Abstract
SKA-31, an activator of endothelial KCa2.3 and KCa3.1 channels, reduces systemic blood pressure in mice and dogs, however, its effects in larger mammals are not well known. We therefore examined the hemodynamic effects of SKA-31, along with sodium nitroprusside (SNP), in anesthetized, juvenile male domestic pigs. Experimentally, continuous measurements of left ventricular (LV), aortic and inferior vena cava (IVC) pressures, along with flows in the ascending aorta, carotid artery, left anterior descending coronary artery and renal artery, were performed during acute administration of SKA-31 (0.1, 0.3, 1.0, 3.0 and 5.0 mg/ml/kg) and a single dose of SNP (5.0 μg/ml/kg). SKA-31 dose-dependently reduced mean aortic pressure (mPAO), with the highest dose decreasing mPAO to a similar extent as SNP (−23±3 and −28±4 mmHg, respectively). IVC pressure did not change. Systemic conductance and conductance in coronary and carotid arteries increased in response to SKA-31 and SNP, but renal conductance was unaffected. There was no change in either LV stroke volume (SV) or heart rate (versus the preceding control) for any infusion. With no change in SV, drug-evoked decreases in LV stroke work (SW) were attributed to reductions in mPAO (SW vs. mPAO, r2 = 0.82, P < 0.001). In summary, SKA-31 dose-dependently reduced mPAO by increasing systemic and arterial conductances. Primary reductions in mPAO by SKA-31 largely account for associated decreases in SW, implying that SKA-31 does not directly impair cardiac contractility.
Keywords: Endothelium, blood pressure, conductance, hemodynamics, KCa channel
1. Introduction
The vascular endothelium plays a critical role in the regulation of blood pressure and blood flow distribution by controlling the intraluminal diameter of conduit and small resistance arteries. This dynamic regulation occurs via the activation of distinct vasodilatory mechanisms in the endothelium that reduce contractile tone in the surrounding vascular smooth muscle, leading to increased intraluminal diameter, arterial conductance and blood flow. Major pathways contributing to endothelium-dependent vasodilation include the de novo synthesis of nitric oxide, prostacyclin and the generation of a hyperpolarizing electrical signal that acts on vascular smooth muscle. Endothelium-dependent hyperpolarization (EDH) is generated primarily via the activation of endothelial small- and intermediate-conductance, Ca2+-activated K+ channels (KCa2.3 and KCa3.1 channels, respectively) and is transmitted via myoendothelial gap junction connections to the adjacent smooth muscle, where it causes membrane hyperpolarization and reduced Ca2+ influx via voltage-gated Ca2+ channels. Small-molecule activators of KCa2.3 and KCa3.1 channels evoke direct hyperpolarization of endothelial cells [1–5], relax myogenically active resistance arteries [1,6] increase coronary flow in isolated heart preparations [7] and lower blood pressure in normo- and hypertensive mice [2,5]. In conscious dogs, bolus administration of a KCa channel activator transiently lowers systemic blood pressure [4]. In contrast, genetic knockout of endothelial KCa channels in mice leads to elevated systemic blood pressure and impairs or abolishes stimulus-evoked vasodilatory processes in isolated arteries and tissues [8]. Endothelial KCa channel activity may also be important in disease settings, as a KCa channel activator is able to restore agonist-evoked vasodilatory responses in the coronary circulation of a rodent model of type II diabetes exhibiting endothelial dysfunction [9].
To advance our knowledge of the in vivo cardiovascular effects of endothelial KCa channel activators, the goal of the present study was to investigate the systemic hemodynamic effects of SKA-31, a recently described, second-generation KCa channel activator [2], in a large animal model, the anesthetized, instrumented pig. Our results demonstrate that bolus intravenous injections of SKA-31 dose-dependently lower mean aortic pressure and increase systemic conductance to levels comparable to those elicited by the nitrovasodilator sodium nitroprusside (SNP). SKA-31 increased arterial conductance in coronary and carotid arteries, indicating that SKA-31 may have broad vasodilatory action in the vasculature. Neither SKA-31 nor SNP appeared to directly alter myocardial contractility. In summary, our data demonstrate that SKA-31 effectively lowers systemic blood pressure and increases arterial conductance in the peripheral circulation of the anesthetized pig. These observations suggest that SKA-31 may also be an effective vasodilator in the human vasculature.
2. Methods and Materials
The experimental protocols used in this study were approved by the University of Calgary Animal Care Committee, and conform to the NIH-published Guide for the Care and Use of Laboratory Animals (8th edition, 2011), and are further consistent with those of the American Physiological Society.
2.1 Animal preparation
Seven male domestic pigs (25–30 kg body weight, average weight 27 kg, 16–18 weeks of age) were studied. Pigs were pre-medicated with an intramuscular injection of ketamine hydrochloride (600 mg), fentanyl citrate (2 mg), and midazolam (10 mg). A 20-gauge catheter was inserted into an ear vein and anesthesia was induced with sodium thiopental (25 mg/kg). Anesthesia (level 3) was maintained with a continuous intravenous (I.V.) infusion containing a mixture of fentanyl citrate (0.04 mg/ml), midazolam (0.025 mg/ml) and ketamine hydrochloride (0.3 mg/ml) at a rate of 100 ml/hour. Both isoflurane (less than 1% in the ventilator) and lidocaine (3 bolus intravenous administrations, 1 mg/kg, 5 min apart, followed by an I.V. infusion of 0.75 – 1.0 mg/min) were used as required. The drug infusion rates were adjusted as necessary to ensure deep sedation without spontaneous respiratory effort. The animals were intubated with a cuffed endotracheal tube and ventilated with constant-volume ventilator (Harvard Apparatus, Millis, MA) with a 50% oxygen -50% nitrous oxide mixture. Tidal volume and respiratory rate were adjusted to maintain physiological values of blood gases and pH in accordance with recommended ventilation parameters for large animals [10]. PaCO2 was maintained between 35 and 45 mmHg.
A median sternotomy was performed and the hearts were delivered from the pericardium through a base-to-apex incision. Sonomicrometry crystals (Sonometrics, London, ON) were implanted in the left ventricular endocardium and mid-wall of the septum to measure the minor-axis septum-to-left ventricular free wall and left ventricular antero-posterior dimensions [11–13]. Ultrasonic flow probes (Transonic Systems, Ithaca, NY) were placed on the ascending aorta, descending aorta (just above diaphragm), inferior vena cava (IVC) (just above the diaphragm), right carotid artery, left renal artery, and left anterior descending coronary artery. Thin walled 7-French fluid-filled catheters connected to pressure transducers (model P23 ID; Statham Gould, Oxnard, CA) were inserted into the left ventricle (LV) (PLV; retrograde through the left carotid artery), aorta (PAO; retrograde through the right femoral artery) and IVC (PIVC; through the right jugular vein). An intravenous line was placed in the left external jugular vein for volume loading (PentaspanTM, 10% pentastarch in 0.9% NaCl) to replenish fluid loss during surgery. A thin-walled catheter was connected to the intravenous line for bolus infusions. Arterial samples for blood-gas analysis were obtained from a side-port on the aortic catheter. Body temperature was monitored with a rectal thermometer. After instrumentation, the heart was returned to the pericardium, which was closed with individual sutures, taking care not to compromise pericardial volume [14]. A single-lead electrocardiogram (ECG) was recorded.
