Abstract
Vectorial capacity is a mathematical approximation of the efficiency of vector-borne disease transmission, measured as the number of new infections disseminated per case per day by an insect vector. Multiple elements of mosquito biology govern their vectorial capacity, including survival, population densities, feeding preferences, and vector competence. Intriguingly, biological pathways essential to mosquito reproductive fitness directly or indirectly influence a number of these elements. Here, we explore this complex interaction, focusing on how the interplay between mating and blood feeding in female Anopheles not only shapes their reproductive success but also influences their ability to sustain Plasmodium parasite development. Central to malaria transmission, mosquito reproductive biology has recently become the focus of research strategies aimed at malaria control, and we discuss promising new methods based on the manipulation of key reproductive steps. In light of widespread resistance to all public health–approved insecticides targeting mosquito reproduction may prove crucial to the success of malaria-eradication campaigns.
In female mosquitoes, reproductive pathways regulating oogenesis also impact malaria parasite development. Mosquito reproduction may therefore be an appropriate target for malaria-eradication efforts.
PLASMODIUM SPOROGONIC DEVELOPMENT: A PROCESS INTIMATELY TIED TO MOSQUITO REPRODUCTION
Transmission of human Plasmodium parasites, the causative agents of malaria, relies on the infectious bite of female Anopheles mosquitoes. In the mosquito midgut, sporogonic development starts within minutes of a female taking a blood meal on an infected vertebrate host. At the same time, nutrients derived from the digestion of the blood meal accumulate in the ovaries during the process of oogenesis, which after 2–3 days will culminate in ovulation and oviposition. Sporogony and oogenesis are, therefore, temporally and physiologically coupled in the female mosquito (Fig. 1).
Figure 1.
Plasmodium sporogony and anopheline oogenesis are temporally and physiologically tied in the mosquito. When a female Anopheles mosquito takes a blood meal from a malaria-infected host, two processes immediately begin within the midgut: Plasmodium gametogenesis, and digestion of the blood bolus for the production of nutrients vital for oogenesis. Within the first 3 days after feeding, a female will have completed her first gonotrophic cycle (GC) consisting of blood feeding, egg development, and oviposition. During this same 72-h period, the Plasmodium gametes will have fused to produce first a zygote and, subsequently, a motile ookinete form, which traverses the midgut epithelium before developing into an oocyst. Over the course of the next 10 days, the oocysts will produce transmission-stage sporozoites, whereas the female will undergo additional gonotrophic cycles, potentially becoming infectious on sporozoites reaching her salivary glands. BF, Blood feeding.
At the start of sporogonic development, male and female gametocytes—the sexual stage of the Plasmodium parasite—are ingested and enter the mosquito midgut where they immediately form gametes, triggered by a drop in surrounding temperature and by the presence of other mosquito-specific factors (Billker et al. 1997, 1998; McRobert et al. 2008; Kuehn and Pradel 2010). After escaping the enveloping erythrocyte, male gametocytes rapidly exflagellate to produce eight motile microgametes, which fuse with female macrogametes to form a diploid zygote. During the next 18–24 h, as the female digests the blood bolus in her midgut, the Plasmodium zygote will transform into a motile ookinete, which traverses the peritrophic matrix and midgut epithelium to reach the basal lamina. Here, the ookinete will encyst, differentiating into an oocyst. The initiation of oocyst development coincides with the completion of oogenesis in the mosquito ovaries, and if inseminated, the female will seek a suitable site to oviposit her eggs. Over the next 8–15 days (depending on Plasmodium species and extrinsic factors), the oocyst undergoes rapid growth and cellular division to produce thousands of infective sporozoites. After rupture of the oocyst wall, the mature sporozoites will migrate to and invade the salivary glands, ready to be injected into the host when the female takes her next blood meal. During this period, the female mosquito may undergo up to three additional gonotrophic cycles consisting of a blood meal, egg development, and oviposition (Fig. 1). This lengthy sporogonic cycle means that only a small proportion of female mosquitoes will actually survive long enough in nature to become infective with Plasmodium sporozoites and transmit disease (Charlwood et al. 1997; Killeen et al. 2000). Therefore, control interventions that target female mosquito life span are particularly successful in reducing malaria transmission in the field (Macdonald 1956; Smith et al. 2012).
Our understanding of the processes regulating egg development in the mosquito is largely derived from studies in the arboviral vector Aedes aegypti (reviewed in Attardo et al. 2005; Hansen et al. 2014). Oogenesis starts with the synthesis and secretion of yolk protein precursors (YPPs) and deposition into the developing oocytes. After a blood meal, the mosquito brain is triggered to release ovary ecdysteroidogenic hormone that stimulates the ovaries to produce the steroid hormone ecdysone (E). The latter, in turn, is hydroxylated to produce the active 20-hydroxyecdysone (20E) form in the fat body. A potent transcriptional regulator, 20E induces the expression of YPPs, including vitellogenin (Vg) and the lipid transporter lipophorin (Lp), which provision the developing oocytes with lipids. 20E and YPPs are integral to egg development in the malaria vector Anopheles gambiae (Atella et al. 2006; Bai et al. 2010), with 20E also fundamental to oogenesis in a number of insect species, including the fruit fly (Drosophila melanogaster) and the silk moth (Bombyx mori) (Swevers and Iatrou 2003; Belles and Piulachs 2015), suggesting conservation of this hormonally controlled process.
Interestingly, the YPPs that facilitate oogenesis also promote Plasmodium development via mechanisms that include protection from the mosquito’s innate immune response (Vlachou et al. 2005; Mendes et al. 2008; Rono et al. 2010). RNAi-based knockdown of YPP Lp in An. gambiae infected with the rodent parasite Plasmodium berghei resulted in reduced oocyst numbers and abolished egg development (Vlachou et al. 2005). It was subsequently demonstrated that the expression and combined action of Lp and Vg after a blood meal blocks the parasite killing action of the complement-like factor thioester-containing protein 1 (TEP1) (Rono et al. 2010). TEP1 was previously shown to severely impair development of Plasmodium species (Levashina et al. 2001; Blandin et al. 2004), including the most deadly human malaria parasite Plasmodium falciparum (Dong et al. 2009; Garver et al. 2009; Eldering et al. 2016). In the absence of Vg, TEP1 binds more efficiently to the surface of P. berghei ookinetes, resulting in higher killing efficiency. Similarly, Lp knockdown resulted in reduced P. falciparum oocyst load, although the precise mechanism was not confirmed (Mendes et al. 2008). Others have shown incorporation of Lp loaded with lipids into the Plasmodium oocysts in Aedes mosquitoes (Atella et al. 2009) and, although the function of these lipids in the parasite is not known, silencing Lp reduces oocyst size in An. gambiae (Rono et al. 2010). Overall, it appears that malaria parasites have developed a system by which vitellogenic factors produced after a blood meal can be coopted to facilitate Plasmodium development within the mosquito.
