Abstract
Objective
To estimate the lifetime and 12-month prevalence of occupational exposure to body fluids among health-care workers in Africa.
Methods
Embase®, PubMed® and CINAHL databases were systematically searched for studies published between January 2000 and August 2017 that reported the prevalence of occupational exposure to blood or other body fluids among health-care workers in Africa. The continent-wide prevalence of exposure was estimated using random-effects meta-analysis.
Findings
Of the 904 articles identified, 65 studies from 21 African countries were included. The estimated pooled lifetime and 12-month prevalence of occupational exposure to body fluids were 65.7% (95% confidence interval, CI: 59.7–71.6) and 48.0% (95% CI: 40.7–55.3), respectively. Exposure was largely due to percutaneous injury, which had an estimated 12-month prevalence of 36.0% (95% CI: 31.2–40.8). The pooled 12-month prevalence of occupational exposure among medical doctors (excluding surgeons), nurses (including midwives and nursing assistants) and laboratory staff (including laboratory technicians) was 46.6% (95% CI: 33.5–59.7), 44.6% (95% CI: 34.1–55.0) and 34.3% (95% CI: 21.8–46.7), respectively. The risk of exposure was higher among health-care workers with no training on infection prevention and those who worked more than 40 hours per week.
Conclusion
The evidence available suggests that almost one half of health-care workers in Africa were occupationally exposed to body fluids annually. However, a lack of data from some countries was a major limitation. National governments and health-care institutions across Africa should prioritize efforts to minimize occupational exposure among health-care workers.
Résumé
Objectif
Estimer la prévalence au cours de la vie et sur 12 mois de l'exposition professionnelle aux liquides organiques des agents de santé en Afrique.
Méthodes
Nous avons systématiquement recherché dans les bases de données Embase®, PubMed® et CINAHL des études publiées entre janvier 2000 et août 2017 documentant la prévalence de l'exposition professionnelle au sang ou à d'autres liquides organiques des agents de santé en Afrique. La prévalence de l'exposition dans l'ensemble du continent a été estimée à l'aide d'une méta-analyse à effets aléatoires.
Résultats
Sur les 904 articles repérés, 65 études menées dans 21 pays africains ont été sélectionnées. La prévalence combinée au cours de la vie et sur 12 mois de l'exposition professionnelle aux liquides organiques était estimée à 65,7% (intervalle de confiance, IC à 95%: 59,7–71,6) dans le premier cas et 48,0% (IC à 95%: 40,7–55,3) dans le second. L'exposition était en grande partie due à des lésions percutanées, la prévalence sur 12 mois étant estimée à 36,0% (IC à 95%: 31,2-40,8). La prévalence combinée sur 12 mois de l'exposition professionnelle était de 46,6% (IC à 95%: 33,5–59,7) chez les médecins (à l'exception des chirurgiens), de 44,6% (IC à 95%: 34,1–55,0) chez les infirmiers (sages-femmes et infirmiers auxiliaires compris) et de 34,3% (IC à 95%: 21,8–46,7) chez le personnel de laboratoire (techniciens de laboratoire compris). Le risque d'exposition était plus élevé chez les agents de santé n'ayant pas été formés à la prévention des infections et chez ceux qui travaillaient plus de 40 heures par semaine.
Conclusion
D'après les données disponibles, près de la moitié des agents de santé en Afrique sont exposés chaque année aux liquides organiques dans le cadre de leur travail. Le manque de données dans certains pays a néanmoins constitué une limite majeure. Les gouvernements nationaux et les établissements de santé de l'ensemble du continent doivent donner un degré de priorité élevé aux efforts visant à minimiser l'exposition professionnelle des agents de santé.
Resumen
Objetivo
Hacer una estimación del tiempo de vida y la prevalencia de 12 meses de exposición profesional a fluidos corporales entre los profesionales sanitarios en África.
Métodos
Se realizó una búsqueda sistemática en las bases de datos Embase®, PubMed® y CINAHL de estudios publicados entre enero de 2000 y agosto de 2017 que mostraran la prevalencia de la exposición profesional a sangre u otros fluidos corporales entre los profesionales sanitarios en África. La prevalencia de la exposición en todo el continente se estimó utilizando un meta-análisis de efectos aleatorios.
Resultados
De los 904 artículos identificados se incluyeron 65 estudios de 21 países africanos. El tiempo de vida estimado en conjunto y la prevalencia de 12 meses de exposición profesional a fluidos corporales fue de un 65,7% (intervalo de confianza, IC, 95%: 59,7–71,6) y 48,0% (95% IC: 40,7–55,3), respectivamente. La exposición se debía en su mayor parte a heridas percutáneas, con una prevalencia estimada durante 12 meses del 36,0% (95% IC: 31,2–40,8). La prevalencia durante 12 meses de exposición profesional en conjunto entre los médicos (excepto los cirujanos), enfermeras (incluidas las matronas y las auxiliares de enfermería) y el personal de laboratorio (incluidos los técnicos de laboratorio) fue del 46,6% (95% IC: 33,5–59,7), 44,6% (95% IC: 34,1–55,0) y del 34,3% (95% IC: 21,8-46,7), respectivamente. El riesgo de exposición fue más alto entre los profesionales sanitarios sin formación en el ámbito de la prevención de infecciones y entre aquellos que trabajaban más de 40 horas a la semana.
Conclusión
Las pruebas disponibles sugieren que casi la mitad de todos los trabajadores sanitarios en África están expuestos profesionalmente a fluidos corporales cada año. Sin embargo, la falta de datos de algunos países supuso una gran limitación. Por lo tanto, los gobiernos nacionales y las instituciones sanitarias africanas deberían priorizar los esfuerzos para disminuir la exposición entre los profesionales sanitarios.
ملخص
الغرض
تقييم انتشار التعرض لملامسة سوائل الجسم بين العاملين في مجال الرعاية الصحية في أفريقيا في سياق الممارسات المهنية، وذلك على مدار 12 شهرًا وطوال الحياة.
الطريقة
جرى البحث المنهجي في قواعد البيانات Embase® وPubMed® وCINAHL للوصول إلى الدراسات التي سبق نشرها في الفترة بين يناير/كانون الثاني 2000 وأغسطس/آب 2017 والتي تسجل انتشار التعرض لملامسة الدم أو غيره من سوائل الجسم بين العاملين في مجال الرعاية الصحية في أفريقيا في سياق الممارسات المهنية. وتم تقييم انتشار تلك الحالات على نطاق القارة بأكملها باستخدام تحليل تلوي للآثار العشوائية.
النتائج
قمنا بتضمين 65 دراسة من 21 دولة أفريقية من بين 904 مقالات تم تحديدها. بلغت نسبة الحالات المجمعة لانتشار التعرض لسوائل الجسم في سياق الممارسات المهنية على مدى 12 شهرًا أو مدى الحياة وفقًا للتقديرات 65.7% (بنطاق ثقة تبلغ نسبته: 59.7 – 71.6) و48.0% (بنطاق ثقة تبلغ نسبته 95%: 40.7 - 55.3)، على التوالي. وكان التعرض لملامسة تلك السوائل يرجع في الأغلب إلى الإصابات عن طريق الجلد، والتي بلغت نسبة انتشارها على مدى 12 شهرًا 36.0% (بنطاق ثقة تبلغ نسبته 95%: 31.2–40.8). وبلغت النسبة المجمعة لحالات انتشار التعرض لملامسة تلك السوائل في سياق الممارسات المهنية على مدى 12 شهرًا لدى الأطباء المعالجين (باستثناء الجراحين)، والممرضين (بما يشمل القابلات ومساعدي الممرضين) وفريق العمل بالمعامل (بما يشمل فنيي المعامل) 46.6% (بنطاق ثقة تبلغ نسبته 95%: 33.5 – 59.7)، و44.6% (بنطاق ثقة تبلغ نسبته 95%: 34.1 – 55.0) و34.3% (بنطاق ثقة تبلغ نسبته 95%: 21.8 – 46.7)، على التوالي. وارتفع خطر التعرض لملامسة تلك السوائل بين العاملين في مجال الرعاية الصحية الذين لم يتلقوا تدريبًا على الوقاية من العدوى ومن كان منهم يعمل لمدة تزيد عن 40 ساعة في الأسبوع.
الاستنتاج
أشارت الأدلة المتاحة إلى أن ما يقرب من نصف العاملين في مجال الرعاية الصحية في أفريقيا يتعرضون سنويًا لملامسة سوائل الجسم في سياق الممارسات المهنية. وبالرغم من ذلك، كان النقص في البيانات المتعلقة ببعض البلدان عائقًا رئيسيًا. ويجب على الحكومات الوطنية ومؤسسات الرعاية الصحية المنتشرة في أنحاء أفريقيا أن تضع ضمن أولوياتها الجهود الرامية للتقليل إلى أدنى حد من تعرض العاملين في مجال الرعاية الصحية لتلك الحالات في إطار الممارسات المهنية.
摘要
目的
旨在估算非洲医疗护理工作者在工作过程中接触体液的终生和年患病率。
方法
系统地搜索 Embase®、PubMed® 和 CINAHL 数据库中在 2000 年 1 月至 2017 年 8 月间发表的报告非洲医疗护理工作者在工作过程中接触血液或其他体液的研究。采用随机效应元分析估算非洲大陆范围内由于接触而感染的患病率。
结果
在选定的 904 篇文章中,收录来自 21 个国家的 65 份研究。由于在工作中接触体液而感染的终身和年患病率总估计值分别为 65.7%(95% 置信区间,CI: 59.7–71.6)和 48.0% (95% CI: 40.7–55.3)。接触大部分是由于经皮损伤,年患病率汇总估计值为 36.0% (95% CI: 31.2–40.8)。医生(不包括外科医生)、护士(包括助产士和护理员)和实验室人员(包括实验室技术人员)在工作过程中由于接触而感染的汇总年患病率分别为 46.6% (95% CI: 33.5–59.7)、44.6% (95% CI: 34.1–55.0) 和 34.3% (95% CI: 21.8–46.7)。未经感染预防培训和每周工作时间超过 40 小时的医疗护理工作者接触的风险偏高。
结论
现有的证据表明每年约有近半数的非洲医疗护理工作者会在工作过程中接触体液。然而,缺乏一些国家的数据是主要局限因素。非洲各国政府和医疗护理机构应优先考虑将医疗护理工作者在工作过程中接触体液的风险降到最低。
Резюме
Цель
Оценить распространенность случаев профессионального вредного воздействия биологических жидкостей среди медицинских работников в Африке на протяжении жизни и за 12 месяцев.
Методы
Был проведен систематический поиск в базах данных Embase®, PubMed® и CINAHL на предмет исследований, опубликованных в период с января 2000 года по август 2017 года, в которых сообщалось о распространенности случаев профессионального вредного воздействия крови или других биологических жидкостей среди медицинских работников в Африке. Общая распространенность вредного воздействия на континенте оценивалась с помощью метаанализа с использованием модели случайных эффектов.