2.2 Experimental protocol
Simultaneous pressure, dimension and flow measurements were recorded at baseline and during each intervention. After stabilization at an LV end-diastolic pressure (PLVED) of ~10 mmHg (11 ± 1 mmHg), control data were collected for 60 s, immediately preceding a 5-min recording period, during and after drug infusion. Each 20 ml infusion was delivered over a 60-s period and proceeded in ascending order of SKA-31 dosage (0.1, 0.3, 1.0, 3.0, and 5.0 mg/ml/kg) followed by a single dosage of sodium nitroprusside (SNP; 5.0 μg/ml/kg). Washout and recovery periods of 15–20 min were interposed between drug infusions. At the end of the experiment, the animals were sacrificed by a bolus KCl injection and the positions of the sonomicrometry crystals within the myocardium were verified.
SKA-31 was synthesized and tested for identity and purity (NMR and HPLC/MS) as previously described [2]. SKA-31 was dissolved in a vehicle solution comprised of Cremophor EL (10% v/v) and phosphate-buffered saline (PBS) (90% v/v). Briefly, an aliquot of Cremophor EL was first heated in a beaker on a magnetic stir plate to a temperature of ~60°C. The desired amount of solid SKA-31 was then added to the heated Cremophor EL liquid as it was being stirred. Once the added SKA-31 had dissolved completely, heating was stopped and stirring was maintained. The first few milliliters of PBS were then added slowly to the SKA-31/Cremophor EL solution and the remaining amount was added more quickly. The final SKA-31 solution was allowed to cool to room temperature with continuous stirring and appeared slightly yellowish. Solutions of SKA-31 in Cremophor-EL/PBS were freshly prepared for each experiment.
2.3 Data analysis
The conditioned signals were passed through a low-pass filter (100 Hz) and were digitized and recorded at 100 Hz (Sonometrics Corp. acquisition system, London, ON). The digitized data were analyzed on a personal computer using custom software (CV Works, Calgary, AB) developed in our laboratory. Baseline and control data are expressed as mean values for the 60-s period immediately preceding each infusion event. All data associated with administration of drug or control solutions were extracted at the time of greatest decrease in mPAO. If mPAO did not change by at least 5 mmHg during a given intervention, acquired data points were averaged for the first 60 s of that period.
Systemic conductance (Gsystemic, the reciprocal of systemic vascular resistance, SVR) was calculated as mean aortic flow / (mPAO – mPIVC) and expressed as a percent change from the preceding control value. Carotid conductance (Gcarotid), renal conductance (Grenal) and coronary conductance (Gcoronary) were expressed similarly and calculated by respectively substituting mean carotid, renal and coronary flow for mean aortic flow. LV stroke work (SW) was calculated as LV stroke volume (SV) x [mean PLV (systolic) – PLVED], where mean PLV (systolic) was calculated as PAO (diastolic) + 2/3 [PAO (systolic) – PAO (diastolic)]. As an index of LV end-diastolic volume, LV area (ALVED), was calculated as the product of the 2 minor-axis LV dimensions [15,16]. SW and ALVED values following drug infusions are expressed as the percent change from the preceding control values determined using the same calculations.
2.4 Measurements of SKA-31 Concentration in Plasma
Blood samples (~2 ml) were taken via a catheter inserted into the left external jugular vein at various intervals following drug infusion at the same site. Samples were collected in heparinized tubes to prevent coagulation and centrifuged at 400 x g for 20 min at 4°C. The resulting supernatants were then stored at −80°C prior to analysis. Plasma samples were then processed and analyzed in duplicate by HPLC/MS as recently described [4] and SKA-31 concentrations were determined from a standard curve. A semi-logarithmic plot of SKA-31 plasma concentrations vs. time was fitted with the following equation:
Where C0 = the maximal initial concentration of SKA-31 in the plasma calculated from the y-intercept of the fitted line, ke is the rate constant and t is the time interval following SKA-31 infusion. The volume of SKA-31 distribution (VolD) was calculated as follows:
2.5 Statistical analysis
Statistical comparisons were performed using SigmaPlot (Systat Software, Inc. 2012). In Figure 8, a linear correlation was calculated for the percentage changes for mPAO and stroke work during saline, vehicle, and all drug infusions (y = y0+a * x). The Student’s paired t-test was used to test for the significance of changes between a given infusion (i.e. vehicle or drug) and the preceding control period. Repeated-measures ANOVA (Holm-Sidak method) was used to test for the significance of differences between vehicle/SKA-31 infusions and SNP. A P value <0.05 was considered statistically significant. Except where noted, data are presented as mean ± SEM.
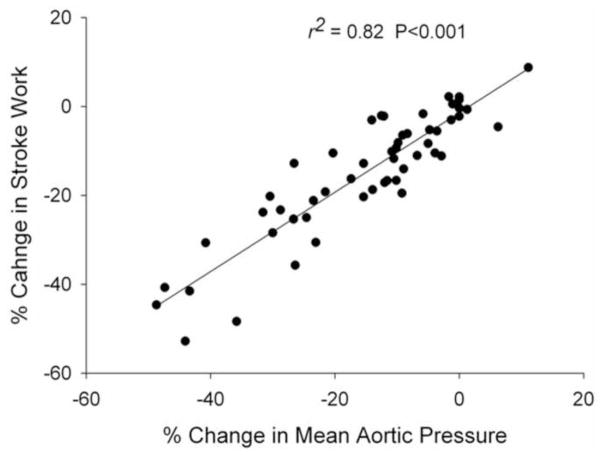
Figure 8.
Scatter plot displaying the relation between observed changes in left ventricular stroke work (SW) and mean aortic pressure (mPAO) following infusions of saline, vehicle, SKA-31 and SNP. Percent changes in mPAO, along with accompanying percent changes in SW, were first calculated in response to each infusion utilized in a given experiment. Data points from all 7 animals were then plotted against each other in a pair-wise fashion, as depicted by the individual symbols on the graph. The straight line through the symbols represents a linear regression fit to the pooled data points (r2 value = 0.82; P < 0.001).
3. Results
Seven anesthetized, juvenile pigs were acutely implanted with blood pressure transducers and Doppler flow probes that allowed us to measure mean aortic and inferior vena cava pressures, systemic conductance and regional conductance in carotid, renal and coronary arteries. Myocardial performance was monitored via a single lead electrocardiogram and implanted sonomicrometry crystals in the myocardium to assess LV dimensions. Table 1 presents average hemodynamic parameters in all 7 animals measured at baseline, following instrumentation and recovery and before the first experimental infusion (saline).
Table 1.
Baseline hemodynamic parameters in anesthetized pigs immediately prior to the control saline infusion at the start of the experiment.
| HR (bpm) | 122±9 |
| SV (ml) | 23±3 |
| PLVED (mmHg) | 11±1 |
| mPAO (mmHg) | 71±6 |
| mPIVC (mmHg) | 7±1 |
HR, heart rate in beats per minute; SV, left ventricular stroke volume; PLVED, end-diastolic left ventricular pressure; mPAO, mean aortic pressure; mPIVC, mean inferior vena cava pressure. Data represent the means ± SEM calculated from 7 pigs in total.
