Abstract
Purpose of Review
Noroviruses are the most common cause of gastroenteritis outbreaks in long-term care facility (LTCFs). This review summarizes the most up-to-date knowledge on norovirus infection in LTCFs with the aim of identifying potential strategies for management.
Recent Findings
LTCF residents are at greater risk of norovirus infection. Early identification of norovirus infection and prompt initiation of appropriate supportive therapy are required to reduce morbidity and mortality. Measures to prevent outbreaks and reduce the risk of norovirus infection in LTCFs include timely diagnosis and implementation of infection control interventions to limit virus transmission.
Summary
Current guidelines for prevention and control are based on generic principles of infection control. Real-time reverse transcription-quantitative polymerase chain reaction assays have been the gold standard for the rapid and sensitive detection of noroviruses. With the recent breakthroughs of human norovirus in vitro culture, doors are now opened to evaluate the efficacy of environmental disinfectants and hand hygiene options. Additionally, development of licensed vaccines against noroviruses may provide another important tool for infection prevention among high-risk individuals.
Keywords: Norovirus, Older adults, Long-term care facilities, Management
Introduction
Human noroviruses are globally important pathogens, contributing substantially to the burden of acute gastroenteritis across all age groups. The World Health Organization recently estimated that noroviruses caused 684 million illnesses and over 200,000 deaths globally in 2010 [1••]. Ahmed et al. conducted a systematic review of the scientific literature published from January 1,2008, to March 8,2014, and found that noroviruses were associated with almost one fifth of all cases of acute gastroenteritis, and the prevalence was higher in high income countries compared to low- and middle-income countries [2••].
Long-term care facilities (LTCFs) are common settings for outbreaks of norovirus infection, where they are responsible for 30–80% of acute gastroenteritis outbreaks [3, 4, 5, 6•]. While noroviruses can cause both sporadic infections and outbreaks in all age groups, older people are at higher risks of hospitalization and death [3], owing to intrinsic factors, such as age-related immunosenescence or the presence of comorbid conditions, which result in more extended symptoms [7]. Additionally, elderly residents of LTCFs are at elevated risks of infection as a result of institutionalized confinement that promotes transmission by sharing rooms and touching common surfaces [8]. This review summarizes the most up-to-date knowledge on norovirus infection in LTCF residents with the aim of identifying potential strategies for management.
Infection in Older Adults and in LTCFs
Norovirus infection generally manifests as a relatively brief, self-limited illness in healthy immunocompetent individuals, although it can cause significant morbidity and mortality in frail elderly adults. Lindasay et al. reviewed 39 studies on risk estimates of norovirus infection and found a high burden of the infection in all ages with the highest rates of hospitalization and death among the elderly [3]. Older people are at higher risk of norovirus-associated hospitalization, resulting in excess hospital stays and greater costs compared to young adults [3]. The overall estimates of disease burden suggest that noroviruses are responsible for approximately 10–20% of gastroenteritis hospitalizations, 10–15% of gastroenteritis deaths, and ≥0.2% of all-cause mortality among older adults in upper-middle-income and high-income countries [9, 10, 11•, 12,13]. Additional data also suggest that noroviruses may trigger severe clinical complications, including acute renal failure, arrhythmia, chronic diarrhea, and severe enteropathy [14, 15].
Noroviruses are the most common cause of gastroenteritis outbreaks in LTCFs [16]. Review of US outbreak surveillance data show that over 60% of all norovirus outbreaks occur in LTCFs [17], while in other high-income countries norovirus outbreaks occur with roughly equal frequency in both acute-care hospitals and LTCFs. The definition of LTCFs differs between studies, but LTCFs generally refer to facilities that provide prolonged care for individuals who required daily living and/or nursing care support. Whereas most community cases of norovirus are self-limiting within 12–60 h, outbreaks of norovirus can significantly impact the institutionalized elderly and cause more severe or prolonged illness [18•, 19,20]. Several factors contribute to the enhanced risk of severe norovirus infection among older adults living in LTCFs, including nutritional status, immunodeficiency or senescence, chronic inflammation, microbiome alterations, and the use of certain medications [21]. Decreased ability to maintain adequate personal hygiene may also increase individual risk among LTCF residents. Environmental factors, such as residence in close, shared quarters, use of shared facilities, and limited ability to isolate infected residents, may contribute to virus transmission in LTCFs. Shared dining facilities may also increase risk for foodborne exposures.
Infection Transmission
Transmission of human noroviruses can occur directly through person-to-person contact, or indirectly through consumption of contaminated food or water, or through contact with contaminated environmental surfaces (Fig. 1). Person-to-person transmission is responsible for >90% of the norovirus outbreaks in healthcare settings, where close living arrangements, shared facilities and contact with visitors and staff increase the risk of norovirus spread from one person to another [17, 22]. Foodborne transmission is another important route for the spread of noroviruses [1••] and can occur when food handlers contaminate food on site or during the earlier steps of food production [23]. An analysis of surveillance data on norovirus outbreaks in the USA, Europe, and New Zealand estimated that about 14% of norovirus outbreaks were attributed to foodborne transmission [24]. Noroviruses can also be transmitted through contaminated environment surfaces and aerosolized particles. Aerosolization of norovirus via vomitus can be particularly problematic in LTCFs, as virus particles can settle on surfaces and survive for long periods of time, leading to environmental contamination for future exposure [25].
Fig. 1. Norovirus transmission in long-term care facilities.
