Abstract
Von Willebrand disease (VWD) is the most common inherited bleeding disorder, yet diagnosis and management remain challenging. Development and use of bleeding assessment tools allows for improved stratification of which patients may require further assessment and which patients are most likely to require treatment of their VWD. New options for laboratory assessment of von Willebrand factor (VWF) activity include a new platelet-binding assay, the VWF:GPIbM, which is subject to less variability than the ristocetin cofactor activity assay, and collagen-binding assays that provide insight into a different function of VWF. Genetic testing may be helpful in some cases where a type 2 VWD variant is suspected but is usually not helpful in type 1 VWD. Finally, treatment options for VWD are reviewed, including the use of recombinant VWF. Despite these advances, still more work is required to improve diagnosis, treatment, and quality of life for affected patients.
Introduction
Von Willebrand disease (VWD) is the most common inherited bleeding disorder, with a reported prevalence of approximately 1 in 1000 persons.1 Quantitative defects include type 1 VWD, with partial deficiency of von Willebrand factor (VWF), and type 3 VWD, with virtually complete deficiency of VWF. Qualitative variants include defects in multimerization (type 2A), spontaneous platelet binding (type 2B), defects in ligand binding with intact multimers (type 2M), and defects in factor VIII (FVIII) binding (type 2N). Type 1 VWD is the most common, accounting for up to 85% of VWD.2 Type 3 is the least common, affecting about 1 in 1 million individuals.3 Type 2 qualitative variants account for the remainder of VWD patients. The possibility and reasonably high frequency of qualitative defects prevent diagnosis of VWD with a single simple assessment of total VWF protein. The frequency of mild bleeding symptoms in the general population also makes choosing which patient to test for VWD a difficult task.
Diagnosis of VWD rests on a history of bleeding symptoms, often with a family history of bleeding symptoms or diagnosed VWD, and confirmatory laboratory testing.3 Typical bleeding includes mucosal bleeding symptoms such as easy bruising, epistaxis, gingival bleeding, surgical bleeding, and heavy menstrual bleeding. Gastrointestinal bleeding is a particular problem for patients with type 2A VWD.4 Type 3 and type 2N VWD patients may have joint bleeds due to low FVIII. There is overlap in the spectrum of normal bleeding with bleeding attributable to defects in VWF, and the diagnosis is not always straightforward. In addition, children and young adults with VWD who have not experienced significant hemostatic challenges may lack a bleeding history. However, recent advances in quantifying bleeding, as well as advances in diagnostic testing, should serve to improve our ability to diagnose patients properly. Treatment of VWD continues to involve use of desmopressin or VWF concentrates, but a recently available recombinant VWF has now been added to our treatment panel.
VWD is of course not the only cause of mucosal bleeding. Acquired VWD, platelet-type VWD, platelet function defects, vascular malformations such as hereditary hemorrhagic telangiectasias, and connective tissue disorders must be considered in the differential diagnosis.
Bleeding assessment tools
Bleeding history is critically important for the diagnosis of VWD. To that end, attempts have been made to quantify reported bleeding symptoms using bleeding assessment tools. The International Society on Thrombosis and Haemostasis (ISTH) has developed and performed initial validation of a bleeding assessment tool for use in screening patients for VWD.5 Normal ranges have been established for children, adult males, and adult females.6 A pediatric bleeding questionnaire (PBQ) has also been shown to have utility as a screening tool for VWD in the pediatric population.7 The PBQ had a high negative predictive value, meaning it was useful to assess which patients did not require further testing. The PBQ had a low positive predictive value. One explanation is the fact that VWD and platelet defects, among other conditions (eg, connective tissue disorders), can present with similar mucosal bleeding symptoms. There is also potential overlap with the wide range of normal bleeding symptoms seen in healthy individuals.8
Bleeding scores in general correlate with severity of VWD. Type 3 patients have the highest bleeding scores, type 2 patients are intermediate, and type 1 patients have the lowest bleeding scores.9 In addition, lower VWF:Ag and FVIII have been associated with increased bleeding scores.10 However, obtaining a bleeding score on a patient who has previously been diagnosed and received treatment may be difficult, as treatment in and of itself will raise the bleeding score. It is possible that history of bleeding is relevant for treatment, as a recent study in adults showed that a bleeding score >10 was highly predictive of need for future treatment.11 This suggests that there is clinical utility in determining bleeding scores both for diagnostic purposes and for treatment purposes.
