Abstract
Transmission of malaria from man to mosquito defines the human infectious reservoir of malaria. At the population level this is influenced by a variety of human, parasite, and mosquito vector factors some or all of which may vary depending on the epidemiological setting. Here, we review our current state of knowledge related to human infectiousness to mosquitoes and how current malaria control strategies might be adapted to focus on reducing this. While much progress has been made in malaria control, we argue that an improved understanding of human infectivity will allow more effective use of current control tools and make elimination a more feasible goal.
The transmission of malaria from humans to mosquitoes is influenced by a variety of factors that vary depending on the epidemiological setting. A better understanding of them may lead to more effective interventions.
Considerable progress has been made in our understanding of the production and maturation of Plasmodium falciparum gametocytes and associated factors related to the biology of malaria transmission at the level of the individual malaria-infected patients (Meibalan and Marti 2016). In this review, we examine how these individual factors combine with epidemiological and entomological elements to define transmission at the population level. The premise is that if we are able to better understand transmission, we can then more effectively target malaria transmission stages in the fraction of the human population that is most important for onward malaria transmission to mosquitoes. Moreover, improving our understanding of malaria transmission at the population level will also allow more accurate monitoring of the effects of control and elimination programs to specifically counter the likelihood of resurgence of malaria in areas that remain receptive to malaria.
THE HUMAN PARASITE RESERVOIR: A MATTER OF SENSITIVITY
Community estimates of parasite carriage by microscopy (as parasite prevalence) are the most widely available malaria metrics and are commonly used to compare malaria transmission intensity between areas (Hay et al. 2009) and compare infection burden between different populations within malaria-endemic regions (Smith et al. 2005). Routine microscopy has a sensitivity in the range of 50–100 parasites/μL of blood (Okell et al. 2012). Rapid diagnostic tests (RDTs), lateral flow devices that are based on histidine-rich protein 2 (HRP2) or Plasmodium lactate dehydrogenase (pLDH) and aldolase (Bell et al. 2006), achieve a similar sensitivity ∼100 parasites/μL (Banoo et al. 2006) and are increasingly widely used for routine malaria diagnosis and epidemiological surveys (Bastiaens et al. 2014). Estimates of parasite prevalence from RDTs are often slightly higher than microscopy because of the persistence of antigen after infection has been cleared (Wu et al. 2015). Molecular methods for malaria detection and species identification include nested polymerase chain reaction (nPCR) (Snounou et al. 1993), quantitative PCR (qPCR), quantitative reverse transcription (qRT)-PCR, and nucleic acid sequence-based amplification (NASBA), and invariably detect a larger number of infections. nPCR, for which most data is available, in conjunction with microscopy data, detects approximately twice as many infections as microscopy (Okell et al. 2012), but the fraction of infections that is undetectable by microscopy varies considerably between endemic areas and population groups. Although parasite prevalence overall is lower, the proportion of infections present at submicroscopic parasite densities is largest in low-endemic areas (Fig. 1). This may reflect the lower density of malaria parasites in low transmission settings that may, in turn, be explained by a lower likelihood of superinfection and a large fraction of monoclonal infections, which are better controlled by the human immune system (Okell et al. 2012). In all areas in which transmission intensity is sufficiently high to elicit an age-dependent acquisition of immunity, adults are more likely to carry submicroscopic infections (Proietti et al. 2011; Nguyen et al. 2012; Mosha et al. 2013). The role of antimalarial immunity in determining the density and detectability of infections is currently incompletely understood. In the context of malaria-elimination initiatives, it is of great relevance to understand the influence of waning residual immunity (that follows successful malaria control) on the likelihood that malaria infections elicit malaria symptoms and reach densities that are detectable by currently available diagnostics (Wu et al. 2015).
Figure 1.
Submicroscopic parasite carriage as a function of malaria transmission intensity. Prevalence of Plasmodium falciparum infections detected by nested polymerase chain reaction (nPCR, x-axis) or microscopy (y-axis) in the same individuals. Each data point and associated confidence intervals represent data from one cross-sectional survey. The dotted line shows the correlation that would be expected if the prevalence of infection detected by both methods was the same. The solid line with shaded area represents the best fit to the data and confidence interval (Okell et al. 2012). (Panel from Okell et al. 2012; adapted, with permission, from the authors.) The shaded arrows in the panel below shows (top) the intensity of malaria transmission, which is typically defined by microscopy and ranges from low to high transmission intensity; (middle) the average parasite density in infections that increases with transmission intensity; and (bottom) the proportion of infections that is submicroscopic. While the proportion of the population that is malaria-infected is lowest in low-endemic settings, the fraction of these infections that is submicroscopic (undetectable by microscopy and rapid diagnostic tests [RDTs]) (Wu et al. 2015) is largest in low-endemic settings.
GAMETOCYTE PRODUCTION AND THE HUMAN INFECTIOUS RESERVOIR
Not surprisingly, in a similar way that molecular diagnostics have identified increased number of malaria infections, molecular assays for the sexual-stage gametocytes have unveiled a previously unappreciated pool of low-density gametocyte carriers (Bousema and Drakeley 2011). The detection of gametocyte-specific mRNA, primarily based on Pfs25 mRNA (Bousema and Drakeley 2011; Wampfler et al. 2013), pfg377, or Pfs230 (Nwakanma et al. 2008), has shown that mature gametocytes can be detected in the majority of symptomatic (Nassir et al. 2005; Nwakanma et al. 2008; Sawa et al. 2013; Eziefula et al. 2014) and asymptomatically P. falciparum–infected individuals (Ouedraogo et al. 2007; Shekalaghe et al. 2007; Nwakanma et al. 2008), even if densities of asexual parasites are below the microscopic threshold for detection (Ouedraogo et al. 2007; Shekalaghe et al. 2007; Wampfler et al. 2013).
The presence of an undetectable gametocyte fraction was assumed before the advent of molecular gametocyte diagnostics as a result of observations of successful mosquito infections from individuals who had no gametocytes detected by microscopy (Boudin et al. 1993b; Bousema et al. 2012a). The proportion of mosquitoes that becomes infected after feeding on these submicroscopic, and therefore low-density, gametocyte carriers is typically two- to fivefold lower than on microscopy-positive gametocyte carriers (Coleman et al. 2004; Schneider et al. 2007; Ouedraogo et al. 2009, 2015; Bousema et al. 2012a; Churcher et al. 2013). However, the high number of these individuals in many endemic settings makes submicroscopic gametocyte carriers potentially significant contributors to malaria transmission (Bousema et al. 2014).