THE COST OF INFECTION: POTENTIAL FOR TRADE-OFFS BETWEEN REPRODUCTION AND IMMUNITY DURING PARASITE DEVELOPMENT
Eliciting an immune response to infection is an energy-demanding process, as is the production of eggs. It has therefore been long postulated that Plasmodium-infected females may be faced with a trade-off of their resources in an effort to temper infection. A number of studies using the rodent malaria parasites Plasmodium yoelii nigeriensis, P. chabaudi and P. berghei have shown that infected females can experience decreased fecundity and fertility compared with noninfected counterparts (Hogg and Hurd 1997; Jahan and Hurd 1997; Ahmed et al. 2001; Ferguson et al. 2003; Olayemi and Ande 2013; reviewed in Hurd 2009). Oocyte resorption, initiated by apoptosis of epithelial cells surrounding the ovarian follicles, rather than a shortage of nutrients to provision-developing eggs, is believed to be the main driver of reduced fecundity in infected females (Hopwood et al. 2001; Hurd 2003; Ahmed and Hurd 2006). The timing of egg resorption coincides with ookinete traversal of the midgut epithelium (Hopwood et al. 2001), which is associated with epithelial cell pathology (Han and Barillas-Mury 2002) and strong immune induction (Dimopoulos 2003; Levashina 2004; Smith et al. 2014), which, in turn, may trigger apoptosis within the ovaries (Ahmed and Hurd 2006).
Yet, these studies suffer from the use of mosquito–parasite pairings that do not occur in nature. In natural pairings, we argue that the coevolution of parasite and vector over tens of thousands of years has led to a more “commensal” relationship in which immune induction is tempered and the potential cost of infection in the mosquito is minimized. Indeed, a number of studies using naturally occurring Anopheles–Plasmodium combinations have failed to show a negative impact of infection on mosquito fecundity (Ferguson et al. 2005; Yaro et al. 2012; Sangare et al. 2013, 2014). With only a modest negative effect on fecundity recorded in one field study in Tanzania, An. gambiae s.l. infected with P. falciparum showed a ∼10% decrease in egg numbers (Hogg and Hurd 1997).
Although the potential trade-off between reproduction and Plasmodium infection remains somewhat unresolved, increasing evidence suggests that the parasite may have developed ways to circumvent damaging its invertebrate host. A recently proposed theory of malaria globalization suggests that Plasmodium parasites have evolved a mechanism to evade triggering the mosquito’s midgut immune response in naturally occurring vector–parasite interactions (Molina-Cruz et al. 2015). This “lock-and-key theory” is based on parasite surface protein Pfs47 (the key), which suppresses the midgut nitration response (Molina-Cruz et al. 2013) through interaction with a yet-undetermined mosquito protein (the lock). It is therefore conceivable that in nonnatural infections the absence of the necessary coevolved “key” leads to immune triggering and, in turn, ovarian apoptosis and a reduction in reproductive fitness as reported in the aforementioned studies.
The mosquito may have also evolved mechanisms to overcome potential fitness costs incurred by Plasmodium infection. This fascinating topic warrants further exploration, although research must be based on Anopheles–Plasmodium combinations that naturally occur in malaria-endemic areas.
THE INTERPLAY BETWEEN MATING AND BLOOD FEEDING SHAPES VECTORIAL CAPACITY VIA THE STEROID HORMONE 20E
A number of studies point to an important role for the steroid hormone 20E during Plasmodium sporogony. 20E is the major ecdysteroid in insects where it regulates molting during juvenile stages (Yamanaka et al. 2013) and, as discussed, is also essential for egg development in many insect species including mosquitoes (Attardo et al. 2005; Belles and Piulachs 2015). Besides the induction of YPPs and their consequent modulation of the mosquito’s complement-like system previously described (Rono et al. 2010), 20E regulates a number of additional genes that can either impair or facilitate parasite development within the female mosquito. In An. gambiae and An. stephensi, the 20E-induced immunomodulatory peroxidase HPX15 (also named IMPer)—alongside a role in ensuring mosquito fertility (Shaw et al. 2014)—protects Plasmodium parasites from the midgut epithelial immune response by catalyzing dityrosine network formation and decreasing epithelial permeability to immune elicitors (Kumar et al. 2010). On the other hand, 20E induces the expression of a number of immune genes with potential roles in curbing Plasmodium infections, including numerous prophenoloxidases (PPOs), which drive the An. gambiae melanization response to pathogens (Ahmed et al. 1999; Muller et al. 1999), and LRIM9 (leucine-rich repeat immune protein 9), an immunity factor that in An. gambiae reduces P. berghei infection intensity through as-yet-unknown mechanisms (Upton et al. 2015). The complex interaction between 20E and the innate immune response is therefore likely to influence the outcome of Plasmodium development in the mosquito.
Moreover, uniquely to anophelines, 20E is also produced by males and transferred to females during copulation. In most Anopheles species, mating occurs at dusk in large swarms formed by tens to hundreds of individuals. Females are attracted to swarms, most likely by a combination of olfactory, audial, and visual cues. On entering, females pair with a male before leaving to complete copulation. The female is inseminated only once in her lifetime (Tripet et al. 2003; Yuval 2006), and she uses sperm from this single copulation event to fertilize all egg batches produced during multiple gonotrophic cycles (Yaro et al. 2006). During this single mating event, females receive sperm into a specialized storage organ—the spermatheca—and seminal fluids secreted by the male accessory glands (MAGs), which in most anopheline species are coagulated to form a gelatinous mating plug deposited in the atrium (uterus) (Giglioli and Mason 1966; Rogers et al. 2009; Mitchell et al. 2015). Male 20E produced in the MAGs (Pondeville et al. 2008) is packaged into the mating plug during copulation and delivered to the female atrium together with seminal proteins and lipids (Baldini et al. 2013; Mitchell et al. 2015). In An. gambiae and other Anopheles species, sexual transfer of male 20E triggers large behavioral and physiological responses paralleled by extensive transcriptional changes that result in a long-term refractoriness to further copulation, increased egg development following a blood meal, induction of oviposition in blood-fed females, and regulation of pathways that ensure fertility (Baldini et al. 2013; Gabrieli et al. 2014a; Shaw et al. 2014).
Sexual delivery of 20E modulates the expression of more than 800 An. gambiae genes in the female reproductive tract (Gabrieli et al. 2014a). Among these genes is the Mating-Induced Stimulator of Oogenesis (MISO), whose transcriptional level is strongly up-regulated by male 20E specifically in the female atrium (Rogers et al. 2008; Baldini et al. 2013). After expression, the interaction between MISO and male 20E activates downstream signaling cascades that ultimately promote oogenesis, via mechanisms that include elevated expression of the lipid transporter Lp and consequent increased lipid uptake into the ovaries (Baldini et al. 2013). The MISO-male 20E interaction therefore couples mating-induced pathways with blood feeding-activated processes, regulating the development of eggs in the ovaries and potentially affecting—based on the role of Lp in Plasmodium development (Vlachou et al. 2005; Mendes et al. 2008; Rono et al. 2010)— parasite survival in the midgut.