There are some limitations to bleeding scores that should be considered. Time and lack of hemostatic challenges with which to measure bleeding are of particular issue in the pediatric population. Inclusion of pediatric-specific questions may be helpful but does not always provide complete reassurance that a given patient will not develop bleeding in the future.9 In addition, some studies are complicated by the fact that bleeding scores rely on the worst historical episode to generate points; a patient who receives a diagnosis of VWD and is subsequently treated for surgery can generate an increased bleeding score independent of their recent symptoms because of that treatment. Because VWF levels increase with age,12 it is possible that a patient might “outgrow” their diagnosis. However, it is also possible that higher VWF levels are required as patients age, so patients with increased VWF levels should be evaluated cautiously and in context of their present and past bleeding history.
Advances in diagnostic testing
VWF and GPIbα
Classic laboratory testing for VWD involves measurement of total VWF protein levels via VWF antigen (VWF:Ag) and measurement of VWF activity via ristocetin cofactor (VWF:RCo). This provides an assessment of VWF function in terms of platelet binding, as VWF binds to platelet glycoprotein Ibα through a binding site in the VWF A1 domain. The VWF:RCo assay, however, has several issues limiting its usefulness. One problem is the high coefficient of variation, with the potential for either falsely high or falsely low results.13 Another issue is the lower limit of detection, usually 10-20 IU/dL. This makes accurate assessment of possible type 2 variants difficult in patients with low VWF:Ag, because the VWF:RCo/VWF:Ag ratio may be difficult to determine. Because the VWF:RCo uses the nonphysiologic agonist ristocetin to bridge VWF and GPIbα, there is the potential for false results due to defects in VWF’s ability to bind ristocetin. The most common of these is the p.D1472H variant, which affects ristocetin binding but not VWF function.14
Fortunately, a new assay is available that avoids the use of ristocetin. The VWF:GPIbM assay, using the terminology recommended by the International Society on Thrombosis and Haemostasis, introduces gain-of-function mutations into GPIbα, allowing it to bind VWF spontaneously in vitro without the requirement for ristocetin.15 The VWF:GPIbM allows greater precision, with a reported lower limit of detection of 2 IU/dL and a reported-within-laboratory coefficient of variation of 5.6%.16 There is reasonable correlation between VWF:RCo and VWF:GPIbM results.15 One study did show increased qualitative VWF defects using the VWF:GPIbM.16 This may be due to use of ristocetin as the “gold standard,” when in reality ristocetin is not the most accurate assay. At the time of writing, commercial availability of VWF:GPIbM assays is limited, but in some countries these have replaced the VWF:RCo entirely. In Europe and Canada, an automated VWF:GPIbM is available,15,16 whereas in the US, an ELISA version is available through the BloodCenter of Wisconsin.17 Both use a combination of 2 gain-of-function GPIbα variants to elicit binding in the absence of ristocetin. It should be noted, however, that neither the VWF:RCo nor the VWF:GPIbM is physiologic, as neither use shear to induce VWF-platelet interactions (Table 1).
Table 1.
Comparison of VWF:RCo and VWF:GPIbM
| VWF:RCo | VWF:GPIbM | |
|---|---|---|
| Lower limit of detection | 10%-20% | <2.2% |
| Coefficient of variation | 20% | 2%-7% |
| Affected by “benign” sequence variations | Yes | No |
| Widely available | Yes | Not yet |
VWF and collagen
VWF has another function in binding exposed collagen at sites of injury, which requires specific testing apart from the platelet-binding assays described above under VWF and GPIbα. Most VWD diagnostic panels do not include any assessment of collagen binding. To further complicate the collagen picture, there are different vascular collagens that interact with VWF and require specific testing. Types I and III collagen bind to the VWF A3 domain.18 Types IV and VI collagen also bind VWF but via the VWF A1 domain.19
All collagen binding is dependent on the presence of high–molecular-weight multimers, but types I and III collagen in particular have been shown to serve as a surrogate for the presence of high–molecular-weight VWF multimers.20 There may be a dual role for collagen-binding assays in VWD diagnosis, in order to evaluate multimer status and in order to screen for a possible collagen-binding defect. Currently, many laboratories can perform type I or III collagen-binding assays, although this is not typically done as part of a first line evaluation. Commercial testing of types IV and VI collagen binding is not currently available, although a kit for type VI collagen is marketed for purchase.
Assays of either type I, type III, or a combination of the two will suffice to detect specific A3 domain collagen-binding variants, of which a handful have been reported to date.21-23 Specific A1 binding defects are more common, although binding to types IV and VI collagen is rarely assessed in clinical practice.24 Research from the Zimmerman Program, a large multicenter United States study on patients with all types of VWD, has shown a relatively high incidence of type IV and VI collagen-binding defects in patients with both type 1 (5%) and type 2M VWD (27%).24 In both cohorts, presence of a collagen-binding variant was associated with an increased bleeding score compared with similar subjects without a collagen-binding defect.