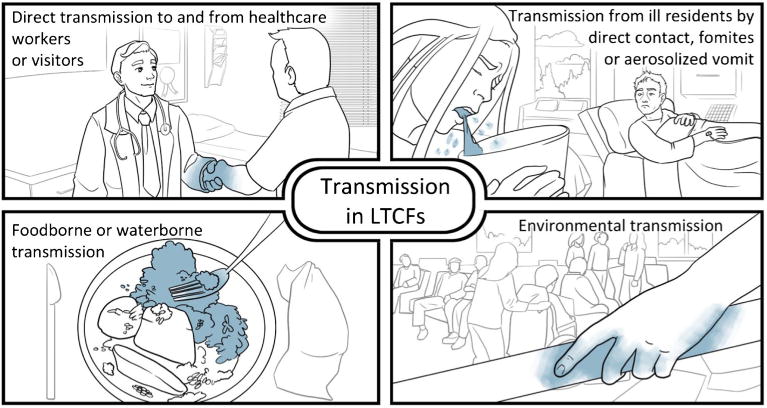
The high shedding titers in feces and vomit [26], low level of infectious dose [27], and environmental stability [28] enable the virus to efficiently transmit via multiple modes. Transmission has also been reported to occur before the onset of symptoms [29], in the postsymptomatic period and during subclinical infections [30]. Investigations of norovirus outbreaks in LTCFs confirmed that infected persons can asymptomatically shed virus at high levels for at least 3 weeks [31••], although reports from nosocomial norovirus outbreaks have shown that symptomatic patients contribute primarily to transmission of infection [32].
Importance of Genetic Diversity and Evolution
Noroviruses, divided into at least six genogroups (GI-GVI) and more than 40 different genotypes (e.g., GII.4), are a genetically diverse group of non-enveloped single-stranded positive-sense RNA viruses [33]. The prevalence of infecting genogroups and genotypes differ between populations and route of transmission [22]. Genogroup I viruses are generally associated with foodborne or waterborne outbreaks [24, 34], while GII.4 viruses are strongly associated with person-to-person transmission and occur predominantly in healthcare and institutional settings [5, 6•, 35, 36]. Infections with GII.4 viruses are more likely to cause severe infections, leading to more hospitalization and deaths than those caused by other GII or GI viruses [37]. Multiple strains of noroviruses can cause human reinfection. Protective immunity to specific types of noroviruses has been reported, but with a limited duration [38].
Despite the extensive genetic diversity, previous data suggest that GII.4 viruses are responsible for the majority of norovirus outbreaks worldwide [39], with a new GII.4 variant strain emerging every 2–4 years [40]. Several mechanisms may enhance GII.4 evolution, including the host herd immunity that drives antigenic drift in the hypervariable P2 domain [41••]. This domain of the viral capsid binds with human histo-blood group antigens (HBGAs), which serve as a point for initial viral attachment [42]. GII.4 viruses can bind a wider range of HBGAs than other genotypes, causing infections to a larger susceptible population [33]. Another explanation for the emergence of novel GII.4 variants is related to homologous recombination, which contributes to the emergence of the recent pandemic GII.4 variants, such as GII.4 New Orleans 2009, and GII.4 Sydney 2012 [43]. The emergence of epidemic strains of noroviruses has contributed to the changing epidemiology of norovirus infection worldwide [44, 45].
Clinical Features and Diagnosis
Noroviruses are highly contagious. Ingestion of a small number of viral particles can lead to infection [27]. The onset of norovirus infection occurs after an average incubation period of 1.2 (range 1–2) days [46]. Vomiting is a cardinal sign of norovirus infection, along with acute onset of other gastrointestinal symptoms including nausea, watery and non-bloody diarrhea, and abdominal cramps. Symptoms often last for 24– 72 h with complete recovery in immunocompetent individuals [19], although older frail people may present with prolonged symptoms and develop complications. One study describing the clinical characteristics of nosocomial outbreaks found that elderly hospitalized patients had prolonged symptoms with norovirus infection, and almost one third of the patients experienced dehydration [47]. Notably, the majority of those study participants (83.9%) had underlying chronic conditions, suggesting that the impact of norovirus infection is more pronounced among older adults with comorbid conditions.
It is difficult to diagnose norovirus gastroenteritis in individual patients on the basis of clinical features alone. The definition for norovirus infection in LTCFs requires the presence of both a compatible clinical presentation and a laboratory confirmation [48]. Historically, human noroviruses could not be cultured in vitro. However, Jones et al. recently published a protocol describing methods for culturing the GII.4-Sydney human norovirus strain directly in human B lymphocytes [49••]. This is a breakthrough research, as for the first time, a human norovirus can be grown in a culture dish. It enables research into the development of antiviral drugs, as well as opens a door to definitively evaluate the efficacy of infection control and prevention options.
Diagnostic methods of norovirus infection have focused on detecting viral RNA or antigen. In recent years, real-time reverse transcription-quantitative polymerase chain reaction (RT-qPCR) assays have become the gold standard for the rapid and sensitive detection of noroviruses in stool, vomitus, foods, water, and environmental specimens [33]. However, virus detection by RT-qPCR does not always correlate with the disease. Infected persons can shed virus for weeks after recovery from clinical symptoms, and noroviruses are also frequently detected in stool samples from asymptomatic patients. Chan et al. analyzed data collected from sporadic cases and speculated a correlation between viral load and virus transmission from infected persons to susceptible hosts through fecal-oral route [50]. This finding indicates that assessment of a possible difference in viral load in samples may be a useful tool to aid clinical interpretation and to assess causal relationship.