Significant divergence in the levels of 20E both produced by the MAGs and transferred to the female on mating is observed across Anopheles species worldwide (Mitchell et al. 2015). However, the function of this steroid hormone appears to be conserved in the genus, with the notable exception of the New World mosquito Anopheles albimanus, which in absence of 20E production in the MAGs must use alternative strategies to regulate oviposition and receptivity to mating (Mitchell et al. 2015). Given the effects of this steroid hormone on multiple pathways that also influence Plasmodium development, heterogeneity in levels of both female and male 20E across different anopheline vectors may result in variation in their ability to transmit malaria. Interestingly, phylogenetic and ancestral state analyses suggest that 20E synthesis in the MAGs and transfer via the mating plug are acquired traits that have evolved in the Anopheles lineage from a plug-less and 20E-less ancestral species, inducing reciprocal adaptation in the 20E-interacting female protein MISO and likely other female reproductive factors (Mitchell et al. 2015). Although a direct link between male-transferred 20E and Plasmodium development in the female remains to be proven, these findings suggest the intriguing hypothesis that the evolution of a mating system based on sexual transfer of this steroid hormone may have affected vectorial capacity in anophelines. Consistent with this hypothesis, the Anopheles species that transfer the highest levels of 20E originate from areas with the largest malaria burden (see WHO 2015) and are among the most efficient vectors of the disease.
TARGETING MATING AND REPRODUCTION FOR MALARIA CONTROL
Current mosquito control methods based on long-lasting insecticide-treated nets (LLINs) and indoor residual spraying (IRS) are severely threatened by the rapid spread of resistance in natural mosquito populations and the lack of new insecticides coming to market (Toé et al. 2014; Hemingway et al. 2016). Of greatest concern are the reports of extensive high-level resistance to pyrethroid insecticides in sub-Saharan Africa (Ranson et al. 2011); pyrethroids are currently the only chemical class approved for use on LLINs, which are by far the most effective intervention against malaria transmission (Bhatt et al. 2015). A further challenge for the success of malaria control lies in those Anopheles populations that predominantly blood feed and rest outdoors, as these mosquitoes evade conventional indoor-based vector-control strategies and represent a significant barrier to malaria eradication (Alonso et al. 2011; Govella and Ferguson 2012).
Reproductive processes offer unexplored opportunities for the implementation of methods that may provide alternatives to, or enhance existing, insecticide interventions. In the mosquito life cycle, sex is a vulnerable step as females mate only once (Tripet et al. 2003; Yuval 2006). Interfering with mating is, therefore, a potential strategy for malaria control: If mating were disrupted or if virgin females were switched to a mated state without being successfully inseminated, the size of natural mosquito populations would be drastically reduced. Alternatively, manipulation of female reproductive physiology, whether in the form of preventing egg development, inhibiting egg laying, or inducing sterility in egg batches, would also provide a powerful means to reduce vectorial capacity.
SIT and Genetic Control Strategies
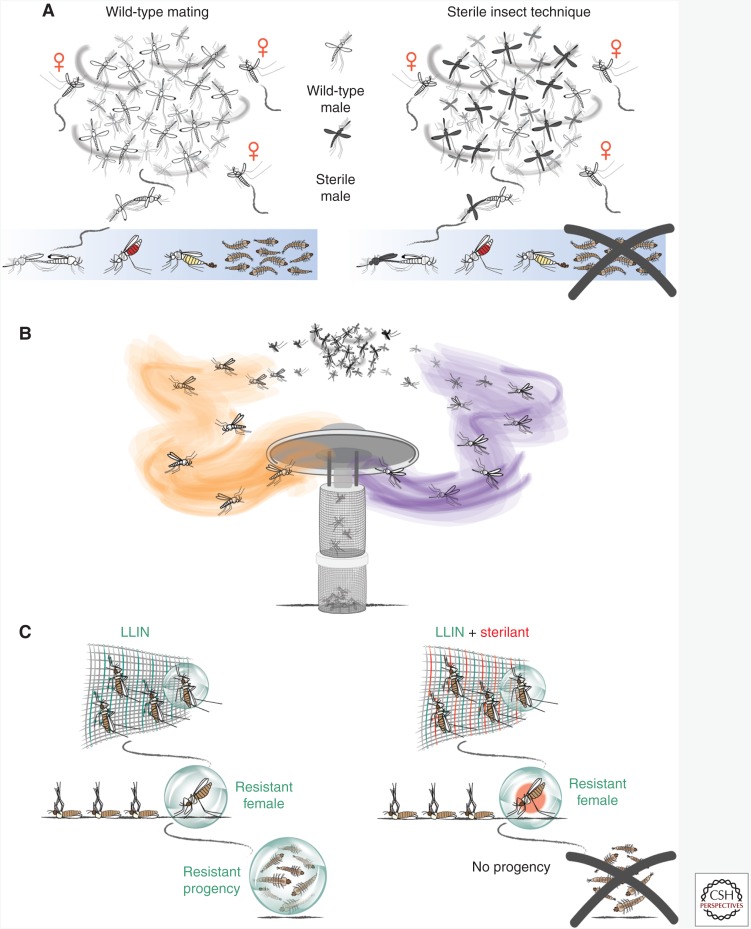
The idea of exploiting mating to control insect populations was first introduced by Raymond Bushland and Edward Knipling in the 1950s, who predicted that releasing mass numbers of sterile insects over a long period of time would collapse field populations (Fig. 2A). This concept, named sterile insect technique (SIT), has since been widely applied to the control of a number of economically important agricultural pests, including the New World screwworm fly (Cochliomyia hominivorax) in the Americas and North Africa (Lindquist et al. 1992; Wyss 2000), the melon fly (Bactrocera cucurbitae) in Japan (Koyama et al. 2004), and the medfly (Ceratitis capitata) in the United States and Central America (Hendrichs et al. 2002). As male mosquitoes do not feed on blood and are therefore not involved in the direct transmission of human disease, they are logical agents for campaigns based on release of sterile adults. However, SIT strategies have met limited success when used against Anopheles populations, with failures attributed to reduced male mating competitiveness resulting from sterilization and mass rearing-associated fitness costs (Dame et al. 2009; Howell and Knols 2009) and the lack of efficient mechanisms to separate females from males before a release (Benedict and Robinson 2003).
Figure 2.
Exploiting anopheline reproductive biology for malaria control. (A) Sterile insect technique (SIT) involves the mass release of sterile males that, if released in high enough numbers, will outcompete normal wild-type males in the mating swarm (right panel) and mate with females. These females will then lay sterile egg batches and not produce any progeny, an effect that will eventually crash the population. (B) Chemical attractants such as aggregation or mating pheromones could be used in mating-disruption strategies to attract male and/or female mosquitoes to traps, diverting individuals from mating swarms. (C) The use of sterilants in combination with insecticides on long-lasting insecticide-treated nets (LLINs) could stop the spread of insecticide resistance. Females impervious to the effects of insecticides on nets (left panel) would not be able to pass genes conferring resistance on to subsequent generations if a sterilant is included on LLINs (right panel).