Patients with increased bleeding scores and unexplained bleeding symptoms may benefit from collagen-binding testing to explore the possibility of an undiagnosed collagen-binding defect in VWF. As noted in the Introduction, however, there are a number of other diagnoses that should be considered, as not all bleeding is due to a defect in VWF.
VWF genetics
The increased availability and lower cost of genetic testing enable increased use in diagnosis of VWD. Genetic testing is certainly simpler than laboratory testing, where a number of different tests on plasma samples are required to make the diagnosis. In addition, plasma VWF levels vary due to other underlying illnesses or stress, whereas VWF genetics should remain stable. However, there are a number of issues with genetic analysis of VWF. First, there is a great deal of variability in the VWF gene in healthy individuals.25 Many variants that were previously called pathogenic have been found in healthy people, some at relatively high frequency, particularly in the African American population and presumably other minority populations. For example, p.M740I was found in 18% of the African American controls, which suggests this is not likely to be the sole cause of VWD in an affected individual.25 Novel variants should be considered with caution, as changes in DNA do not necessarily imply changes to the VWF protein.
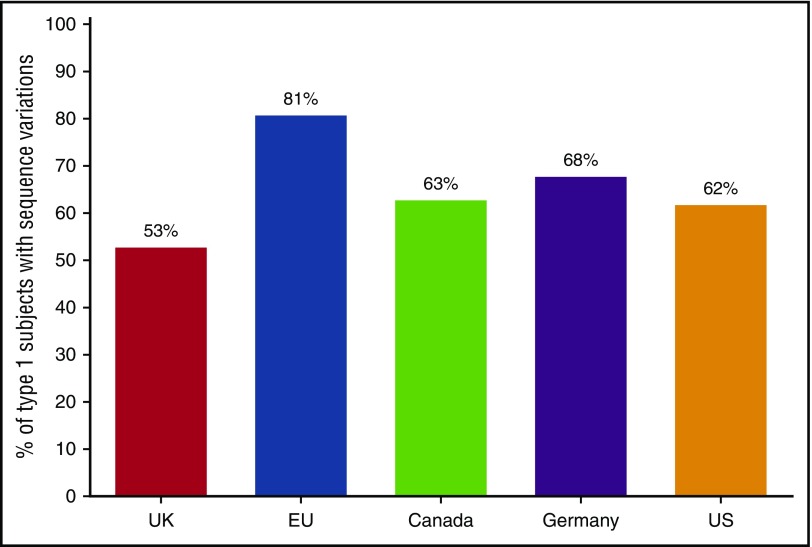
Another impediment to routine use of genetic analysis for VWD is the poor correlation between VWF sequence variants and disease for type 1, the most common VWD type. A large study of VWD subjects in the United States showed a relatively low rate of probably causative VWF variants in those subjects with VWF:Ag >30 IU/dL.26 Figure 1 compares the overall rate of sequence variants in subjects diagnosed as type 1 VWD, with an average rate of approximately 65% across 5 different studies from the United Kingdom,27 Canada,28 Europe,29 and Germany.30 Therefore, at least a third of patients with type 1 VWD will not have a specific genetic variant in VWF. In the Zimmerman program, sequence variants were found in 84% of subjects with VWF levels <30 and 44% in those with levels of 30-50, whereas in the MCMDM-1VWD study from the European Union, sequence variants were found in 83% of subjects with VWF:Ag <30 and 69% of subjects with VWF:Ag 31-45.26,29
Figure 1.
Genetic variants in VWF in type 1 VWD. Frequency of genetic variants in VWF for 5 large population studies of type 1 VWD including the United Kingdom,27 European Union,29 Canada,28 Germany,30 and United States.26 Overall 65% had a variant in VWF found, but 35% did not.
Variants outside the VWF locus may also be responsible for VWF levels. Blood group has been known for years to affect VWF levels.12 More recently, CLEC4M has been shown to affect VWF clearance.31 Other genes implicated in modifying VWF levels include scavenger molecule SCARA5,32 syntaxin binding protein 5 (STXBP5),32 and ubiquitin fold modifier 1 (UFM1).33 Although these have not yet been implicated in VWD, they and other unidentified genes may account for some low VWF levels in patients without a clear genetic diagnosis.
