Abstract
Background
During late summer/fall 2014, pediatric cases of acute flaccid myelitis (AFM) occurred in the United States, coincident with a national outbreak of enterovirus D68 (EV-D68)–associated severe respiratory illness.
Methods
Clinicians and health departments reported standardized clinical, epidemiologic, and radiologic information on AFM cases to the Centers for Disease Control and Prevention (CDC), and submitted biological samples for testing. Cases were ≤21 years old, with acute onset of limb weakness 1 August–31 December 2014 and spinal magnetic resonance imaging (MRI) showing lesions predominantly restricted to gray matter.
Results
From August through December 2014, 120 AFM cases were reported from 34 states. Median age was 7.1 years (interquartile range, 4.8–12.1 years); 59% were male. Most experienced respiratory (81%) or febrile (64%) illness before limb weakness onset. MRI abnormalities were predominantly in the cervical spinal cord (103/118). All but 1 case was hospitalized; none died. Cerebrospinal fluid (CSF) pleocytosis (>5 white blood cells/μL) was common (81%). At CDC, 1 CSF specimen was positive for EV-D68 and Epstein-Barr virus by real-time polymerase chain reaction, although the specimen had >3000 red blood cells/μL. The most common virus detected in upper respiratory tract specimens was EV-D68 (from 20%, and 47% with specimen collected ≤7 days from respiratory illness/fever onset). Continued surveillance in 2015 identified 16 AFM cases reported from 13 states.
Conclusions
Epidemiologic data suggest this AFM cluster was likely associated with the large outbreak of EV-D68–associated respiratory illness, although direct laboratory evidence linking AFM with EV-D68 remains inconclusive. Continued surveillance will help define the incidence, epidemiology, and etiology of AFM.
Keywords: acute flaccid myelitis, limb weakness, polio, enterovirus, surveillance
Since the widespread implementation of polio vaccination starting in the 1950s, acute flaccid paralysis (AFP) due to poliovirus has been eliminated from most countries [1,2]. AFP attributable to spinal cord/anterior horn cell involvement (“nonpoliovirus” anterior myelitis) is thought to occur infrequently, but various nonpolio enteroviruses (eg, enterovirus A71, coxsackie viruses A and B), and other viruses (eg, flaviviruses, herpesviruses, and adenoviruses), have occasionally been reported as etiologies [3–5]. AFP incidence in the United States population <15 years of age is estimated at around 1.4 cases per 100 000 per year [6]; however, systematic surveillance for AFP, specifically with anterior horn cell involvement, is not conducted in the United States.
A report at an April 2014 national US neurology meeting of children in California with a “polio-like” illness generated substantial media attention [7]. In August 2014, physicians at Children’s Hospital Colorado, in Aurora, Colorado, noted an unusual clustering of children with acute limb weakness similar to that described in California [8–11]. Most patients had distinctive abnormalities of the central spinal cord on magnetic resonance imaging (MRI), with gray matter lesions in both anterior and posterior segments of the cord extending multiple levels [12]. Based on these clinical and anatomic characteristics, the illness was referred to as acute flaccid myelitis (AFM), to distinguish it from other forms of AFP [9]. The etiology of the Colorado cases was unclear, although there was a strong temporal association with an increase in severe respiratory disease caused by enterovirus D68 (EV-D68) in Colorado [13]. On 26 September 2014, the Centers for Disease Control and Prevention (CDC) issued a health advisory requesting state and local health departments to report retrospective and prospective AFM cases and send specimens to CDC for testing (http://emergency.cdc.gov/han/han00370.asp). Here, we summarize the epidemiologic, clinical, and laboratory findings of the AFM investigation.
METHODS
Case Information
We defined a case as any person aged ≤21 years, with acute onset of limb weakness starting on or after 1 August 2014, and with a spinal MRI revealing lesions predominantly of the gray matter. Patients with spinal cord trauma or an otherwise known etiology of limb weakness were excluded. Clinicians or public health officials were requested to complete and submit a standardized case report form (CRF) (http://www.cdc.gov/acute-flaccid-myelitis/downloads/patient-summary-form.pdf) including information on patient demographics, symptoms (including respiratory illness or documented fever >100.4°F (38°C) during the 4 weeks before limb weakness onset), clinical presentation, MRI findings, test results from local laboratories, and disposition. We present data for cases with onset through 31 December 2014. Denominators vary due to missing data for some questions.
MRI Review
All CRFs were reviewed by 1 of 2 neurologists (D. M. P., J. J. S.); if MRI findings as reported on the CRF were ambiguous, a copy of the MRI report was requested. If MRI findings remained unclear after review of the report, the MRI images were requested and reviewed by one of the study neurologists.
Laboratory Testing
Specimens sent to the CDC included cerebrospinal fluid (CSF), serum (acute and convalescent were requested), respiratory samples (nasopharyngeal swab, nasopharyngeal aspirate, oropharyngeal swab, or oral swab), and stool (2 stool specimens collected >24 hours apart, per poliovirus testing protocol) [14]. A diagnostic algorithm was established to test for the following pathogens: enteroviruses (enterovirus-specific real-time reverse transcription polymerase chain reaction [rRT-PCR]) [15]), EV-D68–specific real-time RT-PCR (http://www.fda.gov/MedicalDevices/Safety/EmergencySituations/ucm161496.htm) and enterovirus typing by VP1 seminested PCR and sequencing [16]; arboviruses (West Nile virus [immunoglobulin M {IgM} antibody in CSF or serum by IgM-capture enzyme-linked immunosorbent assay {MAC-ELISA}], St Louis encephalitis [IgM antibody in serum by microsphere immunoassay], and LaCrosse viruses [IgM antibody in serum by MAC-ELISA]); herpesviruses (including human herpesviruses 1 and 2, varicella zoster virus, and Epstein-Barr virus by fluorescence resonance energy transfer real-time PCR [FRET-rPCR], human herpesvirus 6A and 6B by conventional rPCR, and cytomegalovirus by TaqMan rPCR); and adenoviruses by rPCR. Expanded viral testing, including a panel of family/genus taxon-specific viral PCR assays and sequencing utilizing generic primers for 12 viral families and metagenomic next-generation sequencing, was conducted on a subset of cases with specimens collected early in the illness. (Full protocols for these CDC in-house PCR and serologic methods are available upon request.) Further screening with electron microscopy was conducted on a convenience sample of 6 CSF specimens.