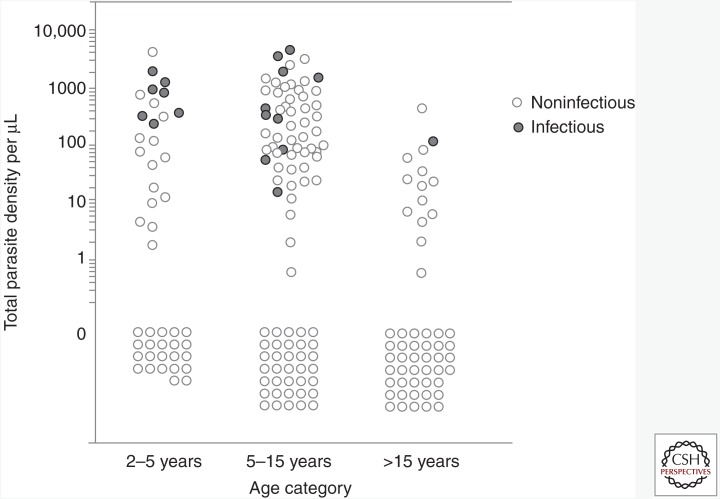
It would seem important that from a public health perspective, a better understanding of the detectability of malaria infections that infect mosquitoes and quantification of the relative contribution of patent and subpatent infections to the human infectious reservoir is required. As examples, two recent studies that determined infectivity in community surveys indicate that onward transmission to mosquitoes is possible from infections that have no detectable gametocytes or asexual parasites by microscopy. In a moderate transmission setting in Senegal, 8% (2/25) of infectious individuals had no parasites detected by microscopy, yet were responsible for 18.2% (12/66) of all mosquito infections. A similar study in a high-endemic setting in Burkina Faso found that 28.7% (25/87) of infectious individuals were parasite-free by microscopy, yet responsible for 17.0% (145/855) of all infected mosquitoes (Ouedraogo et al. 2015). The latter study was the first to use molecular assays in combination with mosquito feeding assays and confirmed that all infectious individuals had parasites detected by RNA-based diagnostics (Ouedraogo et al. 2015). In a setting of intense malaria transmission in Burkina Faso, a substantial proportion of infectious individuals harbored parasite densities below 100 parasites/μL, the density realistically detected by microscopy and RDTs (Fig. 2). These and other findings from transmission studies (Stone et al. 2015) would suggest two relatively clear and distinct paths for diagnostics for research and public health settings. First, more detailed research studies aiming to quantify the human infectious reservoir or quantify the impact of transmission-blocking interventions would benefit from the use of sensitive, quantitative molecular tools to detect asexual parasite populations and gametocytes. In contrast, outside research settings, malaria surveillance and surveys to support and evaluate community interventions would not require gametocyte-specific diagnostics but instead rely on sensitive methods to detect all stages of infection on the assumption that all parasite infections are viable sources of gametocytes and therefore potentially infectious (Bousema et al. 2014).
Figure 2.
Parasite density and infectivity in relation to age in an area of intense malaria transmission. Each dot represents an individual in which parasite carriage was determined and quantified by a quantitative polymerase chain reaction (qPCR) (Hermsen et al. 2001) and onward malaria transmission was determined by membrane feeding assays. Open dots represent uninfected individuals (parasite density equals zero) or malaria-infected individuals who are not infectious to mosquitoes. Closed circles indicate infectious individuals. Parasite density and the likelihood of infecting mosquitoes are highest in children; onward transmission to mosquitoes is commonly observed at densities below the microscopic threshold for detection (Slater et al. 2015).
DEMOGRAPHIC AND ENVIRONMENTAL FACTORS INFLUENCING MALARIA TRANSMISSIBILITY
Many previous studies aiming to determine the human infectious reservoir often selected individuals with microscopically detected gametocytes for transmission experiments (Stone et al. 2015) and, as discussed above, it is now clear that this approach will have missed individuals who harbor submicroscopic gametocyte densities and who are potentially infectious. However, even if individuals are selected randomly from a population to assess their infectivity, these infectivity assessments do not allow drawing firm conclusions on the relative importance of different populations for onward transmission. The selection of study participants often does not take into account the demographic structure of populations. All available studies on the human infectivity to mosquitoes that included a wide age range, observed that the proportion of mosquitoes that become infected in mosquito feeding assays is higher in children, because they have higher gametocyte densities than adults (Muirhead-Thomson 1957; Graves et al. 1988; Githeko et al. 1992; Boudin et al. 1993a; Bonnet et al. 2003; Gaye et al. 2015). Yet, when estimates are adjusted for the demographic composition of the population, the importance of older individuals becomes clearer and in many settings adults comprise >20% of the human infectious reservoir (Muirhead-Thomson 1957; Graves et al. 1988; Githeko et al. 1992; Bonnet et al. 2003; Stone et al. 2015). This contribution may be increased further if, in addition to demographic composition, both accessibility and attractiveness of humans to mosquitoes are also considered. These factors have never been directly incorporated in assessments of the human infectious reservoir, although it is well known that the number of mosquito bites a person experiences is highly variable. This is mostly driven by human behavior and use of protective measures. In areas with good bednet coverage because of the specific targeting of children and pregnant women, net use is highest in young children (<5 years) and lowest in older children and adolescents (Baume and Marin 2007; Bernard et al. 2009; Matovu et al. 2009; Kulkarni et al. 2010). Moreover, older individuals also tend to spend more evening hours awake, unprotected by a net and sometimes outdoors where a relevant, and potentially increasing, proportion of bites of malaria-transmitting mosquitoes occurs (Braimah et al. 2005; Reddy et al. 2011; Moiroux et al. 2012). Adults are also more likely to attract mosquitoes (Muirhead-Thomson 1951; Thomas 1951; Clyde and Shute 1958; Carnevale et al. 1978; Port et al. 1980), a phenomenon probably associated with their larger body weight and surface area (Port et al. 1980) and possibly with body temperature and chemical cues that change with increasing age (Knols et al. 1995; Mukabana et al. 2002; Qiu et al. 2006). The lower intervention use, shorter sleeping times, and increased attractiveness to mosquitoes all make older children and adults considerably more likely to be fed on by anopheline vectors and thereby increase their plausible contribution to the human infectious reservoir for malaria if they are infectious to mosquitoes (Fig. 3).
Figure 3.
The human infectious reservoir for malaria. Individuals in the figure are represented by circles in three age groups: <5 (dark gray), 5–15 (black), and >15 years (light gray). The age-stratified population (A) reflects the abundance of individuals in each group based on a simplified population age structure in sub-Saharan Africa. (B) Speckling within circles represents the presence of Plasmodium falciparum gametocytes by molecular methods. (C) Solid filled circles represent who are infectious to mosquitoes in membrane feeding assays. Many gametocytemic individuals are not infectious to mosquitoes at the moment of sampling. The top pie chart presents the proportional contribution of each age group to the human infectious reservoir for malaria after taking into account the demographic distributions in the population. The bottom pie chart presents the same contribution to the human infectious reservoir for malaria, taking into account differences in mosquito-biting frequency that are related to differences in behavior, protective measures, and attractiveness to mosquitoes because of body size and other host characteristics. (From data in Stone et al. 2015; adapted, with permission, from Elsevier © 2015.)