Genetic analysis is most useful in type 2 VWD. Many type 2 variants, particularly type 2B, have been well characterized, and confirmation of a known genetic variant in VWF will confirm the diagnosis. In addition, many confirmatory plasma tests are not readily available at many centers, whereas genetic testing may be easier to perform. However, caution must again be applied to novel variants, as they may or may not represent true causes of disease. Analysis of type 3 VWD patients may also be helpful for prenatal diagnosis of potentially affected siblings.34 Genetic analysis either specifically for the p.D1472H variant or of VWF exon 28 is helpful when the VWF:RCo/VWF:Ag ratio is decreased in the setting of a normal multimer distribution. Sequencing can either verify that the low ratio is caused by p.D1472H or, in patients with suspected type 2M VWD, reveal a causative variant.
The low VWF conundrum
Recent guidelines have cited a cutoff value of 30 IU/dL for a diagnosis of VWD, leaving patients with levels below the lower limit of the reference range, but higher than 30 IU/dL in the gray zone of “low VWF.”3 As noted in VWF genetics, this group is less likely to have genetic variants, but may still have significant bleeding. This brings up the possibility of low VWF as a risk factor for bleeding, as originally proposed by Sadler.35
Advances in treatment
Current treatment of VWD is summarized in Table 2. Desmopressin is effective in treatment of VWD because it causes release of stored endothelial cell VWF. Testing of VWF is recommended at baseline, then 1 and 4 h following administration to ensure patients have a good response (defined as threefold increase and to hemostatic levels).36 Patients with VWD clearance defects will have an initial response but fall rapidly to a low level, limiting desmopressin’s utility in severe bleeds. Intranasal and IV administration are the most common, but subcutaneous administration has also been used. Typical dosing is 1 spray for patients <50 kg and 2 sprays for patients >50 kg. Side effects include flushing, headache, and tachyphylaxis following repeated dosing due to exhaustion of stores. In addition, there is the potential for hyponatremic seizures. It is recommended that patients have their total fluid intake restricted for 24 hour following each dose, and high-risk patients may require monitoring of sodium levels. Approximately 80% of type 1 patients will have a good response, but some type 1 patients will not respond, particularly those with levels <30 IU/dL.36,37
Table 2.
Comparison of VWD treatment options
| Route of administration | Advantages | Disadvantages | Typical dosing | |
|---|---|---|---|---|
| Desmopressin | Intranasal, IV, SQ | Easily given at home | Fluid restriction required | 0.3 mcg/kg IV or 2 sprays IN (>50 kg)/1 spray IN (<50 kg) |
| Not effective for all VWD types | ||||
| Plasma-derived VWF concentrates | IV | Most products contains both VWF and FVIII | Plasma product | 50-60 ristocetin cofactor activity units/kg for major surgery, depending on baseline VWF level and desired goal level |
| Most products contain both VWF and FVIII | ||||
| Recombinant VWF concentrate | IV | Recombinant, allows titration of FVIII level | May require addition of recombinant FVIII for emergency treatment | 50-80 ristocetin cofactor activity units/kg for major surgery, depending on baseline VWF level and desired goal level; for emergency treatment may require addition of recombinant FVIII depending on patient’s endogenous FVIII level |
| Antifibrinolytics | PO, IV | Easily given at home | May not work for nonmucosal bleeds | Aminocaproic acid: loading dose of 100 mg/kg then 50 mg/kg every 6 h |
| Tranexemic acid: 1500 mg 3 times daily × 5 d for menorrhagia |
IN, intranasal; IV, intravenous; PO, oral
Plasma-derived VWF has been available for decades and is both safe and effective in treating bleeds in VWD. Most currently available concentrates contain both VWF and FVIII, although the ratio varies by product. Humate-P has a VWF:FVIII ratio of approximately 2.4:1, whereas Wilate has a ratio of approximately 1:1. Alphanate also contains both FVIII and VWF but with a VWF:FVIII ratio of 0.5 to 1.38 Wilfactin, which is currently available in Europe, has plasma-derived VWF with very low FVIII. Humate-P has been available since the 1980s and, as detailed in a recent review, has been effective with minimal adverse events.39 One recent surgery study of Humate-P showed that >90% of subjects had good (minor oozing) or excellent (normal hemostasis) results.40 A surgery study using Wilate had >95% of procedures with excellent (no further bleeding) or good (minor bleeding, no additional product needed) responses.41 Wilfactin has also been shown to be safe and effective, although for emergency surgical procedures a dose of FVIII was given along with the VWF concentrate.42
The currently available plasma-derived products appear to have similar efficacy, with the vast majority of VWD patients having an adequate response. Typical treatment doses of plasma-derived VWF depend in part on the patient’s endogenous VWF level but are generally 50 to 60 ristocetin cofactor activity units/kg for major surgery. Repeat dosing is often required given the typical half-life of around 12 hours.40 Risk of viral transmission is always a concern with plasma-derived products but has not been an issue for several decades. Risk of thrombosis may be of increased concern, especially in adult patients, but has not been reported as a significant issue in any of the above studies. Of more concern is the risk of inhibitor formation, particularly in patients with type 3 VWD. Patients with large deletions are most likely to experience inhibitor formation.43