Respiratory Illness Data
Data on respiratory tract specimens testing positive at CDC for EV-D68 were obtained from passive national surveillance [13]. We also examined respiratory illness data from BioSense, a timely, national electronic health information system operated by CDC and used for syndromic surveillance [17, 18]. We used emergency department visits in which dyspnea was diagnosed in persons aged <18 years as a syndromic indicator for when peak EV-D68 circulation occurred nationally in August–October 2014 [13].
Descriptive analyses were performed using SAS version 9.3 software (SAS Institute, Cary, North Carolina). The investigation was determined to be nonresearch public health response by the CDC Human Subjects Review board.
RESULTS
The CDC received 120 case reports meeting AFM case definition with onset 1 August–31 December 2014 (Figure 1; Supplementary Figure 1). During the 5-month period, the crude nationwide AFM incidence among persons ≤21 years was 0.026 cases per 100 000 population per month (0.32 cases/100 000 population/year if this monthly rate applied for 12 months). Cases were reported from 34 states (Figure 2). Median age was 7.1 years (range, 0.4–20.8 years; interquartile range [IQR], 4.8–12.1 years); 71 (59%) were male (Table 1; Supplementary Figure 2). Of 114 patients with information on preceding respiratory illness and fever, 64 (56%) had both fever and respiratory symptoms before limb weakness onset, 28 (25%) experienced only respiratory illness, 10 (9%) experienced only febrile illness, and 12 (11%) experienced neither. Median interval between respiratory or febrile illness onset (whichever occurred first) and limb weakness was 5 days (range, 0–18 days; IQR, 3–9 days).
Figure 1.
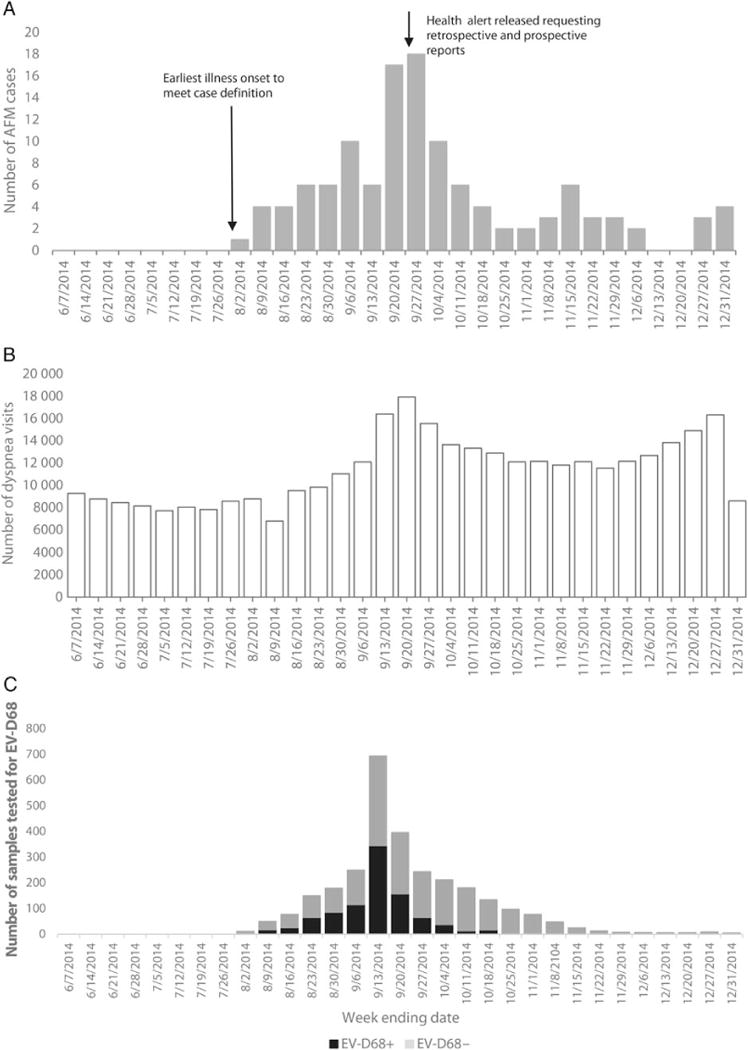
Number of acute flaccid myelitis (AFM) cases by week of limb weakness onset (A), dyspnea visits at hospital emergency departments among children aged <18 years (B), and respiratory specimens tested for enterovirus D68 (EV-D68) at the Centers for Disease Control and Prevention (CDC) by week of sample collection (C), United States, 1 June–31 December 2014. B, BioSense data are combined from 24 states that reported at least 1 AFM case and whose BioSense data (>90% of emergency department visits) came from hospitals that contributed consistently during August–October 2014. BioSense hospitals may be different from the hospitals that reported AFM cases. C, First public health communication informing states that CDC was available to test respiratory specimens for EV-D68 was on 28 August 2014. On 25 September 2014, CDC communications stated that CDC testing would be prioritized to samples from children with severe respiratory disease. Over time, more state laboratories developed capacity to test their samples.
Figure 2.

State distribution of acute flaccid myelitis (AFM) cases, United States, August–December 2014 (N = 120).
Table 1.