Given the rapid spread of noroviruses, especially during outbreaks, timely diagnosis is essential to assist management and implementation of appropriate control measures. Rapid commercial assays, such as enzyme immunoassays (EIAs) have been cleared by the US Food and Drug Administration to detect norovirus antigen in stool samples during outbreaks. However, due to the poor sensitivity of EIAs [51], samples with negative results should be confirmed by a second technique, such as RT-qPCR [52]. Consequently, EIA kits should not replace molecular methods during outbreak investigations, and caution should be used when interpreting test results from sporadic cases [52].
In the absence of laboratory diagnostic tests or delays in obtaining laboratory results, outbreaks of norovirus infection can be identified according to Kaplan criteria [53]. These criteria are based on the clinical and epidemiological profile of illness, which include (1) vomiting in >50% of patients, (2) a mean incubation period of 24–48 h, (3) a mean duration of illness of 12–60 h, and (4) lack of bacterial pathogens in stool culture. The set of Kaplan criteria is highly specific (99%), although with moderate sensitivity (68%) in discriminating outbreaks due to bacteria from those due to norovirus [54]. In LTCF, outbreaks satisfying Kaplan's criteria may justify rapid institution of control measures to limit spread of infection.
Treatment
Currently, there is no specific antiviral therapy available to treat norovirus infection. The management of patients is primarily supportive and focuses on treatment of dehydration and infection control measures to prevent further spread. Dehydration is the most common complication that requires medical care and is especially of concern among LTCF residents with underlying chronic conditions [55]. Patients with comorbidities are often prescribed multiple medications, some of which may have a potential for drug interactions. Therefore, the effect of fluid and electrolyte disturbance on medications should be closely monitored among elderly patients.
Despite recent progress in the development of norovirus vaccines, licensed products are not yet available. Clinical trials have demonstrated safety, immunogenicity, and efficacy of some products [56, 57], although the development of norovirus vaccines is challenging due to the high degree of virus genetic diversity, rapidly evolving new variant strains, and an incomplete understanding of immune correlates of protection [41••, 58••]. The future efficacy of norovirus vaccines may rely on the development of products eliciting a broad cross-protective immune response against heterologous virus [41••]. It is clear that older adults living in LTCFs are at higher risks of norovirus infection and are more likely to have worse outcomes. Therefore, vaccinating LTCFs residents would be beneficial to directly prevent infection transmission and reduce disease burden [59•].
Prevention and Control of Norovirus Outbreaks in LTCFs
The highly infectious nature of noroviruses and their environmental persistence pose multiple challenges to infection management in LTCFs. In 2011, the Centers for Disease Control and Prevention published guidelines providing recommendations for the prevention and control of norovirus gastroenteritis outbreaks in healthcare facilities [60]. Table 1 summarizes the risk-based approach for norovirus infection prevention and management in LTCFs, based on these guidelines and other published recommendations [52, 60, 62, 63, 73•]. The major strategies have included measures for timely diagnosis and implementation of infection control interventions to limit virus transmission.
Table 1. Measures recommended to manage norovirus infection in long-term care facilities.
| Surveillance and diagnosis |
| • Surveillance for infectious gastroenteritis |
| • Access to laboratory facilities capable of timely and accurate diagnosis of infection; |
| • Rapid testing of stool specimens for norovirus |
| • Outbreak notifications to appropriate health departments if norovirus gastroenteritis is suspected |
| Disease control and prevention practices |
| Interruption of person-to-person transmission |
| • Isolation and cohorting of infected persons, if feasible |
| • Minimizing resident transfers |
| • Adherence to personal protective equipment use for persons entering the patient care areas or caring for ill residents |
| • Hand hygiene with soap and water after contact with infected residents, their body substances, or potentially contaminated environment |
| • Informing visitors and residents about importance of hand hygiene to prevent infection spread |
| • Training staff about the transmission, clinical features, diagnosis, management, and prevention of norovirus infection |
| • Minimizing staff working at multiple facilities |
| • Ill staff exclusion until ≥48 h after symptoms resolve |
| Interruption of transmission via contaminated environment |
| • Disinfection and clean areas of any organic material |
| • Disinfection and sterilization using EPA approved products |
| • Restriction of staff working in contaminated areas |
| • Increasing the frequency of cleaning and disinfection of patient care areas and frequently touched surfaces during outbreaks |
| Interruption of transmission via contaminated food and water |
| • Avoiding bare-hand contact with ready-to-eat foods and appropriately hand hygiene practice before preparing foods |
| • Washing fresh foods and cooking shellfish thoroughly |
| • Enhancing cleaning in food facilities and contaminated areas |
| • Exclusion of ill food handlers until ≥48 h after symptoms resolve |
Cohorting and Exclusion
Social distancing measures, such as isolation or cohorting of symptomatic patients, have been successful in limiting norovirus transmission in large outbreaks [61]; however, the appropriate duration of isolation and use of contact precautions are uncertain. Patients may continue to shed norovirus in their stool after resolution of symptoms, and recommendations have been made to minimize contact with patients during the acute phase of illness, and 24–72 h following recovery while patients still shed virus at high levels [52]. This is particularly important during outbreaks in LTCFs to help break the transmission cycle, prevent the amount of secondary transmission, and also decrease the outbreak duration. Most guidelines recommend cohorting patients into groups according to symptomatic, exposed asymptomatic, and unexposed asymptomatic status, with dedicated healthcare staff providing care for infected patients [52, 62, 63]. To minimize the risk of transmission from incubating or asymptomatic cases, such patients should not be transferred to unaffected areas, typically within 48 h after exposure [52].