3.1 SKA-31 Dose Response
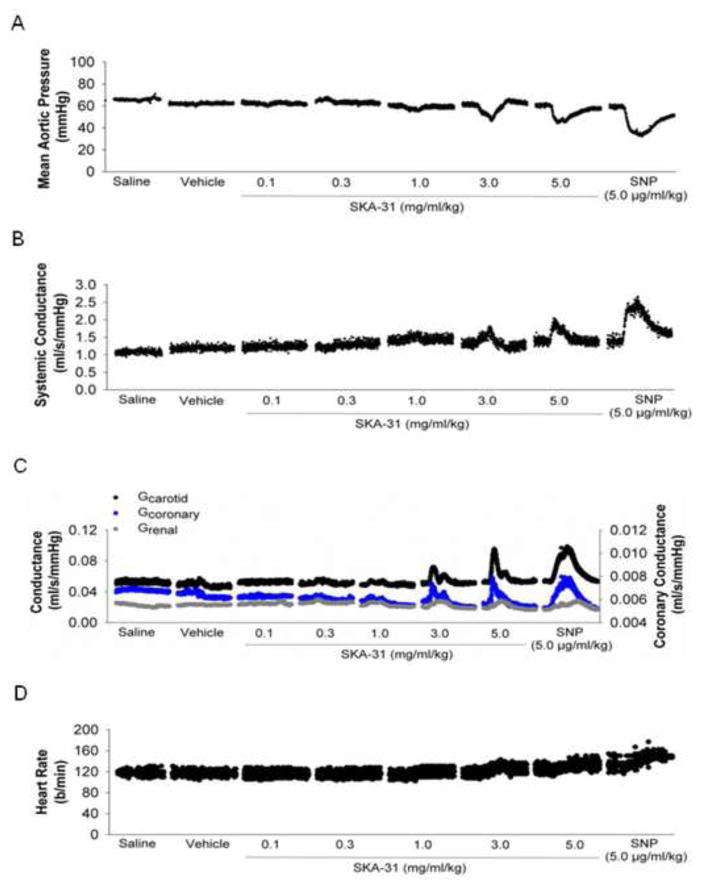
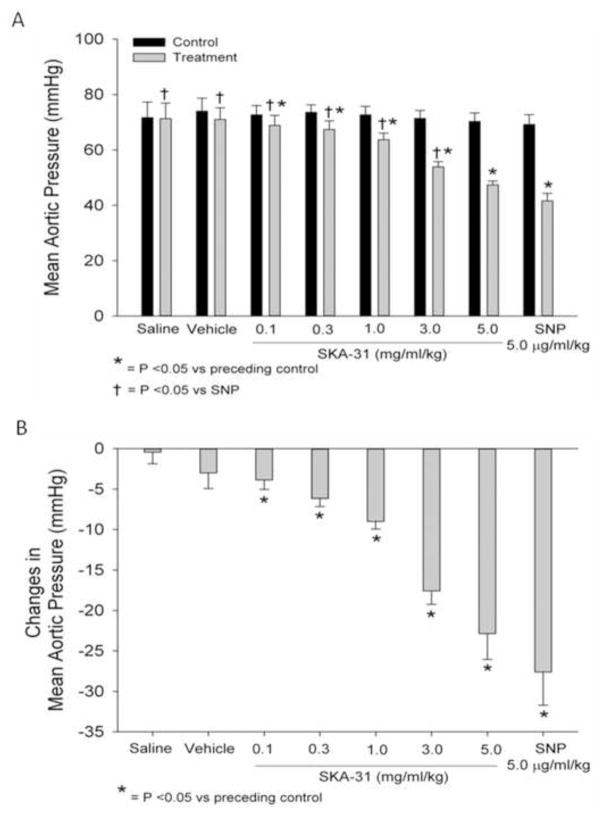
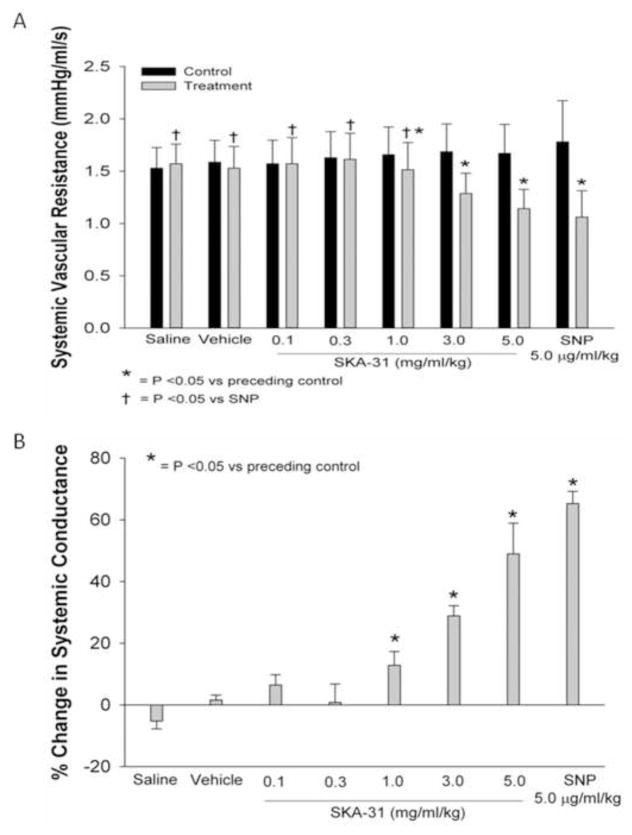
Following surgical interventions, animals were allowed to recover until steady-state basal levels of mean aortic pressure and heart rate were achieved. After a minimum 10 min period of steady-state baseline recording, we commenced with the first (saline) infusion. Figure 1 displays representative tracings of the effect of individual bolus administrations of saline, drug vehicle, SKA-31 (0.1 – 5.0 mg/ml/kg) on mean aortic pressure (mPAO, panel A), systemic conductance (panel B), measured conductance in carotid, coronary and renal arteries (panel C) and heart rate (panel D). While SKA-31 infusions had clear effects on these hemodynamic parameters, neither saline nor vehicle infusions had any observable effects. In an effort to benchmark the effects of SKA-31 on the measured parameters, we infused a single dose of the well characterized nitrovasodilator sodium nitroprusside (SNP) following recovery from the SKA-31 evoked hemodynamic changes. As displayed on the right hand side of Figures 1A–D, SNP infusion (5.0 μg/ml/kg) produced qualitatively similar changes in mPAO, systemic conductance, carotid, coronary and renal artery conductances and heart rate when compared with SKA-31. In case of mPAO (Fig. 1A), intravenous infusion of SKA-31 significantly decreased mPAO in a dose-dependent manner versus each preceding control period, with the greatest decrease occurring after the highest dose (5.0 mg/ml/kg) (Figure 2). SNP infusion also significantly decreased mPAO and this change was comparable to that measured following infusion of 5.0 mg/ml/kg SKA-31.
Figure 1.