Major advances in the field of genetic engineering and the recent availability of genomic data for all major anopheline vectors (Neafsey et al. 2015) provide new opportunities for targeting reproductive processes via genetic manipulation of multiple natural vector populations. Since the generation of the first transgenic Anopheles strains more than a decade ago (Catteruccia et al. 2000), a wide array of gene editing tools with increased precision, flexibility, and level of sophistication have emerged (Urnov et al. 2010; Aryan et al. 2013; Mali et al. 2013). Our ability to introduce desired modifications in the genome while causing minimal perturbation to mosquito physiology has dramatically increased and can be exploited to effectively induce sterility while minimizing fitness costs to the insect. For instance, sterile, spermless males were generated by targeting a gene essential for germ cell development, “zero population growth” (ZPG), and although these males were able to mate successfully and induce refractoriness to further mating in females through the delivery of seminal secretions, mated blood-fed females laid only infertile eggs (Thailayil et al. 2011). Targeting sperm generation via genetic manipulation of zpg or similar genes offers promise as a SIT strategy, considering fertility can be specifically manipulated while minimizing any detrimental effects on male mating competitiveness. A similar strategy to SIT, “release of insects carrying a dominant lethal” (RIDL), eliminates the need for sterilization by producing fertile males whose progeny are killed during larval development (Phuc et al. 2007).
Gene Drives
Sterility or lethal transgenes can also be “driven” through populations using genetic selfish elements that can force their own spread in a non-Mendelian manner (reviewed in Gabrieli et al. 2014b). The most powerful example is CRISPR/Cas9, originally a bacterial-acquired immune defense system. It has been coopted to specifically recognize and cleave target sites in the genome through the action of Cas9 nuclease and small artificial guide RNAs (gRNAs) (Barrangou et al. 2007). When a transgene encoding Cas9 and gRNAs is inserted at the homologous chromosomal locus as their target sequence, DNA repair by homologous recombination results in the transgene being copied into the cleaved locus. When this occurs in the germline of heterozygous parents, the transgene can be inherited in >50% of the progeny, favoring the inheritance of the transgenic cassette (Esvelt et al. 2014). The ability to guarantee inheritance of transgenically encoded traits despite potential fitness defects would enable driving of otherwise highly costly sterility-causing cargoes through wild populations. Indeed, proof-of-principle examples of gene drives in mosquitoes have been generated by multiple laboratories (Gantz et al. 2015; Hammond et al. 2016), and improved “evolutionary stable” systems that prevent insurgence of deleterious mutations that block the drive are likely to emerge in the near future (Esvelt et al. 2014). By exploiting males and their need to find a mate, such genetic control strategies could play a significant role in the path to malaria eradication, contributing to the reduction of residual malaria transmission attributed to outdoor biting and resting mosquitoes that would evade even universal LLIN/IRS coverage.
Targeting Mating Behavior
Considering a female’s lifetime reproductive output is centered on the quality of a single mate, the fitness of the preferred male has important implications for female mate choice decisions. A better understanding of male reproductive biology and the factors ensuring successful mating is therefore essential for SIT- or genetic-based strategies to succeed (Howell and Knols 2009; Lees et al. 2014; Diabate and Tripet 2015). Although the components of precopulatory sexual selection in An. gambiae remain unclear, chemical and acoustic signals appears to play a major role (Polerstock et al. 2002; Howard and Blomquist 2005; Cator et al. 2009, 2010). Before mating, the two sexes engage in a brief period of tarsal interaction, likely facilitating close-range chemical communication (Charlwood and Jones 1979). Longer-range chemical cues such as aggregation pheromones, on the other hand, may play a role in male lekking behavior, as suggested in Aedes mosquitoes (Cabrera and Jaffe 2007; Fawaz et al. 2014). Recent studies have also shown the importance of precopulatory acoustic signaling in the mating success of mosquito vectors including An. gambiae (Cator et al. 2009, 2010; Gibson et al. 2010; Pennetier et al. 2010). Once identified and characterized, these chemical and acoustic cues could be exploited to disrupt mating or be incorporated into traps to attract and kill mosquitoes looking for a mate, thus providing alternative tools to target insecticide-resistant mosquitoes and outdoor biting populations (Fig. 2B).
Recent advances in our understanding of the molecular determinants of female postmating and postblood feeding biology offer additional opportunities for the manipulation of reproductive success of mosquito populations (Rogers et al. 2008; Dottorini et al. 2013; Gabrieli et al. 2014a; Shaw et al. 2014; Mitchell et al. 2015). The steroid hormone 20E, produced by the male and transferred during copulation, has been identified as a major regulator for switching off female’s receptivity to mating (Gabrieli et al. 2014a; Mitchell et al. 2015). If mating refractoriness could be triggered through application of 20E agonists, virgin females could be artificially switched to a mated state in the absence of insemination, dramatically reducing their reproductive output. Recent findings support the use of hormonal agonists as mosquito-control agents. Pyriproxyfen, an analog of the other major insect hormone juvenile hormone, produces both long-term sterility and life-shortening effects in Anopheles mosquitoes (Ohashi et al. 2012; Harris et al. 2013; Koama et al. 2015), with promising results when applied to resting surfaces and bed-nets in semifield trials (Kawada et al. 2014; Lwetoijera et al. 2014). By combining sterilizing compounds with insecticides, mosquitoes resistant to the killing action of the insecticide will be unable to propagate the resistance traits to the next generation, therefore preventing selection and spread of insecticide-resistant alleles in the population, an idea coined as “negative cross resistance” (Fig. 2C) (White et al. 2014).
FUTURE PERSPECTIVES
According to the World Health Organization, “mosquito control is the only intervention that can reduce malaria transmission from very high levels to close to zero” (see WHO 2014). This statement was recently validated by an extensive meta-analysis that established the effect of malaria control interventions on P. falciparum in Africa over the last decade; vector control accounted for 78% of the total 663 million cases averted (Bhatt et al. 2015).
The research community has begun to address the pressing need for new mosquito-control strategies that do not exclusively rely on current insecticides and can target exophilic Anopheles species. Genetic modification of mosquito vectors and, in particular, the use of gene drives that can spread rapidly through insect populations have been a major focus of research efforts (reviewed in Esvelt et al. 2014; Gabrieli et al. 2014b), but have also been met with criticism at both government and community levels (Macer 2006; Lavery et al. 2008; Brown et al. 2014). Given the potential for spread across borders and possible unanticipated ecological and environmental consequences, we believe that the release of gene drives for disease control should be the subject of wide and open debate between the scientific community, national and international funding and governmental agencies, and the general public (Esvelt et al. 2014; Oye and Esvelt 2014; Oye et al. 2014; Akbari et al. 2015). Moreover, two key research priorities are fundamental to the release of successful genetic drives: the generation of stable drives that can withstand the inevitable emergence of mutations; and the development of “counter-drive” mechanisms to reverse possible negative impacts following the release of the initial drive (Esvelt et al. 2014).
Additional strategies that can extend the efficacy of current insecticide-based control are urgently needed. We argue that the opportunities provided by targeting hormonal signaling essential to mosquito reproductive fitness, such as the juvenile hormone- and 20E-regulated pathways, need to be fully explored for the generation of control tools that target both mosquito survival and fertility. Moreover, the conservation of hormonal physiology across major malaria vector species will allow multiple disease vectors to be targeted with a single hormone analog, whereas the aforementioned ability to halt the reproductive output of females resistant to currently used insecticides will reduce the spread of this resistance in the wild.