Environmental Disinfection
Noroviruses are stable and persistent in the environment [64]. Current evidence suggests that environmental contamination with norovirus is common both within and outside outbreak settings [28]. Therefore, environmental cleaning and chemical disinfection are essential to interrupt the chain of virus transmission. To maximize penetration and efficacy, initial cleaning to remove gross organic matter should precede chemical disinfection. CDC recommends sodium hypochlorite at concentration of ≥1000 ppm for disinfection of hard and non-porous environmental surfaces if feasible [52, 65]. The US Environmental Protection Agency (EPA) has published a list of registered disinfectants for use in healthcare settings against noroviruses (https://www.epa.gov/sites/production/files/2016-06/documents/list_g_norovirus.pdf).
Hand Hygiene
Hand hygiene is another key part of interrupting the norovirus transmission cycle, including environmentally mediated transmission as contaminated hands can transfer virus to touched surfaces, or vice versa [66]. Actively promoting adherence to hand hygiene among staff and residents is strongly recommended and should be implemented [52]. Handwashing with soap and water have been reported as preferred means to prevent infection, especially during an outbreak or if there is gross fecal soiling of the hands [8, 52]. The efficacy of alcohol-based sanitizers against noroviruses remains controversial, and further research is required to evaluate the efficacy of alcohol-based hand sanitizers against the virus [67, 68]. As an additional preventive strategy during outbreaks, use of gloves is recommended.
Staff Members
Staff of LTCFs plays an important role in infection transmission. A recent meta-analysis summarizing risk factors of norovirus spread in nursing homes found a positive association between bedside care and the infection [69]. Training staff on the relevant guidelines and personal hygiene practices is important to prevent transmission in LTCFs. Exposure to vomit is another infectious risk [69]. Use of personal protective equipment, including gowns and facial masks, is recommended for staff entering the patient care area or caring for patients with gastroenteritis symptoms to reduce the likelihood of exposure [60]. Ill staff members should not return to work until ≥48 h after symptoms resolve [52, 70]. During outbreaks of norovirus, staff working in multiple facilities may facilitate spread of infection to other LTCF.
Food Safety
While food may become contaminated during production, most norovirus contamination occurs during preparation [71•]. Bare-hand contact by contagious workers with ready-to-eat foods has been frequently identified in the majority of reported foodborne norovirus outbreaks [71•]. Highly infectious noroviruses may be transmitted through contaminated food by ill catering or food service staff in LTCFs. Therefore, food handlers are recommended to adherence to appropriate recommendations for hand washing and avoiding bare-hand contact with ready-to-eat foods (e.g., through use of gloves or utensils). Ill food handlers should not return to work until ≥48 h after symptom resolve [71•]. For asymptomatic food service staff who have tested positive for norovirus, exclusion is recommended [52]. CDC also recommends washing fresh product and thoroughly cooking shellfish as additional specific measures for preventing foodborne norovirus transmission (http://www.cdc.gov/norovirus/preventing-infection.html).
Surveillance and Outbreak Management
Surveillance for norovirus infection is recommended in LTCFs to determine infection rates and outbreaks using the standard case definition [48]. Outbreaks of norovirus infection should be reported to health departments in accordance with local regulations. Outbreak management is a multistage process, including preparedness, identification, response, and evaluation [72]. Guidelines for managing norovirus outbreaks have been published by public health agencies in several high-income countries [60, 62, 63]. Generally, LTCFs should develop outbreak plans outlining management arrangements for outbreaks, which may require involvement of public health agencies. A facility outbreak control team, including physicians, nurses, facility managers, and domestic staff should aim to minimize the early spread of infection. The main approaches to infection control and prevention include implementing policies concerning hand hygiene, patient isolation and cohorting, ill staff exclusion from work, visitor restrictions, food safety, and environmental cleaning and disinfection [52, 60, 62, 63, 73•]. Early detection and isolation of sporadic cases are also recommended to reduce the impact of noroviruses introduced into LTCFs [69].
Conclusions
The key means of managing norovirus infection in LTCFs are well-functioning infection control programs. Current guidelines for prevention and control are generally based on infection control principles, although the efficacy of those control measures is poorly quantified due to the inability to culture the virus. With the recent breakthroughs of human norovirus in vitro culture, doors are now opened to, for example, definitively evaluate the efficacy of environmental disinfectants and hand hygiene options. In addition, there is no specific antiviral therapy available to treat norovirus infection. Therefore, development of licensed vaccines against noroviruses may provide another important tool for infection prevention among high-risk individuals.
Footnotes
Conflict of Interest: Yingxi Chen, Aron Hall and Martyn Kirk declare no conflict of interest.
Compliance with Ethical Standards: Human and Animal Rights and Informed Consent: This article does not contain any studies with human or animal subjects performed by any of the authors.
Human and Animal Rights: All reported studies/experiments with human or animal subjects performed by the authors have been previously published and complied with all applicable ethical standards (including the Helsinki declaration and its amendments, institutional/national research committee standards, and international/national/institutional guidelines).