Demographics and Clinical Findings Among Acute Flaccid Myelitis Cases, United States, August–December 2014 (N = 120)
| Variable (No. With Information) | No. (%) |
|---|---|
| At time of acute illness | |
| Age, median (range; IQR) (n = 119) | 7.1 y (0.4–20.8 y; 4.8–12.1 y) |
| Sex (n = 120) | |
| Male | 71 (59) |
| Female | 49 (41) |
| Race (n = 95) | |
| American Indian or Alaska Native | 1 (1) |
| Asian | 8(8) |
| Black or African American | 7 (7) |
| Native Hawaiian or Pacific Islander | 0(0) |
| White | 79 (83) |
| Ethnicity (n = 88) | |
| Hispanic or Latino | 29 (33) |
| Not Hispanic or Latino | 59 (67) |
| Hospitalized (n = 119) | 118 (99) |
| Respiratory illness preceding limb weakness (n = 118) | 95 (81) |
| Febrile illness preceding limb weakness (n = 115) | 74 (64) |
| Respiratory or febrile illness preceding limb weakness (n = 117) | 105 (90) |
| Limb involvement (n = 119)a | |
| Upper extremity(ies) only | 41 (34) |
| Lower extremity(ies) only | 27 (23) |
| Upperand lowerextremities, but not all 4 extremities | 21 (18) |
| All 4 extremities involved | 30 (25) |
| Cranial nerve findings (n = 120) | |
| Any cranial nerve sign | 34 (28) |
| Facial weakness | 17 (14) |
| Dysphagia | 14 (12) |
| Diplopia/double vision | 10 (9) |
| Dysarthria | 8(7) |
| Facial numbness | 1 (1) |
| Altered mental status (n = 109) | 12 (11) |
| Seizures during illness (n = 116) | 5(4) |
| Received care in intensive care unit (n = 112) | 58 (52) |
| Required mechanical ventilation (n = 109) | 26 (20) |
| Underlying illness (n = 116) | 24 (21) |
| Asthmab | 12 (10) |
| Otherc | 12 (10) |
| At time of follow-up (median 4.2 mo after acute illness) | 56 (49) |
| Functiond | |
| Complete dependence on caretakers | 8 (14) |
| Somewhat functionally impaired | 38 (68) |
| Fully functional | 10 (18) |
| Strength (compared to initial presentation) | |
| As weak | 11 (20) |
| Some improvement | 41 (73) |
| Full recovery | 3 (5) |
| More weak | 1 (2) |
Abbreviation: IQR, interquartile range.
Remaining case had both lower extremities involved, but information was not provided on upper extremities.
One of these cases with asthma was also a former premature infant (26 weeks’ gestation).
Cardiac transplant on immunosuppressive therapy, diabetes mellitus type 1, cerebral palsy with hemiplegia, failure to thrive, post–spinal fusion surgery for scoliosis, postsurgery for Hirschprung disease, former premature infant (32 weeks’ gestation), G6PD deficiency (n = 1 each), attention deficit disorder (n = 4) (categories are all exclusive).
Effect of limb weakness on activities of daily living.
All but 1 patient was hospitalized (Table 1). Twenty percent of patients required mechanical ventilation for neuromuscular respiratory failure. Thirty-four percent had only upper extremity involvement, 23% had only lower extremity involvement, and 43% had both upper and lower extremities involved. Limb weakness was distinctly asymmetrical in 47%, and 30% had weakness restricted to 1 limb. Eighty-one percent had decreased or absent deep-tendon reflexes. Twenty-four percent reported numbness in affected limbs, while 51% reported pain. Cranial nerve dysfunction was evident in 28%, including facial weakness (14%), dysphagia (12%), and diplopia (9%). Signs suggesting cerebral involvement were largely absent, with only 11% demonstrating altered mental status, and 4% with seizures. Twenty-one percent with available information reported an underlying medical condition prior to onset of neurologic illness; the most frequently reported condition was asthma (12/116 [10%]); and 2 patients had an immunosuppressive illness (cardiac transplant and diabetes mellitus type 1).
Treatment and Outcomes
Eighty-five of 98 (87%) patients with any treatment information received some type of immunomodulating therapy for the neurologic illness, including intravenous immunoglobulin (62/85 [73%]), corticosteroids (46 [54%]), plasma exchange (13 [15%]), or other immunosuppressive medication (2 [2%]). We received follow-up information from caregivers on the clinical status of 56 (47%) patients, who reported on function (effect of limb weakness on daily living activities) and limb strength (Table 1). Median interval between limb weakness onset and report of follow-up outcome was 4.2 months (range, 0.8–7.5 months; IQR, 2.8–5.4 months). Of the 56 respondents, 8 patients (14%) were reported as completely dependent on caregivers, 38 (68%) had some degree of functional impairment, requiring assistance for some activities, and 10 (18%) reported being fully functional. Only 3 patients (5%) reported complete recovery of strength; the remainder had some residual weakness. There were no deaths.
MRI Findings
Although the entire spinal cord was not imaged in all patients and not all reports included details on each level, the most common site of involvement was the cervical spinal cord (103/118 [87%]), followed by thoracic (80/100 [80%]) and conus medullaris/cauda equina (36/76 [47%]) (Table 2). Spinal cord parenchymal gadolinium enhancement was seen in 22 of 103 (21%) patients to whom gadolinium was administered, and ventral nerve root enhancement was visualized in 16 of 47 (34%) patients with MRI imaging of the cauda equina and were administered gadolinium. Ninety-seven of 101 (96%) patients whose report included the affected spinal cord levels had >1 spinal segment involved.
Table 2.
Magnetic Resonance Imaging Findings Among Acute Flaccid Myelitis Cases, United States, August–December 2014 (N = 120)
| Finding | No. of Patients (%)a |
|---|---|
| MRI of spinal cord performed | |
| Cervical cord involvement | 103/118 (87)a |
| Thoracic cord involvement | 80/100 (80)a |
| Conus–cauda equina involvement | 36/76 (47)a |
| Ventral nerve enhancement | 16/47 (34)b |
| MRI of brain performed | |
| Cerebral lesions/brain MRI performed | 11/104 (11) |
| Cerebellar lesions/brain MRI performed | 11/104 (11) |
| Brainstem lesions/brain MRI performed | 36/104 (35) |
| Pontine lesions/number with brainstem lesions | 28/36 (78) |
| Medulla lesions/number with brainstem lesions | 27/36 (75) |
| Midbrain lesions/number with brainstem lesions | 10/36 (28) |
Denominator is number of patients reported having that spinal cord level imaged and result provided.