Targeting outdoor biting mosquito populations and preventing residual malaria (defined as malaria transmission occurring despite universal LLINs or IRS) is proving particularly challenging. We believe that novel strategies, in addition to proposed genetic modification, are necessary to control these exophilic populations, and mating biology may prove key in the development of such tools. By bridging the knowledge gap between pre- and postcopulatory mating biology, we can harness essential chemical and acoustic signals for the development of traps that will target these mosquitoes outdoors.
Regardless of the strategies that will be developed, efforts to control malaria vectors must remain a top research and funding priority even in the face of emergencies such as the current Zika outbreak. Given the extraordinary success of mosquito-based interventions to date (Bhatt et al. 2015), “dropping the ball” now on Anopheles mosquito research and control would inevitably lead to disease resurgence, with disastrous consequences for millions worldwide.
CONCLUDING REMARKS
As Plasmodium transmission is contingent on the reproductive needs of its anopheline vector, with the processes of sporogony and oogenesis intimately tied within the female mosquito, it is unsurprising that reproductive pathways regulating oogenesis also impact Plasmodium development. Our increased power to explore the genomes of multiple Anopheles vectors, in combination with an exceptional ability to engineer them, provides unprecedented opportunity to target essential links in both parasite and mosquito reproductive cycles for malaria control. Whether by extending the duration of insecticide effectiveness, enhancing the success of genetic control strategies, or providing novel strategies to disrupt mosquito mating, the study of reproductive biology is fundamental to this next generation of malaria-eradication efforts.
ACKNOWLEDGMENTS
We thank Manuela Bernardi for graphical contributions. We are grateful to members of the Catteruccia Laboratory for useful comments and discussion. We appreciate support from National Institutes of Health (NIH) grants R01-AI104956 and R21-AI117313 to F.C.
Footnotes
Editors: Dyann F. Wirth and Pedro L. Alonso
Additional Perspectives on Malaria: Biology in the Era of Eradication available at www.perspectivesinmedicine.org
REFERENCES
- Ahmed AM, Hurd H. 2006. Immune stimulation and malaria infection impose reproductive costs in Anopheles gambiae via follicular apoptosis. Microbes Infect 8: 308–315. [DOI] [PubMed] [Google Scholar]
- Ahmed A, Martin D, Manetti AG, Han SJ, Lee WJ, Mathiopoulos KD, Muller HM, Kafatos FC, Raikhel A, Brey PT. 1999. Genomic structure and ecdysone regulation of the prophenoloxidase 1 gene in the malaria vector Anopheles gambiae. Proc Natl Acad Sci 96: 14795–14800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed AM, Maingon R, Romans P, Hurd H. 2001. Effects of malaria infection on vitellogenesis in Anopheles gambiae during two gonotrophic cycles. Insect Mol Biol 10: 347–356. [DOI] [PubMed] [Google Scholar]
- Akbari OS, Bellen HJ, Bier E, Bullock SL, Burt A, Church GM, Cook KR, Duchek P, Edwards OR, Esvelt KM, et al. 2015. BIOSAFETY. Safeguarding gene drive experiments in the laboratory. Science 349: 927–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso PL, Brown G, Arevalo-Herrera M, Binka F, Chitnis C, Collins F, Doumbo OK, Greenwood B, Hall BF, Levine MM, et al. 2011. A research agenda to underpin malaria eradication. PLoS Med 8: e1000406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aryan A, Anderson MA, Myles KM, Adelman ZN. 2013. TALEN-based gene disruption in the dengue vector Aedes aegypti. PLoS ONE 8: e60082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atella GC, Silva-Neto MA, Golodne DM, Arefin S, Shahabuddin M. 2006. Anopheles gambiae lipophorin: Characterization and role in lipid transport to developing oocyte. Insect Biochem Mol Biol 36: 375–386. [DOI] [PubMed] [Google Scholar]
- Atella GC, Bittencourt-Cunha PR, Nunes RD, Shahabuddin M, Silva-Neto MA. 2009. The major insect lipoprotein is a lipid source to mosquito stages of malaria parasite. Acta Tropica 109: 159–162. [DOI] [PubMed] [Google Scholar]
- Attardo GM, Hansen IA, Raikhel AS. 2005. Nutritional regulation of vitellogenesis in mosquitoes: Implications for anautogeny. Insect Biochem Mol Biol 35: 661–675. [DOI] [PubMed] [Google Scholar]
- Bai H, Gelman DB, Palli SR. 2010. Mode of action of methoprene in affecting female reproduction in the African malaria mosquito, Anopheles gambiae. Pest Manag Sci 66: 936–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldini F, Gabrieli P, South A, Valim C, Mancini F, Catteruccia F. 2013. The interaction between a sexually transferred steroid hormone and a female protein regulates oogenesis in the malaria mosquito Anopheles gambiae. PLoS Biol 11: e1001695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrangou R, Fremaux C, Deveau H, Richards M, Boyaval P, Moineau S, Romero DA, Horvath P. 2007. CRISPR provides acquired resistance against viruses in prokaryotes. Science 315: 1709–1712. [DOI] [PubMed] [Google Scholar]
- Belles X, Piulachs MD. 2015. Ecdysone signalling and ovarian development in insects: From stem cells to ovarian follicle formation. Biochim Biophys Acta 1849: 181–186. [DOI] [PubMed] [Google Scholar]
- Benedict MQ, Robinson AS. 2003. The first releases of transgenic mosquitoes: An argument for the sterile insect technique. Trends Parasitol 19: 349–355. [DOI] [PubMed] [Google Scholar]
- Bhatt S, Weiss DJ, Cameron E, Bisanzio D, Mappin B, Dalrymple U, Battle KE, Moyes CL, Henry A, Eckhoff PA, et al. 2015. The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature 526: 207–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billker O, Shaw MK, Margos G, Sinden RE. 1997. The roles of temperature, pH and mosquito factors as triggers of male and female gametogenesis of Plasmodium berghei in vitro. Parasitology 115: 1–7. [DOI] [PubMed] [Google Scholar]
- Billker O, Lindo V, Panico M, Etienne AE, Paxton T, Dell A, Rogers M, Sinden RE, Morris HR. 1998. Identification of xanthurenic acid as the putative inducer of malaria development in the mosquito. Nature 392: 289–292. [DOI] [PubMed] [Google Scholar]
- Blandin S, Shiao SH, Moita LF, Janse CJ, Waters AP, Kafatos FC, Levashina EA. 2004. Complement-like protein TEP1 is a determinant of vectorial capacity in the malaria vector Anopheles gambiae. Cell 116: 661–670. [DOI] [PubMed] [Google Scholar]
