Abstract
Objectives
To investigate the impact of sleep quality in hypogonadal symptoms and sexual function in men working non-standard shifts.
Methods
Men treated at a single andrology clinic between July-October 2014 completed questionnaires assessing sleep quality, hypogonadal symptoms (Androgen Deficiency in the Aging Male – ADAM/qADAM), and sexual function (International Index of Erectile Function – IIEF). Serum hormone levels were assessed at the time of survey completion.
Results
182 men were identified as working non-standard shifts (work that starts before 7am or after 2pm, rotates, or regularly includes hours outside of the standard 7am to 6pm work day) with a mean±SD age of 41.1±10.8 years. Of men working non-standard shifts, those with better sleep quality had fewer hypogonadal symptoms and better sexual function. Multivariate regression analysis revealed significant linear associations between sleep quality and qADAM score (p=0.008), positive ADAM responses (p=0.003), and IIEF score (p=0.0004) were observed. When comparing individual groups, men who were “very satisfied” (n=60) with sleep quality had higher qADAM scores than men who were “somewhat dissatisfied” (p=0.02), and men who were “very dissatisfied” had significantly lower IIEF scores than men who were “very satisfied” (p=0.001) and “somewhat satisfied” (p=0.005). No associations between sleep quality and mean serum testosterone (T), free T, estrogen, DHEA, FSH, and LH levels were observed.
Conclusions
Men who work non-standard shifts and have poor sleep quality are at increased risk for hypogonadal symptoms and sexual dysfunction. These effects may be improved with a shift in schedule or techniques to improve sleep quality.
MeSH Key Words: hypogonadism, low testosterone, sleep, shift work disorder, sexual dysfunction
INTRODUCTION
Non-standard work shifts, defined as beginning before 7 a.m. or after 2 p.m., regularly rotating between standard and non-standard formats, or as work hours regularly occurring outside of a standard 7 a.m. to 6 p.m. workday, are increasingly prevalent in the developed world1. Shift workers comprise 15–25% of the workforce and often have suboptimal sleep quality, with 10–32% developing shift work disorder (SWD), a circadian rhythm disorder characterized by insomnia and sleepiness in people who work during normal sleep periods, typically at night2. Patients with SWD often suffer from decreased daytime alertness and cognitive function,3 higher body mass indices (BMIs), lipid abnormalities, and higher rates of hypertension and depression than non-shift workers3, 4. Non-standard shift workers are also prone to gastrointestinal symptoms, breast cancer, diabetes, and the metabolic syndrome5, 6. The mechanisms underlying the sequelae of SWD are unclear, although obesity,7 inflammation,8 and autonomic,9 metabolic,10 and neural dysregulation can all result from sleep impairment.11, 12
Hypogonadism is defined by the presence of low serum testosterone levels and clinical symptoms including decreased libido, erectile dysfunction (ED), lethargy, concentration difficulties, sleep disturbances, and loss of muscle mass.13–15 The incidence of hypogonadism increases as a function of age, which is due in part to a progressive decline in serum testosterone levels beginning at the age of 3016–18, and approximately 4 million American men are currently affected19. Sexual dysfunction, including ED, is found in many hypogonadal men, and is attributed in part to the significant role that testosterone plays in normal sexual function.20
Non-standard shift workers have lower, more variable serum testosterone levels than standard shift workers21. Given the risk of endocrine dysregulation and other sequelae in non-standard shift workers, it is possible that non-standard shift work could increase the risk of hypogonadism and sexual dysfunction in men working these shifts. In this study, we examine the relationship between sleep quality, hypogonadal symptoms and sexual dysfunction in shift workers.
MATERIALS AND METHODS
Study Population
After Institutional Review Board approval, all men presenting to a single academic urology clinic between July and October 2014 were asked to complete an electronic survey about their work schedules, specifically regarding non-standard shift work. Only men working non-standard shifts were asked to indicate the duration of having worked these shifts, and were asked to indicate their satisfaction with sleep quality as “very satisfied,” “somewhat satisfied,” “somewhat dissatisfied,” or “very dissatisfied.” Participants were also asked to complete the validated Androgen Deficiency in the Aging Male (ADAM) and non-validated quantitative ADAM (qADAM) questionnaires22, as well as the validated International Index of Erectile Function (IIEF)23. The ADAM and qADAM are screening tools for hypogonadism; positive ADAM responses are summed to yield a numerical score, and qADAM responses are also summed to yield a numerical score.