Denominator is number of patients reported having lumbosacral MRI performed, gadolinium administered, and result provided.
CSF Characteristics
One hundred twelve patients had CSF parameters reported. The median interval between limb weakness onset and CSF collection was 2 days (range, 0–42 days; IQR, 1–3 days); another 5 patients had CSF collected before their limb weakness onset. Median CSF white blood cell (WBC) count was 44 cells/μL (range, 0–664 cells/μL; IQR, 12–93 cells/μL; Supplementary Table 1). CSF pleocytosis, defined as WBC >5 cells/μL, was present in 91 cases (81%), with most having lymphocytic predominance. Median CSF protein and glucose were 43 (range, 17–921; IQR, 34–60) mg/dL and 59 (range, 37–154; IQR, 53–68) mg/dL, respectively (referent normal values: CSF protein <45 mg/dL; CSF glucose ≥40 mg/dL).
CDC Laboratory Results
CDC laboratories tested CSF from 55 patients (Table 3; Supplementary Figure 3). The median duration between respiratory/febrile illness onset and CSF collection (earliest sample if >1 from a patient) was 7.5 days (IQR, 5–13 days) (Supplementary Figure 4). One CSF specimen tested positive for EV-D68 by rRT-PCR, as well as for Epstein-Barr virus by FRET-rPCR; however, this specimen had >3000 red blood cells/μL, and no corresponding blood sample was available to clarify whether virus was present in blood. No other CSF specimens tested at CDC using pathogen-specific primers or serologic assays produced a positive result. Of 35 CSF specimens tested by metagenomic sequencing, 14 resulted in detection of several different viruses unlikely to be of clinical significance (Table 3). No pathogens were detected in the 6 CSF specimens examined by electron microscopy.
Table 3.
Laboratory Results, Centers for Disease Control and Prevention Laboratories
| Pathogens | CSF | Respiratory | Serum/Plasma | Stool/Rectal Swab |
|---|---|---|---|---|
| Enterovirus/rhinovirus | 1/55 (2) | 24/56 (43)a | 0/43 (0) | 11/54b (20) |
| EV-D68 | 1/1 (100) | 11/23 (48) | … | 0/11 (0) |
| Non-EV-D68 | 0/1 (0) | 12/23 (52) | … | 11/11 (100) |
| Adenoviruses | 0/48 (0) | 0/44 (0) | 1/39 (3) | 5/47 (11) |
| Herpesviruses | ||||
| Herpes simplex virus 1 | 0/41 (0) | 0/35 (0) | 0/33 (0) | … |
| Herpes simplex virus 2 | 0/41 (0) | 0/35 (0) | 0/33 (0) | … |
| Varicella zoster virus | 0/41 (0) | 0/35 (0) | 0/33 (0) | … |
| Epstein-Barr virus | 1/41 (2) | 3/35 (9) | 2/31 (6) | … |
| Human herpesvirus 6A | 0/39 (0) | … | 0/29 (0) | … |
| Human herpesvirus 6B | 0/39 (0) | … | 0/29 (0) | … |
| Cytomegalovirus | 0/38 (0) | … | 0/26 (0) | … |
| Panviral PCR platform | 0/31 (0) | 2/9 (22)c | 1/16 (6)d | 1/7 (14)e |
| Metagenomic next-generation sequencing | 14/35 (40)f | 1/1 (100)g | 7/12 (54)h | … |
| Arboviruses (IgM) | ||||
| West Nile | 0/2 (0) | … | 0/35 (0)i | … |
| St Louis encephalitis | 0/2 (0) | … | 0/35 (0)i | … |
| La Crosse | 0/4 (0) | … | 0/35 (0)i | … |
Data are presented as No. positive/No. tested (% positive).
Abbreviations: …, no specimens tested; CSF, cerebrospinal fluid; EV, enterovirus; IgM, immunoglobulin M; PCR, polymerase chain reaction.
One was enterovirus/rhinovirus (EV/RV) positive but untypeable.
One specimen collected about 2 weeks before the onset of limb weakness, respiratory illness, and fever is not included in this denominator. It was negative for EV/RV.
Herpesviridae (human herpesvirus 7, n = 1) and Herpesviridae (human herpesvirus 6a/b, n = 1).
Parvoviridae (erythrovirus B19, n = 1).
Parvoviridae (mouse PV, n = 1).
GB virus C (n = 8), human rhinovirus 4 (n = 4), transfusion transmitted virus (TTV) (n = 1), GB virus C, and TTV-like mini virus (n = 1).
GB virus C and EV-D68 (n = 1) (by EV/RV real-time reverse transcription polymerase chain reaction, only human rhinovirus A24 was detected in the specimen and hence this specimen is counted as EV-D68 negative in all the calculations in the manuscript).
GB virus C (n = 6), GB virus C, and TTV-like mini virus (n = 1).
Thirty-three patients with 1 serum specimen only: timing of serum collection from limb weakness onset: <3 days, n = 8; 3–10 days, n = 14; ≥11 days, n = 9; unknown, n = 2; 1 patient with serum 2 and 12 days after onset; 1 patient with serum 22 and 43 days after onset.
