant reduction in death/severe disability between the groups, as assessed using modified Rankin Scale scales (mRS), although significant differences in mRS were noted as 90 days.38)
In the ATACH II study, no significant differences in death/severe disability (mRS 4–6) were noted between patients of the drastic SBP reduction group (intensive treatment group: 128.9 ± 16 mmHg) and those of the standard treatment group (141 ± 14.8 mmHg at 2 hours. Furthermore, patients of the intensive treatment group experienced a significantly greater number of severe, treatment-related adverse events,39) suggesting that a target SBP of 140 mmHg may be appropriate for the treatment of SICH.
Prevention of ICH regrowth: hemostatic treatment at acute phase
Injection of activated recombinant factor VII (rFVIIa) has been suggested as a more aggressive treatment for the prevention of hematoma regrowth.17,40) Mayer et al. conducted a double-blind phase 3 trial with 841 patients treated with rFVIIa within 4 hours of stroke onset.41) They observed that hemostatic therapy with rFVIIa reduced growth of the hematoma but did not improve survival or functional outcomes following intracerebral hemorrhage. Similarly, a meta-analysis revealed that treatment with rFVIIa did not significantly reduce death or disability within 90 days of ICH, as assessed using mRS scores (grades 4 to 6). Furthermore, participants undergoing rFVIIa treatment tended to experience a higher number of serious, adverse thromboembolic events. Thus, rFVIIa is not recommended now for the treatment of SICH.42)
However, these clinical data might not be applicable for the patients treated with anticoagulant medication of warfarin and DOAC(NOAC). As anticoagulant-related SICH showed poor outcome,43) reversal agents (treatment) are necessary. The specific reversal agent of vitamin K (anti-warfarin) and Idarucizumab (anti-dabigatoran) are now approved, but now andexanet alfa, and ciraparantag (PER977) (anti-factor Xa inhibitor) are not approved. Besides that, FVIIa, prothrombin complex concentrate (PCC) and fresh frozen plasma (FFP) were used as nonspecific agents (Table 1).13) The large scale prospective studies are necessary to determine whether early administration of these specific/non-specific reversal agents can inhibit hematoma growth and improve outcome of anti-coagulant related SICH.
Prediction of hematoma regrowth
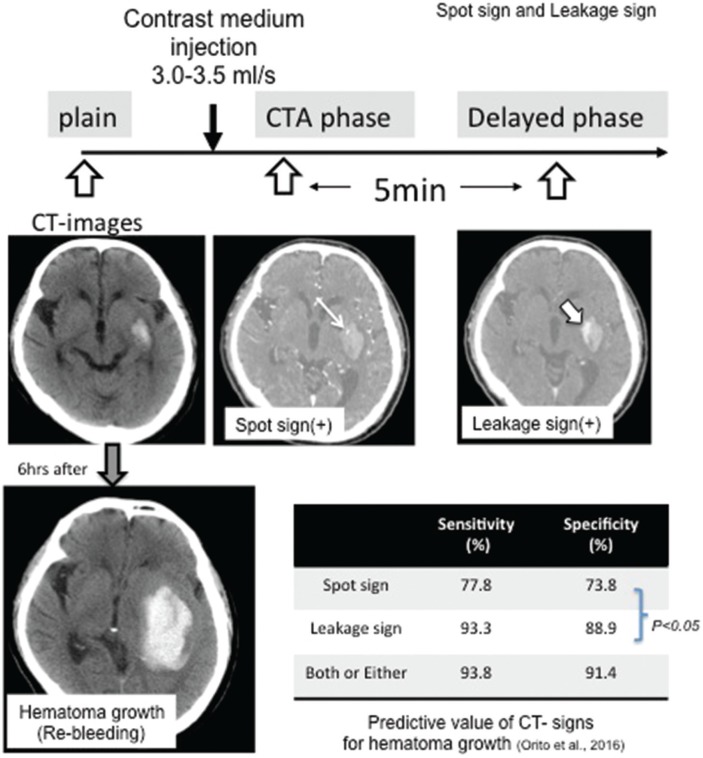
If we could predict SICH regrowth as soon as possible at the first admission, some aggressive treatments can be developed for selective patients. Several prospective studies have been suggested that “spot signs” visualized via computed tomography (CT) using contrast medium may serve as crucial predictors of hematoma regrowth and mortality.44–46) Recently, we revealed that “leakage signs” may also represent significant predictors of hematoma regrowth.47) We performed two CT scans with a rapid infusion of contrast medium: The first scan (CT angiography) was performed for detection of spot signs, while the second scan was performed 5 min after the CTA phase (delayed phase). We utilized an region of interest (ROI) of 10 mm in diameter and compared these two images. Positive leakage signs were defined as >10% increases in Hunsfield units (Fig. 2). The sensitivity and specificity of leakage signs were 93.3% and 88.9%, respectively, which were significantly higher (P < 0.05) than those of spot signs in patients with SICH. Furthermore, leakage sign-negative patients experienced significantly better outcomes than leakage sign-positive patients.47) This non-invasive method for the prediction of SICH regrowth may allow for the identification of patients who require more aggressive treatment prior to the occurrence of serious complications.
Fig. 2.