Результаты
Из 904 выявленных статей в отчет было включено 65 исследований из 21 африканской страны. Предполагаемая распространенность случаев профессионального вредного воздействия биологических жидкостей в течение обобщенной продолжительности жизни и за 12 месяцев составили 65,7% (95%-ный доверительный интервал, ДИ: 59,7–71,6) и 48,0% (95%-ный ДИ: 40,7–55,3) соответственно. Вредное воздействие было в большинстве случаев обусловлено чрескожной травмой, 12-месячная распространенность которой, по оценкам, составила 36,0% (95%-ный ДИ: 31,2–40,8). Суммарная 12-месячная распространенность случаев профессионального вредного воздействия среди врачей (исключая хирургов), медсестер (включая акушерок и помощников медсестер) и сотрудников лаборатории (включая лаборантов) составила 46,6% (95%-ный ДИ: 33,5–59,7), 44,6% (95%-ный ДИ: 34,1–55,0) и 34,3% (95%-ный ДИ: 21,8–46,7) соответственно. Риск вредного воздействия был выше среди медицинских работников, не прошедших обучение по профилактике инфекции, и среди тех, кто работал более 40 часов в неделю.
Вывод
Имеющиеся данные свидетельствуют о том, что в Африке около половины медицинских работников ежегодно подвергаются вредному воздействию биологических жидкостей. Однако отсутствие данных из некоторых стран являлось основным ограничением. Национальным правительствам и учреждениям здравоохранения в Африке следует уделять первоочередное внимание усилиям по минимизации риска профессионального вредного воздействия среди работников здравоохранения.
Introduction
Worldwide, health-care workers risk occupational exposure to blood-borne pathogens through contact with human body fluids. Although about 60 blood-borne infectious pathogens have been identified, including Epstein–Barr virus, most occupation-related, blood-borne infections are due to hepatitis B virus (HBV), hepatitis C virus (HCV) and human immunodeficiency virus (HIV).1,2 However, other blood-borne pathogens still pose a risk: for example, in the 2013–2016 Ebola virus disease outbreak, over 890 health-care workers were infected, with a case fatality rate of 57%.3 Occupational exposure can occur through percutaneous injury (i.e. a needle or sharp object penetrates the skin), mucous membrane exposure (e.g. of the eyes, nose or mouth) and non-intact skin exposure. Percutaneous injury accounts for 66 to 95% of occupational exposures to blood-borne pathogens.4
Little is known about the global burden of percutaneous injury among health-care workers. However, a 2005 report estimated that worldwide more than 3 million occupation-related percutaneous injuries occur annually.4 Moreover, about 40% of HBV and HCV infections and 2.5% of HIV infections in health-care workers were due to percutaneous injuries.5 Hence, each year, percutaneous injury resulted in around 66 000 HBV infections, 16 000 HCV infections and 1000 HIV infections, which together caused about 1100 deaths as well as substantial disability.4 More than 90% of these infections occurred in developing countries, particularly in Africa, where infection is more prevalent and adherence to standard precautions can be poor.5
Given the severe consequences of blood-borne infections, many high-income countries have established surveillance systems to monitor exposure to body fluids in health-care settings.6 These systems help inform policy-makers for reducing the risk of transmission of blood-borne pathogens. In many African countries, such systems are not available and, consequently, exposure to body fluids is rarely monitored. Furthermore, occupational exposure of health-care workers in Africa is generally underreported and poorly documented – one Nigerian study found that up to 97% of exposures were not reported.7
The true incidence of blood and body fluid exposure in Africa is, therefore, uncertain. The 2005 report estimated that the incidence of sharps injuries in individual health-care workers in Africa was 2.10 per annum.4 However, the authors based the estimate on survey findings from eight African countries and did not include data on laboratory technicians or other auxiliary health-care workers. Moreover, the authors obtained the data in hospitals and may not be representative of the diverse range of health-care settings in the continent. A Congolese study found an annual prevalence of occupational exposure to body fluids among health-care workers of 44.9%, with an average of 1.38 exposures per health-care worker per year.8 A Burundian study reported an annual prevalence of 67.6%, with an average of 2.7 exposures per health-care worker per year.9
Here we conducted a systematic review of observational studies to estimate the prevalence of occupational exposure to blood and body fluids among health-care workers in Africa, because a continent-wide estimate would help increase awareness of such exposure and prompt preventative measures.
Methods
We searched the Embase®, CINAHL and PubMed® databases on 1 September 2017 for original research articles published between January 2000 and August 2017 that reported the prevalence of occupational exposure to blood or other body fluids among health-care workers in Africa. The following search terms were combined with others using Boolean operators: “occupational exposure”, “accidental exposure”, “blood”, “body fluid”, “blood-borne pathogens”, “health-care workers”, “health workers”, “health personnel” and “Africa” (Box 1; available at: http://www.who.int/bulletin/volumes/95/12/17-195735). Additional articles were identified by checking reference lists and by Google and Google Scholar searches. There were no language restrictions. The research protocol was registered in the PROSPERO international prospective register of systematic reviews (CRD42017054288).
Box 1. Search strategy, systematic review, blood and body fluid exposure among health-care workers in Africa, 2000–2017.
(Occupation* exposure OR Accident* exposure OR Occupation* disease OR Accidental blood disease* OR Accidental occupational exposure OR Occupational hazard* OR Occupational transmission OR Cross infection).af.
(Blood OR Body fluid* OR blood spill* OR needle injur* OR Blood borne pathogen* OR Sharps* OR Needlestick injur* OR Needle stick OR Blood-borne infection* OR percutaneous injur* OR mucus membrane exposure* OR non-intact skin exposure* OR bite* OR cut* OR Human immunodeficiency virus OR HIV OR Hepatitis B OR Hepatitis C).af.
(Health care worker* OR Nurse* OR Midwive* OR Physician* OR Surgeon* OR Doctor* OR Health personnel OR Health worker* OR Dentist* OR Health staff OR Medical personnel OR Health personnel OR Health officer*).af.
(Africa OR Nigeria OR Senegal OR Morocco OR South Africa OR Ethiopia OR Kenya OR Mauritius OR Mauritania OR Tanzania OR Congo OR Algeria OR Tunisia OR Libya OR Ghana OR Madagascar OR Gabon OR Cameroon OR Mali OR Zimbabwe OR Sudan OR Uganda OR Somalia OR Namibia OR Angola OR Mozambique OR Rwanda OR Eritrea OR Burkina Faso OR Gambia OR Zambia OR Botswana OR Guinea OR Djibouti OR Niger OR Malawi OR Togo OR Liberia OR Benin OR Sierra Leone OR Swaziland OR Côte d’Ivoire OR Chad OR Seychelles OR Cape Verde OR Burundi OR Lesotho).af.
1 AND 2 AND 3 AND 4
Limit 5 to yr = ”2000–Current”
For this review, we considered occupational exposure to body fluids to occur through percutaneous injury, mucous membrane exposure, non-intact skin exposure and bites. We included studies that reported the lifetime or 12-month prevalence of occupational exposure through at least one of these routes. Health-care workers included all paid and unpaid individuals working in a health-care setting who could be exposed to infectious materials, including blood and body fluids. Hence, we included studies that involved doctors, nurses, laboratory technicians, auxiliary health-care workers or students undertaking clinical training or gaining experience in health-care settings. In addition, we included studies if they were observational studies with either a cohort or cross-sectional design. We excluded case reports, case series, case–control studies, qualitative studies, studies with fewer than 100 participants and, because of historic underreporting in Africa, studies that reviewed reported cases of blood and body fluid exposure. Two reviewers independently screened studies against inclusion and exclusion criteria (kappa for inter-rater agreement: 90.8%). Discrepancies were resolved by consensus.
The quality of each study was assessed and the risk of bias was judged using eight parameters, modelled largely on the Joanna Briggs Institute’s critical appraisal framework for prevalence studies: the sampling frame, sample size, sampling strategy, detailed description of research setting and population, response rate (adequate if 60% or higher), reliability of the instrument used, recall bias (12 months or shorter) and statistical analysis methods – failure to satisfy each parameter was scored as 1.10 The risk of bias was classified as either low (total score: 0 to 2), moderate (total score: 3 or 4) or high (total score: 5 to 8).
Two reviewers extracted data from the studies and entered them into Microsoft Excel v. 16.0 (Microsoft Corporation, Redmond, United States of America). The data included: (i) author; (ii) year of publication; (iii) study country; (iv) sample size; (v) response rate; (vi) recall period; (vii) prevalence of blood and body fluid exposure; (viii) prevalence of percutaneous injury; (ix) prevalence of mucous membrane and non-intact skin exposure; (x) prevalence of blood and body fluid exposure by health staff category; and (xi) the proportions of cases due to needle-stick injury, splashes, cuts and bites. Any discrepancy was resolved by consensus.
Two countries, Egypt and Libya, are included in WHO’s Eastern Mediterranean Region, but were classified as African for the purposes of this analysis.
Data analysis
We categorized studies by whether they measured lifetime or 12-month prevalence and by the type of blood and body fluid exposure considered: (i) all types, including percutaneous injury and mucous membrane exposure; or (ii) percutaneous injury only. Generally, we estimated lifetime prevalence using data from studies that reported the proportion of participants exposed to body fluids at any time during their career. Twelve-month prevalence was estimated using data from studies that reported the proportion of participants exposed to body fluids in the preceding 12 months. We derived pooled prevalence estimates of blood and body fluid exposure by random-effects meta-analysis based on the DerSimonian–Laird approach.11 We assessed the robustness of our findings in sensitivity analyses that excluded studies with a high risk of bias.
Interstudy heterogeneity was assessed by Cochran’s Q, which gives values for X2 and P, and the percentage of the total variation across studies due to heterogeneity was estimated using Higgin’s I2 statistic.12 The causes of heterogeneity were explored in subgroup and meta-regression analyses. We considered the covariates: (i) geographical region; (ii) type of health-care facility; (iii) study period; (iv) sampling procedure (i.e. random versus convenience sampling); (v) sample size; (vi) proportion of doctors; (vii) proportion of nurses; (viii) proportion of laboratory staff; and (ix) the risk of bias classification. Only those covariates found to be significant at P < 0.10 were included in the multivariate model. In addition, the pooled prevalence of blood and body fluid exposure in different categories of health-care worker were derived in stratified analyses and the relative risk of occupational exposure between groups was determined by pooling data using a random-effects model. We performed all statistical analyses using Stata version 13.1 (StataCorp LP., College Station, USA).