Using the earliest respiratory specimen for 56 patients that submitted at least 1 specimen, 11 (20%) nasopharyngeal/oropharyngeal specimens were positive for EV-D68 (includes patients from 6 different states), and 12 (21%) were positive for other enteroviruses/rhinoviruses (Table 3; Supplementary Tables 2 and 3). The proportion positive for EV-D68 increased in specimens collected closer to onset of respiratory/febrile illness, with 47% (8/17) of respiratory specimens collected ≤7 days from respiratory/febrile illness onset positive for EV-D68 (Supplementary Table 3). All serum/plasma specimens from 43 patients tested negative for enteroviruses by RT-PCR (Table 3). None of the 35 sera tested positive for IgM antibodies against arboviruses (West Nile, LaCrosse, St Louis encephalitis). None of the 54 stool/rectal swab specimens tested positive for poliovirus by culture or rRT-PCR; EV-D68 was also not detected in stool (Table 3; Supplementary Tables 4 and 5). By family/genus taxon-specific viral PCRs of specimens other than CSF, a few viruses were detected in low frequency.
External Laboratory Results
The reported number of tests conducted on each specimen in laboratories outside of CDC (eg, hospitals, independent clinical laboratories, state laboratories) varied. Three patients were reported to have viral RNA/DNA detected in CSF: 1 each with cytomegalovirus, echovirus 11, and human parechovirus. Among all specific viral testing results reported from any specimen type, only EVD-68 (in respiratory specimens from 8 patients) was detected in ≥2 patients (Supplementary Table 6).
Correlation With National Respiratory Illness
The majority of cases (97 [81%]) experienced limb weakness onset during 1 August–31 October 2014, with peak onset in mid/late September. This coincides with the increase and peak in pediatric emergency department visits for dyspnea collected in BioSense, as well as the number of respiratory samples sent from states to CDC for EV-D68 testing and the proportion testing positive (Figure 1).
DISCUSSION
We report 120 cases of pediatric AFM, most occurring during a national outbreak of EV-D68–associated respiratory illness. Neurologic illness was characterized by asymmetric flaccid limb weakness and spinal MRI findings showing gray matter involvement. Among those with follow-up information, most made some recovery, but few a full recovery. This clinical and neuroimaging phenotype is identical to that observed with poliovirus-associated acute paralysis. No pathogens were consistently detected in CSF or serum.
AFM cases were tightly clustered in time, and anecdotally, clinicians from several locations stated that the number of AFM cases observed over the 5-month period was unprecedented in their experience. However, we cannot confirm whether there was truly an increase in cases over previous years, as surveillance for neither AFM nor AFP is routinely conducted in the United States.
Since the elimination of wild-type poliovirus from the Western Hemisphere in 1991 [19], most AFP cases among all ages worldwide have been attributable to Guillain-Barré syndrome, an immune-mediated condition affecting peripheral nerves and nerve roots (not spinal gray matter). There are other recognized etiologies of AFP, and specifically AFM, including nonpolio enteroviruses [20–22], flaviviruses such as West Nile, St Louis, and Japanese encephalitis viruses [4, 23, 24], herpesviruses [25, 26], adenoviruses [27, 28], and others [3]. Although these pathogens may also be associated with other forms of AFP, including Guillain-Barré syndrome and acute transverse myelitis, AFM caused by these pathogens usually occurs sporadically, with the exception of EV-A71 outbreaks in East Asia [29, 30]. An estimate of the incidence of nonpoliovirus AFM, however, is hampered by lack of systematic surveillance and laboratory testing.
In late summer and early fall 2014, clinicians throughout the country noted an increase in hospitalizations for severe respiratory illness among children, suspected to be associated with EV-D68 [13, 31]. Outbreaks of EV-D68–associated respiratory illness were also reported in other countries in 2014 [32–37]. We demonstrated in several states with BioSense data that the occurrence of AFM aligned with the increase in pediatric emergency department visits for dyspnea and few AFM cases occurred after dyspnea visits declined by early October 2014. We continued to conduct AFM surveillance in 2015, and from 1 January through 31 December, 16 individuals meeting the 2014 case definition were reported from 13 states; 9 cases occurred from August through December. Moreover, passive surveillance at CDC did not identify any cases of EV-D68 respiratory illness in 2015. This temporal association between AFM and EV-D68 respiratory illness in 2014, and the lack of both in 2015, may suggest a causal association, although the association is ecological and surveillance was only done for 2 enterovirus seasons [8, 38].
There is biological plausibility to support such a causal association—other enteroviruses are recognized to cause AFM, most notably poliovirus and enterovirus-A71 [39, 40]. Moreover, EV-D68 has been detected in the CSF of a child with fatal meningomyeloencephalitis, as well as in the blood and upper respiratory tract of patients with AFM [35, 38, 41, 42]. However, despite testing of numerous CSF specimens as part of our national investigation (Supplementary Figure 2), we identified only 1 case with EV-D68 nucleic acid in the CSF, suggesting the presence of the virus within the intrathecal space. However, given the presence of red blood cells in the CSF, it is possible that the blood was the source of the EV-D68 (ie, from a presumptive viremia). Experience with other viruses that cause AFM suggests that while identification of intrathecal presence of virus by culture or PCR is difficult, a certain percentage of cases would be expected to test positive [43–46]. The detection of EV-D68 in CSF could have been hampered by delayed timing of specimen collection in relation to direct viral neuronal invasion and/or the relatively small number of samples to detect a transient presence of virus in CSF. Alternatively, neuronal damage could have been due to a postinfectious immune response; inflammatory damage to gray matter, although less common than to white matter, has increasingly been described in other syndromes [47–49].
While overall only 20% of patients had EV-D68 detected in upper respiratory tract specimens, it is possible that because of the long interval between respiratory illness onset and specimen collection, the virus could no longer be detected; among respiratory specimens collected ≤7 days after respiratory illness/fever onset, 47% were positive for EV-D68. However, detection of viruses in nonsterile sites, such as the upper respiratory tract, in AFM patients has unclear etiologic significance. The lack of EV-D68 in the stool has been shown before [50].