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1••.Kirk MD, Pires SM, Black RE, Caipo M, Crump JA, Devleesschauwer B, et al. World Health Organization estimates of the global and regional disease burden of 22 foodborne bacterial, protozoal, and viral diseases, 2010: a data synthesis. PLoS Med. 2015;12:e1001921. doi: 10.1371/journal.pmed.1001921. Most recent estimates of the global burden of 22 foodborne bacterial, protozoal, and viral infections. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2••.Ahmed SM, Hall AJ, Robinson AE, Verhoef L, Premkumar P, Parashar UD, et al. Global prevalence of norovirus in cases of gastroenteritis: a systematic review and meta-analysis. Lancet Infect Dis. 2014;14:725–30. doi: 10.1016/S1473-3099(14)70767-4. Reviews the global prevalence of norovirus gastroenteritis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lindsay L, Wolter J, De Coster I, Van Damme P, Verstraeten T. A decade of norovirus disease risk among older adults in upper-middle and high income countries: a systematic review. BMC Infect Dis. 2015;15:425. doi: 10.1186/s12879-015-1168-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kirk MD, Fullerton KE, Hall GV, Gregory J, Stafford R, Veitch MG, et al. Surveillance for outbreaks of gastroenteritis in long-term care facilities, Australia, 2002-2008. Clin Infect Dis. 2010;51:907–14. doi: 10.1086/656406. [DOI] [PubMed] [Google Scholar]
- 5.Rosenthal NA, Lee LE, Vermeulen BA, Hedberg K, Keene WE, Widdowson MA, et al. Epidemiological and genetic characteristics of norovirus outbreaks in long-term care facilities, 2003-2006. Epidemiol Infect. 2011;139:286–94. doi: 10.1017/S095026881000083X. [DOI] [PubMed] [Google Scholar]
- 6•.Hall AJ, Wikswo ME, Manikonda K, Roberts VA, Yoder JS, Gould LH. Acute gastroenteritis surveillance through the National Outbreak Reporting System, United States. Emerg Infect Dis. 2013;19:1305–9. doi: 10.3201/eid1908.130482. Demonstrates a more complete characterization of acute gastroenteritis outbreaks, particularly the relative importance of specific transmission modes and settings for norovirus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Payne R, Forde D, Vedio A, Cope A, Pratt G, Tunbridge A. ‘It'sjust a virus’—viral illness in older people: prevention and management. Rev Clin Gerontol. 2013;23:131–41. [Google Scholar]
- 8.Rajagopalan S, Yoshikawa TT. Norovirus infections in long-term care facilities. J Am Geriatr Soc. 2016;64:1097–103. doi: 10.1111/jgs.14085. [DOI] [PubMed] [Google Scholar]
- 9.Haustein T, Harris JP, Pebody R, Lopman BA. Hospital admissions due to norovirus in adult and elderly patients in England. Clin Infect Dis. 2009;49:1890–2. doi: 10.1086/648440. [DOI] [PubMed] [Google Scholar]
- 10.van Asten L, Siebenga J, van den Wijngaard C, Verheij R, van Vliet H, Kretzschmar M, et al. Unspecified gastroenteritis illness and deaths in the elderly associated with norovirus epidemics. Epidemiol. 2011;22:336–43. doi: 10.1097/EDE.0b013e31821179af. [DOI] [PubMed] [Google Scholar]
- 11•.Gastanaduy PA, Hall AJ, Curns AT, Parashar UD, Lopman BA. Burden of norovirus gastroenteritis in the ambulatory setting-United States, 2001-2009. J Infect Dis. 2013;207:1058–65. doi: 10.1093/infdis/jis942. Estimates the burden of norovirus gastroenteritis in ambulatory US patients. [DOI] [PubMed] [Google Scholar]
- 12.Schmid D, Kuo HW, Simons E, Kanitz EE, Wenisch J, Allerberger F, et al. All-cause mortality in hospitalized patients with infectious diarrhea: Clostridium difficile versus other enteric pathogens in Austria from 2008 to 2010. J Infect Public Health. 2014;7:133–44. doi: 10.1016/j.jiph.2013.07.010. [DOI] [PubMed] [Google Scholar]
- 13.Hall AJ, Curns AT, McDonald LC, Parashar UD, Lopman BA. The roles of Clostridium Difficile and norovirus among gastroenteritis-associated deaths in the United States, 1999-2007. Clin Infect Dis. 2012;55:216–23. doi: 10.1093/cid/cis386. [DOI] [PubMed] [Google Scholar]
- 14.Mattner F, Sohr D, Heim A, Gastmeier P, Vennema H, Koopmans M. Risk groups for clinical complications of norovirus infections: an outbreak investigation. Clin Microbiol Infect. 2006;12:69–74. doi: 10.1111/j.1469-0691.2005.01299.x. [DOI] [PubMed] [Google Scholar]
- 15.Woodward JM, Gkrania-Klotsas E, Cordero-Ng AY, Aravinthan A, Bandoh BN, Liu H, et al. The role of chronic norovirus infection in the enteropathy associated with common variable immunodeficiency. Am J Gastroenterol. 2015;110:320–7. doi: 10.1038/ajg.2014.432. [DOI] [PubMed] [Google Scholar]
- 16.Greig JD, Lee MB. Enteric outbreaks in long-term care facilities and recommendations for prevention: a review. Epidemiol Infect. 2009;137:145–55. doi: 10.1017/S0950268808000757. [DOI] [PubMed] [Google Scholar]
- 17.Vega E, Barclay L, Gregoricus N, Shirley SH, Lee D, Vinje J. Genotypic and epidemiologic trends of norovirus outbreaks in the United States, 2009 to 2013. J Clin Microbiol. 2014;52:147–55. doi: 10.1128/JCM.02680-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18•.Trivedi TK, Desai R, Hall AJ, Patel M, Parashar UD, Lopman BA. Clinical characteristics of norovirus-associated deaths: a systematic literature review. Am J Infect Control. 2013;41:654–7. doi: 10.1016/j.ajic.2012.08.002. Reviews of clinical and epidemiologic characteristics of deaths assocaited with norovirus infection. [DOI] [PubMed] [Google Scholar]