Although prophylactic factor dosing is typically associated with hemophilia, there is a role for prophylaxis in VWD. Some patients with type 3 VWD will have significant bleeding such that prophylaxis is useful to prevent recurrence. In addition, secondary prophylaxis is used in certain circumstances (eg, menorrhagia, recurrent gastrointestinal [GI] bleeds). Data from the von Willebrand Disease Prophylaxis Network demonstrate that prophylaxis in severe VWD can reduce both mucosal and joint bleeds.43 Joint bleeds were reduced from an average of 15.6 to an average of 1.3 per year, whereas epistaxis was reduced from 24 to 6.43 Another area where secondary prophylaxis may be required is GI bleeding, which is particularly prevalent in type 2A VWD.4
Recombinant VWF has recently been approved in the United States and has been shown to be effective in treatment of surgery and major bleeds.44 One caveat with its use is that the recombinant VWF preparation does not contain FVIII. Therefore, most patients with low FVIII levels will require concomitant administration of recombinant FVIII along with the initial dose of recombinant VWF. Current dosing recommendations for major surgery include 50 to 80 ristocetin cofactor activity units of recombinant VWF (along with recombinant FVIII if immediate hemostasis is required). Subsequent dosing can use exclusively recombinant VWF, as endogenous FVIII production will maintain normal FVIII levels once VWF is present. Interestingly, in the initial publication on recombinant VWF, 10 initial bleeding treatment doses were given without recombinant FVIII with good results.44 Despite this, the recommendation to infuse FVIII along with the initial dose of VWF remains prudent in most patients with low baseline FVIII levels, but the separation of VWF and FVIII allows for individualized therapy.
Other treatment options include antifibrinolytics as well as hormone therapy for women with heavy menstrual bleeding. Antifibrinolytics such as tranexamic acid or aminocaproic acid have been used with success to treat heavy menstrual bleeding and surgery involving mucosal surfaces (typically tonsillectomy or dental surgery).3 For women with heavy menstrual bleeding, hormone therapy given either as combined estrogen/progesterone pills or via IUD has been shown to be effective at reducing blood loss and maintaining a normal hemoglobin.45 Treatment of heavy menstrual bleeding is of particular importance due to the low quality of life reported by patients.46
GI bleeding represents a specific challenge in the management of VWD. Type 2A VWD patients in particular are subject to an increased frequency of GI bleeding, attributed to increased angiodysplasia in the GI tract.47 Treatment includes blood transfusion acutely and VWF replacement for a prolonged period of time. In cases of refractory bleeding, additional options include octreotide, estrogen, thalidomide, and atorvastatin.48
Who to treat
Although treatment options for VWD remain largely unchanged, save for the new availability of recombinant VWF, there have been improvements in the ability to predict which patients will require treatment. Federici and colleagues recently published an algorithm using bleeding score and VWF level to predict which patients would require treatment of their bleeding symptoms.11 Patients with a bleeding score >10 had the highest incidence of bleeding, regardless of VWD subtype. This study was limited to adults but does provide evidence that bleeding begets more bleeding. This suggests that history of bleeding should be taken into account when planning individualized treatment.
Adequate treatment is key to allowing patients to have the highest possible quality of life. Joint bleeds are associated with decreased health-related quality of life, as is menorrhagia.49,50 Pediatric patients with a diagnosis of VWD also experience a lower quality of life.51 It is to be hoped that improvements in diagnosis and treatment will translate to improvements in quality of life for affected patients. However, more work is needed to identify those patients who will benefit most from treatment without overdiagnosis of VWD.
Summary
VWD is a common and challenging bleeding disorder, given the difficulties in diagnosis and treatment. New options for diagnosis, including use of bleeding assessment tools and new assays for VWF activity, may help alleviate some of these challenges. Because VWD has a major impact on patient quality of life, improved treatment options are always helpful. The addition of recombinant VWF to available therapeutic options will allow clinicians to continue to tailor treatment to optimize outcomes for individual patients. Despite these advances, more work is required to streamline diagnosis and improve treatment of affected patients.
Acknowledgments
V.H.F. would like to acknowledge research funding from the National Institutes of Health (HL126810) and the MACC Fund Center for Cancer and Blood Disorders.
Authorship
Contribution: R.S. and V.H.F. composed and edited the manuscript.