By their nature, cross-sectional surveys are typically time-dependent and reflect a relatively short period or snapshot of population infectivity to mosquitoes. This creates challenges for extrapolating single time-point measurements of infectivity to an overall contribution to transmission. It is clear that infections have differing dynamics in different hosts, which will depend on both intrinsic (e.g., age and immunity as a result of local malaria endemicity) and extrinsic factors (e.g., treatment seeking behavior and treatment). There are very limited data on longitudinal infectiousness of individuals or populations following natural infections. Malaria therapy studies have shown that individuals can be infected for up to a year and be infectious to mosquitoes at multiple time points during this period (Jeffery and Eyles 1955). In the absence of sufficiently detailed data from natural infections, the malaria therapy data form a key component of infectiousness in malaria mathematical models (Ross et al. 2006; Okell et al. 2012; Johnston et al. 2013). However, as discussed above, continual exposure to parasites and the acquisition of immunity will influence asexual and, consequently, sexual parasite levels, which together with factors such as human genetics, exposure to other infections, and nutrition and the restricted demographic range of the participants in the malaria therapy studies limits interpretation from these studies. One of the important suggestions from malaria therapy data that requires further studies in natural infections concerns temporal fluctuations in the infectivity of gametocytes during an infection (Johnston et al. 2013). This corroborates anecdotal reports from natural infections that the likelihood of mosquito infection at a given gametocyte density may fluctuate between seasons and potentially with the duration of malaria infections (Ouedraogo et al. 2015) and would clearly be a very important factor to parametrize malaria transmission models. A recent analysis of case reports and infections post-blood-transfusion has suggested that infections with P. falciparum may last for up to 13 years (Ashley and White 2014). No data on gametocyte carriage or infectivity are available for these and other chronic infections, yet such data may be vitally important in managing malaria resurgence in areas of low transmission.
Broadly speaking, two types of infection scenario can be seen as bookends with individuals transitioning from one to the other as immunity develops. Initially, acute infections in individuals with limited immunity would be expected to result in higher parasite and gametocyte densities infecting high numbers of mosquitoes. These infections would be of relatively short duration curtailed by drugs or, in a worst-case scenario, death. Eventually, infections in immune individuals will achieve low parasite densities and consequently infect few mosquitoes and only sporadically. These infections, however, might be expected to last for several weeks and months as infections are asymptomatic. The infectiousness of any subsequent or superinfection may also depend on when in a transmission season this occurs. Ouédraogo et al. (2015) showed that as parasite and gametocyte densities declined during a transmission season in Burkina Faso, so did infectiousness to mosquitoes. The decline was more pronounced in the youngest age groups, which was hypothesized to be a result of transmission-blocking immunity (discussed below), while the infectiousness of adults remained consistent and low. Longitudinal data such as these need to be interpreted in the context of the likelihood of the infected host being sampled by a mosquito. In Burkina Faso, where malaria transmission vector densities are highly seasonal and in the dry season infectious individuals may rarely be bitten and mosquitoes that do bite may not survive to transmit malaria. Counts of infected mosquitoes can be used to estimate indirect measures of population infectiousness or k (Killeen et al. 2006; Tusting et al. 2014). These measures are perhaps of more use for evaluating entomological factors than for characterizing the human infectious reservoir for malaria as individual human or age-specific contributions cannot be derived. Additionally, there are inconsistencies in methodological approaches used to calculate k, including variation in mosquito trapping techniques that only sample a subset of available mosquitoes, limiting the broader relevance of any estimates.
HUMAN IMMUNE RESPONSES INFLUENCING MALARIA TRANSMISSIBILITY
Human immune responses can influence malaria transmission in several ways. Immune responses that reduce or prevent the establishment of blood-stage parasitemia or reduce the multiplication of the asexual will reduce malaria transmission potential by simply reducing the number of parasites that become gametocytes. Immune responses may also directly target gametocytes or their infectivity to mosquitoes. As described in detail in Meibalan and Marti (2016), early-stage gametocytes of P. falciparum sequester primarily in the bone marrow (Sinden and Smalley 1979). There is incomplete evidence for immune recognition of proteins that are present on the surface of erythrocytes that contain developing gametocytes and may influence gametocyte sequestration (Hayward et al. 1999; Rogers et al. 2000) and maturation (Sutherland 2009; Tonwong et al. 2012). Similarly, naturally acquired antibody responses have been described that recognize erythrocytes containing mature, infectious gametocytes and these antibodies have been hypothesized to play a role in the immune clearance of circulating gametocytes (Saeed et al. 2008). However, experimental evidence for these immune responses to gametocyte-infected erythrocytes is difficult to show and a functional phenotype is only speculative. By comparison, the natural acquisition of antibody responses that influence the infectivity of gametocytes/gametes in mosquitoes is well established. Gametocytes circulate for an average of 4 to 7 days (Bousema et al. 2010), after which gametocytes are removed from the bloodstream in the spleen and gametocyte proteins become accessible to the human immune system. Proteomic analysis has identified >2000 proteins that are expressed in gametocytes of which several hundred that are specific to stage IV and V gametocytes (Lasonder et al. 2002, 2016; Le Roch et al. 2004; Silvestrini et al. 2010). Immune recognition and the functionality of immune responses have been described for only a handful of these proteins. This is due, in part, to a lack of specific reagents to examine immune recognition, which is in turn influenced by the fact that several protein families have complicated tertiary structure. The limited immune responses to gametocyte antigens may be a result of reproductive restraint in which the parasite produces low levels of gametocytes to avoid the induction of effective immunity (Taylor and Read 1997).
The functionality of antibody responses to gametocyte and gamete antigens can be most convincingly assessed in in vitro experiments in which purified IgG is mixed with cultured gametocytes and offered to mosquitoes in the standard membrane feeding assay (SMFA) (Ponnudurai et al. 1989). The SMFA has revealed that naturally acquired antibodies to sexual-stage malaria parasites may inhibit fertilization in the mosquito midgut by inhibiting gamete mobility, reducing contact between male and female gametes or complement-mediated gamete lysis (Vermeulen et al. 1985; Grotendorst et al. 1986; Kaslow et al. 1992; Ranawaka et al. 1994). At present, the most convincing evidence for naturally acquired transmission-reducing immunity (TRI) has been associated with the presence of antibodies to proteins Pfs230 and Pfs48/45 (Rener et al. 1983), which are present on the surface of gametocytes/gametes and play an important role in male microgamete fertility (van Dijk et al. 2001; Eksi et al. 2006). Pfs25 and Pfs28 (Duffy and Kaslow 1997) are candidate proteins for the development of vaccines that elicit TRI but play no relevant role in naturally acquired TRI because these genes are posttranscriptionally repressed until the parasite’s development in the mosquito midgut (Pradel 2007) and proteins are therefore not exposed to the human immune system (Miura et al. 2013). Antibody responses to Pfs8/45 and Pfs230 have been detected in numerous malaria-endemic settings and have been associated with TRI (Premawansa et al. 1994; Healer et al. 1999; Bousema et al. 2006, 2010; Drakeley et al. 2006; Jones et al. 2015) and monoclonal antibodies against several epitopes of these proteins reproducibly inhibit parasite fertilization. However, the role of Pfs45/48 and Pfs230 antibodies in natural malaria transmission remains to be quantified and a possible role of antibody responses to other sexual-stage antigens has been hypothesized but remains to be proven. Despite the need for more research in this area, it is evident that human immune responses can reduce the transmissibility of gametocytes in many African settings (Bousema et al. 2011) and that a fraction of gametocyte carriers, estimated at ∼5%, is capable of completely preventing mosquito infection (Bousema et al. 2006, 2007; Drakeley et al. 2006).