Results
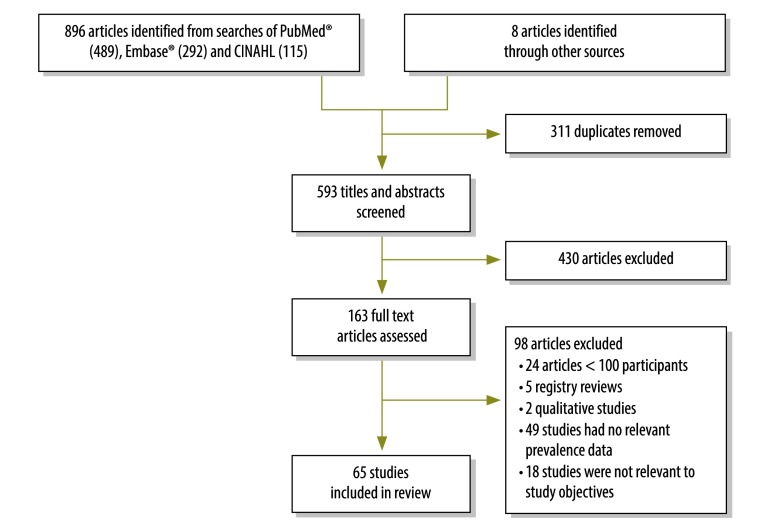
We identified 904 articles through the literature search, of which 65 were eligible for inclusion: they reported on cross-sectional observational studies involving a total of 29 385 health-care workers from 21 African countries (Fig. 1).7–9,13–74 Of the 65 studies, 30 were conducted in eastern Africa, 18 in western Africa, eight in northern Africa, five in southern Africa and four in central Africa (Table 1; available at: http://www.who.int/bulletin/volumes/95/12/17-195735). Thirty-nine studies were done solely among hospital staff, 39 investigated blood and body fluid exposure through all routes and 26 investigated exposure through percutaneous injury only. We found low risk of bias in 37 studies, moderate risk in 25 and high risk in 3; in 44 studies, the increased risk of bias was largely due to sampling bias.
Fig. 1.
Flow diagram, systematic review, blood and body fluid exposure among health-care workers in Africa, 2000–2017
Table 1. Studies identified in the systematic review on blood and body fluid exposure among health-care workers in Africa, 2000–2017.
| Study authors and year | Country and continental region | Data reported | Study participants and settinga | Prevalence of all types of exposure to BBF, % | Prevalence of PCI, % | Risk of biasb |
|---|---|---|---|---|---|---|
| Newsom and Kiwanuka,13 2002 | Uganda, eastern Africa | 12-month prevalence of PCI | 180 doctors, nurses and laboratory staff in Mbarara Teaching Hospital | N/A | 12-month: 55.0 | Low |
| Le Pont et al.,9 2003 | Burundi, central Africa | Lifetime and 12-month prevalence of all types of exposure to BBF and disaggregated PCI data | 219 doctors, nurses, nursing assistants and auxiliary staff in Kamenge University Hospital, Bujumbura | Lifetime: 79.5; 12-month: 67.6 | 12-month: 55.0 | Low |
| Talaat et al.,14 2003 | Egypt, northern Africa | Lifetime prevalence of PCI | 1845 doctors, dentists, nurses and laboratory and auxiliary staff in 98 health-care facilities (i.e. government hospitals, primary care facilities and private facilities) in two Governorates (Nile Delta and Upper Egypt) | N/A | Lifetime: 69.4 | Moderate |
| Bodkin and Bruce,15 2003 | South Africa, southern Africa | 12-month prevalence of PCI | 159 doctors, nurses and medical and nursing students in a teaching hospital in Gauteng | N/A | 12-month: 16.4 | Low |
| Tarantola et al.,16 2005 | Côte d'Ivoire, Mali and Senegal, western Africa | 12-month prevalence of all types of exposure to BBF and disaggregated data on PCI | 1241 doctors, nurses, laboratory staff and clinical students in 43 hospital departments and transfusion clinics in Abidjan (Côte d’Ivoire), Bamako (Mali) and Dakar (Senegal) | 12-month: 45.7 | 12-month: 38.1 | Moderate |
| Ismail et al.,17 2005 | Egypt, northern Africa | Lifetime prevalence of PCI | 1100 doctors and nurses in 7 hospitals and 18 primary health-care centres in Gharbiya Governorate | N/A | Lifetime: 66.2 | Moderate |
| Obi et al.,18 2005 | Nigeria, western Africa | Lifetime prevalence of PCI | 264 surgeons in five tertiary health institutions in south-eastern Nigeria | N/A | Lifetime: 66.7 | Moderate |
| Nsubuga and Jaakkola,19 2005 | Uganda, eastern Africa | Lifetime and 12-month prevalence of PCI | 526 nurses and midwives in Mulago national referral hospital in Kampala, Uganda | N/A | Lifetime: 82; 12-month: 57 | Low |
| National AIDS and STD Control Programme,20 2006 | Kenya, eastern Africa | 12-month prevalence of all types of exposure to BBF | 1897 doctors, clinical officers, nurses, laboratory technicians, social workers and other support staff across a nationally representative sample of 247 health-care facilities | 12-month: 17.0 | ND | Low |
| Ibekwe and Ibeziako,7 2006 | Nigeria, western Africa | Lifetime prevalence of PCI | 246 doctors, nurses, laboratory technicians and ward attendants in University of Nigeria Teaching Hospital, Enugu | N/A | Lifetime: 53.7 | High |
| Braka et al.,21 2006 | Uganda, eastern Africa | Lifetime prevalence of PCI | 311 doctors, dental staff, nurses, laboratory staff, midwives and auxiliary staff in 48 districts in Uganda | N/A | Lifetime: 77.2 | Low |
| Kabbash et al.,22 2007 | Egypt, northern Africa | 12-month prevalence of PCI | 317 doctors and nurses from 32 haemodialysis units in the Nile delta | N/A | 12-month: 48.6 | Low |
| Sofola et al.,23 2007 | Nigeria, western Africa | Lifetime prevalence of all types of exposure to BBF | 153 clinical dental students in four dental training institutions in Lagos, Ibadan, Ife and Benin | Lifetime: 58.8 | ND | Moderate |
| De Villiers et al.,24 2007 | South Africa, southern Africa | Lifetime prevalence of all types of exposure to BBF | 228 doctors in public and private practice in Bloemfontein | Lifetime: 54.2 | ND | Low |
| Taegtmeyer et al.,25 2008 | Kenya, eastern Africa | 12-month prevalence of PCI | 554 doctors, nurses and counsellors in 11 health facilities: two hospitals, eight health centres and one dispensary, Thika District | N/A | 12-month: 30 | Low |
| Laraqui et al.,26 2008 | Morocco, northern Africa | Lifetime and 12-month prevalence of all types of exposure to BBF | 2086 doctors, nurses and laboratory and support staff in 10 hospitals in 10 cities | Lifetime: 76.6; 12-month: 58.9 | ND | Low |
| Okeke et al.,27 2008 | Nigeria, western Africa | Lifetime prevalence of PCI | 346 medical students in a university | N/A | Lifetime:48 | Moderate |
| Manyele et al.,28 2008 | United Republic of Tanzania, eastern Africa | Lifetime prevalence of all types of exposure to BBF and disaggregated data on PCI | 430 nurses and attendants in 14 district, regional and referral hospitals | Lifetime: 74.6 | Lifetime: 52.9 | Moderate |
| Laraqui et al.,29 2009 | Morocco, northern Africa | Lifetime and 12-month prevalence of all types of exposure to BBF | 1002 doctors, nurses and support staff in four hospitals in the cities of Meknes, Taza, Tiznit and Rabat | Lifetime: 89.2; 12-month: 62.8 | ND | Low |
| Reda et al.,30 2010 | Ethiopia, eastern Africa | Lifetime and 12-month prevalence of all types of exposure to BBF and disaggregated data on PCI | 484 doctors, nurses, midwives, laboratory technicians, health officers and assistants in 10 hospitals and 20 health centres, eastern Ethiopia | Lifetime: 85.0; 12-month: 51.2 | Lifetime: 56.2; 12-month: 31.0 | Low |
| Tadesse and Tadesse,31 2010 | Ethiopia, eastern Africa | Lifetime and 12-month prevalence of PCI | 366 nurses and laboratory technicians in 26 health facilities including a university teaching hospital and one private hospital in Awassa City, southern Ethiopia | N/A | Lifetime: 49.2; 12-month: 30.9 | Low |
| Tebeje and Hailu,32 2010 | Ethiopia, eastern Africa | Lifetime prevalence of all types of exposure to BBF and disaggregated data on PCI | 254 doctors, nurses, midwives, laboratory technicians and health officers in government health facilities in Jimma zone and Jimma City | Lifetime: 68.5 | Lifetime: 41.3 | Moderate |
| Azodo,33 2010 | Nigeria, western Africa | Lifetime prevalence of PCI | 300 dentists across Nigeria | N/A | Lifetime: 69.3 | High |
| Hanafi et al.,34 2011 | Egypt, northern Africa | 12-month prevalence of PCI | 645 doctors, nurses and auxiliary staff in University of Alexandria teaching hospitals | N/A | 12-month: 67.9 | Low |
| Nwankwo and Aniebue,35 2011 | Nigeria, western Africa | 12-month prevalence of all types of exposure to BBF | 184 trainee surgeons in three hospitals in Enugu, south-eastern Nigeria | 12-month: 67.5 | ND | Moderate |
| Elduma and Saeed,36 2011 | Sudan, eastern Africa | Lifetime prevalence of PCI | 245 doctors, dentists, nurses and laboratory and support staff in three teaching hospitals, Khartoum | N/A | Lifetime: 51 | Moderate |
| Kumakech et al.,37 2011 | Uganda, eastern Africa | 12-month prevalence of all types of exposure to BBF and disaggregated data on PCI | 224 doctors, nurses, midwives, laboratory staff and medical and nursing students in Mbarara Regional Referral Hospital, south-western Uganda | 12-month: 33.9 | 12-month: 23.6 | Low |
| Ngatu et al.,8 2012 | Democratic Republic of the Congo, central Africa | 12-month prevalence of all types exposure to BBF | 1043 doctors, nurses and laboratory and support staff in four urban and rural hospitals in the southern town of Lubumbashi and the western semirural city of Matadi | 12-month: 44.9 | ND | Moderate |
| Shiferaw et al.,38 2012 | Ethiopia, eastern Africa | 12-month prevalence of all types of exposure to BBF and disaggregated data on PCI | 126 medical waste handlers in three government hospitals in Addis Ababa | 12-month: 67.5 | 12-month: 42.1 | Moderate |
| Pellissier et al.,39 2012 | Niger, western Africa | Lifetime prevalence of PCI | 207 nurses and medical, paramedical, cleaning and administrative staff in Niamey’s National Hospital | N/A | Lifetime: 40.1 | Moderate |
| Owolabi et al.,40 2012 | Nigeria, western Africa | 12-month prevalence of all types of exposure to BBF and disaggregated data on PCI | 230 doctors, nurses and laboratory staff in University of Abuja Teaching Hospital | 12-month: 30.9 | 12-month: 24.8 | Low |