Medical comorbidities, serum testosterone (T), free T (FT), estradiol (E), dehydroepiandrosterone (DHEA), luteinizing hormone (LH) and follicle stimulating hormone (FSH) levels were obtained at the time of questionnaire completion. All laboratory testing was performed by the Laboratory for Male Reproductive Research and Testing at Baylor College of Medicine.
Statistical Analysis
Fisher’s exact test was used to compare comorbidities between men working standard and non-standard shifts. Student’s t-test was used to compare mean IIEF, ADAM, and qADAM scores of non-standard and standard shift workers. Multivariate regression and multivariate analysis of variance (MANOVA) with Sidak post-hoc test were used to evaluate the relationship between sleep quality and ADAM, qADAM, and IIEF scores, as well as serum hormone levels. STATA 12 (StataCorp, College Station, TX) was used for all statistical analyses, with significance considered at p<0.05.
RESULTS
Of men visiting the clinic between July-October 2014, 691 completed all questionnaires and were included in the analysis. Of these men, 494 were identified as working standard shifts with mean±SD age of 46.4±14.7 years, and 182 as working non-standard shifts with a mean±SD age of 41.5±10.8 years (p<0.0001). Comorbidities did not differ significantly between the groups except for a higher incidence of cancer in standard shift workers (p=0.008) (Table 1). Initial analysis of ADAM, qADAM and IIEF scores did not reveal significant differences between men working standard shifts and those working non-standard shifts (Table 2).
Table 1.
Cohort Demographics
| Non-Standard Shift Workers n=182 |
Standard Shift Workers n=494 |
p-value | |
|---|---|---|---|
| Age (mean±SD) | 41.5±10.8 | 46.4±14.7 | 0.00 |
|
|
|||
| Comorbidities (n(%)) | |||
|
|
|||
| Diabetes without neuropathy, retinopathy, or nephropathy | 4 (2.2 %) | 13 (2.63 %) | 1.00 |
|
|
|||
| Diabetes with neuropathy, retinopathy, or nephropathy | 2 (1.10 %) | 7 (1.42 %) | 1.00 |
|
|
|||
| Heart attack (MI) | 0 (0.00 %) | 7 (1.42 %) | 0.20 |
|
|
|||
| Congestive heart failure (CHF) | 1 (0.55 %) | 3 (0.61 %) | 1.00 |
|
|
|||
| Chronic obstructive pulmonary disease | 0 (0.00 %) | 2 (0.40 %) | 1.00 |
|
|
|||
| Connective tissue disease | 0 (0.00 %) | 1 (0.20 %) | 1.00 |
|
|
|||
| Transient ischemic attack | 1 (0.55 %) | 2 (0.40 %) | 1.00 |
|
|
|||
| Stroke (with residual symptoms) | 0 (0.00 %) | 0 (0.00 %) | 1.00 |
|
|
|||
| Peripheral vascular disease | 0 (0.00 %) | 0 (0.00 %) | 1.00 |
|
|
|||
| Dementia | 0 (0.00 %) | 0 (0.00 %) | 1.00 |
|
|
|||
| Chronic kidney disease | 1 (0.55 %) | 2 (0.40 %) | 1.00 |
|
|
|||
| Cancer (excluding leukemia/lymphoma or other blood cancers) | 7 (3.85 %) | 49 (9.92 %) | 0.01 |
|
|
|||
| Leukemia/Lymphoma | 3 (1.65 %) | 3 (0.61 %) | 0.35 |
|
|
|||
| Deep venous thrombosis (DVT) | 1 (0.55 %) | 2 (0.40 %) | 1.00 |
|
|
|||
| Liver disease | 3 (1.65 %) | 3 (0.61 %) | 0.35 |
|
|
|||
| Peptic ulcer disease | 2 (1.10 %) | 3 (0.61 %) | 0.62 |
|
|
|||
| AIDS (not HIV) | 1 (0.55 %) | 1 (0.20 %) | 0.47 |
|
|
|||
| None | 162 (89.0 %) | 409 (82.80 %) | 0.06 |
Table 2.