CONSIDERATIONS FOR THE DEPLOYMENT OF TRANSMISSION-REDUCING INTERVENTIONS
Key considerations for any intervention that aim to reduce transmission are intervention coverage and the duration of the transmission-reducing effects. Coverage is essential in light of the discussion above about the relatively high prevalence of potentially infectious individuals who would be missed by current diagnostics. Recent work on trial design for transmission-blocking vaccines (TBVs) suggests that coverage of 80% is required for a viable vaccine effect (Delrieu et al. 2015) with efficacy further enhanced if the immunity induced lasts for 12 months or longer. This latter point has implications for choice of vaccine antigen and immunization strategies. The impact of all TBV will be dependent on their ability to induce long-lasting TRI. Vaccines based on prefertilization antigens such as Pfs48/45 and Pfs230 may benefit from natural boosting of immune responses because these proteins are expressed in gametocytes and thereby naturally presented to the immune system. TBV based on postfertilization antigens (e.g., Pfs25) would not be boosted as the immune system is not exposed to these antigens and are thereby fully dependent on the immunization approach (Nikolaeva et al. 2015). Antibody levels are crucial for the efficacy of the RTS,S vaccine (White et al. 2015) and the same could be expected for a TBV; the efficacy of naturally induced sexual-stage antibodies is strongly influenced by antibody titer (Bousema et al. 2010).
In the absence of an efficacious TBV, transmission-reducing interventions now commonly rely on the deployment of antimalarial drugs to reduce the human infectious reservoir for malaria. Community-based chemotherapy studies have shown that only treating individuals positive by RDT had a very limited effect on transmission (Tiono et al. 2013; Cook et al. 2015). This limited effect is related to the proportion of infections that are capable of resulting in onward malaria transmission that is undetected and therefore not targeted (Tiono et al. 2013; Cook et al. 2015). In addition, the beneficial prophylactic effect of antimalarial drugs is withheld from a large proportion of the population if treatment is prompted by malaria diagnosis, which may further reduce the effect of screening-and-treatment approaches (Okell et al. 2011). At present, there is little data to indicate what density of parasites a diagnostic test needs to achieve for a screen-and-treat approach to become effective. On one hand, the proportion of populations that is diagnosed with malaria infections is increasing with the improvement of molecular diagnostics that examine larger blood volumes or use more sensitive molecular targets (Bousema et al. 2014; Hofmann et al. 2015). On the other hand, countries are eliminating malaria without a specific focus on these low-density carriers (Bousema et al. 2014; Lin et al. 2014). It appears likely, albeit formally unproven, that malaria elimination can be achieved sooner if field-deployable diagnostics become more sensitive and allow a larger proportion of the parasitemic and infectious human reservoir to be included in interventions.
It is important to note that infections are likely to cluster in a household and these households in turn may also be proximal. Studies in highland Kenya and low-endemic Tanzania found that individuals were 2–6 times more likely to be positive by molecular tests in the household of a person with a positive RDT (Cook et al. 2015; Stresman et al. 2015). This spatial heterogeneity in transmission has long been recognized at a macro scale but is only recently being described at micro scales (Bousema et al. 2012b; Bejon et al. 2014). The clustering of malaria infections in certain geographic areas (Bousema et al. 2012b; Bejon et al. 2014) or demographic populations (Wesolowski et al. 2012; Yangzom et al. 2012; Sturrock et al. 2013) has relevance for both the development of immunity to infection and long-term parasite carriage as well as for targeted control. In some instances in which malaria control nears elimination but isolated pockets of continued transmission persist, the value of targeted interventions is evident. In other settings in which malaria continues to be widespread but heterogeneous, the evidence that supports targeted interventions is less convincing. In these settings, individuals who are disproportionally exposed to (infected and uninfected) mosquitoes may spread malaria transmission to the larger population (Woolhouse et al. 1997). Targeting these areas or individuals theoretically forms a highly efficient approach to malaria control (Bousema et al. 2012b), but many uncertainties exist about the size, locality, detectability, and stability of so-called malaria hot spots (Bejon et al. 2014). One cluster-randomized trial that quantified the effect of hot-spot-targeted interventions on surrounding malaria-endemic communities found very limited evidence for this community effect (Bousema et al. 2016) Uncertainties about the spatial and temporal dynamics of infected mosquitoes and human parasite carriers currently hinder the rational targeting of interventions in areas where malaria transmission is widespread but geographically heterogeneous.
CONCLUSIONS
Control and transmission reduction of malaria presents a uniquely complex challenge given its high transmissibility or R0 (Smith et al. 2005). Notwithstanding this, there are encouraging reports of major reductions in malaria morbidity worldwide (Bhatt et al. 2015) and several countries have recently declared themselves malaria-free. However, lessons from history showed that the Global Malaria Eradication Program had little impact in Africa and, while this was attributed to decreasing efficacy of interventions, there was also major acknowledgment of our lack of fundamental understanding of basic biology. The currently available, field-deployable diagnostic approaches, such as microscopy and RDT, miss a substantial proportion of infectious individuals. This undiagnosed fraction of the human infectious reservoir may limit the impact of malaria interventions that depend on malaria diagnostics and extend the intervention phase that is required before elimination is achieved. TBV would be a highly desirable asset for malaria-elimination efforts and would avoid the challenges experienced by chemotherapy approaches that rely on malaria diagnosis. TBV are receiving significant investment, but their efficacy will depend on the longevity of the induced immune responses and the coverage of individuals transmitting infections to mosquitoes. A better understanding of the dynamics of the human infectious reservoir for malaria in the context of changing mosquito vector populations and other malaria-control interventions is urgently needed to support the implementation of TBV and other transmission-reducing interventions by setting evidence-based targets in terms of spatial and demographic coverage levels and the strength and longevity of the incurred protection.
ACKNOWLEDGMENTS
We thank Bronner Goncalves for discussion and advice and Sophie van Kempen for graphic design. T.B. is supported by the Bill and Melinda Gates Foundation (OPP1034789) and the European Research Council (ERC-2014-StG 639776). C.D. is supported by the Bill and Melinda Gates Foundation (OPP1034789) and the Wellcome Trust (091924).
Footnotes
Editors: Dyann F. Wirth and Pedro L. Alonso
Additional Perspectives on Malaria: Biology in the Era of Eradication available at www.perspectivesinmedicine.org
REFERENCES
*Reference is also in this collection.