Diagnostic evaluation of spot and leakage signs in patients with spontaneous intracerebral hematoma based on the computed tomographic angiography (CTA) and delayed phase computed tomography (CT) images. The Hunsfield unit (HU) values in the ROI were determined in each section of the arterial and delayed phase images, and a >10% increase in HU was considered a positive leakage sign.
Surgical treatment
Surgical treatment of SICH has been performed for quite some time, although the efficacy of surgery for such cases remains controversial. Numerous clinical studies have investigated the effectiveness of ICH surgery following several randomized controlled trials and meta-analyses have also examined the efficacy of craniotomy and other surgical procedures (Table 2).
Table 2.
Recent randomized controlled trials and meta-analyses of surgical treatment for ICH
| Study/Authors year | Target surgery (Compared treatment) | Number of all cases | Evaluation of outcome (time) | Conclusion of surgery-superiority recommendation |
|---|---|---|---|---|
| Craniotomy | ||||
| STICH-I 2005 | Surgery vs conservative treatment | 1033 | Mortality mRS/ BI et al. (6 M) |
better only 1 cm depath hematoma |
| STICH-II 2013 | Craniotomy for superficial lobar ICH vs conservative treatment | 601 | Mortality mRS/ BI et al. (6 M) |
small but relevant survival advantage |
| Pantazis et al. 2006 | early craniotomy (<8 hr) >30 ml SICH vs conservative treatment | 54 | GOS (12 M) | Better in patients 80–30 ml subcortical and putaminal ICH |
| Prasad K et al. | Surgery vs conservative treatment | 2059 | decreased dead/independent rate | |
| Meta-analysis 2008 | 10 RCT | |||
| Gregson BA et al. | Surgery vs conservative treatment | 2186 8 RCT |
GOS, mRS, BI GCS: 9–12, Age: 50–69 |
better early surgery (<8 hr), 20–50 ml |
| Meta-analysis 2012 | ||||
| Moussa WM 2016 | Decompressive craniotomy/dulaplsasty vs simple craniotomy | 40 | GOS (6 M) | Better in special group young/highGCS/ et al. |
| MIS: minimally invasive surgery | ||||
| Zhou X et al. | All MISs | 1995 | BI/mRS (3–12 M) | OR: 0.54 (0.39–0.76) |
| Meta-analysis 2012 | 12 RCT | Mortality | 30–80 y.o., superficial ICH GCS ≥9, 25–40 ml, <72 h |
|
| MISTIE phase 2 2016 | Image guide catheter & alteprase vs conservative treatment | 96 | mRS (6 & 12 M) ICH volume |
Safe surgery, ICH volume reduction frequent asymptomatic hemorrhage |
BI, Barthel Index; GOS, Glasgow Outcome Scale; MIS, minimally invasive surgery; mRS, modified Rankin Scale; SICH, spontaneous intracerebral hematoma.
Surgery I: supratentorial ICH
Pantazis et al.48) conducted a prospective randomized controlled trial in patients of the treatment group underwent early (within 8 hours) hematoma evacuation craniotomy for over hematoma volumes over 30 mL. However, this procedure was only effective for patients with putaminal and subcortical hematomas. Nevertheless, the authors concluded that patients of the surgical group exhibited significantly better functional results (Glasgow Outcome Scale: GOS evaluation) than those of the conservative treatment group, although the early ICH evacuation failed to improve survival rates.
The STICH (Surgical Trial in Intracerebral Haemorrhage) study also compared early surgery with medical management, although the authors reported no significant benefit of early surgery with regard to favorable outcomes (moderate disability or good recovery, mortality, good mRS (0–2).49) However, subgroup analyses of the STICH trial suggested that surgical intervention was associated with improvements for patients with lobar intracerebral hemorrhage, following which the STICH-II trial was conducted. The STICH-II trial further indicated that early surgery does not increase the rate of death or disability at 6 months and may have a small but clinically relevant advantage for patients with spontaneous superficial intracerebral hemorrhage without intraventricular hemorrhage.50)
A systematic review and meta-analysis of ten trials (2,059 patients) in the Cochrane Database51) concluded that surgical intervention for supratentorial SICH was associated with significant improvements in the rate of death/disability when compared with conservative management. However, the reviewers noted that the quality of most trials was acceptable rather than high. Gregson et al.52) performed a meta-analysis of surgery for supratentorial SICH using individual patient data from 8 randomized controlled trials (2,186 cases), revealing that early surgery (<8 hours) was associated with significant improvements in outcomes.49,53–59)
Although craniotomy is not recommended for SICH treatment, research suggests that minimally invasive surgical (MIS) procedures—such as stereotactic aspiration, endoscopic aspiration, and navigation surgery—may be both safe and effective in this patient population. Hattori et al. conducted a randomized controlled trial comparing stereotactic aspiration (n = 121) and medical treatment (n = 121) for putaminal hemorrhage,60) reporting that stereotactic evacuation is beneficial for selected patients (i.e., those whose eyes are closed on admission but will open in response to strong stimuli on admission). In the SICHPA trial, stereotactic aspiration and liquefaction by means of plasminogen activator (urokinase) were performed for 36 patients and 34 non-surgical controls. However, the authors reported no significant reduction in death or disability at 180 days.59) Furthermore, although patients of the surgical group experienced significant reductions in hematoma volume, the re-bleeding rate was significantly higher than in the control group. Auer et al.57) conducted a randomized controlled study in which 50 patients underwent endoscopic hematoma evacuation through a burr hole, while an additional 50 patients received medical treatment. Although overall outcomes were better in the surgical group, patients with putaminal or thalamic hematomas did not experience significant improvements. Although one Japanese study reported the safety and efficacy of endoscopic hematoma evacuation, no randomized controlled trials have been conducted regarding this matter.61)
Zhou et al.62) performed a systematic review and meta-analysis of randomized controlled trials (1,995 patients) treated via MIS58,63–68) and concluded that MIS is more effective than other treatment options for both men and women. The most likely candidates to benefit from MIS are those between 30 and 80 years of age with superficial hematoma, Glasgow Coma Scale (GCS) score >9, hematoma volume between 25 and 40 mL, and treated within 72 hours after the onset of symptoms. Recently, Mould et al. reported on a novel surgical method known as MISTIE: minimally invasive surgery plus recombinant tissue-type plasminogen activator (rt-PA) for intracerebral hemorrhage evacuation. This method was associated with significant reductions in peri-hematomal edema.69) A phase 2 randomized control trial of MISTIE concluded that MISTIE was safe and resulted in decreased hematoma volumes relative to medical treatment, although asymptomatic bleeding was more frequent.70) These developments and MIS techniques may improve treatment outcomes for patients with ICH.