Questionnaire Scores
| Non-Standard Shift Workers n=182 |
Standard Shift Workers n=494 |
p-value | |
|---|---|---|---|
| IIEF Total Score (mean (SD)) | 55.0 (16.6) | 52.5 (17.9) | 0.10 |
|
|
|||
| Erectile dysfunction domain | 25.6 (8.6) | 24.4 (9.4) | 0.14 |
|
|
|||
| Orgasmic function domain | 8.2 (2.1) | 7.9 (2.2) | 0.22 |
|
|
|||
| Sexual desire domain | 7.1 (2.4) | 6.9 (2.5) | 0.33 |
|
|
|||
| Intercourse domain | 10.9(4.2) | 9.9 (4.6) | 0.01 |
|
|
|||
| Overall satisfaction domain | 7.1 (2.5) | 7.0 (2.6) | 0.68 |
|
|
|||
| ADAM Score | 3.6 (3.0) | 3.3 (3.0) | 0.28 |
|
|
|||
| qADAM Score | 36.0 (6.6) | 36.7 (6.5) | 0.17 |
|
|
|||
| Sleep Quality (n(%)) | |||
|
|
|||
| Very Satisfied | 60 (33%) | ND | |
|
|
|||
| Somewhat Satisfied | 61 (34%) | ND | |
|
|
|||
| Somewhat Dissatisfied | 51 (28%) | ND | |
|
|
|||
| Very Dissatisfied | 10 (5%) | ND | |
|
|
|||
ND – No Data
When examining sleep quality, the 188 men working standard shifts rated their satisfaction with sleep quality as “very satisfied” (n=60, 33%), “somewhat satisfied” (n=61, 34%), “somewhat dissatisfied” (n=51, 28%), and “very dissatisfied” (n=10, 5%) (Table 2). Multivariate regression analyses revealed that higher sleep quality was associated with fewer hypogonadal symptoms and better sexual function. Multivariate regression analysis revealed significant linear associations between sleep quality and hypogonadal symptoms as assessed by ADAM (total positive responses 2.4 vs. 3.7 vs. 4.5 vs. 4.5, p=0.003) and qADAM (total score 38.0 vs. 35.9 vs. 34.4 vs. 32.4, p=0.008), indicating that better sleep quality is associated with fewer hypogonadal symptoms. When comparing qADAM and ADAM scores among men with varying sleep qualities using Sidak post-hoc test following MANOVA, those who endorsed being “somewhat dissatisfied” with their sleep quality (n=51) had significantly lower qADAM and ADAM scores compared to those who were “very satisfied” with their sleep quality (n=60) (p<0.05) (Figure 1A, B).
Figure 1. Relationship Between Sleep Quality and Hypogonadal Symptoms.
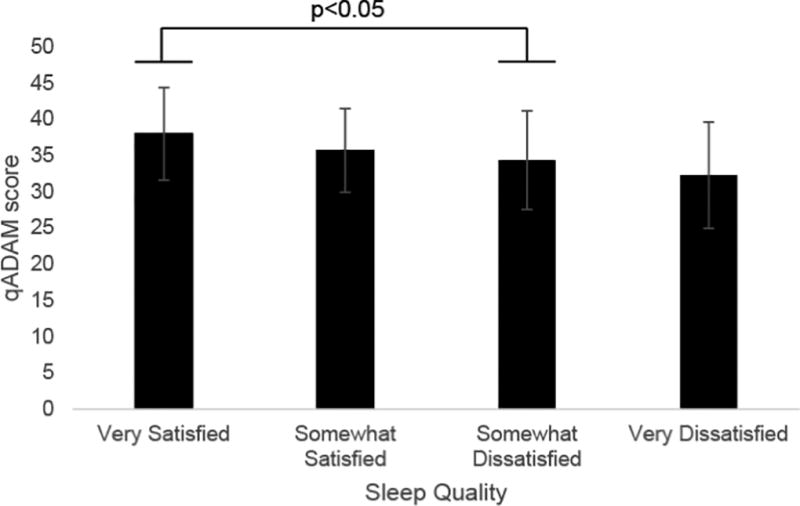
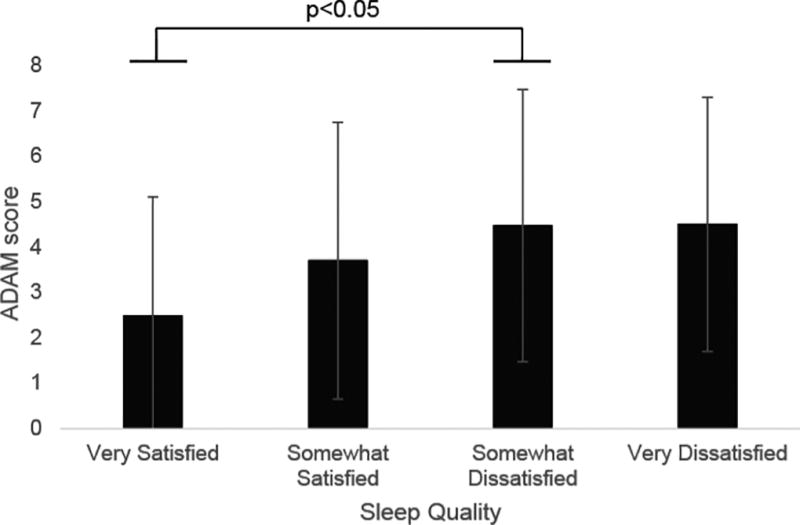
A) Relationship between total qADAM score and sleep quality; B) relationship between ADAM score and sleep quality.