- Ashley EA, White NJ. 2014. The duration of Plasmodium falciparum infections. Malaria J 13: 500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banoo S, Bell D, Bossuyt P, Herring A, Mabey D, Poole F, Smith PG, Sriram N, Wongsrichanalai C, Linke R, et al. 2006. Evaluation of diagnostic tests for infectious diseases: General principles. Nat Rev Microbiol 4: S20–S32. [DOI] [PubMed] [Google Scholar]
- Bastiaens GJ, Bousema T, Leslie T. 2014. Scale-up of malaria rapid diagnostic tests and artemisinin-based combination therapy: Challenges and perspectives in sub-Saharan Africa. PLoS Med 11: e1001590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baume CA, Marin MC. 2007. Intra-household mosquito net use in Ethiopia, Ghana, Mali, Nigeria, Senegal, and Zambia: Are nets being used? Who in the household uses them? Am J Trop Med Hyg 77: 963–971. [PubMed] [Google Scholar]
- Bejon P, Williams TN, Nyundo C, Hay SI, Benz D, Gething PW, Otiende M, Peshu J, Bashraheil M, Greenhouse B, et al. 2014. A micro-epidemiological analysis of febrile malaria in Coastal Kenya showing hotspots within hotspots. eLife 3: e02130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell D, Wongsrichanalai C, Barnwell JW. 2006. Ensuring quality and access for malaria diagnosis: How can it be achieved? Nat Rev Microbiol 4: 682–695. [DOI] [PubMed] [Google Scholar]
- Bernard J, Mtove G, Mandike R, Mtei F, Maxwell C, Reyburn H. 2009. Equity and coverage of insecticide-treated bed nets in an area of intense transmission of Plasmodium falciparum in Tanzania. Malaria J 8: 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatt S, Weiss DJ, Cameron E, Bisanzio D, Mappin B, Dalrymple U, Battle KE, Moyes CL, Henry A, Eckhoff PA, et al. 2015. The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature 526: 207–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnet S, Gouagna LC, Paul RE, Safeukui I, Meunier JY, Boudin C. 2003. Estimation of malaria transmission from humans to mosquitoes in two neighbouring villages in south Cameroon: Evaluation and comparison of several indices. Trans R Soc Trop Med Hyg 97: 53–59. [DOI] [PubMed] [Google Scholar]
- Boudin C, Olivier M, Molez JF, Chiron JP, Ambroise-Thomas P. 1993a. High human malarial infectivity to laboratory-bred Anopheles gambiae in a village in Burkina Faso. Am J Trop Med Hyg 48: 700–706. [DOI] [PubMed] [Google Scholar]
- Boudin C, Olivier M, Molez JF, Chiron JP, Ambroise-Thomas P. 1993b. High human malarial infectivity to laboratory-bred Anopheles gambiae in a village in Burkina Faso. Am J Trop Med Hyg 48: 700–706. [DOI] [PubMed] [Google Scholar]
- Bousema T, Drakeley C. 2011. Epidemiology and infectivity of Plasmodium falciparum and Plasmodium vivax gametocytes in relation to malaria control and elimination. Clin Microbiol Rev 24: 377–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bousema JT, Roeffen W, van der Kolk M, de Vlas SJ, van de Vegte-Bolmer M, Bangs MJ, Teelen K, Kurniawan L, Maguire JD, Baird JK, et al. 2006. Rapid onset of transmission-reducing antibodies in Javanese migrants exposed to malaria in Papua, Indonesia. Am J Trop Med Hyg 74: 425–431. [PubMed] [Google Scholar]
- Bousema JT, Drakeley CJ, Kihonda J, Hendriks JC, Akim NI, Roeffen W, Sauerwein RW. 2007. A longitudinal study of immune responses to Plasmodium falciparum sexual stage antigens in Tanzanian adults. Parasite Immunol 29: 309–317. [DOI] [PubMed] [Google Scholar]
- Bousema T, Okell L, Shekalaghe S, Griffin J, Omar S, Sawa P, Sutherland C, Sauerwein R, Ghani A, Drakeley C. 2010. Revisiting the circulation time of Plasmodium falciparum gametocytes: Molecular detection methods to estimate the duration of gametocyte carriage and the effect of gametocytocidal drugs. Malaria J 9: 136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bousema T, Sutherland CJ, Churcher TS, Mulder B, Gouagna LC, Riley EM, Targett GA, Drakeley CJ. 2011. Human immune responses that reduce the transmission of Plasmodium falciparum in African populations. Int J Parasitol 41: 293–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bousema T, Dinglasan RR, Morlais I, Gouagna LC, van Warmerdam T, Awono-Ambene PH, Bonnet S, Diallo M, Coulibaly M, Tchuinkam T, et al. 2012a. Mosquito feeding assays to determine the infectiousness of naturally infected Plasmodium falciparum gametocyte carriers. PLoS ONE 7: e42821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bousema T, Griffin JT, Sauerwein RW, Smith DL, Churcher TS, Takken W, Ghani A, Drakeley C, Gosling R. 2012b. Hitting hotspots: Spatial targeting of malaria for control and elimination. PLoS Med 9: e1001165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bousema T, Okell L, Felger I, Drakeley C. 2014. Asymptomatic malaria infections: Detectability, transmissibility and public health relevance. Nat Rev Microbiol 12: 833–840. [DOI] [PubMed] [Google Scholar]
- Bousema T, Stresman G, Baidjoe AY, Bradley J, Knight P, Stone W, Osoti V, Makori E, Owaga C, Odongo W, et al. 2016. The impact of hotspot-targeted interventions on malaria transmission in Rachuonyo South District in the Western Kenyan Highlands: A cluster-randomized controlled trial. PLoS Med 13: e1001993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braimah N, Drakeley C, Kweka E, Mosha F, Helinski M, Pates H, Maxwell C, Massawe T, Kenward MG, Curtis C. 2005. Tests of bednet traps (Mbita traps) for monitoring mosquito populations and time of biting in Tanzania and possible impact of prolonged insecticide treated net use. Int J Trop Insect Sci 25: 208–213. [Google Scholar]
- Carnevale P, Frézil JL, Bosseno MF, Le Pont F, Lancien J. 1978. The aggressiveness of Anopheles gambiae A in relation to the age and sex of the human subjects. Bull World Health Organ 56: 147–154. [PMC free article] [PubMed] [Google Scholar]
- Churcher TS, Bousema T, Walker M, Drakeley C, Schneider P, Ouedraogo AL, Basanez MG. 2013. Predicting mosquito infection from Plasmodium falciparum gametocyte density and estimating the reservoir of infection. eLife 2: e00626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clyde DF, Shute GT. 1958. Selective feeding habits of Anophelines amongst Africans of different ages. Am J Trop Med Hyg 7: 543–545. [DOI] [PubMed] [Google Scholar]
- Coleman RE, Kumpitak C, Ponlawat A, Maneechai N, Phunkitchar V, Rachapaew N, Zollner G, Sattabongkot J. 2004. Infectivity of asymptomatic Plasmodium-infected human populations to Anopheles dirus mosquitoes in western Thailand. J Med Entomol 41: 201–208. [DOI] [PubMed] [Google Scholar]