- 19.Lopman BA, Reacher MH, Vipond IB, Sarangi J, Brown DW. Clinical manifestation of norovirus gastroenteritis in health care settings. Clin Infect Dis. 2004;39:318–24. doi: 10.1086/421948. [DOI] [PubMed] [Google Scholar]
- 20.Kaufman SS, Green KY, Korba BE. Treatment of norovirus infections: moving antivirals from the bench to the bedside. Antivir Res. 2014;105:80–91. doi: 10.1016/j.antiviral.2014.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iturriza-Gomara M, Lopman B. Norovirus in healthcare settings. Curr Opin Infect Dis. 2014;27:437–43. doi: 10.1097/QCO.0000000000000094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kroneman A, Verhoef L, Harris J, Vennema H, Duizer E, van Duynhoven Y, et al. Analysis of integrated virological and epidemiological reports of norovirus outbreaks collected within the foodborne viruses in Europe network from 1 July 2001 to 30 June 2006. J Clin Microbiol. 2008;46:2959–65. doi: 10.1128/JCM.00499-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rodriguez-Lazaro D, Cook N, Ruggeri FM, Sellwood J, Nasser A, Nascimento MS, et al. Virus hazards from food, water and other contaminated environments. FEMS Microbiol Rev. 2012;36:786–814. doi: 10.1111/j.1574-6976.2011.00306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Verhoef L, Hewitt J, Barclay L, Ahmed SM, Lake R, Hall AJ, et al. Norovirus genotype profiles associated with foodborne transmission, 1999-2012. Emerg Infect Dis. 2015;21:592–9. doi: 10.3201/eid2104.141073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Repp KK, Keene WE. A point-source norovirus outbreak caused by exposure to fomites. J Infect Dis. 2012;205:1639–41. doi: 10.1093/infdis/jis250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Atmar RL, Opekun AR, Gilger MA, Estes MK, Crawford SE, Neill FH, et al. Norwalk virus shedding after experimental human infection. Emerg Infect Dis. 2008;14:1553–7. doi: 10.3201/eid1410.080117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Teunis PFM, Moe CL, Liu P, Miller S, Lindesmith L, Baric RS, et al. Norwalk virus: how infectious is it? J Med Virol. 2008;80:1468–76. doi: 10.1002/jmv.21237. [DOI] [PubMed] [Google Scholar]
- 28.Lopman B, Gastañaduy P, Park GW, Hall AJ, Parashar UD, Vinje J. Environmental transmission of norovirus gastroenteritis. Curr Opin Virol. 2012;2:96–102. doi: 10.1016/j.coviro.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 29.Ozawa K, Oka T, Takeda N, Hansman GS. Norovirus infections in symptomatic and asymptomatic food handlers in Japan. J Clin Microbiol. 2007;45:3996–4005. doi: 10.1128/JCM.01516-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sukhrie FA, Teunis P, Vennema H, Copra C, Thijs Beersma MFC, Bogerman J, et al. Nosocomial transmission of norovirus is mainly caused by symptomatic cases. Clin Infect Dis. 2012;54:931–7. doi: 10.1093/cid/cir971. [DOI] [PubMed] [Google Scholar]
- 31••.Costantini VP, Cooper EM, Hardaker HL, Lee LE, Bierhoff M, Biggs C, et al. Epidemiologic, virologic, and host genetic factors of norovirus outbreaks in long-term care facilities. Clin Infect Dis. 2016;62:1–10. doi: 10.1093/cid/civ747. Demostrates the epidemiology, virology, and genetic host factors of naturally occurring norovirus outbreaks in LTCF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Teunis PF, Sukhrie FH, Vennema H, Bogerman J, Beersma MF, Koopmans MP. Shedding of norovirus in symptomatic and asymptomatic infections. Epidemiol Infect. 2015;143:1710–7. doi: 10.1017/S095026881400274X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vinje J. Advances in laboratory methods for detection and typing of norovirus. J Clin Microbiol. 2015;53:373–81. doi: 10.1128/JCM.01535-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lysen M, Thorhagen M, Brytting M, Hjertqvist M, Andersson Y, Hedlund KO. Genetic diversity among food-borne and waterborne norovirus strains causing outbreaks in Sweden. J Clin Microbiol. 2009;47:2411–8. doi: 10.1128/JCM.02168-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hall AJ, Eisenbart VG, Etingue AL, Gould LH, Lopman BA, Parashar UD. Epidemiology of foodborne norovirus outbreaks, United States, 2001-2008. Emerg Infect Dis. 2012;18:1566–73. doi: 10.3201/eid1810.120833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bruggink LD, Oluwatoyin O, Sameer R, Witlox KJ, Marshall JA. Molecular and epidemiological features of gastroenteritis outbreaks involving genogroup I norovirus in Victoria, Australia, 2002-2010. J Med Virol. 2012;84:1437–48. doi: 10.1002/jmv.23342. [DOI] [PubMed] [Google Scholar]