Possibly EV-D68 is “necessary but not sufficient” in the pathogenesis of AFM, rather than being truly neuroinvasive, and that other unknown factors, along with EV-D68 infection, are necessary to cause AFM. While the possibility of an autoimmune etiology has also been raised, the clinical and neuroradiographic findings are more consistent with a neuroinvasive viral pathogen. This is suggested by the abrupt limb weakness onset, the predominance of gray (rather than white) matter involvement, and the apparent lack of response to immunosuppressive therapy or significant improvement of neurologic deficits over time [51–53].
Our investigation has several limitations. We utilized a very specific case definition, including MRI findings, to ensure that we identified cases with the same disease process. However, we might have missed some true cases not meeting our strict case definition, underestimating the true illness burden. Several cases reported from Colorado presented with cranial nerve deficits alone [8]; as we limited our surveillance to patients with limb weakness, we would have failed to identify other possible neurologic manifestations of this syndrome. Second, we received CSF specimens for testing at CDC on only 46% of AFM cases. In some cases, timing of specimen collection may not have been optimal to conclusively rule in or rule out particular etiologies (eg, arboviral serologies). Finally, follow-up information was available only on about one-half of patients, and the representativeness of those outcomes is not known.
In the future, it will be important for clinicians to maintain vigilance for AFM and report cases to public health officials, both to better ascertain background incidence of this syndrome and to provide early detection of large outbreak events. Application of new laboratory diagnostic techniques may allow for determination of causative etiologies for AFM. Furthermore, as poliovirus is an important cause of AFM globally, it is critical to document that polio has been ruled out with the involvement of the state/local health department. Documentation should include appropriate specimens for viral isolation, documented poliovirus vaccine history, travel history, or exposure to someone who may have traveled, and 60-day follow-up of the neurologic deficit (http://www.cdc.gov/vaccines/pubs/surv-manual/chpt12-polio.html). Collection of appropriate biological specimens as early as possible will facilitate identification of an etiology of AFM. Cooperation between clinicians and public health officials will be important to better understand the epidemiology and clinical features of AFM.
Supplementary Material
Acknowledgments
The authors acknowledge the input and assistance of the following individuals: Jessica Leung, MPH; Jeanette St Pierre, MPH, MA; Sarah Poser, BS; and Sandra Roush, MT, MPH, from the Centers for Disease Control and Prevention (CDC) who provided valuable support during the CDC acute flaccid myelitis (AFM) national investigation. Samuel Dominguez, MD; Kevin Messacar, MD; and Teri Schreiner, MD, MPH, from Children’s Hospital Colorado, and Keith Van Haren, MD, from Stanford University, provided early identification and characterization of cases and brought national attention to the outbreak. We also thank the state epidemiologists and surveillance officers, and the BioSense community from states affected by AFM, for their support during the national investigation.
Footnotes
Clinical Infectious Diseases®
Published by Oxford University Press for the Infectious Diseases Society of America 2016. This work is written by (a) US Government employee(s) and is in the public domain in the US.
Supplementary Data
Supplementary materials are available at http://cid.oxfordjournals.org. Consisting of data provided by the author to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the author, so questions or comments should be addressed to the author.
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the CDC.
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Hagan JE, Wassilak SG, Craig AS, et al. Progress toward polio eradication—worldwide, 2014–2015. MMWR Morb Mortal Wkly Rep. 2015;64:527–31. [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. Report of the interim meeting of the technical consultative group (TCG) on the global eradication of poliomyelitis. Geneva, Switzerland: WHO; Nov 9–11, 2002. 2003. [Google Scholar]
- 3.Solomon T, Willison H. Infectious causes of acute flaccid paralysis. Curr Opin Infect Dis. 2003;16:375–81. doi: 10.1097/00001432-200310000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Solomon T, Kneen R, Dung NM, et al. Poliomyelitis-like illness due to Japanese encephalitis virus. Lancet. 1998;351:1094–7. doi: 10.1016/S0140-6736(97)07509-0. [DOI] [PubMed] [Google Scholar]
- 5.Sejvar JJ, Bode AV, Marfin AA, et al. West Nile virus-associated flaccid paralysis. Emerg Infect Dis. 2005;11:1021–7. doi: 10.3201/eid1107.040991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zangwill KM, Yeh SH, Wong EJ, et al. Paralytic syndromes in children: epidemiology and relationship to vaccination. Pediatr Neurol. 2010;42:206–12. doi: 10.1016/j.pediatrneurol.2009.10.012. [DOI] [PubMed] [Google Scholar]
- 7.Roux A, Lulu S, Waubant E, et al. A polio-like syndrome in California: clinical, radiologic, and serologic evaluation of five children identified by a statewide laboratory over a twelve-months period. Neurology. 2014;82(10 suppl):3, 335. [Google Scholar]