Surgery II: posterior fossa ICH
Although no randomized controlled trials have been conducted regarding cerebellar hematoma, some studies have suggested that patients with ICH >3 cm in diameter have better outcomes with surgical decompression.71) Morioka et al.72) examined 1,010 cases of SICH in Japan and concluded that surgically-treated patients with cerebellar hemorrhage exhibited significantly greater improvements in National Institutes of Health Stroke Scale (NIHSS) or Japan Stroke Scale (JSS) scores when compared with medically-treated patients.
Surgery-III: Decompressive craniotomy
Decompressive craniotomy and expansive duraplasty (DC/ED) is sometimes performed for severe cases of SICH, although the efficacy of DC/ED has not been established. Recently, Moussa and Khedr conducted a randomized controlled trial comparing DC/ED for intracerebral hematoma and simple hematoma evacuation surgery.73) They concluded that DC/ED improved outcomes in a selected group of patients (younger age, smaller hematoma, subcortical hematoma), although the number of patients included in the trial was rather low.
Surgery IV: Intraventricular hematoma
SICH may result in complications such as intraventricular hemorrhage (IVH), which may further lead to obstructive hydrocephalus (Fig. 1). IVH and obstructive hydrocephalus are independent predictors of poor outcome in SICH.15–17) External drainage of cerebrospinal fluid and hemorrhage via ventricular catheter can reduce intracranial pressure, decreasing the risk of cerebral herniation.74) However, infection and re-bleeding may occur.
Naff et al. reported that urokinase injection during ventricular drainage was effective in reducing the drainage period,75) while Staykov et al. demonstrated the surgical superiority of intraventricular fibrinolysis and lumber drainage in a randomized controlled trial.76) In contrast, other researchers have reported good clinical outcomes for endoscopic management of ICH in patients with obstructive hydrocephalus.77,78)
Thus, surgical evacuation for supratentorial ICH remains controversial despite the abundance of clinical studies. AHA/ASA guidelines79) do not clearly recommend craniotomy for the treatment of supratentorial SICH, and the use of MIS with/without thrombolytics remains uncertain. However, the guidelines note some exceptions: Patients with cerebellar hemorrhage experiencing neurological deterioration, brainstem compression, and/or hydrocephalus from ventricular obstruction should undergo surgical removal of SICH as soon as possible. Supratentorial SICH evacuation in such patients may be regarded as a life-saving procedure. Decompressive craniotomy may also reduce mortality in patients with supratentorial SICH.
Future Consideration
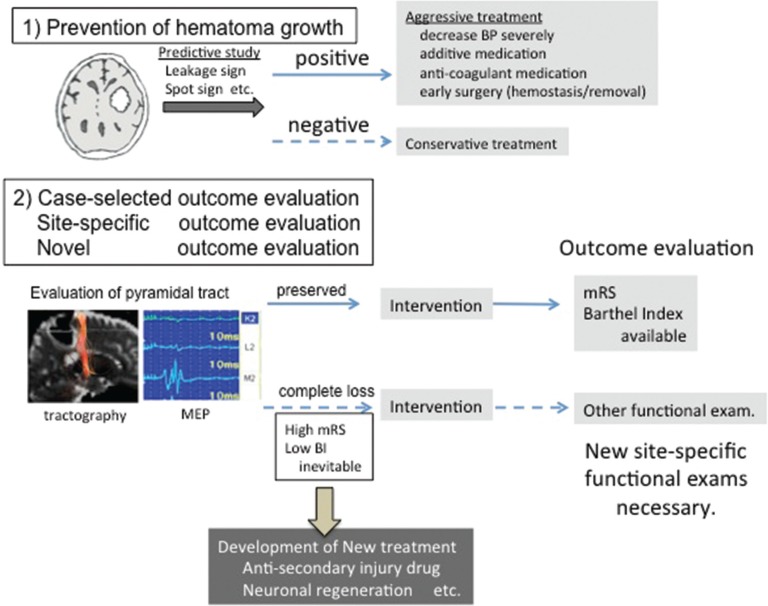
Although many clinical studies have been conducted, few effective treatments have been established for SICH. However, prevention of hematoma regrowth may significantly improve outcomes. Prediction of ICH regrowth may also allow for more aggressive treatment of selected patients at risk for neurological deterioration. The combination of leakage and spot signs is currently the most effective method for predicting ICH regrowth, with high sensitivity and specificity, although the use of contrast medium and additional CT scanning (increased x-ray exposure) are required. Additional aggressive treatments include (a) drastic reductions in BP; (b) additional medications; (c) short-acting coagulant medication (higher dose or new medication); and (d) early surgical treatment to stop bleeding, even in cases of small hematoma (Fig. 3). Although these methods have not been proven significantly effective to date, these measures may be beneficial in patients prone to hematoma regrowth.