- Cook J, Xu W, Msellem M, Vonk M, Bergstrom B, Gosling R, Al-Mafazy AW, McElroy P, Molteni F, Abass AK, et al. 2015. Mass screening and treatment on the basis of results of a Plasmodium falciparum–specific rapid diagnostic test did not reduce malaria incidence in Zanzibar. J Infect Dis 211: 1476–1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delrieu I, Leboulleux D, Ivinson K, Gessner BD; Malaria Transmission Blocking Vaccine Technical Consultation Group. 2015. Design of a phase III cluster randomized trial to assess the efficacy and safety of a malaria transmission blocking vaccine. Vaccine 33: 1518–1526. [DOI] [PubMed] [Google Scholar]
- Drakeley CJ, Bousema JT, Akim NI, Teelen K, Roeffen W, Lensen AH, Bolmer M, Eling W, Sauerwein RW. 2006. Transmission-reducing immunity is inversely related to age in Plasmodium falciparum gametocyte carriers. Parasite Immunol 28: 185–190. [DOI] [PubMed] [Google Scholar]
- Duffy PE, Kaslow DC. 1997. A novel malaria protein, Pfs28, and Pfs25 are genetically linked and synergistic as falciparum malaria transmission-blocking vaccines. Infect Immun 65: 1109–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eksi S, Czesny B, van Gemert GJ, Sauerwein RW, Eling W, Williamson KC. 2006. Malaria transmission-blocking antigen, Pfs230, mediates human red blood cell binding to exflagellating male parasites and oocyst production. Mol Microbiol 61: 991–998. [DOI] [PubMed] [Google Scholar]
- Eziefula AC, Bousema T, Yeung S, Kamya M, Owaraganise A, Gabagaya G, Bradley J, Grignard L, Lanke KHW, Wanzira H, et al. 2014. Single-dose primaquine for clearance of P. falciparum gametocytes in children with uncomplicated malaria in Uganda: A randomised controlled double-blinded dose-ranging trial. Lancet Infect Dis 14: 130–139. [DOI] [PubMed] [Google Scholar]
- Gaye A, Bousema T, Libasse G, Ndiath MO, Konaté L, Jawara M, Faye O, Sokhna C. 2015. Infectiousness of the human population to Anopheles arabiensis by direct skin feeding in an area hypoendemic for malaria in Senegal. Am J Trop Med Hyg 92: 648–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Githeko AK, Brandling-Bennett AD, Beier M, Atieli F, Owaga M, Collins FH. 1992. The reservoir of Plasmodium falciparum malaria in a holoendemic area of western Kenya. Trans R Soc Trop Med Hyg 86: 355–358. [DOI] [PubMed] [Google Scholar]
- Graves PM, Burkot TR, Carter R, Cattani JA, Lagog M, Parker J, Brabin BJ, Gibson FD, Bradley DJ, Alpers MP. 1988. Measurement of malarial infectivity of human populations to mosquitoes in the Madang area, Papua New Guinea. Parasitology 96: 251–263. [DOI] [PubMed] [Google Scholar]
- Grotendorst CA, Carter R, Rosenberg R, Koontz LC. 1986. Complement effects on the infectivity of Plasmodium gallinaceum to Aedes aegypti mosquitoes. I: Resistance of zygotes to the alternative pathway of complement. J Immunol 136: 4270–4274. [PubMed] [Google Scholar]
- Hay SI, Guerra CA, Gething PW, Patil AP, Tatem AJ, Noor AM, Kabaria CW, Manh BH, Elyazar IR, Brooker S, et al. 2009. A world malaria map: Plasmodium falciparum endemicity in 2007. PLoS Med 6: e1000048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward RE, Tiwari B, Piper KP, Baruch DI, Day KP. 1999. Virulence and transmission success of the malarial parasite Plasmodium falciparum. Proc Natl Acad Sci 96: 4563–4568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Healer J, McGuinness D, Carter R, Riley E. 1999. Transmission-blocking immunity to Plasmodium falciparum in malaria-immune individuals is associated with antibodies to the gamete surface protein Pfs230. Parasitology 119: 425–433. [DOI] [PubMed] [Google Scholar]
- Hermsen CC, Telgt DS, Linders EH, van de Locht LA, Eling WM, Mensink EJ, Sauerwein RW. 2001. Detection of Plasmodium falciparum malaria parasites in vivo by real-time quantitative PCR. Mol Biochem Parasitol 118: 247–251. [DOI] [PubMed] [Google Scholar]
- Hofmann N, Mwingira F, Shekalaghe S, Robinson LJ, Mueller I, Felger I. 2015. Ultra-sensitive detection of Plasmodium falciparum by amplification of multi-copy subtelomeric targets. PLoS Med 12: e1001788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffery GM, Eyles DE. 1955. Infectivity to mosquitoes of Plasmodium falciparum as related to gametocyte density and duration of infection. Am J Trop Med Hyg 4: 781–789. [DOI] [PubMed] [Google Scholar]
- Johnston GL, Smith DL, Fidock DA. 2013. Malaria’s missing number: Calculating the human component of R0 by a within-host mechanistic model of Plasmodium falciparum infection and transmission. PLoS Comput Biol 9: e1003025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S, Grignard L, Nebie I, Chilongola J, Dodoo D, Sauerwein R, Theisen M, Roeffen W, Singh SK, Singh RK, et al. 2015. Naturally acquired antibody responses to recombinant Pfs230 and Pfs48/45 transmission blocking vaccine candidates. J Infect 71: 117–127. [DOI] [PubMed] [Google Scholar]
- Kaslow DC, Bathurst IC, Barr PJ. 1992. Malaria transmission-blocking vaccines. Trends Biotechnol 10: 388–391. [DOI] [PubMed] [Google Scholar]
- Killeen GF, Ross A, Smith T. 2006. Infectiousness of malaria-endemic human populations to vectors. Am J Trop Med Hyg 75: 38–45. [DOI] [PubMed] [Google Scholar]
- Knols BGJ, de Jong R, Takken W. 1995. Differential attractiveness of isolated humans to mosquitoes in Tanzania. Trans R Soc Trop Med Hyg 89: 604–606. [DOI] [PubMed] [Google Scholar]
- Kulkarni MA, Eng JV, Desrochers RE, Cotte AH, Goodson JL, Johnston A, Wolkon A, Erskine M, Berti P, Rakotoarisoa A, et al. 2010. Contribution of integrated campaign distribution of long-lasting insecticidal nets to coverage of target groups and total populations in malaria-endemic areas in Madagascar. Am J Trop Med Hy 82: 420–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasonder E, Ishihama Y, Andersen JS, Vermunt AM, Pain A, Sauerwein RW, Eling WM, Hall N, Waters AP, Stunnenberg HG, et al. 2002. Analysis of the Plasmodium falciparum proteome by high-accuracy mass spectrometry. Nature 419: 537–542. [DOI] [PubMed] [Google Scholar]
- Lasonder E, Rijpma SR, van Schaijk BC, Hoeijmakers WA, Kensche PR, Gresnigt MS, Italiaander A, Vos MW, Woestenenk R, Bousema T, et al. 2016. Integrated transcriptomic and proteomic analyses of P. falciparum gametocytes: Molecular insight into sex-specific processes and translational repression. Nucleic Acids Res 44: 6087–6101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Roch KG, Johnson JR, Florens L, Zhou Y, Santrosyan A, Grainger M, Yan SF, Williamson KC, Holder AA, Carucci DJ, et al. 2004. Global analysis of transcript and protein levels across the Plasmodium falciparum life cycle. Genome Res 14: 2308–2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JT, Saunders DL, Meshnick SR. 2014. The role of submicroscopic parasitemia in malaria transmission: What is the evidence? Trends Parasitol 30: 183–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matovu F, Goodman C, Wiseman V, Mwengee W. 2009. How equitable is bed net ownership and utilisation in Tanzania? A practical application of the principles of horizontal and vertical equity. Malaria J 8: 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Meibalan E, Marti M. 2016. Biology of malaria transmission. Cold Spring Harb Perspect Med 10.1101/cshperspect.a025452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura K, Takashima E, Deng B, Tullo G, Diouf A, Moretz SE, Nikolaeva D, Diakite M, Fairhurst RM, Fay MP, et al. 2013. Functional comparison of Plasmodium falciparum transmission-blocking vaccine candidates by the standard membrane-feeding assay. Infect Immun 81: 4377–4382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moiroux N, Gomez MB, Pennetier C, Elanga E, Djènontin A, Chandre F, Djègbé I, Guis H, Corbel V. 2012. Changes in Anopheles funestus biting behavior following universal coverage of long-lasting insecticidal nets in Benin. J Infect Dis 206: 1622–1629. [DOI] [PubMed] [Google Scholar]