| Odongkara et al.,41 2012 | Uganda, eastern Africa | Lifetime prevalence of all types of exposure to BBF | 235 doctors, nurses and laboratory staff in Gulu Regional Referral Hospital and St. Mary's Hospital Lacor, northern Uganda | Lifetime: 46 | ND | Moderate |
| Noubiap et al.,42 2013 | Cameroon, central Africa | Lifetime prevalence of all types of exposure to BBF | 111 clinical medical students of the Faculty of Medicine and Biomedical Sciences of the University of Yaoundé | Lifetime: 55.9 | ND | Moderate |
| Zawilla and Ahmed,43 2013 | Egypt, northern Africa | 12-month prevalence of PCI | 1036 health-care workers in Cairo University Hospitals | N/A | 12-month: 40 | Low |
| Mathewos et al.,44 2013 | Ethiopia, eastern Africa | Lifetime prevalence of all types of exposure to BBF | 195 doctors, nurses, laboratory technicians, midwives, anaesthetists, heath officers and physiotherapists in Gondar University Hospital | Lifetime: 33.8 | ND | Low |
| Yimechew and Tadese Ejigu,45 2013 | Ethiopia, eastern Africa | Lifetime and 12-month prevalence of all types of exposure to BBF and disaggregated data on PCI | 285 doctors, nurses, laboratory staff, auxiliary staff and medical students in the University of Gondar Hospital | Lifetime: 70.2; 12-month: 62.9 | 12-month: 41 | Low |
| Mbaisi et al.,46 2013 | Kenya, eastern Africa | 12-month prevalence of all types of exposure to BBF and disaggregated data on PCI | 305 doctors, clinical officers, nurses, laboratory personnel, mortuary attendants, housekeeping staff and clinical students in Rift Valley Provincial General Hospital | 12-month: 25 | 12-month: 19 | Low |
| Osazuwa-Peters et al.,47 2013 | Nigeria, western Africa | 12-month prevalence of PCI | 144 medical and dental house officers in three government hospitals in Edo State | N/A | 12-month: 56.9 | Low |
| Bagny et al.,48 2013 | Togo, western Africa | Lifetime prevalence of all types of exposure to BBF | 155 nurses in Lome Campus Teaching Hospital | Lifetime: 34.8 | ND | Moderate |
| Mashoto et al.,49 2013 | United Republic of Tanzania, eastern Africa | 12-month prevalence of all types of exposure to BBF and disaggregated data on PCI | 401 doctors, dentists, dental assistants, clinical officers, nurses, laboratory staff, radiologists, physiotherapists and health attendants in Tumbi and Dodoma regional hospitals | 12-month: 47.9 | 12-month: 39.1 | Low |
| Zoungrana et al.,50 2014 | Burkina Faso, western Africa | Lifetime prevalence of all types of exposure to BBF | 275 student nurses and midwives in the medical ward of the Bobo-Dioulasso teaching hospital | Lifetime: 29.1 | ND | Moderate |
| Beyera and Beyen,51 2014 | Ethiopia, eastern Africa | 12-month prevalence of all types of exposure to BBF and disaggregated data on PCI | 401 doctors, anaesthetists, nurses, laboratory staff, health officer and cleaners in four public health institutions (one hospital and three health centres) in Gondar city | 12-month: 40.4 | 12-month: 22.9 | Low |
| Yenesew and Fekadu,52 2014 | Ethiopia, eastern Africa | Lifetime and 12-month prevalence of all types of exposure to BBF and disaggregated data on PCI | 317 nurses, health officers, health assistants, doctors, laboratory technicians and dentists in health-care facilities, Bahir Dar town | Lifetime: 76.0; 12-month: 65.9 | Lifetime: 45.9; 12-month: 29.0 | Low |
| Aynalem Tesfay and Dejenie Habtewold,53 2014 | Ethiopia, eastern Africa | 12-month prevalence of all types of exposure to BBF and disaggregated data on PCI | 211 doctors, nurses, midwives, health officers and laboratory technicians in two hospitals and two health centres in Debre Berhan town, Amhara region | 12-month: 56.7 | 12-month: 31.5 | Low |
| Beyene and Tadesse,54 2014 | Ethiopia, eastern Africa | 12-month prevalence of all types of exposure to BBF | 532 health-care workers in two hospitals and six health centres run by the government in Hawassa Town, southern Ethiopia | 12-month: 51.9 | ND | Low |
| Ajibola et al.,55 2014 | Nigeria, western Africa | Lifetime prevalence of PCI | 300 doctors and nurses in Lagos University Teaching Hospital | N/A | Lifetime: 47.3 | Moderate |
| Amira and Awobusuyi,56 2014 | Nigeria, western Africa | Lifetime and 12-month prevalence of PCI | 102 doctors, nurses, dialysis technicians and auxiliary health staff in four (two government and two private) dialysis units in Lagos | N/A | Lifetime: 40.2; 12-month: 24.5 | Moderate |
| Ogoina et al.,57 2014 | Nigeria, western Africa | Lifetime prevalence of all types of exposure to BBF | 230 doctors, nurses and laboratory staff in two tertiary hospitals in north-central and south-south Nigeria | Lifetime: 84 | ND | Moderate |
| Mbah,58 2014 | South Africa, southern Africa | 12-month prevalence of all types of exposure to BBF | 515 doctors and nurses in public, primary health-care settings in subdistrict F of Johannesburg metropolitan district | 12-month: 25.2 | ND | Low |
| Bekele et al.,59 2015 | Ethiopia, eastern Africa | Lifetime prevalence of PCI | 340 doctors, anaesthetists, health officers, nurses, midwives, laboratory personnel, laundry workers and waste handlers in four hospitals in Bale zone, south-east Ethiopia | N/A | Lifetime: 37.1 | Low |
| Burmen and Osoga,60 2015 | Kenya, eastern Africa | Lifetime prevalence of all types of exposure to BBF | 116 laboratory staff | Lifetime: 77 | ND | High |
| Arheiam and Ingafou,61 2015 | Libya, northern Africa | 12-month prevalence of PCI | 340 dental practitioners | N/A | 12-month: 35.1 | Low |
| Kone and Malle,62 2015 | Mali, western Africa | Lifetime prevalence of all types of exposure to BBF | 128 doctors, nurses and students in a public hospital in Ségou, south-western Mali. | Lifetime: 64.1 | ND | Moderate |
| Kateera et al.,63 2015 | Rwanda, eastern Africa | Lifetime prevalence of PCI | 378 doctors, nurses and laboratory and support staff in the University Teaching Hospital of Butare, Huye District, Southern Province, Rwanda | N/A | Lifetime: 57.1 | Moderate |
| Chalya et al.,64 2015 | United Republic of Tanzania, eastern Africa | 12-month prevalence of all types of exposure to BBF and disaggregated data on PCI | 436 doctors, nurses, laboratory staff and auxiliary health workers in Bugando Medical Centre, Mwanza | 12-month: 48.6 | 12-month: 31.7 | Low |
| Mponela et al.,65 2015 | United Republic of Tanzania, eastern Africa | 12-month prevalence of all types of exposure to BBF and disaggregated data on PCI | 291 doctors, dental staff, nurses, laboratory staff, medical attendants and cleaners in one referral and two district hospitals, Mbeya region | 12-month: 35.1 | 12-month: 22.0 | Low |
| Kassa et al.,66 2016 | Botswana, southern Africa | Lifetime prevalence of all types of exposure to BBF | 1624 doctors, nurses and laboratory technicians in three public hospitals: a referral hospital and two district hospitals | Lifetime: 67.2 | ND | Moderate |
| Kaweti and Abegaz,67 2016 | Ethiopia, eastern Africa | Lifetime and 12-month prevalence of PCI | 496 doctors, nurses, laboratory technicians and cleaners in two public hospitals: Hawassa Referral and Adare District hospitals | N/A | Lifetime: 46; 12-month: 28 | Low |
| Oluwatosin et al.,68 2016 | Nigeria, western Africa | Lifetime prevalence of PCI | 642 doctors, nurses, laboratory workers and health attendants in two specialist hospitals in Ondo State | N/A | Lifetime: 55.8 | Moderate |
| Nmadu et al.,69 2016 | Nigeria, western Africa | Lifetime prevalence of all types of exposure to BBF | 172 nurses, midwives, community health workers and laboratory technicians in 14 primary health-care centres in Kaduna State | Lifetime: 68.9 | ND | Low |
| Makhado and Davhana-Maselesele,70 2016 | South Africa, southern Africa | 12-month prevalence of all types of exposure to BBF | 233 nurses in a regional hospital in Limpopo Province | 12-month: 43 | ND | Low |
| Lahuerta et al.,71 2016 | United Republic of Tanzania, eastern Africa | Lifetime prevalence of all types of exposure to BBF and disaggregated data on PCI | 973 doctors, nurses, dentists, students, cleaners and other support workers in three public hospitals | Lifetime: 79 | Lifetime: 37 | Low |
| Shindano et al.,72 2017 | Democratic Republic of the Congo, central Africa | 12-month prevalence of all types of exposure to BBF | 217 doctors and nurses in Bukavu, an eastern town in the Democratic Republic of the Congo | 12-month: 42.8 | ND | Low |
| Sharew et al.,73 2017 | Ethiopia, eastern Africa | 12-month prevalence of PCI | 195 nurses, midwives, laboratory staff, doctors, health officers and anaesthetists in two hospitals in Debre Berhan town, north-eastern Ethiopia | N/A | 12-month: 32.8 | Low |
| Laisser and Ng’Home,74 2017 | United Republic of Tanzania, eastern Africa | 12-month prevalence of all types of exposure to BBF and disaggregated data on PCI | 277 doctors, nurses and laboratory and auxiliary staff in 31 private and public health facilities in Kahama District, north-western United Republic of Tanzania | 12-month: 59.2 | 12-month: 34.7 | Moderate |
AIDS: acquired immunodeficiency syndrome; BBF: blood and body fluid; N/A: not applicable; ND: not determined; PCI: percutaneous injury; STD: sexually transmitted disease.
a All studies were cross-sectional.
b The risk of bias was assessed as described in the methods.