Fig. 3.
Issues and future directions regarding SICH treatment. (1) Prevention of hematoma growth; using predictive methods (leakage sign and/or spot sign) for hematoma growth, patient selection. Aggressive treatments might improve overall outcomes. (2) Re-evaluation or new method of outcome analysis is necessary High mRS/Low Barthel Index scores are inevitable in patients with complete destruction of the pyramidal tract at admission, even if the hematoma is small.
In the aspect of surgical treatment, the adaptation of craniotomy has become to be limited after several clinical studies. In contrast, the development of safer MIS combined with endoscopy and navigation system might have a possibility of acceptance in future. However, precise identification of bleeding point and secure hemostasis using the imaging technique such as spot sign and leakage sign are necessary.
SICH is heterogeneous with regard to hemorrhagic sites, eloquency, etc. However, in most clinical studies, evaluations of treatment outcome are based on mRS, BI (Barthel Index), or GOS scores, which are influenced by the presence of severe hemiparesis. Even in patients with small lesions, hematomas of the internal capsule that produce complete hemiparesis would result in low scores at six months.
Future studies aimed at evaluating the efficacy of treatment for the recovery of motor function should also interpret the results with caution when including patients with complete pyramidal tract injury, as these patients would not show recovery of motor function even following an optimal surgery.
Case-selected or site-specific evaluation of outcomes is also necessary. Diffusion tensor tractography80,81) or motor evoked potentials82,83) have been reported as good predictors of motor function recovery, though alternative measures are necessary for patients with complete pyramidal tract injury (Fig. 3). Furthermore, the development of novel treatment strategies84) (i.e., facilitating neuronal regeneration) for the patients with poor outcomes remains necessary.
Conclusion
Although the efficacy of surgical evacuation has not been well established despite the abundance of randomized controlled trials and reviews, reducing systolic BP in the acute phase of SICH (120–140 mmHg) may improve outcomes. However, new methods of evaluating outcomes in patients with severe hemiparesis are required. Furthermore, aggressive treatment for selected cases may be necessary to improve overall outcomes and prevent SICH regrowth, such as in those with positive spot or leakage signs.
Grant Support
This work was supported by Grants-in-Aid for Scientific Research from the Ministry of Education, Sports, Science and Culture of Japan.
Footnotes
Conflicts of Interest Disclosure
The author declares no conflict of interest regarding this review article.
References
- 1). Qureshi AI, Mendelow AD, Hanley DF: Intracerebral haemorrhage. Lancet 373: 1632– 1644, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2). Qureshi AI, Tuhrim S, Broderick JP, Batjer HH, Hondo H, Hanley DF: Spontaneous intracerebral hemorrhage. N Engl J Med 344: 1450– 1460, 2001. [DOI] [PubMed] [Google Scholar]
- 3). Morgenstern LB, Hemphill JC, Anderson C, et al. American heart association stroke council and council on cardiovascular nursing : Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 41: 2108– 2129, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4). Kubo M, Kiyohara Y, Kato I, et al. : Trends in the incidence, mortality, and survival rate of cardiovascular disease in a Japanese community: the Hisayama study. Stroke 34: 2349– 2354, 2003. [DOI] [PubMed] [Google Scholar]
- 5). Jiang B, Wang WZ, Chen H, et al. : Incidence and trends of stroke and its subtypes in China: results from three large cities. Stroke 37: 63– 68, 2006. [DOI] [PubMed] [Google Scholar]
- 6). Rothwell PM, Coull AJ, Giles MF, et al. : Oxford vascular Study: Change in stroke incidence, mortality, case-fatality, severity, and risk factors in oxfordshire, UK from 1981 to 2004 (oxford vascular study). Lancet 363: 1925– 1933, 2004. [DOI] [PubMed] [Google Scholar]
- 7). Gotoh S, Hata J, Ninomiya T, et al. : Trends in the incidence and survival of intracerebral hemorrhage by its location in a Japanese community. Circ J 78: 403– 409, 2014. [DOI] [PubMed] [Google Scholar]
- 8). Hankey GJ, Sudlow CL, Dunbabin DW: Thienopyridines or aspirin to prevent stroke and other serious vascular events in patients at high risk of vascular disease? A systematic review of the evidence from randomized trials. Stroke 31: 1779– 1784, 2000. [DOI] [PubMed] [Google Scholar]