An inverse linear association was observed between sleep quality and sexual function measured by IIEF (total score 59.4 vs. 56.5 vs. 51.5 vs. 38.0 for ‘Very Satisfied,’ ‘Somewhat Satisfied,’ ‘Somewhat Dissatisfied,’ or ‘Very Dissatisfied,’ p=0.0004), directly linking sleep quality with sexual function. When evaluating sexual function among men with different sleep qualities, those reporting “very satisfied” and “somewhat satisfied” sleep quality (n=121) had significantly higher IIEF scores compared with those reporting “very unsatisfactory” sleep quality (n=10) (p<0.05) (Figure 2A). All IIEF domains (erectile function, orgasmic function, sexual desire, intercourse satisfaction, and overall satisfaction) also demonstrated a direct relationship between sleep quality and sexual function (p<0.05 for each). Men who were “very satisfied” with their sleep had significantly higher scores than men who were “very dissatisfied” with their sleep in every IIEF domain except for sexual desire (Figure 2B).
Figure 2. Relationship Between Sleep Quality and Sexual Function.
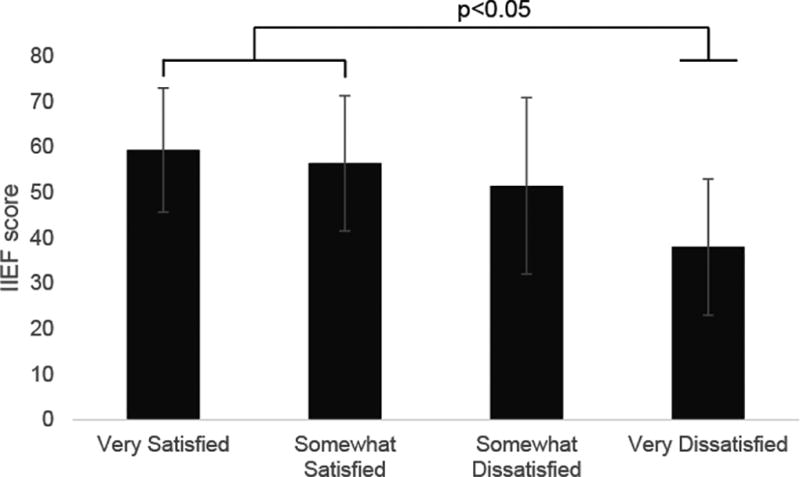
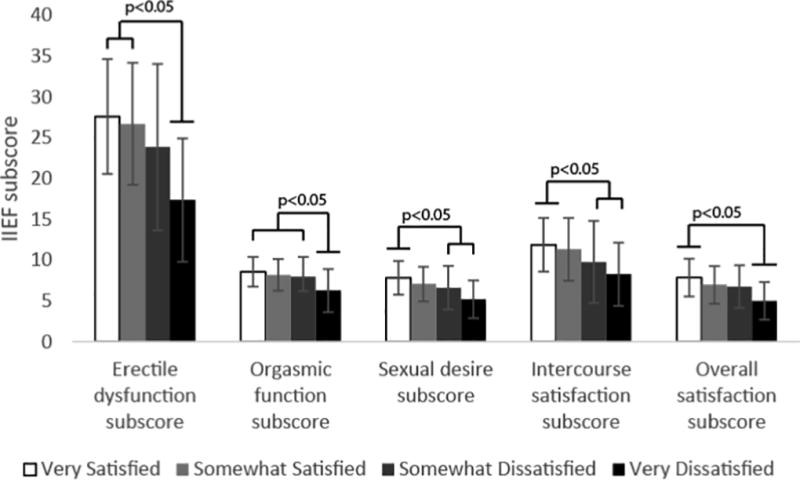
B) Relationship between total IIEF score and sleep quality; B) relationship between sleep quality and individual IIEF domain scores. NS = no statistical significance
Of the men working non-standard shifts, no association between sleep quality and mean±SD serum T, FT, E, DHEA, FSH, and LH levels was observed (Table 3). Overall serum hormone values were comparable between men working standard and non-standard shifts (Tables 3 and 4). Of men working standard shifts, 222 of 494 (44.9%) had a history of testosterone therapy (TTh) or had taken medications that alter hormone levels such as clomiphene, anastrozole, or HCG. Similarly, of men working non-standard shifts, 80 of 182 (44.0%) had a history of therapy that could alter serum hormone levels at the time of survey completion. No significant differences in hormone levels or survey responses were observed between men with a history of use of the above medications and those without (Table 4). When comparing men on and off medical therapy for hypogonadism, those on therapy had overall higher serum testosterone levels, with corresponding differences in other hormone levels, as expected (Table 4). Of men working non-standard shifts with no history of TTh or use of other hormone-altering medications, there was no significant relationship between sleep quality and hormonal levels (Table 5). Of note, sleep quality data were not available for men working standard shifts, as a function of our questionnaire workflow, limiting our ability to evaluate the link between sleep quality and hormone levels in this cohort of men.