Representative data from one pig demonstrating the rapid and reversible effects of SKA-31 and sodium nitroprusside (SNP) following acute intravenous infusion on mean aortic pressure (mPAO) (panel A), systemic vascular conductance (panel B), measured conductance in coronary, carotid and renal arteries (panel C) and heart rate (panel D). In each panel, the sections of continuous data points displayed represent 5-min epochs that were extracted from the master data record and illustrate the basal levels and evoked changes in the measured parameters in response to the infusions. The horizontal bars and labels provided beneath each panel specify the experimental infusion for the 5-min section of data appearing immediately above each description. Note that all displayed data were acquired simultaneously during the experiment. Individual infusions were separated by a 15–20 min recovery period (indicated by the breaks between the sections of data points) and control hemodynamic data were acquired for the first 1–2 min period immediately prior to a given infusion, once a steady baseline was clearly apparent (not shown).
Figure 2.
Quantification of mean aortic pressure (mPAO) under control conditions and following acute infusion of SKA-31 (0.1 – 5.0 mg/ml/kg) and SNP (5.0 μg/ml/kg) (panel A). Panel B quantifies the drug-evoked changes in mPAO relative to the preceding control value for each experimental condition. N = 7 animals for both panels A and B.
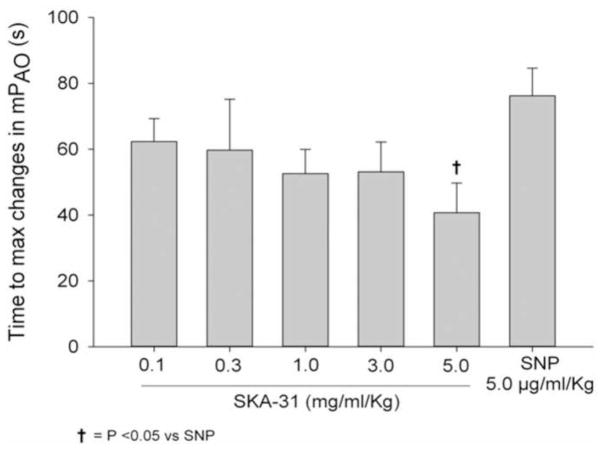
As quantified in Figure 3, the time to peak response for the SKA-31 induced decrease in mPAO was slowest at 0.1 mg/ml/kg drug administration and became faster with increasing dosages. At a dosage of 5.0 mg/ml/kg, SKA-31 infusion resulted in a significantly faster decline in mPAO compared with SNP.
Figure 3.
Quantification of the time to maximal change in mean aortic pressure (mPAO) following intravenous infusion of either SKA-31 (0.1 – 5.0 mg/ml/kg) or SNP (5.0 μg/ml/kg). Administration of either saline or drug vehicle did not evoke measurable changes in mPAO. The response evoked by 5.0 mg/ml/kg SKA-31 was significantly faster than that elicited by SNP, as determined by two-way ANOVA; P < 0.05, n = 7 animals.
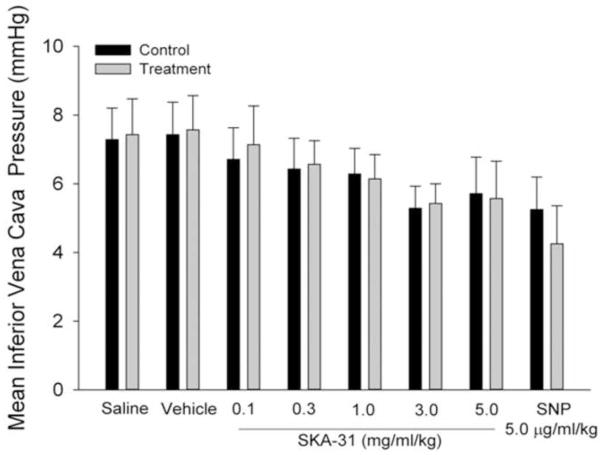
In contrast to the observed decreases in mPAO, SKA-31 did not significantly alter mean inferior vena cava pressure (mPIVC), compared with preceding control values (Figure 4). In the case of SNP, we did observe a trend toward lower mPIVC, although this change did not reach statistical significance.
Figure 4.
Lack of effect of SKA-31 (0.1 – 5.0 mg/ml/kg) on mean inferior vena cava pressure (mPIVC) following acute administration. Histogram displays mPIVC values recorded in response to infusions of saline, drug vehicle and the indicated dosages of SKA-31 and SNP. Values for baseline mPIVC (control) immediately preceding each infusion are designated by the black bars.
3.2 Conductance and Resistance
Figure 5 shows the effect of SKA-31 and SNP administration on absolute changes in systemic vascular resistance (SVR) (Fig. 5A), along with the calculated percent changes in systemic conductance (Fig. 5B). We observed no changes in SVR versus the preceding control values following infusions of saline, drug vehicle or lower dosages of SKA-31 (0.1 and 0.3 mg/ml/kg), whereas dosages of 1.0, 3.0 and 5.0 mg/ml/kg each significantly decreased systemic resistance. SKA-31 at the highest dosage decreased SVR to a level comparable to that evoked by 5.0 μg/ml/kg SNP. Predictably, the inverse relationships were observed for drug-induced changes in systemic conductance (Fig. 5B).
Figure 5.
Acute administration of SKA-31 and sodium nitroprusside (SNP) reduce systemic vascular resistance (SVR). Panel A displays absolute values for SVR recorded prior to a given drug infusion and following SKA-31 and SNP infusions at the indicated dosages. For the latter data, measurements were taken during the peak change in SVR. Panel B displays the calculated percentage change in systemic vascular resistance under each infusion condition compared with the preceding control.
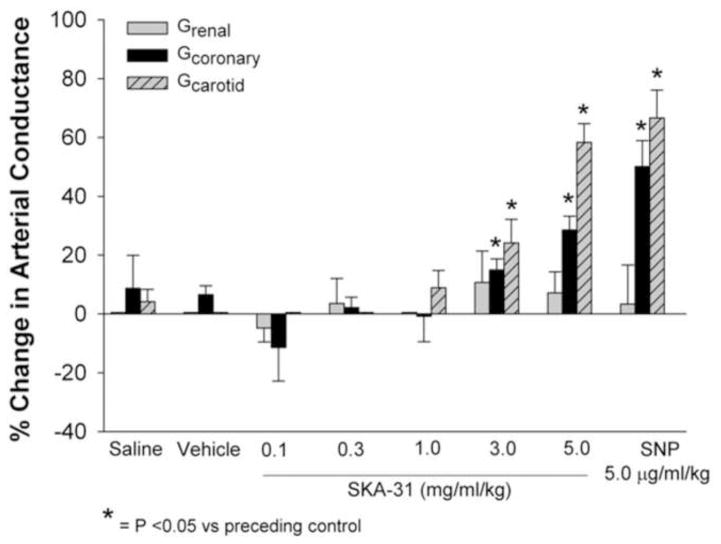
In addition to its impact on systemic conductance, we also examined the effect of SKA-31 on blood flow in select vascular regions. As shown in Figures 1C and 6, SKA-31 and SNP infusions produced qualitatively similar effects on conductance in the right carotid artery (Gcarotid), left anterior descending coronary artery (Gcoronary), and left renal artery (Grenal). SKA-31 increased Gcarotid at dosages of 3.0 and 5.0 mg/ml/kg and produced a maximal change in conductance similar to that observed with 5.0 μg/ml/kg SNP. In the left anterior descending coronary artery, SKA-31 also significantly increased Gcoronary at doses of 3.0 and 5.0 mg/ml; the increase evoked by the latter dose approximated that observed with SNP. Interestingly, neither SKA-31 nor SNP significantly increased blood flow in the renal artery (compared with the preceding control conductance values) and vasodilatory responses in this artery were generally blunted compared with carotid and coronary vessels (Fig. 1C).