- 9). Toyoda K, Yasaka M, Iwade K, et al. Bleeding with Antithrombotic Therapy (BAT) Study Group : Dual antithrombotic therapy increases severe bleeding events in patients with stroke and cardiovascular disease: a prospective, multicenter, observational study. Stroke 39: 1740– 1745, 2008. [DOI] [PubMed] [Google Scholar]
- 10). Butler AC, Tait RC: Management of oral anticoagulant-induced intracranial haemorrhage. Blood Rev 12: 35– 44, 1998. [DOI] [PubMed] [Google Scholar]
- 11). Linkins LA, Choi PT, Douketis JD: Clinical impact of bleeding in patients taking oral anticoagulant therapy for venous thromboembolism: a meta-analysis. Ann Intern Med 139: 893– 900, 2003. [DOI] [PubMed] [Google Scholar]
- 12). Steinberg BA: How I use anticoagulation in atrial fibrillation. Blood 128: 2891– 2898, 2016. [DOI] [PubMed] [Google Scholar]
- 13). Ruff CT, Giugliano RP, Antman EM: Management of bleeding with non-vitamin k antagonist oral anticoagulants in the era of specific reversal agents. Circulation 134: 248– 261, 2016. [DOI] [PubMed] [Google Scholar]
- 14). Davis SM, Broderick J, Hennerici M, et al. Recombinant activated factor VII intracerebral hemorrhage trial investigators : Hematoma growth is a determinant of mortality and poor outcome after intracerebral hemorrhage. Neurology 66: 1175– 1181, 2006. [DOI] [PubMed] [Google Scholar]
- 15). Bhattathiri PS, Gregson B, Prasad KS, Mendelow AD, STICH Investigators : Intraventricular hemorrhage and hydrocephalus after spontaneous intracerebral hemorrhage: results from the STICH trial. Acta Neurochir Suppl 96: 65– 68, 2006. [DOI] [PubMed] [Google Scholar]
- 16). Gujjar AR, Deibert E, Manno EM, Duff S, Diringer MN: Mechanical ventilation for ischemic stroke and intracerebral hemorrhage: indications, timing, and outcome. Neurology 51: 447– 451, 1998. [DOI] [PubMed] [Google Scholar]
- 17). Mayer SA, Brun NC, Begtrup K, et al. : Recombinant activated factor VII intracerebral hemorrhage trial investigators: recombinant activated factor VII for acute intracerebral hemorrhage. N Engl J Med 352: 777– 785, 2005. [DOI] [PubMed] [Google Scholar]
- 18). Alvarez-Sabín J, Delgado P, Abilleira S, et al. : Temporal profile of matrix metalloproteinases and their inhibitors after spontaneous intracerebral hemorrhage: relationship to clinical and radiological outcome. Stroke 35: 1316– 1322, 2004. [DOI] [PubMed] [Google Scholar]
- 19). Aronowski J, Hall CE: New horizons for primary intracerebral hemorrhage treatment: experience from preclinical studies. Neurol Res 27: 268– 279, 2005. [DOI] [PubMed] [Google Scholar]
- 20). Hua Y, Wu J, Keep RF, Nakamura T, Hoff JT, Xi G: Tumor necrosis factor-alpha increases in the brain after intracerebral hemorrhage and thrombin stimulation. Neurosurgery 58: 542– 550, 2006. [DOI] [PubMed] [Google Scholar]
- 21). Gong C, Boulis N, Qian J, Turner DE, Hoff JT, Keep RF: Intracerebral hemorrhage-induced neuronal death. Neurosurgery 48: 875– 882; discussion 882–883, 2001. [DOI] [PubMed] [Google Scholar]
- 22). Matz PG, Lewén A, Chan PH: Neuronal, but not microglial, accumulation of extravasated serum proteins after intracerebral hemolysate exposure is accompanied by cytochrome c release and DNA fragmentation. J Cereb Blood Flow Metab 21: 921– 928, 2001. [DOI] [PubMed] [Google Scholar]
- 23). Yang S, Nakamura T, Hua Y, et al. : The role of complement C3 in intracerebral hemorrhage-induced brain injury. J Cereb Blood Flow Metab 26: 1490– 1495, 2006. [DOI] [PubMed] [Google Scholar]
- 24). Inaji M, Tomita H, Tone O, Tamaki M, Suzuki R, Ohno K: Chronological changes of perihematomal edema of human intracerebral hematoma. Acta Neurochir Suppl 86: 445– 448, 2003. [DOI] [PubMed] [Google Scholar]
- 25). Butcher KS, Baird T, MacGregor L, Desmond P, Tress B, Davis S: Perihematomal edema in primary intracerebral hemorrhage is plasma derived. Stroke 35: 1879– 1885, 2004. [DOI] [PubMed] [Google Scholar]
- 26). Kidwell CS, Chalela JA, Saver JL, et al. : Comparison of MRI and CT for detection of acute intracerebral hemorrhage. JAMA 292: 1823– 1830, 2004. [DOI] [PubMed] [Google Scholar]
- 27). Chalela JA, Kidwell CS, Nentwich LM, et al. : Magnetic resonance imaging and computed tomography in emergency assessment of patients with suspected acute stroke: a prospective comparison. Lancet 369: 293– 298, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28). Kortman HG, Smit EJ, Oei MT, Manniesing R, Prokop M, Meijer FJ: 4D-CTA in neurovascular disease: a review. AJNR Am J Neuroradiol 36: 1026– 1033, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29). Bokura H, Saika R, Yamaguchi T, et al. : Microbleeds are associated with subsequent hemorrhagic and ischemic stroke in healthy elderly individuals. Stroke 42: 1867– 1871, 2011. [DOI] [PubMed] [Google Scholar]