Table 3.
Serum Hormone Levels as a Function of Sleep Quality in Non-Standard Shift Workers
| Sleep Quality | T (ng/dL) Mean (SD) |
FT (pg/mL) Mean (SD) |
E (pg/mL) Mean (SD) |
DHEA (ng/mL) Mean (SD) |
FSH (pg/mL) Mean (SD) |
LH (pg/mL) Mean (SD) |
|---|---|---|---|---|---|---|
| Very Satisfied | 647.32 (401.1) | 17.4 (12.6) | 4.5 (2.8) | 2388.1 (1391.1) | 3.5 (4.6) | 2.8 (4.6) |
|
|
||||||
| Somewhat Satisfied | 589.3 (411.6) | 13.9 (10.9) | 4.3 (2.6) | 1892.7 (1122.4) | 3.1 (5.4) | 1.7 (2.1) |
|
|
||||||
| Somewhat Dissatisfied | 607.2 (380.5) | 15.7 (11.1) | 5.0 (3.0) | 2465.7 (1333.0) | 2.0 (3.0) | 1.6 (2.3) |
|
|
||||||
| Very Dissatisfied | 607.7 (490.8) | 14.5 (12.2) | 4.6 (3.2) | 2209.0 (918.3) | 2.4 (2.3) | 1.8 (1.5) |
|
|
||||||
| p-value | 0.91 | 0.54 | 0.70 | 0.25 | 0.53 | 0.28 |
|
|
||||||
Table 4.
Serum Hormone Levels of Standard and Non-Standard Shift Workers as a Function of Testosterone Therapy
| T (ng/dL) mean±SD |
FT (pg/mL) mean±SD |
E (pg/mL) mean±SD |
DHEA (ng/mL) mean±SD |
FSH (pg/mL) mean±SD |
LH (pg/mL) mean±SD |
|
|---|---|---|---|---|---|---|
| Standard Shift Workers | ||||||
| TTh | 709.5 (445.1) | 17.0 (12.3) | 4.6 (2.7) | 2154.3 (1556.4) | 3.0 (6.4) | 1.9 (3.9) |
|
|
||||||
| No TTh | 403.9 (301.8) | 8.6 (8.1) | 3.4 (1.5) | 2339.9 (1357.7) | 4.9 (5.0) | 6.6 (29.3) |
|
|
||||||
| p-value | 0.00 | 0.00 | 0.00 | 0.39 | 0.01 | 0.02 |
|
|
||||||
| TTh + No TTh | 615.3 (401.8) | 14.4 (11.8) | 4.3 (2.5) | 2203.8 (1505.5) | 3.6 (6.1) | 3.4 (16.8) |
|
|
||||||
| Non-Standard Shift Workers | ||||||
|
|
||||||
| TTh | 730.0 (420.3) | 18.7 (12.4) | 5.1 (3.0) | 2151.2 (1123.0) | 1.7 (5.15) | 1.1 (1.9) |
|
|
||||||
| No TTh | 371.0 (206.3) | 8.6 (4.8) | 3.4 (1.7) | 2441.8 (1688.2) | 5.6 (5.1) | 4.2 (4.3) |
|
|
||||||
| p-value | 0.00 | 0.00 | 0.001 | 0.34 | 0.00 | 0.00 |
|
|
||||||
| TTh & No TTh | 609.1 (428.0) | 15.6 (11.6) | 4.6 (2.8) | 2212.7 (1259.8) | 2.9 (4.5) | 2.0 (3.2) |
Table 5.