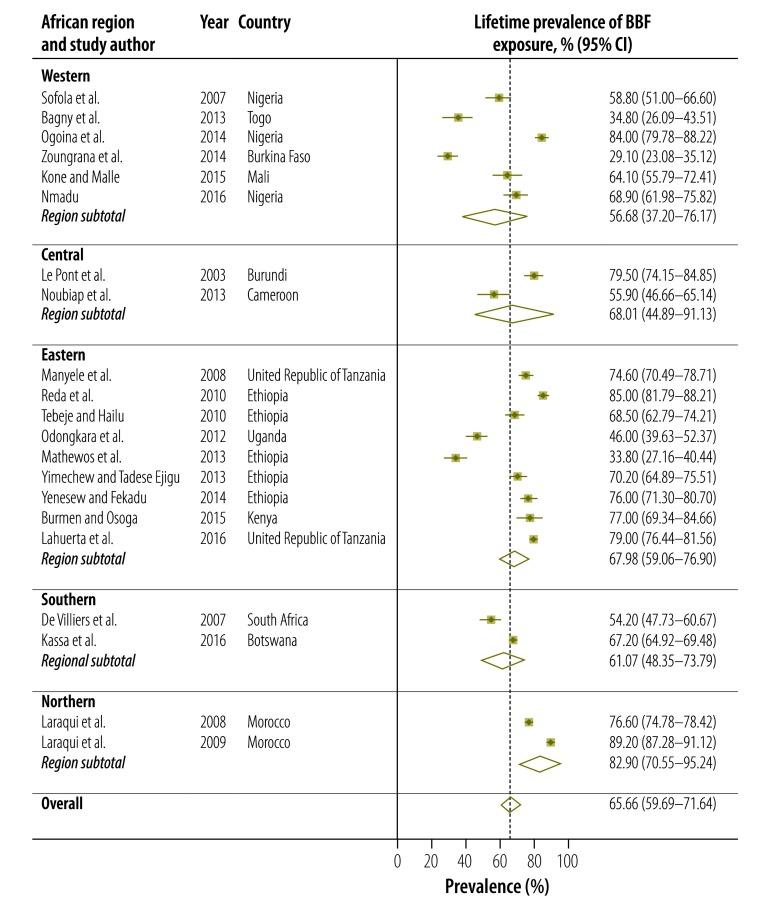
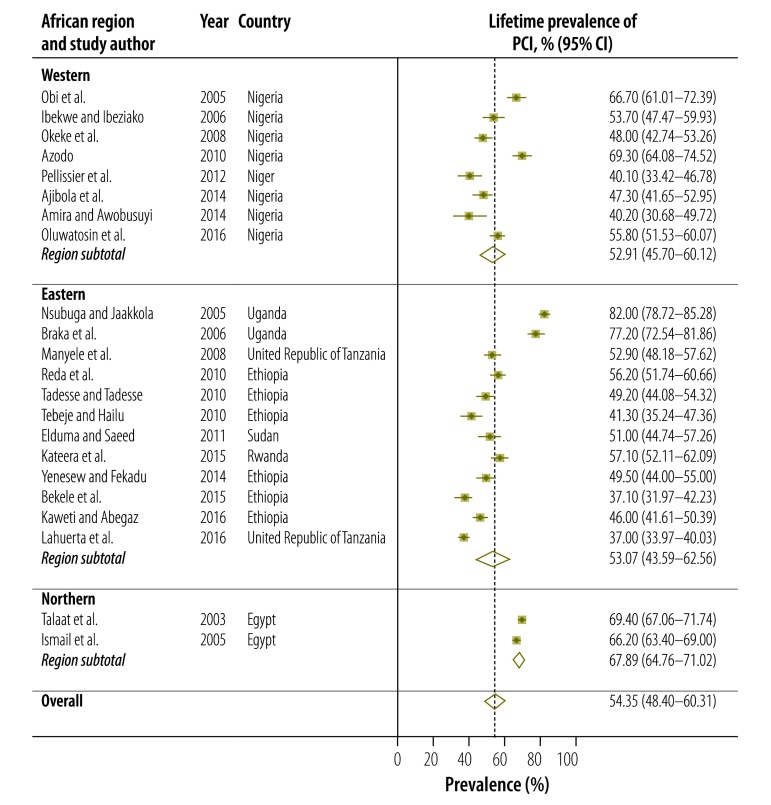
Twenty-one studies presented data on the lifetime prevalence of all types of occupational exposure to blood and body fluids, including percutaneous injury and mucous membrane exposure, among health-care workers in Africa (Table 1; available at: http://www.who.int/bulletin/volumes/95/12/17-195735). Lifetime prevalence varied widely from 29.1% (95% confidence interval, CI: 23.1–35.1) in Burkina Faso50 to 89.2% (95% CI: 87.3–91.1) in Morocco (Fig. 2).29 Overall, the estimated pooled lifetime prevalence was 65.7% (95% CI: 59.7–71.6). The regional prevalence estimate was highest for northern Africa: 82.9% (95% CI: 70.6–95.2). For percutaneous injury only, the lifetime prevalence ranged from 37.0% (95% CI: 34.0–40.0) in a Tanzanian study71 to 82.0% (95% CI: 78.7–85.3) in a Ugandan study (Fig. 3).19 Overall, the estimated pooled lifetime prevalence of percutaneous injury was 54.4% (95% CI: 48.4–60.3). After excluding studies with a high risk of bias, the estimated pooled lifetime prevalence of all types of exposure to blood and body fluids and of percutaneous injury was 65.1% (95% CI: 59.0–71.3) and 53.6% (95% CI: 47.3–60.0), respectively, figures which were comparable to the overall pooled estimates.
Fig. 2.
Meta-analysis, lifetime prevalence of blood and body fluid exposure among health-care workers in Africa, by region, 2002–2017
BBF: blood and body fluid; CI: confidence interval.
Note: The dashed vertical line presents the overall estimated prevalence.
Fig. 3.
Meta-analysis, lifetime prevalence of percutaneous injury among health-care workers in Africa, by region, 2000–2017
CI: confidence interval; PCI: percutaneous injury.
Note: The dashed vertical line represents the overall estimated prevalence.
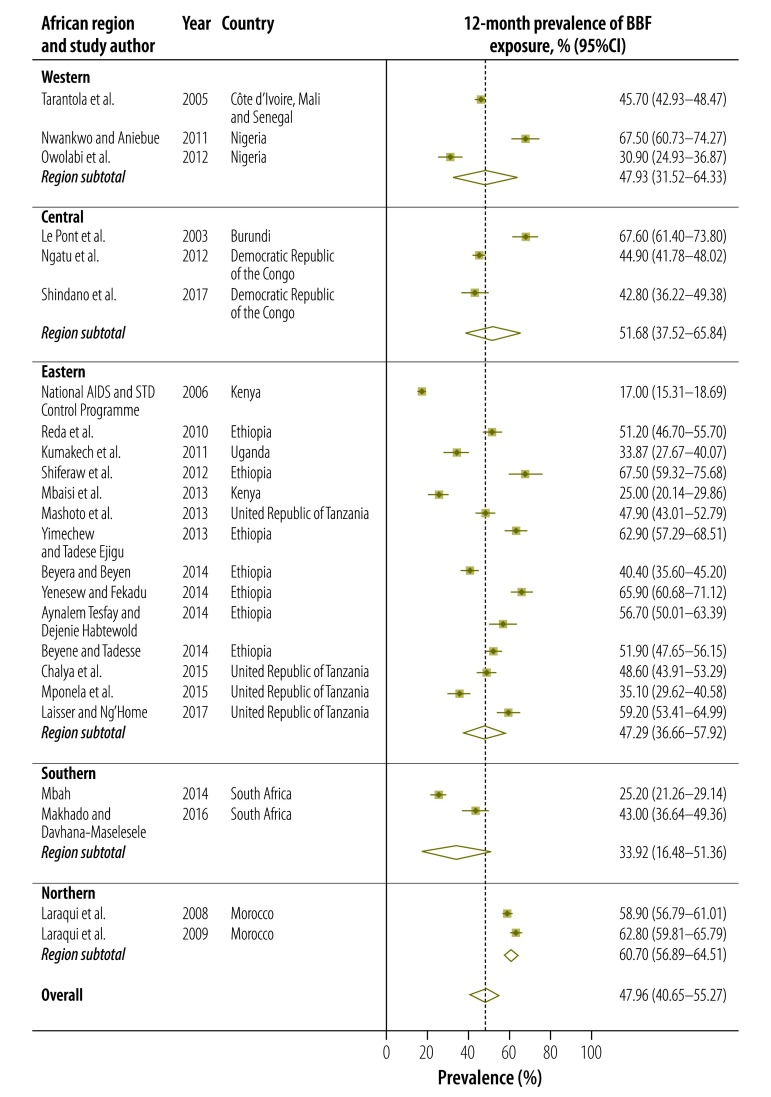
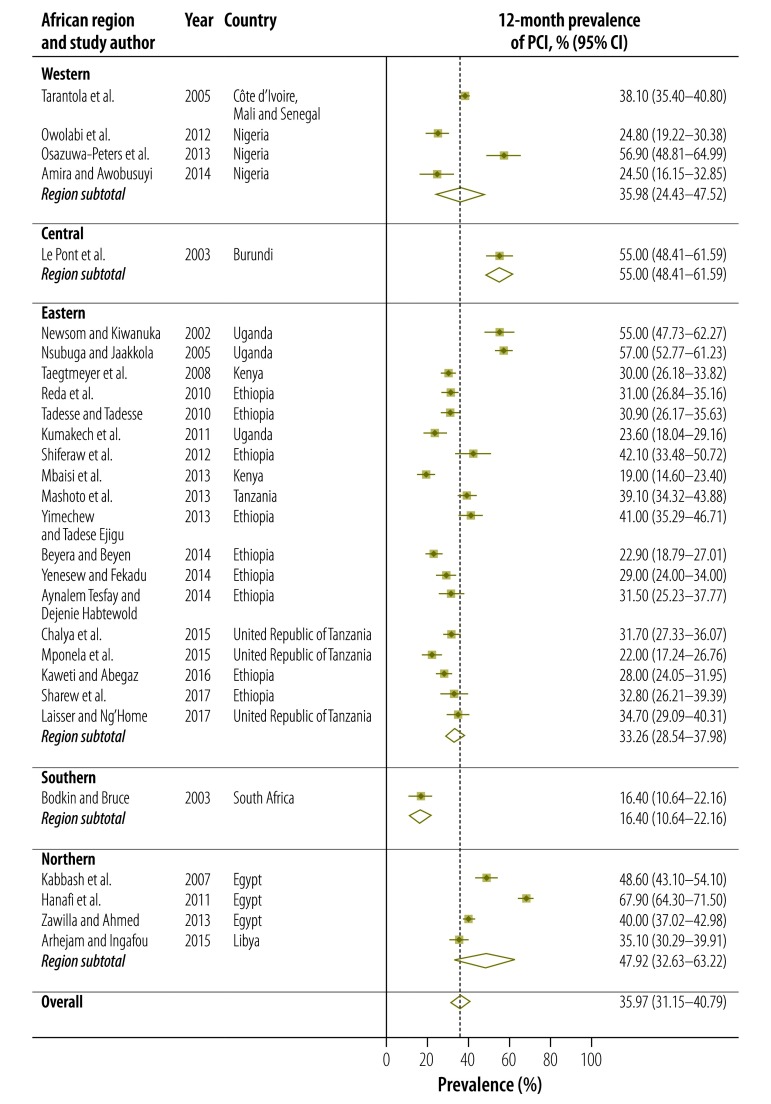
The 12-month prevalence of all types of occupational exposure to blood and body fluids ranged from 17.0% (95% CI: 15.3–18.7) in a Kenyan study20 to 67.6% (95% CI: 61.4–73.8) in a Burundian study (Fig. 4).9 The estimated pooled 12-month prevalence was 48.0% (95% CI: 40.7–55.3). Regional pooled estimates ranged from 33.9% (95% CI: 16.5–51.4) in southern Africa to 60.7% (95% CI: 56.9–64.5) in northern Africa. Twenty-eight studies reported the 12-month prevalence of percutaneous injury: it ranged from 16.4% (95% CI: 10.6–22.2) to 67.9% (95% CI: 64.3–71.5; Fig. 5). The pooled estimate was 36.0% (95% CI: 31.2–40.8). Seven studies provided disaggregated data on the 12-month prevalence of mucous membrane exposure: the pooled estimate was 18.2% (95% CI: 12.6–23.7).
Fig. 4.
Meta-analysis, 12-month prevalence of blood and body fluid exposure among health-care workers in Africa, by region, 2002–2017
AIDS: acquired immunodeficiency syndrome; BBF: blood and body fluid; CI: confidence interval; STD: sexually transmitted disease.
Note: The dashed vertical line represents the overall estimated prevalence.
Fig. 5.
Meta-analysis, 12-month prevalence of percutaneous injury among health-care workers in Africa, by region, 2000–2017
CI: confidence interval; PCI: percutaneous injury.