Conflict-of-interest disclosure: R.S. declares no competing financial interest. V.H.F. has consulted for CSL Behring and Shire. Off-label drug use: None disclosed.
Correspondence: Veronica H. Flood, Comprehensive Center for Bleeding Disorders, 8739 Watertown Plank Rd, PO Box 2178, Milwaukee, WI, 53201-2178; e-mail: vflood@mcw.edu.
References
- 1.Bowman M, Hopman WM, Rapson D, Lillicrap D, James P. The prevalence of symptomatic von Willebrand disease in primary care practice. J Thromb Haemost. 2010;8(1):213-216. [DOI] [PubMed] [Google Scholar]
- 2.Robertson J, Lillicrap D, James PD. Von Willebrand disease. Pediatr Clin North Am. 2008;55(2):377-392. [DOI] [PubMed] [Google Scholar]
- 3.Nichols WL, Hultin MB, James AH, et al. von Willebrand disease (VWD): evidence-based diagnosis and management guidelines, the National Heart, Lung, and Blood Institute (NHLBI) Expert Panel report (USA). Haemophilia. 2008;14(2):171-232. [DOI] [PubMed] [Google Scholar]
- 4.Castaman G, Federici AB, Tosetto A, et al. Different bleeding risk in type 2A and 2M von Willebrand disease: a 2-year prospective study in 107 patients. J Thromb Haemost. 2012;10(4):632-638. [DOI] [PubMed] [Google Scholar]
- 5.Rodeghiero F, Tosetto A, Abshire T, et al. ; ISTH/SSC joint VWF and Perinatal/Pediatric Hemostasis Subcommittees Working Group. ISTH/SSC bleeding assessment tool: a standardized questionnaire and a proposal for a new bleeding score for inherited bleeding disorders. J Thromb Haemost. 2010;8(9):2063-2065. [DOI] [PubMed] [Google Scholar]
- 6.Elbatarny M, Mollah S, Grabell J, et al. ; Zimmerman Program Investigators. Normal range of bleeding scores for the ISTH-BAT: adult and pediatric data from the merging project. Haemophilia. 2014;20(6):831-835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Biss TT, Blanchette VS, Clark DS, et al. Quantitation of bleeding symptoms in children with von Willebrand disease: use of a standardized pediatric bleeding questionnaire. J Thromb Haemost. 2010;8(5):950-956. [DOI] [PubMed] [Google Scholar]
- 8.Rydz N, James PD. The evolution and value of bleeding assessment tools. J Thromb Haemost. 2012;10(11):2223-2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sanders YV, Fijnvandraat K, Boender J, et al. ; WiN Study Group. Bleeding spectrum in children with moderate or severe von Willebrand disease: Relevance of pediatric-specific bleeding. Am J Hematol. 2015;90(12):1142-1148. [DOI] [PubMed] [Google Scholar]
- 10.De Wee EM, Sanders YV, Mauser-Bunschoten EP, et al. ; WiN study group. Determinants of bleeding phenotype in adult patients with moderate or severe von Willebrand disease. Thromb Haemost. 2012;108(4):683-692. [DOI] [PubMed] [Google Scholar]
- 11.Federici AB, Bucciarelli P, Castaman G, et al. The bleeding score predicts clinical outcomes and replacement therapy in adults with von Willebrand disease. Blood. 2014;123(26):4037-4044. [DOI] [PubMed] [Google Scholar]
- 12.Gill JC, Endres-Brooks J, Bauer PJ, Marks WJ Jr, Montgomery RR. The effect of ABO blood group on the diagnosis of von Willebrand disease. Blood. 1987;69(6):1691-1695. [PubMed] [Google Scholar]
- 13.Kitchen S, Jennings I, Woods TA, Kitchen DP, Walker ID, Preston FE. Laboratory tests for measurement of von Willebrand factor show poor agreement among different centers: results from the United Kingdom National External Quality Assessment Scheme for Blood Coagulation. Semin Thromb Hemost. 2006;32(5):492-498. [DOI] [PubMed] [Google Scholar]
- 14.Flood VH, Gill JC, Morateck PA, et al. Common VWF exon 28 polymorphisms in African Americans affecting the VWF activity assay by ristocetin cofactor. Blood. 2010;116(2):280-286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patzke J, Budde U, Huber A, et al. Performance evaluation and multicentre study of a von Willebrand factor activity assay based on GPIb binding in the absence of ristocetin. Blood Coagul Fibrinolysis. 2014;25(8):860-870. [DOI] [PubMed] [Google Scholar]
- 16.Graf L, Moffat KA, Carlino SA, et al. Evaluation of an automated method for measuring von Willebrand factor activity in clinical samples without ristocetin. Int J Lab Hematol. 2014;36(3):341-351. [DOI] [PubMed] [Google Scholar]