- 30). Diringer MN, Edwards DF: Admission to a neurologic/neurosurgical intensive care unit is associated with reduced mortality rate after intracerebral hemorrhage. Crit Care Med 29: 635– 640, 2001. [DOI] [PubMed] [Google Scholar]
- 31). Ng I, Lim J, Wong HB: Effects of head posture on cerebral hemodynamics: its influences on intracranial pressure, cerebral perfusion pressure, and cerebral oxygenation. Neurosurgery 54: 593– 597; discussion 598, 2004. [DOI] [PubMed] [Google Scholar]
- 32). Kollmar R, Staykov D, Dörfler A, Schellinger PD, Schwab S, Bardutzky J: Hypothermia reduces perihemorrhagic edema after intracerebral hemorrhage. Stroke 41: 1684– 1689, 2010. [DOI] [PubMed] [Google Scholar]
- 33). Staykov D, Wagner I, Volbers B, Doerfler A, Schwab S, Kollmar R: Mild prolonged hypothermia for large intracerebral hemorrhage. Neurocrit Care 18: 178– 183, 2013. [DOI] [PubMed] [Google Scholar]
- 34). Koga M, Toyoda K, Yamagami H, et al. Stroke acute management with urgent risk-factor assessment and improvement study investigators : Systolic blood pressure lowering to 160 mmHg or less using nicardipine in acute intracerebral hemorrhage: a prospective, multicenter, observational study (the stroke acute management with urgent risk-factor assessment and improvement-intracerebral hemorrhage study). J Hypertens 30: 2357– 2364, 2012. [DOI] [PubMed] [Google Scholar]
- 35). Sakamoto Y, Koga M, Yamagami H, et al. Samurai study investigators : Systolic blood pressure after intravenous antihypertensive treatment and clinical outcomes in hyperacute intracerebral hemorrhage: the stroke acute management with urgent risk-factor assessment and improvement-intracerebral hemorrhage study. Stroke 44: 1846– 1851, 2013. [DOI] [PubMed] [Google Scholar]
- 36). Wang X, Arima H, Heeley E, et al. INTERACT2 Investigators : Magnitude of blood pressure reduction and clinical outcomes in acute intracerebral hemorrhage: intensive blood pressure reduction in acute cerebral hemorrhage trial study. Hypertension 65: 1026– 1032, 2015. [DOI] [PubMed] [Google Scholar]
- 37). Tanaka E, Koga M, Kobayashi J, et al. : Blood pressure variability on antihypertensive therapy in acute intracerebral hemorrhage: the stroke acute management with urgent risk-factor assessment and improvement-intracerebral hemorrhage study. Stroke 45: 2275– 2279, 2014. [DOI] [PubMed] [Google Scholar]
- 38). Anderson CS, Heeley E, Huang Y, et al. INTERACT2 Investigators : Rapid blood-pressure lowering in patients with acute intracerebral hemorrhage. N Eng J Med 368: 2355– 2365, 2013. [DOI] [PubMed] [Google Scholar]
- 39). Qureshi AI, Palesch YY, Barsan WG, et al. ATACH-2 trial investigators and the neurological emergency treatment trials network : Intensive blood-pressure lowering in patients with acute cerebral hemorrhage. N Engl J Med 375: 1033– 1043, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40). Mayer SA, Brun NC, Broderick J, et al. United States NovoSeven ICH Trial Investigators : Recombinant activated factor VII for acute intracerebral hemorrhage: US phase IIA trial. Neurocrit Care 4: 206– 214, 2006. [DOI] [PubMed] [Google Scholar]
- 41). Mayer SA, Brun NC, Begtrup K, et al. FAST Trial Investigators : Efficacy and safety of recombinant activated factor VII for acute intracerebral hemorrhage. N Engl J Med 358: 2127– 2137, 2008. [DOI] [PubMed] [Google Scholar]
- 42). Al-Shahi Salman R: Haemostatic drug therapies for acute spontaneous intracerebral haemorrhage. Cochrane Database Syst Rev 4: CD005951, 2009. [DOI] [PubMed] [Google Scholar]
- 43). Purrucker JC, Haas K, Rizos T, et al. : Early clinical and radiological course, management, and outcome of intracerebral hemorrhage related to new oral anticoagulants. JAMA Neurol 73: 169– 177, 2016. [DOI] [PubMed] [Google Scholar]
- 44). d’Esterre CD, Chia TL, Jairath A, Lee TY, Symons SP, Aviv RI: Early rate of contrast extravasation in patients with intracerebral hemorrhage. AJNR Am J Neuroradiol 32: 1879– 1884, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45). Demchuk AM, Dowlatshahi D, Rodriguez-Luna D, et al. PREDICT/Sunnybrook ICH CTA study group : Prediction of haematoma growth and outcome in patients with intracerebral haemorrhage using the CT-angiography spot sign (PREDICT): a prospective observational study. Lancet Neurol 11: 307– 314, 2012. [DOI] [PubMed] [Google Scholar]
- 46). Dowlatshahi D, Brouwers HB, Demchuk AM, et al. : Predicting intracerebral hemorrhage growth with the spot sign: the effect of onset-to-scan time. Stroke 47: 695– 700, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47). Orito K, Hirohata M, Nakamura Y, et al. : Leakage sign for primary intracerebral hemorrhage: a novel predictor of hematoma growth. Stroke 47: 958– 963, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48). Pantazis G, Tsitsopoulos P, Mihas C, Katsiva V, Stavrianos V, Zymaris S: Early surgical treatment vs conservative management for spontaneous supratentorial intracerebral hematomas: a prospective randomized study. Surg Neurol 66: 492– 501; discussion 501–502, 2006. [DOI] [PubMed] [Google Scholar]