- 37.Desai R, Hembree CD, Handel A, Matthews JE, Dickey BW, McDonald S, et al. Severe outcomes are associated with genogroup 2 genotype 4 norovirus outbreaks: a systematic literature review. Clin Infect Dis. 2012;55:189–93. doi: 10.1093/cid/cis372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ayukekbong JA, Fobisong C, Tah F, Lindh M, Nkuo-Akenji T, Bergstrom T. Pattern of circulation of norovirus GII strains during natural infection. J Clin Mcrobiol. 2014;52:4253–9. doi: 10.1128/JCM.01896-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Siebenga JJ, Vennema H, Zheng DP, Vinje J, Lee BE, Pang XL, et al. Norovirus illness is a global problem: emergence and spread of norovirus GII.4 variants, 2001-2007. J Infect Dis. 2009;200:802–12. doi: 10.1086/605127. [DOI] [PubMed] [Google Scholar]
- 40.Zheng DP, Widdowson MA, Glass RI, Vinje J. Molecular epidemiology of genogroup II-genotype 4 noroviruses in the United States between 1994 and 2006. J Clin Microbiol. 2010;48:168–77. doi: 10.1128/JCM.01622-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41••.de Graaf M, van Beek J, Koopmans MP. Human norovirus transmission and evolution in a changing world. Nat Rev Microbiol. 2016;14:421–33. doi: 10.1038/nrmicro.2016.48. Most recent review describing the transmission, pathogenesis and evolution of human noroviruses, and considering the ongoing risk of norovirus outbreaks, along with the future prospects for treatment. [DOI] [PubMed] [Google Scholar]
- 42.Lindesmith LC, Beltramello M, Donaldson EF, Corti D, Swanstrom J, Debbink K, et al. Immunogenetic mechanisms driving norovirus GII.4 antigenic variation. PLoS Pathog. 2012;8:e1002705. doi: 10.1371/journal.ppat.1002705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Eden JS, Tanaka MM, Boni MF, Rawlinson WD, White PA. Recombination within the pandemic norovirus GII.4 lineage. J Virol. 2013;87:6270–82. doi: 10.1128/JVI.03464-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hasing ME, Lee BE, Preiksaitis JK, Tellier R, Honish L, Senthilselvan A, et al. Emergence of a new norovirus GII.4 variant and changes in the historical biennial pattern of norovirus outbreak activity in Alberta, Canada, from 2008 to 2013. J Clin Microbiol. 2013;51:2204–11. doi: 10.1128/JCM.00663-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Allen DJ, Adams NL, Aladin F, Harris JP, Brown DW. Emergence of the GII-4 Norovirus Sydney 2012 strain in England, winter 2012-2013. PLoS One. 2014;9:e88978. doi: 10.1371/journal.pone.0088978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee RM, Lessler J, Lee RA, Rudolph KE, Reich NG, Perl TM, et al. Incubation period of viral gastroenteritis: a systematic review. BMC Infect Dis. 2013;13:446. doi: 10.1186/1471-2334-13-446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tsang OTY, Wong ATY, Chow CB, Yung RWH, Lim WWL, Liu SH. Clinical characteristics of nosocomial norovirus outbreaks in Hong Kong. J Hosp Infect. 2008;69:135–40. doi: 10.1016/j.jhin.2008.03.015. [DOI] [PubMed] [Google Scholar]
- 48.Stone ND, Ashraf MS, Calder J, Crnich CJ, Crossley K, Drinka PJ, et al. Surveillance definitions of infections in long-term care facilities: revisiting the McGeer criteria. Infect Control Hosp Epidemiol. 2012;33:965–77. doi: 10.1086/667743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49••.Jones MK, Grau KR, Costantini V, Kolawole AO, de Graaf M, Freiden P, et al. Human norovirus culture in B cells. Nat Protoc. 2015;10:1939–47. doi: 10.1038/nprot.2015.121. Describes the first cell culture system for a human norovirus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chan MC, Sung JJ, Lam RK, Chan PK, Lee NL, Lai RW, et al. Fecal viral load and norovirus-associated gastroenteritis. Emerg Infect Dis. 2006;12:1278–80. doi: 10.3201/eid1208.060081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wilhelmi de Cal I, Revilla A, del Alamo JM, Roman E, Moreno S, Sanchez-Fauquier A. Evaluation of two commercial enzyme immunoassays for the detection of norovirus in faecal samples from hospitalised children with sporadic acute gastroenteritis. Clin Microbiol Infect. 2007;13:341–3. doi: 10.1111/j.1469-0691.2006.01594.x. [DOI] [PubMed] [Google Scholar]
- 52.Updated norovirus outbreak management and disease prevention guidelines. MMWR Recomm Rep. 2011;60:1–18. [PubMed] [Google Scholar]
- 53.Kaplan JE, Gary GW, Baron RC, Singh N, Schonberger LB, Feldman R, et al. Epidemiology of Norwalk gastroenteritis and the role of Norwalk virus in outbreaks of acute nonbacterial gastroenteritis. Ann Intern Med. 1982;96:756–61. doi: 10.7326/0003-4819-96-6-756. [DOI] [PubMed] [Google Scholar]
- 54.Turcios RM, Widdowson MA, Sulka AC, Mead PS, Glass RI. Reevaluation of epidemiological criteria for identifying outbreaks of acute gastroenteritis due to norovirus: United States, 1998-2000. Clin Infect Dis. 2006;42:964–9. doi: 10.1086/500940. [DOI] [PubMed] [Google Scholar]