- Mosha JF, Sturrock HJ, Greenhouse B, Greenwood B, Sutherland CJ, Gadalla N, Atwal S, Drakeley C, Kibiki G, Bousema T, et al. 2013. Epidemiology of subpatent Plasmodium falciparum infection: Implications for detection of hotspots with imperfect diagnostics. Malaria J 12: 221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muirhead-Thomson RC. 1951. Distribution of anopheline mosquito bites among different age groups. BMJ 1: 1114–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muirhead-Thomson RC. 1957. The malarial infectivity of an African village population to mosquitoes (Anopheles gambiae); a random xenodiagnostic survey. Am J Trop Med Hyg 6: 971–979. [DOI] [PubMed] [Google Scholar]
- Mukabana W, Takken W, Coe R, Knols B. 2002. Host-specific cues cause differential attractiveness of Kenyan men to the African malaria vector Anopheles gambiae. Malaria J 1: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassir E, Abdel-Muhsin AM, Suliaman S, Kenyon F, Kheir A, Geha H, Ferguson HM, Walliker D, Babiker HA. 2005. Impact of genetic complexity on longevity and gametocytogenesis of Plasmodium falciparum during the dry and transmission-free season of eastern Sudan. Int J Parasitol 35: 49–55. [DOI] [PubMed] [Google Scholar]
- Nguyen HV, van den Eede P, van Overmeir C, Thang ND, Hung le X, D’Alessandro U, Erhart A. 2012. Marked age-dependent prevalence of symptomatic and patent infections and complexity of distribution of human Plasmodium species in central Vietnam. Am J Trop Med Hyg 87: 989–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolaeva D, Draper SJ, Biswas S. 2015. Toward the development of effective transmission-blocking vaccines for malaria. Exp Rev Vaccines 14: 653–680. [DOI] [PubMed] [Google Scholar]
- Nwakanma D, Kheir A, Sowa M, Dunyo S, Jawara M, Pinder M, Milligan P, Walliker D, Babiker HA. 2008. High gametocyte complexity and mosquito infectivity of Plasmodium falciparum in the Gambia. Int J Parasitol 38: 219–227. [DOI] [PubMed] [Google Scholar]
- Okell LC, Griffin JT, Kleinschmidt I, Hollingsworth TD, Churcher TS, White MJ, Bousema T, Drakeley CJ, Ghani AC. 2011. The potential contribution of mass treatment to the control of Plasmodium falciparum malaria. PLoS ONE 6: e20179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okell LC, Bousema T, Griffin JT, Ouedraogo AL, Ghani AC, Drakeley CJ. 2012. Factors determining the occurrence of submicroscopic malaria infections and their relevance for control. Nat Commun 3: 1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouédraogo AL, Schneider P, de Kruijf M, Nebie I, Verhave JP, Cuzin-Ouattara N, Sauerwein RW. 2007. Age-dependent distribution of Plasmodium falciparum gametocytes quantified by Pfs25 real-time QT-NASBA in a cross-sectional study in Burkina Faso. Am J Trop Med Hyg 76: 626–630. [PubMed] [Google Scholar]
- Ouédraogo AL, Bousema T, Schneider P, de Vlas SJ, Ilboudo-Sanogo E, Cuzin-Ouattara N, Nebie I, Roeffen W, Verhave JP, Luty AJ, et al. 2009. Substantial contribution of submicroscopical Plasmodium falciparum gametocyte carriage to the infectious reservoir in an area of seasonal transmission. PLoS ONE 4: e8410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouédraogo AL, Goncalves BP, Gneme A, Wenger EA, Guelbeogo MW, Ouedraogo A, Gerardin J, Bever CA, Lyons H, Pitroipa X, et al. 2015. Dynamics of the human infectious reservoir for malaria determined by mosquito feeding assays and ultrasensitive malaria diagnosis in Burkina Faso. J Infect Dis 213: 90–99. [DOI] [PubMed] [Google Scholar]
- Ponnudurai T, Lensen AH, Van Gemert GJ, Bensink MP, Bolmer M, Meuwissen JH. 1989. Infectivity of cultured Plasmodium falciparum gametocytes to mosquitoes. Parasitology 98: 165–173. [DOI] [PubMed] [Google Scholar]
- Port GR, Boreham PFL, Bryan JH. 1980. The relationship of host size to feeding by mosquitoes of the Anopheles gambiae Giles complex (Diptera: Culicidae). Bull Entomol Res 70: 133–144. [Google Scholar]
- Pradel G. 2007. Proteins of the malaria parasite sexual stages: Expression, function and potential for transmission blocking strategies. Parasitology 134: 1911–1929. [DOI] [PubMed] [Google Scholar]
- Premawansa S, Gamage-Mendis A, Perera L, Begarnie S, Mendis K, Carter R. 1994. Plasmodium falciparum malaria transmission-blocking immunity under conditions of low endemicity as in Sri Lanka. Parasite Immunol 16: 35–42. [DOI] [PubMed] [Google Scholar]
- Proietti C, Pettinato DD, Kanoi BN, Ntege E, Crisanti A, Riley EM, Egwang TG, Drakeley C, Bousema T. 2011. Continuing intense malaria transmission in northern Uganda. Am J Trop Med Hyg 84: 830–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu YT, Smallegange RC, Van Loon JJA, Ter Braak CJF, Takken W. 2006. Interindividual variation in the attractiveness of human odours to the malaria mosquito Anopheles gambiae s.s. Med Vet Entomol 20: 280–287. [DOI] [PubMed] [Google Scholar]
- Ranawaka GR, Alejo-Blanco AR, Sinden RE. 1994. Characterization of the effector mechanisms of a transmission-blocking antibody upon differentiation of Plasmodium berghei gametocytes into ookinetes in vitro. Parasitology 109: 11–17. [DOI] [PubMed] [Google Scholar]
- Reddy M, Overgaard H, Abaga S, Reddy V, Caccone A, Kiszewski A, Slotman M. 2011. Outdoor host seeking behaviour of Anopheles gambiae mosquitoes following initiation of malaria vector control on Bioko Island, Equatorial Guinea. Malaria J 10: 184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rener J, Graves PM, Carter R, Williams JL, Burkot TR. 1983. Target antigens of transmission-blocking immunity on gametes of Plasmodium falciparum. J Exp Med 158: 976–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers NJ, Hall BS, Obiero J, Targett GA, Sutherland CJ. 2000. A model for sequestration of the transmission stages of Plasmodium falciparum: Adhesion of gametocyte-infected erythrocytes to human bone marrow cells. Infect Immun 68: 3455–3462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross A, Killeen G, Smith T. 2006. Relationships between host infectivity to mosquitoes and asexual parasite density in Plasmodium falciparum. Am J Trop Med Hyg 75: 32–37. [DOI] [PubMed] [Google Scholar]