Note: The dashed vertical line represents the overall estimated prevalence.
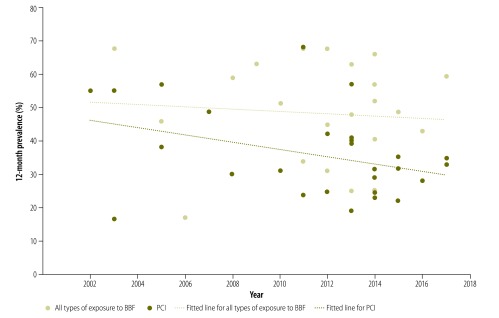
In Fig. 6, the slopes of the fitted lines suggest that the 12-month prevalence of both all types of exposure to blood and body fluids and of percutaneous injury decreased only gradually over the study period. The estimated pooled 12-month prevalence for studies published between 2010 and 2017 was 47.3% (95% CI: 41.5–53.1) for all types of exposure and 33.7% (95% CI: 28.2–39.2) for percutaneous injury (Table 2). These estimates were comparable to the overall estimated pooled 12-month prevalence for all types of exposure and percutaneous injury, which were 48.0% (95% CI: 40.7–55.3) and 36.0% (95% CI: 31.2–40.8), respectively.
Fig. 6.
Trend in 12-month prevalence of blood and body fluid exposure and percutaneous injury among health-care workers in Africa, 2000–2017
BBF: blood and body fluid; PCI: percutaneous injury.
Note: The fitted lines were derived by linear regression.
Table 2. Subgroup meta-analysis, blood and body fluid exposure and percutaneous injury among health-care workers in Africa, 2000–2017.
| Subgroup | Blood and body fluid exposure |
Percutaneous injury |
|||||
|---|---|---|---|---|---|---|---|
| Pooled 12-month prevalence, % (95% CI) | No. studies included | Study heterogeneity, I2,% (P-value) | Pooled 12-month prevalence, % (95% CI) | No. studies included | Study heterogeneity, I2,% (P-value) | ||
| African region | |||||||
| Western | 47.9 (31.5–64.3) | 3 | 96.8 (< 0.001) | 36.0 (24.4–47.5) | 4 | 94.1 (< 0.001) | |
| Central | 51.7 (37.5–65.8) | 3 | 95.5 (< 0.001) | 55.0 (48.4–61.6) | 1 | N/A | |
| Eastern | 47.3 (36.7–57.9) | 14 | 98.7 (< 0.001) | 33.3 (28.5–38.0) | 18 | 94.0 (< 0.001) | |
| Southern | 33.9 (16.5–51.4) | 2 | 95.4 (< 0.001) | 16.4 (10.6–22.2) | 1 | N/A | |
| Northern | 60.7 (56.9–64.5) | 2 | 77.0 (0.037) | 47.9 (32.6–63.2) | 4 | 98.3 (< 0.001) | |
| Study period | |||||||
| 2000–2009 | 50.3 (29.2–71.4) | 5 | 99.7 (< 0.001) | 42.8 (32.7–52.8) | 7 | 97.0 (< 0.001) | |
| 2010–2017 | 47.3 (41.5–53.1) | 19 | 95.9 (< 0.001) | 33.7 (28.2–39.2) | 21 | 96.3 (< 0.001) | |
| Type of health-care facility | |||||||
| Hospital | 49.7 (42.8–56.6) | 14 | 97.0 (< 0.001) | 39.2 (31.6–46.9) | 17 | 97.6 (< 0.001) | |
| Mixeda | 47.8 (34.5–61.1) | 9 | 99.1 (< 0.001) | 31.5 (28.1–34.9) | 9 | 81.7 (< 0.001) | |
| Risk of bias | |||||||
| Low | 45.7 (36.7–54.6) | 19 | 98.9 (< 0.001) | 36.2 (30.5–41.8) | 24 | 97.0 (< 0.001) | |
| Moderate | 56.4 (47.8–65.0) | 5 | 94.5 (< 0.001) | 35.2 (29.6–40.9) | 4 | 73.3 (0.011) | |
BBF: blood and body fluid; CI: confidence interval; N/A: not applicable; PCI: percutaneous injury.
a Both hospitals and primary care facilities.
Overall, substantial heterogeneity was observed among the studies for the estimated 12-month prevalence of all types of exposure to blood and body fluids (X2: 1816.5; P < 0.001; I2: 98.7%) and of percutaneous injury only (X2: 780.9; P < 0.001; I2: 96.5%). Meta-regression analysis showed that, of all the covariates explored in the bivariate analyses, only geographical region had a P-value less than 0.10: (P = 0.0874) and geographical region explained 17.6% of the between-study variation in the estimated 12-month prevalence of percutaneous injury.
Subgroup analyses
As many of the studies included disaggregated data, we were able to estimate: (i) the pooled 12-month prevalence of occupational exposure to blood and body fluids by job category; and (ii) the relative risk of all types of exposure to blood and body fluids or of percutaneous injury between various demographic groups, which were distinguished, for example, by job category, gender, years of working experience or receipt of training on prevention of blood and body fluid exposure (details available from the corresponding author). The estimated pooled 12-month prevalence of exposure to blood and body fluids for medical doctors (excluding surgeons), nursing staff (including midwives and nursing assistants) and laboratory staff (including laboratory technicians) was 46.6% (95% CI: 33.5–59.7), 44.6% (95% CI: 34.1–55.0) and 34.3% (95% CI: 21.8–46.7), respectively. Moreover, when data on percutaneous injuries were included, there was no significant difference in the risk of all types of occupational exposure between these job categories: the relative risk (RR) was 1.108 (95% CI: 0.926–1.326) for doctors versus nursing staff, 1.267 (95% CI: 0.733–2.193) for doctors versus laboratory staff and 1.332 (95% CI: 0.947–1.874) for nursing staff versus laboratory staff. Nor was there a significant difference in risk between males and females (RR: 0.886; 95% CI: 0.692–1.133).
In addition, when data on percutaneous injuries were included, there was no significant difference in the risk of all types of occupational exposure between health-care workers with 5 years or less working experience and those with more than 5 years (RR: 0.999; 95% CI: 0.831–1.202). In contrast, health-care workers who worked 40 hours or more per week were significantly more likely to be exposed than those who worked fewer hours (RR: 2.221; 95% CI: 1.001–4.926). Six studies reported on health-care workers who had received training on infection prevention and occupational exposure to blood and body fluids. The risk of occupational exposure in the preceding 12 months among health-care workers without training was significantly higher than in trained staff (RR: 1.791, 95% CI: 1.234–2.071).
Discussion
We found a high lifetime and 12-month prevalence of occupational exposure to blood and body fluids among health-care workers in Africa: about two thirds were exposed during their entire career and almost one half were exposed each year. Most exposure was due to percutaneous injury, which had an estimated 12-month prevalence of 36.0%. Direct comparison of our findings with those in other continents was difficult because of a lack of similar, continent-wide systematic reviews and meta-analyses. Nevertheless, the high prevalence of percutaneous injury among health-care workers in Africa has serious implications because most occupational exposure to blood-borne viruses, such as HBV and HIV, occurs via this route. This can have implications for the exposed health-care worker’s health, the transmission of blood-borne viruses to patients and the availability of scarce human resources for health care in Africa.
We found a variation in health-care workers’ exposure to blood and body fluids across Africa. Occupational exposure to blood and body fluids and percutaneous injury were consistently more frequent in northern Africa and less frequent in southern Africa. The reason for these regional differences is not clear. One possible explanation is that blood and body fluid exposure was underreported in some studies, which is likely. Alternatively, our findings may reflect regional differences in the level of knowledge of occupational exposure or in adherence to standard precautions.
Our meta-analysis found that the 12-month prevalence of blood and body fluid exposure differed little between various professions and there was no significant difference in risk. A critical appraisal of the literature showed that these figures may have been influenced by differences in study methods and in the categorization of health-care workers, but most discrepancies observed were linked to the underreporting of blood and body fluid exposure.75,76 In contrast, we found that the risk of blood and body fluid exposure was higher among health-care workers who had received no training on infection prevention, which is unsurprising because training improves knowledge and preventive practice. Furthermore, the risk of occupational exposure was also increased among staff who worked more than 40 hours per week. The acute shortage of health-care workers in Africa may, therefore, have contributed to the present findings.4 Inadequate staffing often results in a high patient-to-staff ratio, which may in turn lead to staff having to work longer hours to bridge gaps in personnel.77 Although longer hours can bring additional rewards for health-care workers, levels of stress and fatigue can increase, which may result in overworked staff becoming less alert and more susceptible to exposure to blood and body fluids.77 Our findings may therefore indicate the need not only to promote the safety and well-being of existing health-care workers in Africa, but also to address the acute shortage of health-care workers across the continent.
Our study also highlights the need to step-up efforts to reduce occupational exposure to blood and body fluids – particularly via percutaneous injury – among health-care workers in Africa. Percutaneous injury could be prevented by practical interventions such as safety engineered devices, including needleless intravenous systems, auto-disable syringes and blunt suture needles. However, our findings suggest that it may be more cost–effective to address factors contributing to increased exposure in the continent, such as a lack of training and long work hours. Regular in-service training for health-care workers could help promote standard precautions for preventing the transmission of blood-borne infection, such as hand hygiene, the use of personal protective equipment and techniques for minimizing the manipulation of sharps, including the avoidance of needle recapping. In addition to training health-care workers, a holistic strategy is needed to address the acute shortage of health-care workers in the continent and to monitor staff workload. Furthermore, standard precautions could be supplemented by educating health-care workers to take responsibility for their own health and safety and for that of others who may be affected by their actions at work. Finally, governments should provide policies and support systems for the surveillance, reporting and management of occupational exposure to blood and body fluids among health-care workers.
This study has some limitations. First, the cross-sectional design of the studies reviewed does not allow causal relationships to be established. Second, because the studies reviewed were based on self-reported retrospective data, they may be prone to recall and social desirability biases. Therefore, it is likely that exposure was underreported in many studies. Third, our review included single or limited reports from some countries and many reports concerned regional studies that were not nationally representative of the study countries. These factors may affect the generalizability of our findings. Furthermore, our review would have benefited from the inclusion of studies from Guinea, Liberia and Sierra Leone, where there was substantial transmission of Ebola virus infection among health-care workers during the recent outbreak. However, no studies of the prevalence of occupational exposure to blood and body fluids among health-care workers in these countries have been published. Future research in these countries should investigate occupational exposure to blood and body fluids and the circumstances in which it occurs to inform policy and practice. Nevertheless, our study provides an insight into the burden of occupational exposure to blood and body fluids among health-care workers in Africa and could prompt the development of appropriate policies, systems and processes in the continent.
Competing interests:
None declared.