Hormone levels in non-standard shift workers not on TTh as a function of sleep quality
| T (ng/dL) mean±SD |
FT (pg/mL) mean±SD |
E (pg/mL) mean±SD |
DHEA (ng/mL) mean±SD |
FSH (pg/mL) mean±SD |
LH (pg/mL) mean±SD |
|
|---|---|---|---|---|---|---|
| Very Satisfied | 341.2 (202.3) | 8.3 (4.7) | 3.35 (1.93) | 3428.5 (1967.7) | 7.0 (4.4) | 6.0 (6.1) |
|
|
||||||
| Somewhat Satisfied | 397.2 (251.5) | 9.3 (6.7) | 3.42 (1.7) | 1586.5 (1173.7) | 4.5 (7.2) | 2.1 (1.9) |
|
|
||||||
| Somewhat Dissatisfied | 402.6 (177.0) | 8.4 (2.4) | 3.6 (1.4) | 2874.4 (1856.0) | 5.0 (3.4) | 4.3 (2.1) |
|
|
||||||
| Very Dissatisfied | 181.0 (0.0) | 5.8 (0.0) | 3.0 (0.0) | 2911.0 (0.0) | 3.0 (0.0) | 3.0 (0.0) |
|
|
||||||
| p-value | 0.75 | 0.88 | 0.96 | 0.17 | 0.38 | 0.26 |
|
|
||||||
DISCUSSION
Non-standard work shifts have been associated with numerous negative effects on the endocrine system. Working non-standard shifts can lower 6-sulfatoxymelatonin and melatonin levels, and may increase the risk of cancer24. Non-standard shift work is also associated with alterations in postprandial hormone levels including insulin, proinsulin, and glucose-dependent insulinotropic polypeptide25. In this study, we focus on the impact of sleep on hypogonadal and sexual symptoms, often associated with alterations in the hypothalamic-pituitary-gonadal (HPG) axis. Lower serum testosterone levels have been observed in the setting of sleep restriction and fragmentation that corrupts diurnal GnRH release, and thus testosterone production26–28. Sleep fragmentation often results in the loss of the first phase of REM sleep, when testosterone levels are highest during a sleep cycle, and can explain the lower, more variable testosterone levels previously observed in non-standard shift workers21, 26, 29. In our cohort, determination of the effects of non-standard shift work on serum hormone levels was limited due to a significant proportion of men already being on TTh.
When we compared hypogonadal and sexual symptoms assessed using ADAM, qADAM, and IIEF questionnaires between standard and non-standard shift workers, no differences between these groups as a whole were observed, although this could have been influenced by the significant number of men on TTh at the time of survey completion. However, when we examined the relationship between sleep quality and hypogonadal symptoms and sexual dysfunction in non-standard shift workers, we observed a significant inverse relationship between sleep quality, hypogonadal symptoms and sexual dysfunction, with worse sleep quality associated with more hypogonadal symptoms and sexual dysfunction, suggesting that sleep quality may have a significant impact on these symptoms, independent of serum hormone levels.
Among men working non-standard shifts, men with better sleep quality had fewer hypogonadal symptoms and better sexual function than those with worse sleep quality. Better sleep quality is associated with higher testosterone levels, and may translate into fewer hypogonadal symptoms and less sexual dysfunction30. One study of 368 Swedish non-standard shift workers showed that those who were dissatisfied with their work hours had low testosterone levels5. In another study of 531 Chinese men, short sleep duration was associated with lower testosterone and free testosterone levels, and lower masturbation rates, suggestive of decreased libido31. Our findings suggest that sleep quality increases the risk for hypogonadal symptoms and sexual dysfunction, and that this is independent of serum hormone levels.
The present study is limited by several factors. First, the men surveyed were visiting a urology clinic because they were seeking treatment for infertility, prostate cancer, and hypogonadism, among other conditions. Thus, while we were able to compare standard and non-standard shift workers, a healthy normal male cohort was not available for comparison, making the data susceptible to selection bias. Second, sleep quality data were lacking for standard shift workers, limiting our ability to assess differences in sleep quality between standard and non-standard shift workers. Men were also asked to self-report sleep quality, a necessarily subjective measure which may have introduced bias into the study; a more objective measure would have been formal study of REM and non-REM sleep. Third, not all patients underwent testing for the serum hormones, and many men were already on treatment for hypogonadism, limiting our ability to objectively evaluate the relationship between serum hormones levels and sleep quality; this may have resulted in artificially uniform distributions for serum hormone levels among the study subjects.
Nevertheless, this work does link sleep quality directly with hypogonadal symptoms and sexual dysfunction, and suggests that sleep quality may more potently affect these parameters than serum hormone levels.