Figure 6.
Quantification of evoked changes in arterial conductance calculated for the carotid, coronary and renal arteries in response to infusions of saline, drug vehicle, SKA-31 (0.1 – 5.0 mg/ml/kg) and SNP (5.0 μg/ml/kg). Histogram displays the percentage change in conductance in each artery evoked by administered drugs relative to the preceding control value for each infusion. Asterisks indicate a statistically significant difference compared with the baseline conductance value preceding a given infusion.
3.3 Cardiac Function
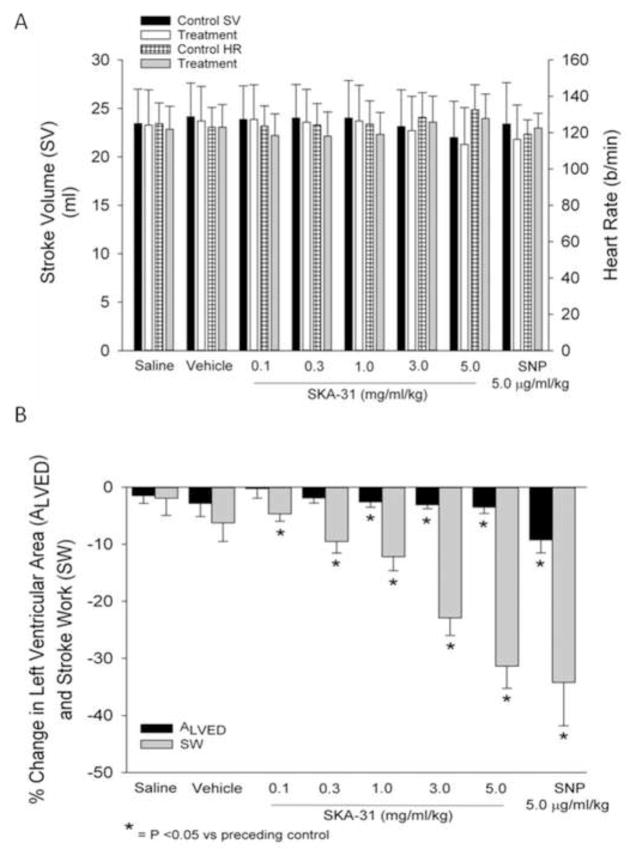
As depicted in Figure 7A, infusions of SKA-31 and SNP did not produce significant changes in either cardiac stroke volume (SV) or heart rate (HR) under our experimental conditions. We further examined potential drug-induced changes in the left ventricular end diastolic area (ALVED), as measured by sonomicrometry crystals implanted in the septal wall and LV endocardium, and the calculated LV stroke work (SWLV); both of these parameters are expressed as the percent change from the respective preceding control value. Over the dosage range of 1.0 to 5.0 mg/ml/kg, SKA-31 produced very modest decreases in ALVED (< 5% below control), whereas SNP reduced ALVED by an average of 8% compared with control. In contrast to the slight decreases observed in ALVED, SKA-31 evoked a clear, dose-dependent reduction in SWLV over the range of 0.1 to 5.0 mg/ml/kg and produced a similar maximal decrease at a dosage of 5.0 mg/ml/kg (−29%) as that observed following SNP infusion (−32%).
Figure 7.
Quantification of the effects of acute administration of saline, drug vehicle, SKA-31 (0.1 – 5.0 mg/ml/kg) or SNP (5.0 μg/ml/kg) on left ventricular stroke volume and heart rate (panel A). No significant changes were noted for either stroke volume or heart rate in response to a given infusion compared with the values measured during the preceding control period. The histogram in panel B displays the percentage changes in left ventricular area (ALVED) and stroke work (SW), relative to the baseline values measured during the control period preceding each indicated infusion.
To evaluate in greater depth the observed decreases in SWLV following SKA-31 and SNP administrations, we plotted the calculated percent changes in SWLV versus the observed percent changes in mean aortic pressure (mPAO). The scatter plot in Figure 8 shows the relation between SWLV and mPAO, based on the pooled data derived from all infusions of saline, vehicle, SKA-31 and SNP for the 7 animals employed in our study. Importantly, the calculated r2 value of 0.82 for the linear regression line indicates that more than 80% of the variance in SWLV can be explained by the variance in mPAO. Using a similar approach, we also plotted the percent changes in ALVED versus mPAO for all animals and infusions (i.e. saline, vehicle, drug) examined. Linear regression analysis of this relation yielded a r2 value of only 0.24, indicating that no more than 25% of the variance in ALVED can be explained by the variance in mPAO (P<0.001; data not shown). Based on these results and the fact that drug infusions did not change stroke volume under any condition (see Figure 7A), we conclude that neither SKA-31 nor SNP directly impaired cardiac contractility.
3.4 Plasma Concentrations of SKA-31 Following Acute Infusion
In a separate group of 3 anesthetized and instrumented animals, we analyzed the plasma concentrations of SKA-31 at select time points following acute intravenous infusion of a 3.0 mg/ml/kg SKA-31 bolus dose. Blood samples were withdrawn from the left jugular vein at ~1 min, 35 min and 75 min following complete infusion of the drug. The average free plasma concentration of SKA-31 measured at each of the above time points (n = 3) was 77.5 ± 27.7 μM, 27.3 ± 3.8 μM and 27.4 ± 4.9 μM, respectively. The volume of distribution for SKA-31 calculated from a semi-logarithmic plot of average SKA-31 plasma concentrations versus sampling times was 0.19 L/kg.
4. Discussion
Using an anesthetized and instrumented porcine model, we have provided the first detailed description of the systemic hemodynamic actions of SKA-31, a small molecule activator of KCa 2.x and 3.1 channels [2], on key cardiovascular parameters in a large animal and how these actions compare with those of SNP, an established nitrovasodilator and blood pressure-lowering agent. As shown in Figures 1 and 2, intravenous administration of SKA-31 dose-dependently evoked significant decreases in mean aortic pressure, with the highest dose utilized in our study (5.0 mg/ml/kg SKA-31) producing a similar decrease in mPAO as that observed with SNP (−23±3 and −28±4 mmHg, respectively) (Fig. 2B). In previous studies [2,5,8], acute in vivo administration of SKA-31 was shown to lower blood pressure in both normotensive and hypertensive mice and, more recently, Köhler and colleagues [4] have reported that acute infusion of SKA-31 (0.4 and 2.0 mg/kg) transiently decreases systemic blood pressure in conscious dogs. We also noted that the decrease in mPAO evoked by 5.0 mg/ml/kg SKA-31 was more rapid compared with SNP (Fig. 3), even though both agents lowered mean aortic pressure to a similar extent (Fig. 2). The slower time course of the SNP-mediated drop in mPAO may reflect the fact that SNP requires vascular conversion/decomposition to release nitric oxide and induce subsequent cellular actions in arterial smooth muscle [17], while SKA-31 directly hyperpolarizes the endothelium by activating KCa channels. Collectively, these observations are in agreement with the reported vasodilatory actions of SKA-31 in the intact coronary [7] and skeletal muscle circulations [5,8] of rodents and the systemic circulation of the dog [4].