- 49). Mendelow AD, Gregson BA, Fernandes HM, et al. STICH investigators : Early surgery versus initial conservative treatment in patients with spontaneous supratentrial intracranial haematoma in the international surgical trial in intracerebral haemorrhage (STICH): a randomized trial. Lancet 365: 387–397, 2005. [DOI] [PubMed] [Google Scholar]
- 50). Mendelow AD, Gregson BA, Rowan EN, Murray GD, Gholkar A, Mitchell PM, STICH II Investigators : Early surgery versus initial conservative treatment in patients with spontaneous supratentrial lobar intracranial hematoma (STICH II): a randomized trial. Lancet 382: 397–408, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51). Prasad K, Mendelow AD, Gregson B: Surgery for primary supratentorial intracerebral haemorrhage. Cochrane Database Syst Rev 4: CD000200, 2008. [DOI] [PubMed] [Google Scholar]
- 52). Gregson BA, Broderick JP, Auer LM, et al. : Individual patient data subgroup meta-analysis of surgery for spontaneous supratentorial intracerebral hemorrhage. Stroke 43: 1496– 1504, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53). Juvela S, Heiskanen O, Poranen A, et al. : The treatment of spontaneous intracerebral hemorrhage. A prospective randomized trial of surgical and conservative treatment. J Neurosurg 70: 755– 758, 1989. [DOI] [PubMed] [Google Scholar]
- 54). Morgenstern LB, Frankowski RF, Shedden P, Pasteur W, Grotta JC: Surgical treatment for intracerebral hemorrhage (STICH): a single-center, randomized clinical trial. Neurology 51: 1359– 1363, 1998. [DOI] [PubMed] [Google Scholar]
- 55). Zuccarello M, Brott T, Derex L, et al. : Early surgical treatment for supratentorial intracerebral hemorrhage: a randomized feasibility study. Stroke 30: 1833– 1839, 1999. [DOI] [PubMed] [Google Scholar]
- 56). Chen X, Yang H, Cheng Z: A prospective randomized trial of surgical and conservative treatment of hypertensive intracerebral haemorrhage. Acta Acad Shanghai Med 19: 237– 240, 1992. [Google Scholar]
- 57). Auer LM, Deinsberger W, Niederkorn K, et al. : Endoscopic surgery versus medical treatment for spontaneous intracerebral hematoma: a randomized study. J Neurosurg 70: 530– 535, 1989. [DOI] [PubMed] [Google Scholar]
- 58). Wang WZ, Jiang B, Liu HM, et al. : Minimally invasive craniopuncture therapy vs. conservative treatment for spontaneous intracerebral hemorrhage: results from a randomized clinical trial in China. Int J Stroke 4: 11–16, 2009. [DOI] [PubMed] [Google Scholar]
- 59). Teernstra OP, Evers SM, Lodder J, Leffers P, Franke CL, Blaauw G: Multicenter randomized controlled trial (SICHPA): stereotactic treatment of intracerebral hematoma by means of a plasminogen activator: a multicenter randomized controlled trial (SICHPA). Stroke 34: 968– 974, 2003. [DOI] [PubMed] [Google Scholar]
- 60). Hattori N, Katayama Y, Maya Y, Gatherer A: Impact of stereotactic hematoma evacuation on activities of daily living during the chronic period following spontaneous putaminal hemorrhage: a randomized study. J Neurosurg 101: 417– 420, 2004. [DOI] [PubMed] [Google Scholar]
- 61). Nishihara T, Morita A, Teraoka A, Kirino T: Endoscopy-guided removal of spontaneous intracerebral hemorrhage: comparison with computer tomography-guided stereotactic evacuation. Childs Nerv Syst 23: 677– 683, 2007. [DOI] [PubMed] [Google Scholar]
- 62). Zhou X, Chen J, Li Q, et al. : Minimally invasive surgery for spontaneous supratentorial intracerebral hemorrhage: a meta-analysis of randomized controlled trials. Stroke 43: 2923– 2930, 2012. [DOI] [PubMed] [Google Scholar]
- 63). Zhou H, Zhang Y, Liu L, et al. : A prospective controlled study: minimally invasive stereotactic puncture therapy versus conventional craniotomy in the treatment of acute intracerebral hemorrhage. BMC Neurol 11: 76, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64). Kim YZ, Kim KH: Even in patients with a small hemorrhagic volume, stereotactic-guided evacuation of spontaneous intracerebral hemorrhage improves functional outcome. J Korean Neurosurg Soc 46: 109– 115, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65). Miller CM, Vespa P, Saver JL, et al. : Image-guided endoscopic evacuation of spontaneous intracerebral hemorrhage. Surg Neurol 69: 441– 446; discussion 446, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66). Sun H, Liu H, Li D, et al. : An effective treatment for cerebral hemorrhage: minimally invasive craniopuncture combined with urokinase infusion therapy. Neurol Res 32: 371– 377, 2010. [DOI] [PubMed] [Google Scholar]