- 55.Goller JL, Dimitriadis A, Tan A, Kelly H, Marshall JA. Long-term features of norovirus gastroenteritis in the elderly. J Hosp Infect. 2004;58:286–91. doi: 10.1016/j.jhin.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 56.Lindesmith LC, Ferris MT, Mullan CW, Ferreira J, Debbink K, Swanstrom J, et al. Broad blockade antibody responses in human volunteers after immunization with a multivalent norovirus VLP candidate vaccine: immunological analyses from a phase I clinical trial. PLoS Med. 2015;12:e1001807. doi: 10.1371/journal.pmed.1001807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Atmar RL, Baehner F, Cramer JP, Song E, Borkowski A, Mendelman PM. Rapid Responses to Two Virus-Like Particle Norovirus Vaccine Candidate Formulations in Healthy Adults: A Randomized Controlled Trial. J Infect Dis. :2016. doi: 10.1093/infdis/jiw259. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58••.Lopman BA, Steele D, Kirkwood CD, Parashar UD. The vast and varied global burden of norovirus: prospects for prevention and control. PLoS Med. 2016;13:e1001999. doi: 10.1371/journal.pmed.1001999. Reviews the evidence for the global burden of norovirus and discusses the prospects for norovirus vaccine development. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59•.Aliabadi N, Lopman BA, Parashar UD, Hall AJ. Progress toward norovirus vaccines: considerations for further development and implementation in potential target populations. Expert Rev Vaccines. 2015;14:1241–53. doi: 10.1586/14760584.2015.1073110. Discusses recent data on human norovirus vaccine development and potential targets for implementation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.MacCannell T, Umscheid CA, Agarwal RK, Lee I, Kuntz G, Stevenson KB. Guideline for the prevention and control of norovirus gastroenteritis outbreaks in healthcare settings. Infect Control Hosp Epidemiol. 2011;32:939–69. doi: 10.1086/662025. [DOI] [PubMed] [Google Scholar]
- 61.Hansen S, Stamm-Balderjahn S, Zuschneid I, Behnke M, Ruden H, Vonberg RP, et al. Closure of medical departments during nosocomial outbreaks: data from a systematic analysis of the literature. J Hosp Infect. 2007;65:348–53. doi: 10.1016/j.jhin.2006.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Communicable Diseases Network Australia, Guidelines for the public health management of gastroenteritis outbreaks due to norovirus or suspected viral agents in Australia. 2010 [Google Scholar]
- 63.Party NW. Guidelines for the management of norovirus outbreaks in acute and community health and social care settings. London: Health Protection Agency; 2012. [Google Scholar]
- 64.D'Souza DH, Sair A, Williams K, Papafragkou E, Jean J, Moore C, et al. Persistence of caliciviruses on environmental surfaces and their transfer to food. Int J Food Microbiol. 2006;108:84–91. doi: 10.1016/j.ijfoodmicro.2005.10.024. [DOI] [PubMed] [Google Scholar]
- 65.Park GW, Sobsey MD. Simultaneous comparison of murine norovirus, feline calicivirus, coliphage MS2, and GII.4 norovirus to evaluate the efficacy of sodium hypochlorite against human norovirus on a fecally soiled stainless steel surface. Foodborne Pathog Dis. 2011;8:1005–10. doi: 10.1089/fpd.2010.0782. [DOI] [PubMed] [Google Scholar]
- 66.Otter JA, Yezli S, French GL. The role played by contaminated surfaces in the transmission of nosocomial pathogens. Infect Control Hosp Epidemiol. 2011;32:687–99. doi: 10.1086/660363. [DOI] [PubMed] [Google Scholar]
- 67.Park GW, Barclay L, Macinga D, Charbonneau D, Pettigrew CA, Vinje J. Comparative efficacy of seven hand sanitizers against murine norovirus, feline calicivirus, and GII.4 norovirus. J Food Prot. 2010;73:2232–8. doi: 10.4315/0362-028x-73.12.2232. [DOI] [PubMed] [Google Scholar]
- 68.Liu P, Yuen Y, Hsiao HM, Jaykus LA, Moe C. Effectiveness of liquid soap and hand sanitizer against Norwalk virus on contaminated hands. Appl Environ Microbiol. 2010;76:394–9. doi: 10.1128/AEM.01729-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Petrignani M, van Beek J, Borsboom G, Richardus JH, Koopmans M. Norovirus introduction routes into nursing homes and risk factors for spread: a systematic review and meta-analysis of observational studies. J Hosp Infect. 2015;89:163–78. doi: 10.1016/j.jhin.2014.11.015. [DOI] [PubMed] [Google Scholar]
- 70.Friesema IH, Vennema H, Heijne JC, de Jager CM, Morroy G, van den Kerkhof JH, et al. Norovirus outbreaks in nursing homes: the evaluation of infection control measures. Epidemiol Infect. 2009;137:1722–33. doi: 10.1017/S095026880900274X. [DOI] [PubMed] [Google Scholar]
- 71•.Hall AJ, Wikswo ME, Pringle K, Gould LH, Parashar UD. Vital signs: foodborne norovirus outbreaks - United States, 2009-2012. MMWRMorb Mortal Wkly Rep. 2014;63:491–5. Analysis of surveillance data suggests that food workers were associated with 70% of foodborne norovirus outbreaks reported to the National Outbreak Reporting System in the US. [PMC free article] [PubMed] [Google Scholar]
- 72.Connolly MA. Communicable disease control in emergencies: a field manual. World Health Organization; p. 2005. [Google Scholar]
- 73•.Barclay L, Park GW, Vega E, Hall AJ, Parashar U, Vinjé J, et al. Infection control for norovirus. Clin Infect. 2014;20:731–40. doi: 10.1111/1469-0691.12674. Reviews of infection prevention and control for norovirus. [DOI] [PMC free article] [PubMed] [Google Scholar]