SKA-31 had no significant effect on mean inferior vena cava pressure (mPIVC) (Fig. 4). In the case of SNP, we did observe a trend towards lower mPIVC, which would be in agreement with the known clinical effects of SNP to lower central venous pressure, due to its ability to increase venous capacitance [18]. One possible reason for our observation is that mPIVC was already quite low under basal experimental conditions (~8 mmHg) and a further drug-induced decrease in mPIVC may have been difficult to detect in our anesthetized pigs. Although KCa2.3 and KCa3.1 channel mRNA and whole cell K+ currents have been reported in venous endothelial cells (e.g. HUVECs) [1,3,19], we are unaware of data describing a direct vasodilatory effect of KCa channel activators on veins or the venous circulation.
SKA-31 dosages of 1.0 to 5.0 mg/ml/kg increased systemic arterial conductance, with the highest dose producing an increase in conductance similar to that induced by SNP (Figure 5B). We also observed increases in both carotid and coronary arterial conductances at 3.0 and 5.0 mg/ml/kg SKA-31 (Figure 6), which were similar to those observed with SNP at the highest dosage of SKA-31. Interestingly, renal conductance appeared to be unaffected by either SKA-31 or SNP. In the case of SNP, this is somewhat unexpected, as other investigators have reported that renal arteries are sensitive to nitrovasodilators [20,21]. The renal microcirculation is known to exhibit strong autoregulatory behavior [22,23], which is critical for ensuring adequate blood flow to glomerular units and protecting them from arterial pressure-induced damage. One possible explanation for this apparent insensitivity of the renal conductance to SKA-31 and SNP is that the renal circulation may have already been near-maximally dilated, due to a combination of intrinsic autoregulation and the somewhat lower mPAO present in our anesthetized pigs. Alternatively, it is possible that reduced arterial resistance triggered an increase in peripheral sympathetic tone to counteract reduced blood pressure, which then limited renal arterial dilation. However, this possibility is less likely, as we observed no concomitant increase in heart rate with declines in mPAO, which one would anticipate with the activation of a baroreceptor feedback mechanism acting on the heart.
4.1 Cardiac Function
As small-conductance Ca2+-activated K+ channels have been reported in the atria and pacemaker/conducting cells of murine and human cardiac tissue [24–27] and thus may be activated in response to systemic SKA-31 administration, we recorded various indices of myocardial performance during SKA-31 infusions. Importantly, we observed no significant change in left ventricular stroke volume following administration of either SKA-31 or SNP. In contrast to Köhler and coworkers [4], who reported a pronounced increase in heart rate (HR) following acute SKA-31 infusion, we did not detect a significant change in HR in response to SKA-31 or SNP infusions in the anesthetized pig (Fig. 7). The difference in HR responses in these two studies could be attributed to the difference in experimental models, as Köhler and colleagues examined conscious dogs (presumably with unsuppressed baroreceptor reflexes providing autonomic nerve input to the heart) versus our anesthetized, instrumented pig model. Importantly, the absence of SKA-31 induced changes in HR observed in our study strongly suggests that plasma levels of SKA-31 sufficient to evoke substantial decreases in blood pressure do not directly impact either pacemaker function or action potential propagation in the heart, as revealed under conditions of minimal baroreceptor reflex activity.
Neither SKA-31 nor SNP significantly reduced central venous pressure (Fig. 4). One possible explanation is that the relative magnitude of the arterial and venous effects of these vasodilatory agents may differ [18,28–30] or an increase in total venous capacitance may have been limited by a modest elevation in arterial capacitance as a result of evoked vasodilation. Hemodynamically, a minor, undetected rise in total venous capacitance could explain the slight decrease we observed in ALVED, our measure of left ventricular end-diastolic volume, in response to SKA-31 and more so to SNP (Fig. 7B). Since left ventricular stroke work (SW) is a function of both ventricular volume and pressure, the decreases observed in SW following administration of SKA-31 and SNP could be explained, in part, by the minor reduction in ALVED. However, further analysis of these data clearly showed that changes in ALVED accounted for less than 25% of the variance in SW, whereas changes in mPAO accounted for more than 80%. Thus, the observed decreases in SW could be largely attributed to the reductions in mPAO associated with drug administration (Figure 8). Furthermore, the observed reductions in mPAO, indicative of left ventricular afterload, would be expected to offset the slight decreases in ALVED observed with SKA-31 and SNP administrations, and the balance of these two effects would tend to maintain stroke volume near control levels in the presence of either SKA-31 or SNP (Fig. 7A).
Our attempt to explore the pharmacokinetic behavior of SKA-31 revealed that its plasma levels measured 35–75 min following intravenous infusion of a 3.0 mg/ml/kg dose were higher than the reported EC50 values of SKA-31 for KCa3.1 channels (~0.3 μM) and KCa2.3 channels (~2 μM) [2], suggesting that sustained activation of these endothelial channels might be anticipated. However, the absence of prolonged hypotension following administration of SKA-31 in our anesthetized pigs suggests that the relationship between the free plasma concentration of SKA-31 (plasma protein binding of SKA-31 in mice and dogs is reported to be 35–40%) [2,4] and its vasodilatory actions may not be a direct one and may be complicated by the availability of additional drug binding sites or a more complex whole body distribution pattern.
Another explanation for the relatively short-lived hemodynamic response following SKA-31 infusion could be a “desensitization” of the pharmacological targets for SKA-31 actions. Both KCa3.1 and KCa2.3 channels are subject to regulation by intracellular second messengers or protein kinases and phosphatases. For example, phosphorylation of channel-associated calmodulin by casein kinase 2 reduces the affinity of KCa2 channels for the membrane phospholipid PIP2 [31] and likely contributes to the inhibition of KCa2 channel activity by Gq-associated G-protein coupled receptors. KCa3.1 activity is increased by phosphorylation of His358 in the channel’s C-terminus through the histidine kinase nucleoside diphosphate kinase B (NDPK-B) [32], while the PI3P phosphatase myotubalarin related protein 6 (MTMR6) and the histidine phosphatase phosphohistidine phosphatase-1 (PHPT-1) inhibit KCa3.1 function in T-cells [33,34]. Additionally, KCa3.1 currents can be regulated by cAMP-dependent protein kinase (PKA) via Ser phosphorylation sites in the C-terminus [35–37], which may impair endothelium-dependent vasodilation [38].