- 67). Cho DY, Chen CC, Chang CS, Lee WY, Tso M: Endoscopic surgery for spontaneous basal ganglia hemorrhage: comparing endoscopic surgery, stereotactic aspiration, and craniotomy in noncomatose patients. Surg Neurol 65: 547– 555; discussion 555–556, 2006. [DOI] [PubMed] [Google Scholar]
- 68). Zuccarello M, Brott T, Derex L, et al. : Early surgical treatment for supratentorial intracerebral hemorrhage: a randomized feasibility study. Stroke 30: 1833– 1839, 1999. [DOI] [PubMed] [Google Scholar]
- 69). Mould WA, Carhuapoma JR, Muschelli J, et al. MISTIE investigators : Minimally invasive surgery plus recombinant tissue-type plasminogen activator for intracerebral hemorrhage evacuation decreases perihematomal edema. Stroke 44: 627– 634, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70). Hanley DF, Thompson RE, Muschelli J, et al. MISTIE Investigators : Safety and efficacy of minimally invasive surgery plus alteplase in intracerebral haemorrhage evacuation (MISTIE): a randomised, controlled, open-label, phase 2 trial. Lancet Neurol 15: 1228– 1237, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71). van Loon J, Van Calenbergh F, Goffin J, Plets C: Controversies in the management of spontaneous cerebellar haemorrhage. A consecutive series of 49 cases and review of the literature. Acta Neurochir (Wien) 122: 187– 193, 1993. [DOI] [PubMed] [Google Scholar]
- 72). Morioka J, Fujii M, Kato S, et al. Japan Standard Stroke Registry Group (JSSR) : Surgery for spontaneous intracerebral hemorrhage has greater remedial value than conservative therapy. Surg Neurol 65: 67– 72; discussion 72–73, 2006. [DOI] [PubMed] [Google Scholar]
- 73). Moussa WM, Khedr W: Decompressive craniectomy and expansive duraplasty with evacuation of hypertensive intracerebral hematoma, a randomized controlled trial. Neurosurg Rev 40: 115– 127, 2017. [DOI] [PubMed] [Google Scholar]
- 74). Liliang PC, Liang CL, Lu CH, et al. : Hypertensive caudate hemorrhage prognostic predictor, outcome, and role of external ventricular drainage. Stroke 32: 1195– 1200, 2001. [DOI] [PubMed] [Google Scholar]
- 75). Naff NJ, Hanley DF, Keyl PM, et al. : Intraventricular thrombolysis speeds blood clot resolution: results of a pilot, prospective, randomized, double-blind, controlled trial. Neurosurgery 54: 577– 583; discussion 583–584, 2004. [DOI] [PubMed] [Google Scholar]
- 76). Staykov D, Kuramatsu JB, Bardutzky J, et al. : Efficacy and safety of combined intraventricular fibrinolysis with lumbar drainage for prevention of permanent shunt dependency after intracerebral hemorrhage with severe ventricular involvement: a randomized trial and individual patient data meta-analysis. Ann Neurol 81: 93– 103, 2017. [DOI] [PubMed] [Google Scholar]
- 77). Zhang Z, Li X, Liu Y, Shao Y, Xu S, Yang Y: Application of neuroendoscopy in the treatment of intraventricular hemorrhage. Cerebrovasc Dis 24: 91– 96, 2007. [DOI] [PubMed] [Google Scholar]
- 78). Yadav YR, Mukerji G, Shenoy R, Basoor A, Jain G, Nelson A: Endoscopic management of hypertensive intraventricular haemorrhage with obstructive hydrocephalus. BMC Neurol 7: 1, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79). Hemphill JC, Greenberg SM, Anderson CS, et al. American heart association stroke council; Council on cardiovascular and stroke nursing; Council on Clinical Cardiology : Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the american heart association/american stroke association. Stroke 46: 2032– 2060, 2015. [DOI] [PubMed] [Google Scholar]
- 80). Kusano Y, Seguchi T, Horiuchi T, et al. : Prediction of functional outcome in acute cerebral hemorrhage using diffusion tensor imaging at 3T: a prospective study. AJNR Am J Neuroradiol 30: 1561– 1565, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81). Yoshioka H, Horikoshi T, Aoki S, et al. : Diffusion tensor tractography predicts motor functional outcome in patients with spontaneous intracerebral hemorrhage. Neurosurgery 62: 97– 103; discussion 103, 2008. [DOI] [PubMed] [Google Scholar]
- 82). Ikedo T, Nakamura K, Sano N, et al. : Intraoperative transcranial motor-evoked potentials predict motor function outcome in intracerebral hemorrhage surgery. World Neurosurg 90: 518– 523, 2016. [DOI] [PubMed] [Google Scholar]
- 83). Nagao S, Kawai N: Prediction of motor function by magnetic brain stimulation in patients with intracerebral hematoma. Neurol Med Chir (Tokyo) 32: 268– 274, 1992. [DOI] [PubMed] [Google Scholar]
- 84). Zhu J, Xiao Y, Li Z, et al. : Efficacy of surgery combined with autologous bone marrow stromal cell transplantation for treatment of intracerebral hemorrhage. Stem Cells Int 2015: 318269, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]