Conclusions
Men who work non-standard shifts and have poor sleep quality are at increased risk for hypogonadal symptoms and sexual dysfunction, as measured by ADAM, qADAM, and IIEF questionnaires. The relationship between sleep quality and hypogonadal symptoms/sexual dysfunction may be independent of testosterone. These effects may be improved with a shift in schedule or techniques to improve sleep quality.
References
- 1.Presser HB. Job, family, and gender: Determinants of nonstandard work schedules among employed Americans in 1991. Demography. 1995;32:577–598. [PubMed] [Google Scholar]
- 2.Wright KP, Jr, Bogan RK, Wyatt JK. Shift work and the assessment and management of shift work disorder (SWD) Sleep medicine reviews. 2013;17:41–54. doi: 10.1016/j.smrv.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 3.Vogel M, Braungardt T, Meyer W, Schneider W. The effects of shift work on physical and mental health. Journal of neural transmission. 2012;119:1121–1132. doi: 10.1007/s00702-012-0800-4. [DOI] [PubMed] [Google Scholar]
- 4.Froy O. Metabolism and circadian rhythms--implications for obesity. Endocrine reviews. 2010;31:1–24. doi: 10.1210/er.2009-0014. [DOI] [PubMed] [Google Scholar]
- 5.Axelsson J, Åkerstedt T, Kecklund G, Lindqvist A, Attefors R. Hormonal changes in satisfied and dissatisfied shift workers across a shift cycle. Journal of Applied Physiology. 2003;95:2099–2105. doi: 10.1152/japplphysiol.00231.2003. [DOI] [PubMed] [Google Scholar]
- 6.Knutsson A. Health disorders of shift workers. Occupational Medicine. 2003;53:103–108. doi: 10.1093/occmed/kqg048. [DOI] [PubMed] [Google Scholar]
- 7.Patel SR, Hu FB. Short sleep duration and weight gain: a systematic review. Obesity. 2008;16:643–653. doi: 10.1038/oby.2007.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grandner MA, Sands-Lincoln MR, Pak VM, Garland SN. Sleep duration, cardiovascular disease, and proinflammatory biomarkers. Nature and science of sleep. 2013;5:93–107. doi: 10.2147/NSS.S31063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mullington JM, Simpson NS, Meier-Ewert HK, Haack M. Sleep loss and inflammation. Best practice & research. Clinical endocrinology & metabolism. 2010;24:775–784. doi: 10.1016/j.beem.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gacci M, Vignozzi L, Sebastianelli A, et al. Metabolic syndrome and lower urinary tract symptoms: the role of inflammation. Prostate cancer and prostatic diseases. 2013;16:101–106. doi: 10.1038/pcan.2012.44. [DOI] [PubMed] [Google Scholar]
- 11.Drake CL, Roehrs T, Richardson G, Walsh JK, Roth T. Shift work sleep disorder: prevalence and consequences beyond that of symptomatic day workers. Sleep. 2004;27:1453–1462. doi: 10.1093/sleep/27.8.1453. [DOI] [PubMed] [Google Scholar]
- 12.Meyer JH, Ginovart N, Boovariwala A, et al. Elevated monoamine oxidase a levels in the brain: an explanation for the monoamine imbalance of major depression. Archives of general psychiatry. 2006;63:1209–1216. doi: 10.1001/archpsyc.63.11.1209. [DOI] [PubMed] [Google Scholar]
- 13.Wang C, Nieschlag E, Swerdloff R, et al. Investigation, treatment, and monitoring of late-onset hypogonadism in males: ISA, ISSAM, EAU, EAA, and ASA recommendations. Journal of andrology. 2009;30:1–9. doi: 10.2164/jandrol.108.006486. [DOI] [PubMed] [Google Scholar]
- 14.Wu FC, Tajar A, Beynon JM, et al. Identification of late-onset hypogonadism in middle-aged and elderly men. The New England journal of medicine. 2010;363:123–135. doi: 10.1056/NEJMoa0911101. [DOI] [PubMed] [Google Scholar]
- 15.Araujo AB, Esche GR, Kupelian V, et al. Prevalence of symptomatic androgen deficiency in men. The Journal of clinical endocrinology and metabolism. 2007;92:4241–4247. doi: 10.1210/jc.2007-1245. [DOI] [PubMed] [Google Scholar]
- 16.Moskovic DJ, Araujo AB, Lipshultz LI, Khera M. The 20-year public health impact and direct cost of testosterone deficiency in U.S. men. J Sex Med. 2013;10:562–569. doi: 10.1111/j.1743-6109.2012.02944.x. [DOI] [PubMed] [Google Scholar]