- Brown DM, Alphey LS, McKemey A, Beech C, James AA. 2014. Criteria for identifying and evaluating candidate sites for open-field trials of genetically engineered mosquitoes. Vector Borne Zoonotic Dis 14: 291–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabrera M, Jaffe K. 2007. An aggregation pheromone modulates lekking behavior in the vector mosquito Aedes aegypti (Diptera: Culicidae). J Am Mosq Control Assoc 23: 1–10. [DOI] [PubMed] [Google Scholar]
- Cator LJ, Arthur BJ, Harrington LC, Hoy RR. 2009. Harmonic convergence in the love songs of the dengue vector mosquito. Science 323: 1077–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cator LJ, Ng’Habi KR, Hoy RR, Harrington LC. 2010. Sizing up a mate: Variation in production and response to acoustic signals in Anopheles gambiae. Behav Ecol 21: 1033–1039. [Google Scholar]
- Catteruccia F, Nolan T, Loukeris TG, Blass C, Savakis C, Kafatos FC, Crisanti A. 2000. Stable germline transformation of the malaria mosquito Anopheles stephensi. Nature 405: 959–962. [DOI] [PubMed] [Google Scholar]
- Charlwood JD, Jones MDR. 1979. Mating behaviour in the mosquito, Anopheles gambiae s.l. Physiol Entomol 4: 111–120. [Google Scholar]
- Charlwood JD, Smith T, Billingsley PF, Takken W, Lyimo EOK, Meuwissen JHET. 1997. Survival and infection probabilities of anthropophagic anophelines from an area of high prevalence of Plasmodium falciparum in humans. Bull Entomol Res 87: 445–453. [Google Scholar]
- Dame DA, Curtis CF, Benedict MQ, Robinson AS, Knols BG. 2009. Historical applications of induced sterilisation in field populations of mosquitoes. Malaria J 8: S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diabate A, Tripet F. 2015. Targeting male mosquito mating behaviour for malaria control. Parasit Vectors 8: 347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimopoulos G. 2003. Insect immunity and its implication in mosquito–malaria interactions. Cell Microbiol 5: 3–14. [DOI] [PubMed] [Google Scholar]
- Dong Y, Manfredini F, Dimopoulos G. 2009. Implication of the mosquito midgut microbiota in the defense against malaria parasites. PLoS Pathog 5: e1000423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dottorini T, Persampieri T, Palladino P, Baker DA, Spaccapelo R, Senin N, Crisanti A. 2013. Regulation of Anopheles gambiae male accessory gland genes influences postmating response in female. FASEB J 27: 86–97. [DOI] [PubMed] [Google Scholar]
- Eldering M, Morlais I, van Gemert GJ, van de Vegte-Bolmer M, Graumans W, Siebelink-Stoter R, Vos M, Abate L, Roeffen W, Bousema T, et al. 2016. Variation in susceptibility of African Plasmodium falciparum malaria parasites to TEP1 mediated killing in Anopheles gambiae mosquitoes. Sci Rep 6: 20440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esvelt KM, Smidler AL, Catteruccia F, Church GM. 2014. Concerning RNA-guided gene drives for the alteration of wild populations. eLife 10.7554/eLife.03401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fawaz EY, Allan SA, Bernier UR, Obenauer PJ, Diclaro JW II. 2014. Swarming mechanisms in the yellow fever mosquito: Aggregation pheromones are involved in the mating behavior of Aedes aegypti. J Vector Ecol 39: 347–354. [DOI] [PubMed] [Google Scholar]
- Ferguson HM, Rivero A, Read AF. 2003. The influence of malaria parasite genetic diversity and anaemia on mosquito feeding and fecundity. Parasitology 127: 9–19. [DOI] [PubMed] [Google Scholar]
- Ferguson HM, Gouagna LC, Obare P, Read AF, Babiker H, Githure J, Beier JC. 2005. The presence of Plasmodium falciparum gametocytes in human blood increases the gravidity of Anopheles gambiae mosquitoes. Am J Trop Med Hyg 73: 312–320. [PubMed] [Google Scholar]
- Gabrieli P, Kakani EG, Mitchell SN, Mameli E, Want EJ, Mariezcurrena Anton A, Serrao A, Baldini F, Catteruccia F. 2014a. Sexual transfer of the steroid hormone 20E induces the postmating switch in Anopheles gambiae. Proc Natl Acad Sci 111: 16353–16358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabrieli P, Smidler A, Catteruccia F. 2014b. Engineering the control of mosquito-borne infectious diseases. Genome Biol 15: 535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gantz VM, Jasinskiene N, Tatarenkova O, Fazekas A, Macias VM, Bier E, James AA. 2015. Highly efficient Cas9-mediated gene drive for population modification of the malaria vector mosquito Anopheles stephensi. Proc Natl Acad Sci 112: E6736–E6743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garver LS, Dong Y, Dimopoulos G. 2009. Caspar controls resistance to Plasmodium falciparum in diverse anopheline species. PLoS Pathog 5: e1000335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson G, Warren B, Russell IJ. 2010. Humming in tune: Sex and species recognition by mosquitoes on the wing. J Assoc Res Otolaryngol 11: 527–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giglioli MEC, Mason GF. 1966. The mating plug in anopheline mosquitoes. Proc R Entomol Soc Lond Ser A Gen Entomol 41: 123–129. [Google Scholar]
- Govella NJ, Ferguson H. 2012. Why use of interventions targeting outdoor biting mosquitoes will be necessary to achieve malaria elimination. Front Physiol 3: 199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond A, Galizi R, Kyrou K, Simoni A, Siniscalchi C, Katsanos D, Gribble M, Baker D, Marois E, Russell S, et al. 2016. A CRISPR-Cas9 gene drive system targeting female reproduction in the malaria mosquito vector Anopheles gambiae. Nat Biotechnol 34: 78–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han YS, Barillas-Mury C. 2002. Implications of Time Bomb model of ookinete invasion of midgut cells. Insect Biochem Mol Biol 32: 1311–1316. [DOI] [PubMed] [Google Scholar]
- Hansen IA, Attardo GM, Rodriguez SD, Drake LL. 2014. Four-way regulation of mosquito yolk protein precursor genes by juvenile hormone-, ecdysone-, nutrient-, and insulin-like peptide signaling pathways. Front Physiol 5: 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris C, Lwetoijera DW, Dongus S, Matowo NS, Lorenz LM, Devine GJ, Majambere S. 2013. Sterilising effects of pyriproxyfen on Anopheles arabiensis and its potential use in malaria control. Parasites Vectors 6: 144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemingway J, Ranson H, Magill A, Kolaczinski J, Fornadel C, Gimnig J, Coetzee M, Simard F, Roch DK, Hinzoumbe CK, et al. 2016. Averting a malaria disaster: Will insecticide resistance derail malaria control? Lancet 387: 1785–1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrichs J, Robinson A, Cayol J, Enkerlin W. 2002. Medfly area-wide sterile insect technique programmes for prevention, suppression or eradication: The importance of mating behavior studies. Fla Entomol 85: 1–13. [Google Scholar]
- Hogg JC, Hurd H. 1997. The effects of natural Plasmodium falciparum infection on the fecundity and mortality of Anopheles gambiae s.l. in north east Tanzania. Parasitology 114: 325–331. [DOI] [PubMed] [Google Scholar]