References
- 1.Tarantola A, Abiteboul D, Rachline A. Infection risks following accidental exposure to blood or body fluids in health care workers: a review of pathogens transmitted in published cases. Am J Infect Control. 2006. August;34(6):367–75. 10.1016/j.ajic.2004.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Elseviers MM, Arias-Guillén M, Gorke A, Arens HJ. Sharps injuries amongst healthcare workers: review of incidence, transmissions and costs. J Ren Care. 2014. September;40(3):150–6. 10.1111/jorc.12050 [DOI] [PubMed] [Google Scholar]
- 3.Ngatu NR, Kayembe NJ, Phillips EK, Okech-Ojony J, Patou-Musumari M, Gaspard-Kibukusa M, et al. Epidemiology of ebolavirus disease (EVD) and occupational EVD in health care workers in sub-Saharan Africa: need for strengthened public health preparedness. J Epidemiol. 2017. October;27(10):455–61. 10.1016/j.je.2016.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prüss-Ustün A, Rapiti E, Hutin Y. Estimation of the global burden of disease attributable to contaminated sharps injuries among health-care workers. Am J Ind Med. 2005. December;48(6):482–90. 10.1002/ajim.20230 [DOI] [PubMed] [Google Scholar]
- 5.The world health report 2002 – reducing risks, promoting healthy life. Geneva: World Health Organization; 2002. Available from: http://www.who.int/whr/2002/en/ [cited 2017 Sep 22].
- 6.Dement JM, Epling C, Ostbye T, Pompeii LA, Hunt DL. Blood and body fluid exposure risks among health care workers: results from the Duke Health and Safety Surveillance System. Am J Ind Med. 2004. December;46(6):637–48. 10.1002/ajim.20106 [DOI] [PubMed] [Google Scholar]
- 7.Ibekwe RC, Ibeziako N. Hepatitis B vaccination status among health workers in Enugu, Nigeria. Niger J Clin Pract. 2006. June;9(1):7–10. [PubMed] [Google Scholar]
- 8.Ngatu NR, Phillips EK, Wembonyama OS, Hirota R, Kaunge NJ, Mbutshu LH, et al. Practice of universal precautions and risk of occupational blood-borne viral infection among Congolese health care workers. Am J Infect Control. 2012. February;40(1):68–70.e1. 10.1016/j.ajic.2011.01.021 [DOI] [PubMed] [Google Scholar]
- 9.Le Pont F, Hatungimana V, Guiguet M, Ndayiragije A, Ndoricimpa J, Niyongabo T, et al. Burhop Research Group. Assessment of occupational exposure to human immunodeficiency virus and hepatitis C virus in a referral hospital in Burundi, Central Africa. Infect Control Hosp Epidemiol. 2003. October;24(10):717–8. 10.1086/502908 [DOI] [PubMed] [Google Scholar]
- 10.Munn Z, Moola S, Lisy K, Riitano D, Tufanaru C. Methodological guidance for systematic reviews of observational epidemiological studies reporting prevalence and cumulative incidence data. Int J Evid-Based Healthc. 2015. September;13(3):147–53. 10.1097/XEB.0000000000000054 [DOI] [PubMed] [Google Scholar]
- 11.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986. September;7(3):177–88. 10.1016/0197-2456(86)90046-2 [DOI] [PubMed] [Google Scholar]
- 12.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003. September 6;327(7414):557–60. 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Newsom DH, Kiwanuka JP. Needle-stick injuries in an Ugandan teaching hospital. Ann Trop Med Parasitol. 2002. July;96(5):517–22. 10.1179/000349802125001186 [DOI] [PubMed] [Google Scholar]
- 14.Talaat M, Kandeel A, El-Shoubary W, Bodenschatz C, Khairy I, Oun S, et al. Occupational exposure to needlestick injuries and hepatitis B vaccination coverage among health care workers in Egypt. Am J Infect Control. 2003. December;31(8):469–74. 10.1016/j.ajic.2003.03.003 [DOI] [PubMed] [Google Scholar]
- 15.Bodkin C, Bruce J. Health professionals’ knowledge of prevention strategies and protocol following percutaneous injury. Curationis. 2003. December;26(4):22–8. 10.4102/curationis.v26i4.868 [DOI] [PubMed] [Google Scholar]
- 16.Tarantola A, Koumaré A, Rachline A, Sow PS, Diallo MB, Doumbia S, et al. ; Groupe d’Etude des Risques d’Exposition des Soignants aux agents infectieux (GERES). A descriptive, retrospective study of 567 accidental blood exposures in healthcare workers in three West African countries. J Hosp Infect. 2005. July;60(3):276–82. 10.1016/j.jhin.2004.11.025 [DOI] [PubMed] [Google Scholar]
- 17.Ismail NA, Aboul Ftouh AM, El Shoubary WH. Safe injection practice among health care workers, Gharbiya, Egypt. J Egypt Public Health Assoc. 2005;80(5-6):563–83. [PubMed] [Google Scholar]
- 18.Obi SN, Waboso P, Ozumba BC. HIV/AIDS: occupational risk, attitude and behaviour of surgeons in southeast Nigeria. Int J STD AIDS. 2005. May;16(5):370–3. [DOI] [PubMed] [Google Scholar]
- 19.Nsubuga FM, Jaakkola MS. Needle stick injuries among nurses in sub-Saharan Africa. Trop Med Int Health. 2005. August;10(8):773–81. 10.1111/j.1365-3156.2005.01453.x [DOI] [PubMed] [Google Scholar]
- 20.Preparedness for HIV/AIDS service delivery: the 2005 Kenya health workers survey. Nairobi: Kenya Ministry of Health; 2006. Available from: http://pdf.usaid.gov/pdf_docs/Pnadk460.pdf [cited 2017 Oct 2].
- 21.Braka F, Nanyunja M, Makumbi I, Mbabazi W, Kasasa S, Lewis RF. Hepatitis B infection among health workers in Uganda: evidence of the need for health worker protection. Vaccine. 2006. November 17;24(47-48):6930–7. 10.1016/j.vaccine.2006.08.029 [DOI] [PubMed] [Google Scholar]
- 22.Kabbash IA, El-Sayed NM, Al-Nawawy AN, Abou Salem Mel-S, El-Deek B, Hassan NM. Risk perception and precautions taken by health care workers for HIV infection in haemodialysis units in Egypt. East Mediterr Health J. 2007. Mar-Apr;13(2):392–407. [PubMed] [Google Scholar]
- 23.Sofola OO, Folayan MO, Denloye OO, Okeigbemen SA. Occupational exposure to bloodborne pathogens and management of exposure incidents in Nigerian dental schools. J Dent Educ. 2007. June;71(6):832–7. [PubMed] [Google Scholar]
- 24.De Villiers HC, Nel M, Prinsloo EA. Occupational exposure to bloodborne viruses amongst medical practitioners in Bloemfontein, South Africa. S Afr Fam Pract. 2007;49(3):14–c. 10.1080/20786204.2007.10873522 [DOI] [Google Scholar]
- 25.Taegtmeyer M, Suckling RM, Nguku PM, Meredith C, Kibaru J, Chakaya JM, et al. Working with risk: occupational safety issues among healthcare workers in Kenya. AIDS Care. 2008. March;20(3):304–10. 10.1080/09540120701583787 [DOI] [PubMed] [Google Scholar]
- 26.Laraqui O, Laraqui S, Tripodi D, Zahraoui M, Caubet A, Verger C, et al. [Assessing knowledge, attitude, and practice on occupational blood exposure in caregiving facilities in Morocco] (in French). Med Mal Infect. 2008. December;38(12):658–66. 10.1016/j.medmal.2008.09.009 [DOI] [PubMed] [Google Scholar]
- 27.Okeke EN, Ladep NG, Agaba EI, Malu AO. Hepatitis B vaccination status and needle stick injuries among medical students in a Nigerian university. Niger J Med. 2008. Jul-Aug;17(3):330–2. 10.4314/njm.v17i3.37404 [DOI] [PubMed] [Google Scholar]
- 28.Manyele SV, Ngonyani HA, Eliakimu E. The status of occupational safety among health service providers in hospitals in Tanzania. Tanzan J Health Res. 2008. July;10(3):159–65. 10.4314/thrb.v10i3.14356 [DOI] [PubMed] [Google Scholar]
- 29.Laraqui O, Laraqui S, Laraqui S, Tripodi D, Ouazzani LC, Caubet A, et al. [Evaluation of knowledge, attitudes and practices in the health care setting in Morocco with regard to hepatitis B and C]. Sante Publique. 2009. May-Jun;21(3):271–86. French. [PubMed] [Google Scholar]
- 30.Reda AA, Fisseha S, Mengistie B, Vandeweerd JM. Standard precautions: occupational exposure and behaviour of health care workers in Ethiopia. PLoS One. 2010. December 23;5(12):e14420. 10.1371/journal.pone.0014420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tadesse M, Tadesse T. Epidemiology of needlestick injuries among health-care workers in Awassa City, southern Ethiopia. Trop Doct. 2010. April;40(2):111–3. 10.1258/td.2009.090191 [DOI] [PubMed] [Google Scholar]
- 32.Tebeje B, Hailu C. Assessment of HIV post-exposure prophylaxis use among health workers of governmental health institutions in Jimma Zone, Oromiya Region, southwest Ethiopia. Ethiop J Health Sci. 2010. March;20(1):55–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Azodo C. Occupational risk of HIV infection among Nigerian dentists. Int J Infect Dis. 2010;14:e73 10.1016/j.ijid.2010.02.1652 [DOI] [Google Scholar]
- 34.Hanafi MI, Mohamed AM, Kassem MS, Shawki M. Needlestick injuries among health care workers of University of Alexandria Hospitals. East Mediterr Health J. 2011. January;17(1):26–35. [PubMed] [Google Scholar]
- 35.Nwankwo TO, Aniebue UU. Percutaneous injuries and accidental blood exposure in surgical residents: awareness and use of prophylaxis in relation to HIV. Niger J Clin Pract. 2011. Jan-Mar;14(1):34–7. 10.4103/1119-3077.79237 [DOI] [PubMed] [Google Scholar]
- 36.Elduma AH, Saeed NS. Hepatitis B virus infection among staff in three hospitals in Khartoum, Sudan, 2006–07. East Mediterr Health J. 2011. June;17(6):474–8. [PubMed] [Google Scholar]
- 37.Kumakech E, Achora S, Berggren V, Bajunirwe F. Occupational exposure to HIV: a conflict situation for health workers. Int Nurs Rev. 2011. December;58(4):454–62. 10.1111/j.1466-7657.2011.00887.x [DOI] [PubMed] [Google Scholar]
- 38.Shiferaw Y, Abebe T, Mihret A. Sharps injuries and exposure to blood and bloodstained body fluids involving medical waste handlers. Waste Manag Res. 2012. December;30(12):1299–305. 10.1177/0734242X12459550 [DOI] [PubMed] [Google Scholar]
- 39.Pellissier G, Yazdanpanah Y, Adehossi E, Tosini W, Madougou B, Ibrahima K, et al. Is universal HBV vaccination of healthcare workers a relevant strategy in developing endemic countries? The case of a university hospital in Niger. PLoS One. 2012;7(9):e44442. 10.1371/journal.pone.0044442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Owolabi RS, Alabi P, Ajayi S, Daniel O, Ogundiran A, Akande TM, et al. Knowledge and practice of post-exposure prophylaxis (PEP) against HIV infection among health care providers in a tertiary hospital in Nigeria. J Int Assoc Physicians AIDS Care (Chic). 2012. May-Jun;11(3):179–83. 10.1177/1545109711401409 [DOI] [PubMed] [Google Scholar]