Finally, the ability of a KCa channel activator to lower blood pressure more effectively in hypertensive versus normotensive mice [2,5] suggests that this class of compound may be beneficial in the acute or chronic treatment of elevated blood pressure. Our results showing that SKA-31 reduces blood pressure and increases systemic conductance in the pig suggest that translational studies examining the potential blood pressure-lowering actions of a KCa channel activator in a large animal model of hypertension or vascular disease are likely feasible.
4.2 Summary and Conclusions
The results of our study demonstrate that the KCa channel activator SKA-31 effectively and reversibly increases systemic conductance in a dose-dependent manner and lowers mean aortic pressure in a large animal model. The observed hemodynamic actions of SKA-31 closely mimic those evoked by SNP. SKA-31 did not directly affect cardiac contractility, nor did it appear to impact heart rate or excitability. The common and overlapping cardiovascular responses to SKA-31 and SNP are consistent with the conclusion that SKA-31 acts primarily on blood vessels to evoke its effects on the systemic vasculature. Given that SKA-31 is strictly an endothelium-dependent vasodilator [6], endothelial KCa channel activators may be useful as an alternative pharmacologic strategy to evoke acute arterial vasodilation in situations where the hemodynamic actions of SNP may not be desirable or effective (e.g. nitrate tolerance).
Acknowledgments
The authors would like to acknowledge the excellent surgical expertise of Ms. Cheryl Meek throughout this study. This work was supported by research funding to A.P. Braun (Canadian Institutes of Health Research MOP 97901), to H. Wulff (National Institutes of Health NS072585) and to J.V. Tyberg (Kidney Foundation of Canada/Pfizer Canada).
Abbreviations
- G
conductance
- HR
heart rate
- IVC
inferior vena cava
- KCa channel
calcium-activated K+ channel
- mPAO
mean aortic pressure
- mPIVC
mean inferior vena caval pressure
- PBS
phosphate-buffered saline
- PLVED
left ventricular end-diastolic pressure
- SKA-31
naphthol[1,2-d]thiazol-2-ylamine
- SNP
sodium nitroprusside
- SV
stroke volume
- SVR
systemic vascular resistance
- SW
stroke work
- VolD
volume of distribution
Footnotes
Conflict of Interest: On behalf of the all authors, the corresponding author states that no conflicts of interest exist.
References
- 1.Sheng J-Z, Ella S, Davis MJ, Hill MA, Braun AP. Openers of SKCa and IKCa channels enhance agonist-evoked endothelial nitric oxide synthesis and arteriolar dilation. FASEB J. 2009;23:1138–1145. doi: 10.1096/fj.08-120451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sankaranarayanan A, Raman G, Busch C, Schultz T, Zimin PI, Hoyer J, et al. Naphthol[1,2-d]thiazol-2-ylamine (SKA-31), a new activator of KCa2 and KCa3. 1 potassium channels, potentiates the endothelium-derived hyperpolarizing factor response and lowers blood pressure. Mol Pharmacol. 2009;75:281–295. doi: 10.1124/mol.108.051425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stankevicius E, Dalsgaard T, Kroigaard C, Beck L, Boedtkjer E, Misfeldt M, et al. Opening of small and intermediate calcium-activated potassium channels induces relaxation mainly mediated by nitric-oxide release in large arteries and endothelium-derived hyperpolarizing factor in small arteries from rat. J Pharmacol Exp Ther. 2011;339:842–850. doi: 10.1124/jpet.111.179242. [DOI] [PubMed] [Google Scholar]
- 4.Damkjaer M, Nielsen G, Bodendiek S, Staehr M, Gramsbergen JB, de Wit C, et al. Pharmacological activation of KCa3.1/KCa2. 3 channels produces endothelial hyperpolarization and lowers blood pressure in conscious dogs. Br J Pharmacol. 2012;165:223–234. doi: 10.1111/j.1476-5381.2011.01546.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Radtke J, Schmidt K, Wulff H, de Wit C. Activation of KCa3. 1 by SKA-31 induces arteriolar dilatation and lowers blood pressure in normo- and hypertensive connexin40-deficient mice. Br J Pharmacol. 2013;170:293–303. doi: 10.1111/bph.12267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mishra RC, Wulff H, Hill MA, Braun AP. Inhibition of myogenic tone in rat cremaster and cerebral arteries by SKA-31, an activator of endothelial KCa2.3 and KCa3.1 channels. J Cardiovasc Pharmacol. 2015 doi: 10.1097/FJC.0000000000000252. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mishra RC, Belke D, Wulff H, Braun AP. SKA-31, a novel activator of SKCa and IKCa channels, increases coronary flow in male and female rat hearts. Cardiovasc Res. 2013;97:339–348. doi: 10.1093/cvr/cvs326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brähler S, Kaistha A, Schmidt VJ, Wölfle SE, Busch C, Kaistha BP, et al. Genetic deficit of SK3 and IK1 channels disrupts the endothelium-derived hyperpolarizing factor vasodilator pathway and causes hypertension. Circulation. 2009;119:2323–2332. doi: 10.1161/CIRCULATIONAHA.108.846634. [DOI] [PubMed] [Google Scholar]
- 9.Mishra RC, Wulff H, Cole WC, Braun AP. A pharmacologic activator of endothelial KCa channels enhances coronary flow in the hearts of type 2 diabetic rats. J Mol Cell Cardiol. 2014;72:364–373. doi: 10.1016/j.yjmcc.2014.04.013. [DOI] [PubMed] [Google Scholar]
- 10.Kirk RW. Current Veterinary Therapy IX: Small Animal Practice. W.B. Saunders; 1986. Short-term ventilatory support. [Google Scholar]
- 11.Moore TD, Frenneaux MP, Sas R, Atherton JJ, Morris-Thurgood JA, Smith ER, et al. Ventricular interaction and external constraint account for decreased stroke work during volume loading in CHF. Am J Physiol Heart Circ Physiol. 2001;281:H2385–H2391. doi: 10.1152/ajpheart.2001.281.6.H2385. [DOI] [PubMed] [Google Scholar]
- 12.Feneley MP, Elbeery JR, Gaynor JW, Gall SA, Davis JW, Rankin JS. Ellipsoidal shell subtraction model of right ventricular volume. Comparison with regional free wall dimensions as indexes of right ventricular function. Circ Res. 1990;67:1427–1436. doi: 10.1161/01.RES.67.6.1427. [DOI] [PubMed] [Google Scholar]
- 13.Belenkie I, Dani R, Smith ER, Tyberg JV. Effects of volume loading during experimental acute pulmonary embolism. Circulation. 1989;80:178–188. doi: 10.1161/01.CIR.80.1.178. [DOI] [PubMed] [Google Scholar]