- Hopwood JA, Ahmed AM, Polwart A, Williams GT, Hurd H. 2001. Malaria-induced apoptosis in mosquito ovaries: A mechanism to control vector egg production. J Exp Biol 204: 2773–2780. [DOI] [PubMed] [Google Scholar]
- Howard RW, Blomquist GJ. 2005. Ecological, behavioral, and biochemical aspects of insect hydrocarbons. Annu Rev Entomol 50: 371–393. [DOI] [PubMed] [Google Scholar]
- Howell PI, Knols BG. 2009. Male mating biology. Malaria J 8: S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurd H. 2003. Manipulation of medically important insect vectors by their parasites. Annu Rev Entomol 48: 141–161. [DOI] [PubMed] [Google Scholar]
- Hurd H. 2009. Evolutionary drivers of parasite-induced changes in insect life-history traits from theory to underlying mechanisms. Adv Parasitol 68: 85–110. [DOI] [PubMed] [Google Scholar]
- Jahan N, Hurd H. 1997. The effects of infection with Plasmodium yoelii nigeriensis on the reproductive fitness of Anopheles stephensi. Ann Trop Med Parasitol 91: 365–369. [DOI] [PubMed] [Google Scholar]
- Kawada H, Dida GO, Ohashi K, Kawashima E, Sonye G, Njenga SM, Mwandawiro C, Minakawa N. 2014. A small-scale field trial of pyriproxyfen-impregnated bed nets against pyrethroid-resistant Anopheles gambiae s.s. in western Kenya. PLoS ONE 9: e111195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killeen GF, McKenzie FE, Foy BD, Schieffelin C, Billingsley PF, Beier JC. 2000. A simplified model for predicting malaria entomologic inoculation rates based on entomologic and parasitologic parameters relevant to control. Am J Trop Med Hyg 62: 535–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koama B, Namountougou M, Sanou R, Ndo S, Ouattara A, Dabire RK, Malone D, Diabate A. 2015. The sterilizing effect of pyriproxyfen on the malaria vector Anopheles gambiae: Physiological impact on ovaries development. Malaria J 14: 101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama J, Kakinohana H, Miyatake T. 2004. Eradication of the melon fly, Bactrocera cucurbitae, in Japan: Importance of behavior, ecology, genetics, and evolution. Annu Rev Entomol 49: 331–349. [DOI] [PubMed] [Google Scholar]
- Kuehn A, Pradel G. 2010. The coming-out of malaria gametocytes. J Biomed Biotechnol 2010: 976827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Molina-Cruz A, Gupta L, Rodrigues J, Barillas-Mury C. 2010. A peroxidase/dual oxidase system modulates midgut epithelial immunity in Anopheles gambiae. Science 327: 1644–1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavery JV, Harrington LC, Scott TW. 2008. Ethical, social, and cultural considerations for site selection for research with genetically modified mosquitoes. Am J Trop Med Hyg 79: 312–318. [PubMed] [Google Scholar]
- Lees RS, Knols B, Bellini R, Benedict MQ, Bheecarry A, Bossin HC, Chadee DD, Charlwood J, Dabire RK, Djogbenou L, et al. 2014. Review: Improving our knowledge of male mosquito biology in relation to genetic control programmes. Acta Tropica 132: S2–S11. [DOI] [PubMed] [Google Scholar]
- Levashina EA. 2004. Immune responses in Anopheles gambiae. Insect Biochem Mol Biol 34: 673–678. [DOI] [PubMed] [Google Scholar]
- Levashina EA, Moita LF, Blandin S, Vriend G, Lagueux M, Kafatos FC. 2001. Conserved role of a complement-like protein in phagocytosis revealed by dsRNA knockout in cultured cells of the mosquito, Anopheles gambiae. Cell 104: 709–718. [DOI] [PubMed] [Google Scholar]
- Lindquist DA, Abusowa M, Hall MJ. 1992. The New World screwworm fly in Libya: A review of its introduction and eradication. Med Vet Entomol 6: 2–8. [DOI] [PubMed] [Google Scholar]
- Lwetoijera DW, Harris C, Kiware SS, Killeen GF, Dongus S, Devine GJ, Majambere S. 2014. Comprehensive sterilization of malaria vectors using pyriproxyfen: A step closer to malaria elimination. Am J Trop Med Hyg 90: 852–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald G. 1956. Epidemiological basis of malaria control. Bull World Health Organ 15: 613–626. [PMC free article] [PubMed] [Google Scholar]
- Macer DRJ. 2006. Ethics and community engagement for GM insect vector release. In Genetically modified mosquitoes for malaria control (ed. Boete C), pp. 152–165. Landes Bioscience, Austin, TX. [Google Scholar]
- Mali P, Esvelt KM, Church GM. 2013. Cas9 as a versatile tool for engineering biology. Nat Methods 10: 957–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRobert L, Taylor CJ, Deng W, Fivelman QL, Cummings RM, Polley SD, Billker O, Baker DA. 2008. Gametogenesis in malaria parasites is mediated by the cGMP-dependent protein kinase. PLoS Biol 6: e139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendes AM, Schlegelmilch T, Cohuet A, Awono-Ambene P, De Iorio M, Fontenille D, Morlais I, Christophides GK, Kafatos FC, Vlachou D. 2008. Conserved mosquito/parasite interactions affect development of Plasmodium falciparum in Africa. PLoS Pathog 4: e1000069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell SN, Kakani EG, South A, Howell PI, Waterhouse RM, Catteruccia F. 2015. Mosquito biology. Evolution of sexual traits influencing vectorial capacity in anopheline mosquitoes. Science 347: 985–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina-Cruz A, Garver LS, Alabaster A, Bangiolo L, Haile A, Winikor J, Ortega C, van Schaijk BC, Sauerwein RW, Taylor-Salmon E, et al. 2013. The human malaria parasite Pfs47 gene mediates evasion of the mosquito immune system. Science 340: 984–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina-Cruz A, Canepa GE, Kamath N, Pavlovic NV, Mu J, Ramphul UN, Ramirez JL, Barillas-Mury C. 2015. Plasmodium evasion of mosquito immunity and global malaria transmission: The lock-and-key theory. Proc Natl Acad Sci 112: 15178–15183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller HM, Dimopoulos G, Blass C, Kafatos FC. 1999. A hemocyte-like cell line established from the malaria vector Anopheles gambiae expresses six prophenoloxidase genes. J Biol Chem 274: 11727–11735. [DOI] [PubMed] [Google Scholar]
- Neafsey DE, Waterhouse RM, Abai MR, Aganezov SS, Alekseyev MA, Allen JE, Amon J, Arca B, Arensburger P, Artemov G, et al. 2015. Mosquito genomics. Highly evolvable malaria vectors: The genomes of 16 Anopheles mosquitoes. Science 347: 1258522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohashi K, Nakada K, Ishiwatari T, Miyaguchi J, Shono Y, Lucas JR, Mito N. 2012. Efficacy of pyriproxyfen-treated nets in sterilizing and shortening the longevity of Anopheles gambiae (Diptera: Culicidae). J Med Entomol 49: 1052–1058. [DOI] [PubMed] [Google Scholar]