- 17.Flood VH, Gill JC, Morateck PA, et al. Gain-of-function GPIb ELISA assay for VWF activity in the Zimmerman Program for the Molecular and Clinical Biology of VWD. Blood. 2011;117(6):e67-e74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pareti FI, Niiya K, McPherson JM, Ruggeri ZM. Isolation and characterization of two domains of human von Willebrand factor that interact with fibrillar collagen types I and III. J Biol Chem. 1987;262(28):13835-13841. [PubMed] [Google Scholar]
- 19.Saelman EU, Nieuwenhuis HK, Hese KM, et al. Platelet adhesion to collagen types I through VIII under conditions of stasis and flow is mediated by GPIa/IIa (alpha 2 beta 1-integrin). Blood. 1994;83(5):1244-1250. [PubMed] [Google Scholar]
- 20.Favaloro EJ. An update on the von Willebrand factor collagen binding assay: 21 years of age and beyond adolescence but not yet a mature adult. Semin Thromb Hemost. 2007;33(8):727-744. [DOI] [PubMed] [Google Scholar]
- 21.Ribba AS, Loisel I, Lavergne JM, et al. Ser968Thr mutation within the A3 domain of von Willebrand factor (VWF) in two related patients leads to a defective binding of VWF to collagen. Thromb Haemost. 2001;86(3):848-854. [PubMed] [Google Scholar]
- 22.Riddell AF, Gomez K, Millar CM, et al. Characterization of W1745C and S1783A: 2 novel mutations causing defective collagen binding in the A3 domain of von Willebrand factor. Blood. 2009;114(16):3489-3496. [DOI] [PubMed] [Google Scholar]
- 23.Flood VH, Lederman CA, Wren JS, et al. Absent collagen binding in a VWF A3 domain mutant: utility of the VWF:CB in diagnosis of VWD. J Thromb Haemost. 2010;8(6):1431-1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Flood VH, Schlauderaff AC, Haberichter SL, et al. ; Zimmerman Program Investigators. Crucial role for the VWF A1 domain in binding to type IV collagen. Blood. 2015;125(14):2297-2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bellissimo DB, Christopherson PA, Flood VH, et al. VWF mutations and new sequence variations identified in healthy controls are more frequent in the African-American population. Blood. 2012;119(9):2135-2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Flood VH, Christopherson PA, Gill JC, et al. Clinical and laboratory variability in a cohort of patients diagnosed with type 1 VWD in the United States. Blood. 2016;127(20):2481-2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cumming A, Grundy P, Keeney S, et al. ; UK Haemophilia Centre Doctors’ Organisation. An investigation of the von Willebrand factor genotype in UK patients diagnosed to have type 1 von Willebrand disease. Thromb Haemost. 2006;96(5):630-641. [PubMed] [Google Scholar]
- 28.James PD, Notley C, Hegadorn C, et al. The mutational spectrum of type 1 von Willebrand disease: Results from a Canadian cohort study. Blood. 2007;109(1):145-154. [DOI] [PubMed] [Google Scholar]
- 29.Goodeve A, Eikenboom J, Castaman G, et al. Phenotype and genotype of a cohort of families historically diagnosed with type 1 von Willebrand disease in the European study, Molecular and Clinical Markers for the Diagnosis and Management of Type 1 von Willebrand Disease (MCMDM-1VWD). Blood. 2007;109(1):112-121. [DOI] [PubMed] [Google Scholar]
- 30.Yadegari H, Driesen J, Pavlova A, Biswas A, Hertfelder HJ, Oldenburg J. Mutation distribution in the von Willebrand factor gene related to the different von Willebrand disease (VWD) types in a cohort of VWD patients. Thromb Haemost. 2012;108(4):662-671. [DOI] [PubMed] [Google Scholar]
- 31.Rydz N, Swystun LL, Notley C, et al. The C-type lectin receptor CLEC4M binds, internalizes, and clears von Willebrand factor and contributes to the variation in plasma von Willebrand factor levels. Blood. 2013;121(26):5228-5237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smith NL, Chen MH, Dehghan A, et al. ; Wellcome Trust Case Control Consortium. Novel associations of multiple genetic loci with plasma levels of factor VII, factor VIII, and von Willebrand factor: The CHARGE (Cohorts for Heart and Aging Research in Genome Epidemiology) Consortium. Circulation. 2010;121(12):1382-1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van Loon J, Dehghan A, Weihong T, et al. Genome-wide association studies identify genetic loci for low von Willebrand factor levels. Eur J Hum Genet. 2016;24(7):1035-1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Swystun LL, James PD. Genetic diagnosis in hemophilia and von Willebrand disease. Blood Rev. 2017;31(1):47-56. [DOI] [PubMed] [Google Scholar]