- Saeed M, Roeffen W, Alexander N, Drakeley CJ, Targett GA, Sutherland CJ. 2008. Plasmodium falciparum antigens on the surface of the gametocyte-infected erythrocyte. PloS ONE 3: e2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawa P, Shekalaghe SA, Drakeley CJ, Sutherland CJ, Mweresa CK, Baidjoe AY, Manjurano A, Kavishe RA, Beshir KB, Yussuf RU, et al. 2013. Malaria transmission after artemether-lumefantrine and dihydroartemisinin-piperaquine: A randomized trial. J Infect Dis 207: 1637–1645. [DOI] [PubMed] [Google Scholar]
- Schneider P, Bousema JT, Gouagna LC, Otieno S, van dV, Omar SA, Sauerwein RW. 2007. Submicroscopic Plasmodium falciparum gametocyte densities frequently result in mosquito infection. Am J Trop Med Hyg 76: 470–474. [PubMed] [Google Scholar]
- Shekalaghe SA, Bousema JT, Kunei KK, Lushino P, Masokoto A, Wolters LR, Mwakalinga S, Mosha FW, Sauerwein RW, Drakeley CJ. 2007. Submicroscopic Plasmodium falciparum gametocyte carriage is common in an area of low and seasonal transmission in Tanzania. Trop Med Int Health 12: 547–553. [DOI] [PubMed] [Google Scholar]
- Silvestrini F, Lasonder E, Olivieri A, Camarda G, van Schaijk B, Sanchez M, Younis Younis S, Sauerwein R, Alano P. 2010. Protein export marks the early phase of gametocytogenesis of the human malaria parasite Plasmodium falciparum. Mol Cell Proteomics 9: 1437–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinden RE, Smalley ME. 1979. Gametocytogenesis of Plasmodium falciparum in vitro: The cell cycle. Parasitology 79: 277–296. [DOI] [PubMed] [Google Scholar]
- Slater HC, Ross A, Ouedraogo AL, White LJ, Nguon C, Walker PG, Ngor P, Aguas R, Silal SP, Dondorp AM, et al. 2015. Assessing the impact of next-generation rapid diagnostic tests on Plasmodium falciparum malaria elimination strategies. Nature 528: S94–101. [DOI] [PubMed] [Google Scholar]
- Smith DL, Dushoff J, Snow RW, Hay SI. 2005. The entomological inoculation rate and Plasmodium falciparum infection in African children. Nature 438: 492–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snounou G, Viriyakosol S, Jarra W, Thaithong S, Brown KN. 1993. Identification of the four human malaria parasite species in field samples by the polymerase chain reaction and detection of a high prevalence of mixed infections. Mol Biochem Parasitol 58: 283–292. [DOI] [PubMed] [Google Scholar]
- Stone W, Goncalves BP, Bousema T, Drakeley C. 2015. Assessing the infectious reservoir of falciparum malaria: Past and future. Trends Parasitol 31: 287–296. [DOI] [PubMed] [Google Scholar]
- Stresman GH, Baidjoe AY, Stevenson J, Grignard L, Odongo W, Owaga C, Osoti V, Makori E, Shagari S, Marube E, et al. 2015. Focal screening to identify the subpatent parasite reservoir in an area of low and heterogeneous transmission in the Kenya highlands. J Infect Dis 212: 1768–1777. [DOI] [PubMed] [Google Scholar]
- Sturrock HJ, Hsiang MS, Cohen JM, Smith DL, Greenhouse B, Bousema T, Gosling RD. 2013. Targeting asymptomatic malaria infections: Active surveillance in control and elimination. PLoS Med 10: e1001467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland CJ. 2009. Surface antigens of Plasmodium falciparum gametocytes—A new class of transmission-blocking vaccine targets? Mol Biochem Parasitol 166: 93–98. [DOI] [PubMed] [Google Scholar]
- Taylor LH, Read AF. 1997. Why so few transmission stages? Reproductive restraint by malaria parasites. Parasitol Today 13: 135–140. [DOI] [PubMed] [Google Scholar]
- Thomas TCE. 1951. Biting activity of Anopheles gambiae. BMJ 2: 1402–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiono AB, Ouedraogo A, Ogutu B, Diarra A, Coulibaly S, Gansane A, Sirima SB, O’Neil G, Mukhopadhyay A, Hamed K. 2013. A controlled, parallel, cluster-randomized trial of community-wide screening and treatment of asymptomatic carriers of Plasmodium falciparum in Burkina Faso. Malaria J 12: 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonwong N, Sattabongkot J, Tsuboi T, Iriko H, Takeo S, Sirichaisinthop J, Udomsangpetch R. 2012. Natural infection of Plasmodium falciparum induces inhibitory antibodies against gametocyte development in human hosts. Jpn J Infect Dis 65: 152–156. [PubMed] [Google Scholar]
- Tusting LS, Bousema T, Smith DL, Drakeley C. 2014. Measuring changes in Plasmodium falciparum transmission: Precision, accuracy and costs of metrics. Adv Parasitol 84: 151–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dijk MR, Janse CJ, Thompson J, Waters AP, Braks JA, Dodemont HJ, Stunnenberg HG, van Gemert GJ, Sauerwein RW, Eling W. 2001. A central role for P48/45 in malaria parasite male gamete fertility. Cell 104: 153–164. [DOI] [PubMed] [Google Scholar]
- Vermeulen AN, Ponnudurai T, Beckers PJ, Verhave JP, Smits MA, Meuwissen JH. 1985. Sequential expression of antigens on sexual stages of Plasmodium falciparum accessible to transmission-blocking antibodies in the mosquito. J Exp Med 162: 1460–1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wampfler R, Mwingira F, Javati S, Robinson L, Betuela I, Siba P, Beck HP, Mueller I, Felger I. 2013. Strategies for detection of Plasmodium species gametocytes. PLoS ONE 8: e76316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesolowski A, Eagle N, Tatem AJ, Smith DL, Noor AM, Snow RW, Buckee CO. 2012. Quantifying the impact of human mobility on malaria. Science 338: 267–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White MT, Verity R, Griffin JT, Asante KP, Owusu-Agyei S, Greenwood B, Drakeley C, Gesase S, Lusingu J, Ansong D, et al. 2015. Immunogenicity of the RTS,S/AS01 malaria vaccine and implications for duration of vaccine efficacy: Secondary analysis of data from a phase 3 randomised controlled trial. Lancet Infect Dis 15: 1450–1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolhouse ME, Dye C, Etard JF, Smith T, Charlwood JD, Garnett GP, Hagan P, Hii JL, Ndhlovu PD, Quinnell RJ, et al. 1997. Heterogeneities in the transmission of infectious agents: Implications for the design of control programs. Proc Natl Acad Sci 94: 338–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L, van den Hoogen LL, Slater H, Walker PGT, Ghani A, J DC, Okell LC. 2015. Detecting asymptomatic Plasmodium falciparum infections to inform control and elimination strategies—How do current diagnostics compare? Nature 528: S86–93. [DOI] [PubMed] [Google Scholar]
- Yangzom T, Gueye CS, Namgay R, Galappaththy GN, Thimasarn K, Gosling R, Murugasampillay S, Dev V. 2012. Malaria control in Bhutan: Case study of a country embarking on elimination. Malaria J 11: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]