- Olayemi IK, Ande AT. 2013. Plasmodium parasite-infection in the malaria vector mosquito, Anopheles gambiae (Diptera: Culicidae). Eur J Biotechnol Biosci 1: 6–11. [Google Scholar]
- Oye KA, Esvelt KM. 2014. Gene drives raise dual-use concerns—Response. Science 345: 1010–1011. [DOI] [PubMed] [Google Scholar]
- Oye KA, Esvelt K, Appleton E, Catteruccia F, Church G, Kuiken T, Lightfoot SB, McNamara J, Smidler A, Collins JP. 2014. Biotechnology. Regulating gene drives. Science 345: 626–628. [DOI] [PubMed] [Google Scholar]
- Pennetier C, Warren B, Dabire KR, Russell IJ, Gibson G. 2010. “Singing on the wing” as a mechanism for species recognition in the malarial mosquito Anopheles gambiae. Curr Biol 20: 131–136. [DOI] [PubMed] [Google Scholar]
- Phuc HK, Andreasen MH, Burton RS, Vass C, Epton MJ, Pape G, Fu G, Condon KC, Scaife S, Donnelly CA, et al. 2007. Late-acting dominant lethal genetic systems and mosquito control. BMC Biol 5: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polerstock AR, Eigenbrode SD, Klowden MJ. 2002. Mating alters the cuticular hydrocarbons of female Anopheles gambiae sensu stricto and Aedes aegypti (Diptera: Culcidae). J Med Entomol 39: 545–552. [DOI] [PubMed] [Google Scholar]
- Pondeville E, Maria A, Jacques JC, Bourgouin C, Dauphin-Villemant C. 2008. Anopheles gambiae males produce and transfer the vitellogenic steroid hormone 20-hydroxyecdysone to females during mating. Proc Natl Acad Sci 105: 19631–19636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranson H, N’Guessan R, Lines J, Moiroux N, Nkuni Z, Corbel V. 2011. Pyrethroid resistance in African anopheline mosquitoes: What are the implications for malaria control? Trends Parasitol 27: 91–98. [DOI] [PubMed] [Google Scholar]
- Rogers DW, Whitten MM, Thailayil J, Soichot J, Levashina EA, Catteruccia F. 2008. Molecular and cellular components of the mating machinery in Anopheles gambiae females. Proc Natl Acad Sci 105: 19390–19395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers DW, Baldini F, Battaglia F, Panico M, Dell A, Morris HR, Catteruccia F. 2009. Transglutaminase-mediated semen coagulation controls sperm storage in the malaria mosquito. PLoS Biol 7: e1000272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rono MK, Whitten MM, Oulad-Abdelghani M, Levashina EA, Marois E. 2010. The major yolk protein vitellogenin interferes with the anti-Plasmodium response in the malaria mosquito Anopheles gambiae. PLoS Biol 8: e1000434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangare I, Michalakis Y, Yameogo B, Dabire R, Morlais I, Cohuet A. 2013. Studying fitness cost of Plasmodium falciparum infection in malaria vectors: Validation of an appropriate negative control. Malaria J 12: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangare I, Dabire R, Yameogo B, Da DF, Michalakis Y, Cohuet A. 2014. Stress dependent infection cost of the human malaria agent Plasmodium falciparum on its natural vector Anopheles coluzzii. Infect Genet Evol 25: 57–65. [DOI] [PubMed] [Google Scholar]
- Shaw WR, Teodori E, Mitchell SN, Baldini F, Gabrieli P, Rogers DW, Catteruccia F. 2014. Mating activates the heme peroxidase HPX15 in the sperm storage organ to ensure fertility in Anopheles gambiae. Proc Natl Acad Sci 111: 5854–5859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DL, Battle KE, Hay SI, Barker CM, Scott TW, McKenzie FE. 2012. Ross, Macdonald, and a theory for the dynamics and control of mosquito-transmitted pathogens. PLoS Pathog 8: e1002588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RC, Vega-Rodriguez J, Jacobs-Lorena M. 2014. The Plasmodium bottleneck: Malaria parasite losses in the mosquito vector. Mem Instit Oswaldo Cruz 109: 644–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swevers L, Iatrou K. 2003. The ecdysone regulatory cascade and ovarian development in lepidopteran insects: Insights from the silkmoth paradigm. Insect Biochem Mol Biol 33: 1285–1297. [DOI] [PubMed] [Google Scholar]
- Thailayil J, Magnusson K, Godfray HCJ, Crisanti A, Catteruccia F. 2011. Spermless males elicit large-scale female responses to mating in the malaria mosquito Anopheles gambiae. Proc Natl Acad Sci 108: 13677–13681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toé KH, Jones CM, N’Fale S, Ismail HM, Dabiré RK, Ranson H. 2014. Increased pyrethroid resistance in malaria vectors and decreased bed net effectiveness, Burkina Faso. Emerg Infect Dis 20: 1691–1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripet F, Toure YT, Dolo G, Lanzaro GC. 2003. Frequency of multiple inseminations in field-collected Anopheles gambiae females revealed by DNA analysis of transferred sperm. Am J Trop Med Hyg 68: 1–5. [PubMed] [Google Scholar]
- Upton LM, Povelones M, Christophides GK. 2015. Anopheles gambiae blood feeding initiates an anticipatory defense response to Plasmodium berghei. J Innate Immun 7: 74–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urnov FD, Rebar EJ, Holmes MC, Zhang HS, Gregory PD. 2010. Genome editing with engineered zinc finger nucleases. Nat Rev Genet 11: 636–646. [DOI] [PubMed] [Google Scholar]
- Vlachou D, Schlegelmilch T, Christophides GK, Kafatos FC. 2005. Functional genomic analysis of midgut epithelial responses in Anopheles during Plasmodium invasion. Curr Biol 15: 1185–1195. [DOI] [PubMed] [Google Scholar]
- White MT, Lwetoijera D, Marshall J, Caron-Lormier G, Bohan DA, Denholm I, Devine GJ. 2014. Negative cross resistance mediated by co-treated bed nets: A potential means of restoring pyrethroid-susceptibility to malaria vectors. PLoS ONE 9: e95640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO. 2014. World malaria report 2014. World Health Organization, Geneva. [Google Scholar]
- WHO. 2015. World malaria report 2015. World Health Organization, Geneva. [Google Scholar]
- Wyss JH. 2000. Screwworm eradication in the Americas. Ann NY Acad Sci 916: 186–193. [DOI] [PubMed] [Google Scholar]
- Yamanaka N, Rewitz KF, O’Connor MB. 2013. Ecdysone control of developmental transitions: Lessons from Drosophila research. Annu Rev Entomol 58: 497–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaro AS, Dao A, Adamou A, Crawford JE, Traore SF, Toure AM, Gwadz R, Lehmann T. 2006. Reproductive output of female Anopheles gambiae (Diptera: Culicidae): Comparison of molecular forms. J Med Entomol 43: 833–839. [DOI] [PubMed] [Google Scholar]
- Yaro AS, Toure AM, Guindo A, Coulibaly MB, Dao A, Diallo M, Traore SF. 2012. Reproductive success in Anopheles arabiensis and the M and S molecular forms of Anopheles gambiae: Do natural sporozoite infection and body size matter? Acta Tropica 122: 87–93. [DOI] [PubMed] [Google Scholar]
- Yuval B. 2006. Mating systems of blood-feeding flies. Annu Rev Entomol 51: 413–440. [DOI] [PubMed] [Google Scholar]