- 35.Sadler JE. Von Willebrand disease type 1: a diagnosis in search of a disease. Blood. 2003;101(6):2089-2093. [DOI] [PubMed] [Google Scholar]
- 36.Federici AB. The use of desmopressin in von Willebrand disease: the experience of the first 30 years (1977-2007). Haemophilia. 2007;14(suppl 1):5-14. [DOI] [PubMed] [Google Scholar]
- 37.Ben-Ami T, Revel-Vilk S. The use of DDAVP in children with bleeding disorders. Pediatr Blood Cancer. 2012;60(suppl 1):S41-S43. [DOI] [PubMed] [Google Scholar]
- 38.Mannucci PM, Chediak J, Hanna W, et al. ; Alphanate Study Group. Treatment of von Willebrand disease with a high-purity factor VIII/von Willebrand factor concentrate: a prospective, multicenter study. Blood. 2002;99(2):450-456. [DOI] [PubMed] [Google Scholar]
- 39.Miesbach W, Krekeler S, Wolf Z, Seifried E. Clinical use of Haemate® P in von Willebrand disease: a 25-year retrospective observational study. Thromb Res. 2015;135(3):479-484. [DOI] [PubMed] [Google Scholar]
- 40.Gill JC, Shapiro A, Valentino LA, et al. von Willebrand factor/factor VIII concentrate (Humate-P) for management of elective surgery in adults and children with von Willebrand disease. Haemophilia. 2011;17(6):895-905. [DOI] [PubMed] [Google Scholar]
- 41.Windyga J, von Depka-Prondzinski M. European Wilate® Study Group. Efficacy and safety of a new generation von Willebrand factor/factor VIII concentrate (Wilate®) in the management of perioperative haemostasis in von Willebrand disease patients undergoing surgery. Thromb Haemost. 2011;105(6):1072-1079. [DOI] [PubMed] [Google Scholar]
- 42.Borel-Derlon A, Federici AB, Roussel-Robert V, et al. Treatment of severe von Willebrand disease with a high-purity von Willebrand factor concentrate (Wilfactin): a prospective study of 50 patients. J Thromb Haemost. 2007;5(6):1115-1124. [DOI] [PubMed] [Google Scholar]
- 43.Abshire TC, Federici AB, Alvárez MT, et al. ; VWD PN. Prophylaxis in severe forms of von Willebrand’s disease: results from the von Willebrand Disease Prophylaxis Network (VWD PN). Haemophilia. 2012;19(1):76-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gill JC, Castaman G, Windyga J, et al. Hemostatic efficacy, safety, and pharmacokinetics of a recombinant von Willebrand factor in severe von Willebrand disease. Blood. 2015;126(17):2038-2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.James AH. Women and bleeding disorders. Haemophilia. 2010;16(suppl 5):160-167. [DOI] [PubMed] [Google Scholar]
- 46.Rae C, Furlong W, Horsman J, et al. Bleeding disorders, menorrhagia and iron deficiency: impacts on health-related quality of life. Haemophilia. 2012;19(3):385-391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Randi AM. Endothelial dysfunction in von Willebrand disease: angiogenesis and angiodysplasia. Thromb Res. 2016;141(suppl 2):S55-S58. [DOI] [PubMed] [Google Scholar]
- 48.Franchini M, Mannucci PM. Gastrointestinal angiodysplasia and bleeding in von Willebrand disease. Thromb Haemost. 2014;112(3):427-431. [DOI] [PubMed] [Google Scholar]
- 49.van Galen KP, Sanders YV, Vojinovic U, et al. ; WiN Study Group. Joint bleeds in von Willebrand disease patients have significant impact on quality of life and joint integrity: a cross-sectional study. Haemophilia. 2015;21(3):e185-e192. [DOI] [PubMed] [Google Scholar]
- 50.Govorov I, Ekelund L, Chaireti R, et al. Heavy menstrual bleeding and health-associated quality of life in women with von Willebrand’s disease. Exp Ther Med. 2016;11(5):1923-1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.De Wee EM, Fijnvandraat K, de Goede-Bolder A, et al. ; WiN Study Group. Impact of von Willebrand disease on health-related quality of life in a pediatric population. J Thromb Haemost. 2011;9(3):502-509. [DOI] [PubMed] [Google Scholar]