- 8.Messacar K, Schreiner TL, Maloney JA, et al. A cluster of acute flaccid paralysis and cranial nerve dysfunction temporally associated with an outbreak of enterovirus D68 in children in Colorado, USA. Lancet. 2015;385:1662–71. doi: 10.1016/S0140-6736(14)62457-0. [DOI] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention. Notes from the field: acute flaccid myelitis among persons aged ≤21 years—United States, August 1-November 13, 2014. MMWR Morb Mortal Wkly Rep. 2015;63:1243–4. [PMC free article] [PubMed] [Google Scholar]
- 10.Pastula DM, Aliabadi N, Haynes AK, et al. Acute neurologic illness of unknown etiology in children—Colorado, August–September 2014. MMWR Morb Mortal Wkly Rep. 2014;63:901–2. [PMC free article] [PubMed] [Google Scholar]
- 11.Ayscue P, Van Haren K, Sheriff H, et al. Acute flaccid paralysis with anterior myelitis - California, June 2012–June 2014. MMWR Morb Mortal Wkly Rep. 2014;63:903–6. [PMC free article] [PubMed] [Google Scholar]
- 12.Maloney JA, Mirsky DM, Messacar K, et al. MRI findings in children with acute flaccid paralysis and cranial nerve dysfunction occurring during the 2014 enterovirus D68 outbreak. AJNR Am J Neuroradiol. 2015;36:245–50. doi: 10.3174/ajnr.A4188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Midgley CM, Jackson MA, Selvarangan R, et al. Severe respiratory illness associated with enterovirus D68—Missouri and Illinois, 2014. MMWR Morb Mortal Wkly Rep. 2014;63:798–9. [PMC free article] [PubMed] [Google Scholar]
- 14.Centers for Disease Control and Prevention. Progress toward interruption of wild poliovirus transmission—worldwide, 2009. MMWR Morb Mortal Wkly Rep. 2010;59:545–50. [PubMed] [Google Scholar]
- 15.Oberste MS, Penaranda S, Rogers SL, et al. Comparative evaluation of TaqMan real-time PCR and semi-nested VP1 PCR for detection of enteroviruses in clinical specimens. J Clin Virol. 2010;49:73–4. doi: 10.1016/j.jcv.2010.06.022. [DOI] [PubMed] [Google Scholar]
- 16.Nix WA, Oberste MS, Pallansch MA. Sensitive, seminested PCR amplification of VP1 sequences for direct identification of all enterovirus serotypes from original clinical specimens. J Clin Microbiol. 2006;44:2698–704. doi: 10.1128/JCM.00542-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bradley CA, Rolka H, Walker D, Loonsk J. BioSense: implementation of a national early event detection and situational awareness system. MMWR Morb Mortal Wkly Rep. 2005;54(suppl):11–9. [PubMed] [Google Scholar]
- 18.Tokars JI, English R, McMurray P, Rhodes B. Summary of data reported to CDC’s national automated biosurveillance system, 2008. BMC Med Inform Decis Mak. 2010;10:30. doi: 10.1186/1472-6947-10-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Certification of poliomyelitis eradication—the Americas, 1994. MMWR Morb Mortal Wkly Rep. 1994;43:720–2. [PubMed] [Google Scholar]
- 20.Hsueh C, Jung SM, Shih SR, et al. Acute encephalomyelitis during an outbreak of enterovirus type 71 infection in Taiwan: report of an autopsy case with pathologic, immunofluorescence, and molecular studies. Mod Pathol. 2000;13:1200–5. doi: 10.1038/modpathol.3880222. [DOI] [PubMed] [Google Scholar]
- 21.Jang S, Suh SI, Ha SM, et al. Enterovirus 71-related encephalomyelitis: usual and unusual magnetic resonance imaging findings. Neuroradiology. 2012;54:239–45. doi: 10.1007/s00234-011-0921-8. [DOI] [PubMed] [Google Scholar]
- 22.Takimoto S, Waldman EA, Moreira RC, et al. Enterovirus 71 infection and acute neurological disease among children in Brazil (1988–1990) Trans R Soc Trop Med Hyg. 1998;92:25–8. doi: 10.1016/s0035-9203(98)90939-7. [DOI] [PubMed] [Google Scholar]
- 23.Chung CC, Lee SS, Chen YS, et al. Acute flaccid paralysis as an unusual presenting symptom of Japanese encephalitis: a case report and review of the literature. Infection. 2007;35:30–2. doi: 10.1007/s15010-007-6038-7. [DOI] [PubMed] [Google Scholar]
- 24.Sejvar JJ, Leis AA, Stokic DS, et al. Acute flaccid paralysis and West Nile virus infection. Emerg Infect Dis. 2003;9:788–93. doi: 10.3201/eid0907.030129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wong M, Connolly AM, Noetzel MJ. Poliomyelitis-like syndrome associated with Epstein-Barr virus infection. Pediatr Neurol. 1999;20:235–7. doi: 10.1016/s0887-8994(98)00142-8. [DOI] [PubMed] [Google Scholar]
- 26.Sabin AB, Wright AM. Acute ascending myelitis following a monkey bite, with the isolation of a virus capable of reproducing the disease. J Exp Med. 1934;59:115–36. doi: 10.1084/jem.59.2.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ivanova OE, Yurashko OV, Eremeeva TP, et al. Adenovirus isolation rates in acute flaccid paralysis patients. J Med Virol. 2012;84:75–80. doi: 10.1002/jmv.22265. [DOI] [PubMed] [Google Scholar]
- 28.Cho TA, Vaitkevicius H. Infectious myelopathies. Continuum (Minneap Minn) 2012;18:1351–73. doi: 10.1212/01.CON.0000423851.63017.2a. [DOI] [PubMed] [Google Scholar]
- 29.Komatsu H, Shimizu Y, Takeuchi Y, et al. Outbreak of severe neurologic involvement associated with enterovirus 71 infection. Pediatr Neurol. 1999;20:17–23. doi: 10.1016/s0887-8994(98)00087-3. [DOI] [PubMed] [Google Scholar]
- 30.Wang S-M, Liu C-C, Tseng H-W, et al. Clinical spectrum of enterovirus 71 infection in children in southern Taiwan, with an emphasis on neurological complications. Clin Infect Dis. 1999;29:184–90. doi: 10.1086/520149. [DOI] [PubMed] [Google Scholar]