- 14.Scott-Douglas NW, Traboulsi M, Smith ER, Tyberg JV. Experimental instrumentation and left ventricular pressure-strain relationship. Am J Physiol Heart Circ Physiol. 1991;261:H1693–H1697. doi: 10.1152/ajpheart.1991.261.6.H1693. [DOI] [PubMed] [Google Scholar]
- 15.Appleyard RF, Glantz SA. Two dimensions describe left ventricular volume change during hemodynamic transients. Am J Physiol Heart Circ Physiol. 1990;258:H277–H284. doi: 10.1152/ajpheart.1990.258.1.H277. [DOI] [PubMed] [Google Scholar]
- 16.Suga H, Sagawa K. Assessment of absolute volume from diameter of the intact canine left ventricular cavity. J Appl Physiol. 1974;36:496–499. doi: 10.1152/jappl.1974.36.4.496. [DOI] [PubMed] [Google Scholar]
- 17.Feelisch M. The use of nitric oxide donors in pharmacological studies. Naunyn-Schmiedeberg's Arch Pharmacol. 1998;358:113–122. doi: 10.1007/PL00005231. [DOI] [PubMed] [Google Scholar]
- 18.Wang SY, Scott-Douglas NW, Manyari DE, Tyberg JV. Arterial versus venous changes in vascular capacitance during nitroprusside infusion: A vascular modelling study. Can J Physiol Pharmacol. 1999;77:131–137. doi: 10.1139/y99-013. [DOI] [PubMed] [Google Scholar]
- 19.Sheng J-Z, Braun AP. Small- and intermediate-conductance Ca2+-activated K+ channels directly control agonist-evoked nitric oxide synthesis in human vascular endothelial cells. Am J Physiol Cell Physiol. 2007;293:C458–C467. doi: 10.1152/ajpcell.00036.2007. [DOI] [PubMed] [Google Scholar]
- 20.Hoffend J, Cavarape A, Endlich K, Steinhausen M. Influence of endothelium-derived relaxing factor on renal microvessels and pressure-dependent vasodilation. Am J Physiol Renal,Fluid Electrolyte Physiol. 1993;265:F285–F292. doi: 10.1152/ajprenal.1993.265.2.F285. [DOI] [PubMed] [Google Scholar]
- 21.Gustafsson F, Holstein-Rathlou NH. Conducted vasomotor responses in arterioles: Characteristics, mechanisms and physiological significance. Acta Physiol Scand. 1999;167:11–21. doi: 10.1046/j.1365-201x.1999.00582.x. [DOI] [PubMed] [Google Scholar]
- 22.Loutzenhiser R, Bidani AK, Wang X. Systolic pressure and the myogenic response of the renal afferent arteriole. Acta Physiol Scand. 2004;181:407–413. doi: 10.1111/j.1365-201X.2004.01312.x. [DOI] [PubMed] [Google Scholar]
- 23.Cupples WA. Interactions contributing to kidney blood flow auatoregulation. Current Opinion in Nephrology and Hypertension. 2007;16:39–45. doi: 10.1097/MNH.0b013e3280117fc7. [DOI] [PubMed] [Google Scholar]
- 24.Tuteja D, Xu D, Timofeyev V, Lu L, Sharma D, Zhang Z, et al. Differential expression of small-conductance Ca2+-activated K+ channels SK1, SK2 and SK3 in mouse atrial and ventricular myocytes. Am J Physiol Heart Circ Physiol. 2005;289:H2714–H2723. doi: 10.1152/ajpheart.00534.2005. [DOI] [PubMed] [Google Scholar]
- 25.Zhang Q, Timofeyev V, Lu L, Li N, Singapuri A, Long MK, et al. Functional roles of a Ca2+-activated K+ channel in atrioventricular nodes. Circ Res. 2008;102:465–471. doi: 10.1161/CIRCRESAHA.107.161778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu Y, Tuteja D, Zhang Z, Xu D, Zhang Y, Rodriguez J, et al. Molecular identification and functional roles of a Ca2+-activated K+ channel in human and mouse hearts. J Biol Chem. 2003;278:49085–49094. doi: 10.1074/jbc.M307508200. [DOI] [PubMed] [Google Scholar]
- 27.Chang PC, Turker I, Lopshire JC, Masroor S, Nguyen BL, Tao W, et al. Heterogeneous upregulation of apamin-sensitive potassium currents in failing human ventricles. Journal of the American Heart Association. 2012;1:e004713. doi: 10.1161/JAHA.112.004713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chihara E, Manyari DE, Isaac DL, Tyberg JV. Comparative effects of nitroglycerin on intestinal vascular capacitance and conductance. Canandian Journal of Cardiology. 2002;18:165–174. [PubMed] [Google Scholar]
- 29.Semeniuk LM, Belenkie I, Tyberg JV. Acute effects of toborinone on vascular capacitance and conductance in experimental heart failure. Circulation. 1998;98:58–63. doi: 10.1161/01.CIR.98.1.58. [DOI] [PubMed] [Google Scholar]
- 30.Isaac DL, Belenkie I, Tyberg JV. Vascular and cardiac effects of amlodipine in acute heart failure in dogs. Canandian Journal of Cardiology. 1998;14:1375–1382. [PubMed] [Google Scholar]
- 31.Zhang M, Meng XY, Cui M, Pascal JM, Logothetis DE, Zhang JF. Selective phosphorylation modulates the PIP2 sensitivity of the CaM-SK channel complex. Nature Chemical Biology. 2014;10:753–759. doi: 10.1038/nchembio.1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Srivastava S, Li Z, Ko K, Choudhury P, Albaqumi M, Johnson AK, et al. Histidine phosphorylation of the potassium channel KCa3.1 by nucleoside diphosphoate kinase B is required for activation of KCa3. 1 and CD4 T cells. Molecular Cell. 2006;24:665–675. doi: 10.1016/j.molcel.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 33.Srivastava S, Li Z, Lin L, Liu G, Ko K, Coetzee WA, et al. The phosphatidylinositol 3-phosphate phosphatase myotubularin-related protein 6 (MTMR6) is a negative regulator of the Ca2+-activated K+ channel KCa3. 1 Molec. Cell Biol. 2005;25:3630–3638. doi: 10.1128/MCB.25.9.3630-3638.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Srivastava S, Zhdanova O, Di L, Li Z, Albaqumi M, Wulff H, et al. Protein histidine phosphatase 1 negatively regulates CD4 T cells by inhibiting the K+ channel KCa3. 1. Proc Natl Acad Sci USA. 2008;105:14442–14446. doi: 10.1073/pnas.0803678105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gerlach AC, Gangopadhyay NN, Devor DC. Kinase-dependent regulation of the intermediate condutance, calcium-dependent potassium channel, hIK1. J Biol Chem. 2000;275:585–598. doi: 10.1074/jbc.275.1.585. [DOI] [PubMed] [Google Scholar]
- 36.Neylon CB, D'Souza T, Reinhart PH. Protein kinase A inhibits intermediate conductance Ca2+-activated K+ channels expressed in Xenopus oocytes. Pflügers Arch. 448:613–620. doi: 10.1007/s00424-004-1302-5. [DOI] [PubMed] [Google Scholar]
- 37.Wong R, Schlichter LC. PKA reduces the rat and human KCa3. 1 current, CaM binding and Ca2+ signaling, which requires Ser332/334 in the CaM-binding C terminus. J Neurosci. 2014;34:13371–13383. doi: 10.1523/JNEUROSCI.1008-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yarova PL, Smirnov SV, Dora KA, Garland CJ. β1-Adrenoceptor stimulation suppresses endothelial IKCa-channel hyperpolarization and associated dilatation in resistance arteries. Br J Pharmacol. 2013;169:875–886. doi: 10.1111/bph.12160. [DOI] [PMC free article] [PubMed] [Google Scholar]