- 41.Odongkara BM, Mulongo G, Mwetwale C, Akasiima A, Muchunguzi HV, Mukasa S, et al. Prevalence of occupational exposure to HIV among health workers in northern Uganda. Int J Risk Saf Med. 2012;24(2):103–13. [DOI] [PubMed] [Google Scholar]
- 42.Noubiap JJ, Nansseu JR, Kengne KK, Tchokfe Ndoula S, Agyingi LA. Occupational exposure to blood, hepatitis B vaccine knowledge and uptake among medical students in Cameroon. BMC Med Educ. 2013. November 8;13(1):148. 10.1186/1472-6920-13-148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zawilla NH, Ahmed D. Sharps injuries among health care workers in Cairo University Hospitals. Int J Risk Saf Med. 2013;25(2):79–92. [DOI] [PubMed] [Google Scholar]
- 44.Mathewos B, Birhan W, Kinfe S, Boru M, Tiruneh G, Addis Z, et al. Assessment of knowledge, attitude and practice towards post exposure prophylaxis for HIV among health care workers in Gondar, north west Ethiopia. BMC Public Health. 2013. May 25;13(1):508. 10.1186/1471-2458-13-508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yimechew Z, Tiruneh G, Ejigu T. Occupational exposures to blood and body fluids (BBFs) among health care workers and medical students in University of Gondar Hospital, northwest of Ethiopia. Glob J Med Res. 2013;13(3):17–23. [Google Scholar]
- 46.Mbaisi EM, Ng’ang’a Z, Wanzala P, Omolo J. Prevalence and factors associated with percutaneous injuries and splash exposures among health-care workers in a provincial hospital, Kenya, 2010. Pan Afr Med J. 2013;14:10. 10.11604/pamj.2013.14.10.1373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Osazuwa-Peters N, Obarisiagbon A, Azodo CC, Ehizele AO, Obuekwe ON. Occupational exposure to sharp injuries among medical and dental house officers in Nigeria. Int J Occup Med Environ Health. 2013. April;26(2):283–90. 10.2478/s13382-013-0098-y [DOI] [PubMed] [Google Scholar]
- 48.Bagny A, Bouglouga O, Djibril M, Lawson A, Laconi Kaaga Y, Hamza Sama D, et al. [Knowledge, attitudes, and practices relative to the risk of transmission of hepatitis B and C viruses in a hospital in Togo]. Med Sante Trop. 2013. Jul-Sep;23(3):300–3. French. [DOI] [PubMed] [Google Scholar]
- 49.Mashoto KO, Mubyazi GM, Mohamed H, Malebo HM. Self-reported occupational exposure to HIV and factors influencing its management practice: a study of healthcare workers in Tumbi and Dodoma Hospitals, Tanzania. BMC Health Serv Res. 2013. July 17;13(1):276. 10.1186/1472-6963-13-276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zoungrana J, Yaméogo TM, Kyelem CG, Aba YT, Sawadogo A, Millogo A. Connaissances, attitudes et pratiques des élèves des formations paramédicales face aux accidents d’exposition au sang au CHU Sanou-Sourô de Bobo-Dioulasso (Burkina Faso). Med Sante Trop. 2014. Jul-Sep;24(3):258–62. [French.] [DOI] [PubMed] [Google Scholar]
- 51.Beyera GK, Beyen TK. Epidemiology of exposure to HIV/AIDS risky conditions in healthcare settings: the case of health facilities in Gondar City, north west Ethiopia. BMC Public Health. 2014. December 16;14(1):1283. 10.1186/1471-2458-14-1283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yenesew MA, Fekadu GA. Occupational exposure to blood and body fluids among health care professionals in Bahir Dar town, northwest Ethiopia. Saf Health Work. 2014. March;5(1):17–22. 10.1016/j.shaw.2013.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Aynalem Tesfay F, Dejenie Habtewold T. Assessment of prevalence and determinants of occupational exposure to HIV infection among healthcare workers in selected health institutions in Debre Berhan town, North Shoa Zone, Amhara Region, Ethiopia, 2014. Aids Res Treat. 2014;2014:731848. 10.1155/2014/731848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Beyene T, Tadesse S. Predictors of occupational exposure to HIV infection among healthcare workers in southern Ethiopia. Int J Infect Control. 2014;10(3):2. [Google Scholar]
- 55.Ajibola S, Akinbami A, Elikwu C, Odesanya M, Uche E. Knowledge, attitude and practices of HIV post exposure prophylaxis amongst health workers in Lagos University Teaching Hospital. Pan Afr Med J. 2014. October 20;19:172. 10.11604/pamj.2014.19.172.4718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Amira CO, Awobusuyi JO. Needle-stick injury among health care workers in hemodialysis units in Nigeria: a multi-center study. Int J Occup Environ Med. 2014. January;5(1):1–8. [PMC free article] [PubMed] [Google Scholar]
- 57.Ogoina D, Pondei K, Adetunji B, Chima G, Isichei C, Gidado S. Prevalence and determinants of occupational exposures to blood and body fluids among health workers in two tertiary hospitals in Nigeria. Afr J Infect Dis. 2014;8(2):50–4. 10.4314/ajid.v8i2.7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mbah CC. Reporting of accidental occupational exposures to blood and body fluids by doctors and nurses in the public primary health care setting of sub district F of Johannesburg metropolitan district. Johannesburg: University of the Witwatersrand; 2014. Available from: http://wiredspace.wits.ac.za/handle/10539/15293 [cited 2017 Oct 2].
- 59.Bekele T, Gebremariam A, Kaso M, Ahmed K. Attitude, reporting behaviour and management practice of occupational needle stick and sharps injuries among hospital healthcare workers in Bale zone, southeast Ethiopia: a cross-sectional study. J Occup Med Toxicol. 2015. December 3;10(1):42. 10.1186/s12995-015-0085-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Burmen BK, Osoga J. Quantifying the magnitude of hazardous incidents among laboratory staff in Kenya; preliminary results of a national health care workers survey, 2014-2015. Antimicrob Resist Infect Control. 2015. June 16;4(1) Suppl 1:97 10.1186/2047-2994-4-S1-P97 [DOI] [Google Scholar]
- 61.Arheiam A, Ingafou M. Self-reported occupational health problems among Libyan dentists. J Contemp Dent Pract. 2015. January 1;16(1):31–5. 10.5005/jp-journals-10024-1631 [DOI] [PubMed] [Google Scholar]
- 62.Koné MC, Mallé KK. [Blood exposure accidents: knowledge and practices of hospital health workers in Mali]. Bull Soc Pathol Exot. 2015. December;108(5):369–72. French. [DOI] [PubMed] [Google Scholar]
- 63.Kateera F, Walker TD, Mutesa L, Mutabazi V, Musabeyesu E, Mukabatsinda C, et al. Hepatitis B and C seroprevalence among health care workers in a tertiary hospital in Rwanda. Trans R Soc Trop Med Hyg. 2015. March;109(3):203–8. 10.1093/trstmh/trv004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chalya PL, Seni J, Mushi MF, Mirambo MM, Jaka H, Rambau PF, et al. Needle-stick injuries and splash exposures among health-care workers at a tertiary care hospital in north-western Tanzania. Tanzan J Health Res. 2015;17(2) [Google Scholar]
- 65.Mponela MJ, Oleribe OO, Abade A, Kwesigabo G. Post exposure prophylaxis following occupational exposure to HIV: a survey of health care workers in Mbeya, Tanzania, 2009–2010. Pan Afr Med J. 2015. May 15;21:32. 10.11604/pamj.2015.21.32.4996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kassa G, Selenic D, Lahuerta M, Gaolathe T, Liu Y, Letang G, et al. Occupational exposure to bloodborne pathogens among health care workers in Botswana: reporting and utilization of postexposure prophylaxis. Am J Infect Control. 2016. August 1;44(8):879–85. 10.1016/j.ajic.2016.01.027 [DOI] [PubMed] [Google Scholar]
- 67.Kaweti G, Abegaz T. Prevalence of percutaneous injuries and associated factors among health care workers in Hawassa Referral and Adare District hospitals, Hawassa, Ethiopia, January 2014. BMC Public Health. 2016. January 5;16(1):8. 10.1186/s12889-015-2642-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Oluwatosin O, Oladapo M, Asuzu M. Needlestick injuries among health care workers in Ondo State, Nigeria. Int J Med Public Health. 2016;6(1):31 10.4103/2230-8598.179757 [DOI] [Google Scholar]
- 69.Nmadu AG, Sabitu K, Joshua IA. Occupational exposure to blood and body fluids among primary health-care workers in Kaduna State, Nigeria. J Med Trop. 2016;18(2):79 10.4103/2276-7096.192223 [DOI] [Google Scholar]
- 70.Makhado L, Davhana-Maselesele M. Knowledge and uptake of occupational post-exposure prophylaxis amongst nurses caring for people living with HIV. Curationis. 2016. March 29;39(1):1593. 10.4102/curationis.v39i1.1593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lahuerta M, Selenic D, Kassa G, Mwakitosha G, Hokororo J, Ngonyani H, et al. Reporting and case management of occupational exposures to blood-borne pathogens among healthcare workers in three healthcare facilities in Tanzania. J Infect Prev. 2016;17(4):153–60. 10.1177/1757177416645343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shindano TA, Bahizire E, Fiasse R, Horsmans Y. Knowledge, attitudes, and practices of health-care workers about viral hepatitis B and C in south Kivu. Am J Trop Med Hyg. 2017. February 8;96(2):400–4. 10.4269/ajtmh.16-0287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sharew NT, Mulu GB, Habtewold TD, Gizachew KD. Occupational exposure to sharps injury among healthcare providers in Ethiopia regional hospitals. Ann Occup Environ Med. 2017. March 23;29(1):7. 10.1186/s40557-017-0163-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Laisser RM, Ng’home JF. Reported incidences and factors associated with percutaneous injuries and splash exposures among healthcare workers in Kahama District, Tanzania. Tanzan J Health Res. 2017;19(1) 10.4314/thrb.v19i1.4 [DOI] [Google Scholar]
- 75.Nguyen M, Paton S, Koch J. Update-surveillance of health care workers exposed to blood, body fluids and bloodborne pathogens in Canadian hospital settings: 1 April, 2000, to 31 March, 2002. Can Commun Dis Rep. 2003. December 15;29(24):209–13. [PubMed] [Google Scholar]
- 76.Shokuhi Sh, Gachkar L, Alavi-Darazam I, Yuhanaee P, Sajadi M. Occupational exposure to blood and body fluids among health care workers in teaching hospitals in Tehran, Iran. Iran Red Crescent Med J. 2012. July;14(7):402–7. [PMC free article] [PubMed] [Google Scholar]
- 77.Clarke SP, Sloane DM, Aiken LH. Effects of hospital staffing and organizational climate on needlestick injuries to nurses. Am J Public Health. 2002. July;92(7):1115–9. 10.2105/AJPH.92.7.1115 [DOI] [PMC free article] [PubMed] [Google Scholar]