- 31.Oermann CM, Schuster JE, Conners GP, et al. Enterovirus D68: a focused review and clinical highlights from the 2014 United States outbreak. Ann Am Thorac Soc. 2015;12:775–81. doi: 10.1513/AnnalsATS.201412-592FR. [DOI] [PubMed] [Google Scholar]
- 32.Gimferrer L, Campins M, Codina MG, et al. First enterovirus D68 (EV-D68) cases detected in hospitalised patients in a tertiary care university hospital in Spain, October 2014. Enferm Infecc Microbiol Clin. 2015;33:585–9. doi: 10.1016/j.eimc.2015.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Imamura T, Oshitani H. Global reemergence of enterovirus D68 as an important pathogen for acute respiratory infections. Rev Med Virol. 2015;25:102–14. doi: 10.1002/rmv.1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meijer A, Benschop KS, Donker GA, van der Avoort HG. Continued seasonal circulation of enterovirus D68 in the Netherlands, 2011–2014. Euro Surveill. 2014;19:20935. doi: 10.2807/1560-7917.es2014.19.42.20935. [DOI] [PubMed] [Google Scholar]
- 35.Pfeiffer HC, Bragstad K, Skram MK, et al. Two cases of acute severe flaccid myelitis associated with enterovirus D68 infection in children, Norway, autumn 2014. Euro Surveill. 2015;20:21062. doi: 10.2807/1560-7917.es2015.20.10.21062. [DOI] [PubMed] [Google Scholar]
- 36.Poelman R, Scholvinck EH, Borger R, et al. The emergence of enterovirus D68 in a Dutch university medical center and the necessity for routinely screening for respiratory viruses. J Clin Virol. 2015;62:1–5. doi: 10.1016/j.jcv.2014.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Skowronski DM, Chambers C, Sabaiduc S, et al. Systematic community- and hospital-based surveillance for enterovirus-D68 in three Canadian provinces, August to December 2014. Euro Surveill. 2015;20:1–14. doi: 10.2807/1560-7917.ES.2015.20.43.30047. [DOI] [PubMed] [Google Scholar]
- 38.Greninger AL, Naccache SN, Messacar K, et al. A novel outbreak enterovirus D68 strain associated with acute flaccid myelitis cases in the USA (2012–14): a retrospective cohort study. Lancet Infect Dis. 2015;15:671–82. doi: 10.1016/S1473-3099(15)70093-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Khetsuriani N, Lamonte-Fowlkes A, Oberst S, Pallansch MA. Enterovirus surveillance—United States, 1970–2005. MMWR Surveill Summ. 2006;55:1–20. [PubMed] [Google Scholar]
- 40.Ooi MH, Wong SC, Lewthwaite P, et al. Clinical features, diagnosis, and management of enterovirus 71. Lancet Neurol. 2010;9:1097–105. doi: 10.1016/S1474-4422(10)70209-X. [DOI] [PubMed] [Google Scholar]
- 41.Kreuter JD, Barnes A, McCarthy JE, et al. A fatal central nervous system enterovirus 68 infection. Arch Pathol Lab Med. 2011;135:793–6. doi: 10.5858/2010-0174-CR.1. [DOI] [PubMed] [Google Scholar]
- 42.Lang M, Mirand A, Savy N, et al. Acute flaccid paralysis following enterovirus D68 associated pneumonia, France, 2014. Euro Surveill. 2014;19:20952. doi: 10.2807/1560-7917.es2014.19.44.20952. [DOI] [PubMed] [Google Scholar]
- 43.Leparc I, Aymard M, Fuchs F. Acute, chronic and persistent enterovirus and poliovirus infections: detection of viral genome by seminested PCR amplification in culture-negative samples. Mol Cell Probes. 1994;8:487–95. doi: 10.1006/mcpr.1994.1070. [DOI] [PubMed] [Google Scholar]
- 44.Rocchi G, Andreoni G. Detection of polioviruses in human cerebrospinal fluid. Arch Gesamte Virusforsch. 1968;25:359–62. doi: 10.1007/BF01556564. [DOI] [PubMed] [Google Scholar]
- 45.Dierssen U, Rehren F, Henke-Gendo C, Harste G, Heim A. Rapid routine detection of enterovirus RNA in cerebrospinal fluid by a one-step real-time RT-PCR assay. J Clin Virol. 2008;42:58–64. doi: 10.1016/j.jcv.2007.11.016. [DOI] [PubMed] [Google Scholar]
- 46.Briese T, Glass WG, Lipkin WI. Detection of West Nile virus sequences in cerebrospinal fluid. Lancet. 2000;355:1614–5. doi: 10.1016/s0140-6736(00)02220-0. [DOI] [PubMed] [Google Scholar]
- 47.Kawachi I, Masatoyo N. Significance of gray matter brain lesions in multiple sclerosis and neuromyelitis optica. Neuropathology. 2015;35:481–6. doi: 10.1111/neup.12216. [DOI] [PubMed] [Google Scholar]
- 48.Lucchinetti CF, et al. A role for humoral mechanisms in the pathogenesis of Devic’s neuromyelitis optica. Brain. 2002;125:1450–61. doi: 10.1093/brain/awf151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Morris NA, Kaplan TB, Linnoila J, Cho T. HSV encephalitis-induced anti-NMDAR encephalitis in a 67-year-old woman: report of a case and review of the literature. J Neurovirol. 2016;22:33–7. doi: 10.1007/s13365-015-0364-9. [DOI] [PubMed] [Google Scholar]
- 50.Oberste MS, Maher K, Schnurr D, et al. Enterovirus 68 is associated with respiratory illness and shares biological features with both the enteroviruses and the rhinoviruses. J Gen Virol. 2004;85:2577–84. doi: 10.1099/vir.0.79925-0. [DOI] [PubMed] [Google Scholar]
- 51.Sejvar JJ, Kohl KS, Bilynsky R, et al. Encephalitis, myelitis, and acute disseminated encephalomyelitis (ADEM): case definitions and guidelines for collection, analysis, and presentation of immunization safety data. Vaccine. 2007;25:5771–92. doi: 10.1016/j.vaccine.2007.04.060. [DOI] [PubMed] [Google Scholar]
- 52.Storch-Hagenlocher B, Griffin DE, Einhäupl KM, Hacke W. Neurocritical Care. Berlin, Germany: Springer Berlin Heidelberg; 1994. Acute disseminated encephalomyelitis (parainfectious and postvaccinal encephalitis) pp. 493–9. [Google Scholar]
- 53.Wingerchuk DM. Postinfectious encephalomyelitis. Curr Neurol Neurosci Rep. 2003;3:256–64. doi: 10.1007/s11910-003-0086-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