- 17.Feldman HA, Longcope C, Derby CA, et al. Age trends in the level of serum testosterone and other hormones in middle-aged men: longitudinal results from the Massachusetts male aging study. The Journal of clinical endocrinology and metabolism. 2002;87:589–598. doi: 10.1210/jcem.87.2.8201. [DOI] [PubMed] [Google Scholar]
- 18.Harman SM, Metter EJ, Tobin JD, Pearson J, Blackman MR Baltimore Longitudinal Study of A. Longitudinal effects of aging on serum total and free testosterone levels in healthy men. Baltimore Longitudinal Study of Aging. The Journal of clinical endocrinology and metabolism. 2001;86:724–731. doi: 10.1210/jcem.86.2.7219. [DOI] [PubMed] [Google Scholar]
- 19.Seftel A. Male hypogonadism. Part I: Epidemiology of hypogonadism. International journal of impotence research. 2006;18:115–120. doi: 10.1038/sj.ijir.3901397. [DOI] [PubMed] [Google Scholar]
- 20.Pastuszak AW. Current Diagnosis and Management of Erectile Dysfunction. Current Sexual Health Reports. 2014;6:164–176. doi: 10.1007/s11930-014-0023-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Touitou Y, Motohashi Y, Reinberg A, et al. Effect of shift work on the night-time secretory patterns of melatonin, prolactin, cortisol and testosterone. European journal of applied physiology and occupational physiology. 1990;60:288–292. doi: 10.1007/BF00379398. [DOI] [PubMed] [Google Scholar]
- 22.Mohamed O, Freundlich R, Dakik H, et al. The quantitative ADAM questionnaire: a new tool in quantifying the severity of hypogonadism. International journal of impotence research. 2010;22:20–24. doi: 10.1038/ijir.2009.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rosen RC, Riley A, Wagner G, Osterloh IH, Kirkpatrick J, Mishra A. The international index of erectile function (IIEF): a multidimensional scale for assessment of erectile dysfunction. Urology. 1997;49:822–830. doi: 10.1016/s0090-4295(97)00238-0. [DOI] [PubMed] [Google Scholar]
- 24.Mirick DK, Bhatti P, Chen C, Nordt F, Stanczyk FZ, Davis S. Night shift work and levels of 6-sulfatoxymelatonin and cortisol in men. Cancer Epidemiology Biomarkers & Prevention. 2013;22:1079–1087. doi: 10.1158/1055-9965.EPI-12-1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ribeiro D, Hampton S, Morgan L, Deacon S, Arendt J. Altered postprandial hormone and metabolic responses in a simulated shift work environment. Journal of Endocrinology. 1998;158:305–310. doi: 10.1677/joe.0.1580305. [DOI] [PubMed] [Google Scholar]
- 26.Luboshitzky R, Zabari Z, Shen-Orr Z, Herer P, Lavie P. Disruption of the Nocturnal Testosterone Rhythm by Sleep Fragmentation in Normal Men. The Journal of Clinical Endocrinology & Metabolism. 2001;86:1134–1139. doi: 10.1210/jcem.86.3.7296. [DOI] [PubMed] [Google Scholar]
- 27.Leproult R, Van Cauter E. Effect of 1 Week of Sleep Restriction on Testosterone Levels in Young Healthy Men. JAMA. 2011;305:2173–2174. doi: 10.1001/jama.2011.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Luboshitzsky R, Herer P, Levi M, Shen-Orr Z, Lavie P. Relationship Between Rapid Eye Movement Sleep and Testosterone Secretion in Normal Men. Journal of Andrology. 1999;20:731–737. [PubMed] [Google Scholar]
- 29.Kang JI, Ham BK, Oh MM, Kim JJ, Moon DG. Correlation between serum total testosterone and the AMS and IIEF questionnaires in patients with erectile dysfunction with testosterone deficiency syndrome. Korean journal of urology. 2011;52:416–420. doi: 10.4111/kju.2011.52.6.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boloña ER, Uraga MV, Haddad RM, et al. Mayo Clinic Proceedings. Vol. 82. Elsevier; 2007. Testosterone use in men with sexual dysfunction: a systematic review and meta-analysis of randomized placebo-controlled trials; pp. 20–28. [DOI] [PubMed] [Google Scholar]
- 31.Goh VHH, Tong TYY. Sleep, sex steroid hormones, sexual activities, and aging in Asian men. Journal of andrology. 2010;31:131–137. doi: 10.2164/jandrol.109.007856. [DOI] [PubMed] [Google Scholar]


