Abstract
Polarized exocytosis is generally considered as the multistep vesicular trafficking process in which membrane-bounded carriers are transported from the Golgi or endosomal compartments to specific sites of the plasma membrane. Polarized exocytosis in cells is achieved through the coordinated actions of membrane trafficking machinery and cytoskeleton orchestrated by signaling molecules such as the Rho family of small GTPases. Elucidating the molecular mechanisms of polarized exocytosis is essential to our understanding of a wide range of pathophysiological processes from neuronal development to tumor invasion.
Proteins and lipids are asymmetrically distributed on the surfaces of eukaryotic cells (e.g., epithelial and migrating cells). The main trafficking pathways and regulators that establish and maintain this distribution are conserved.
Most, if not all, eukaryotic cells are asymmetrical in shape. Polarized distribution of proteins and lipids on the plasma membrane not only confers diverse identities to eukaryotic cells, but also is essential for their physiological functions. The establishment and maintenance of cell polarity require polarized exocytosis. The incorporation of proteins and lipids to specific domains of the cell surface is, in principle, mediated by directional transport, docking, and fusion of vesicles to the plasma membrane.
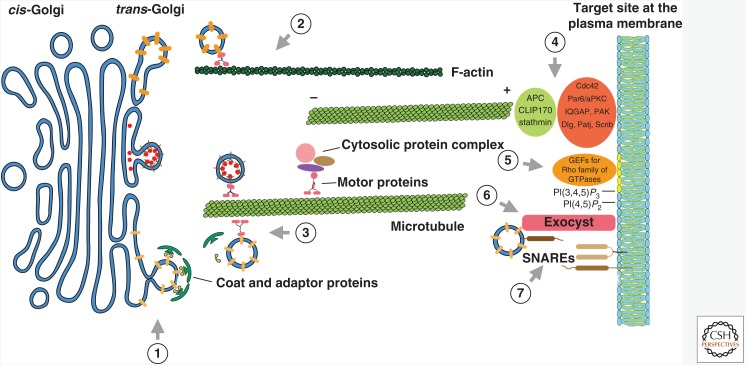
Polarized exocytosis has been studied in a diverse array of eukaryotic systems from the budding yeast to neurons. It is now accepted that the core machineries and general mechanisms are mostly conserved (Fig. 1). Usually, polarized exocytosis is initiated in response to a spatial cue, which ranges from a chemical gradient to mechanical force. This is followed by the establishment of a polarity axis, upon which the cytoskeletons are assembled. The polarity axis is often enforced or stabilized through signaling amplification and positive feedback loops. Cargo proteins contained in vesicles or tubular carriers are transported from a donor compartment (e.g., trans-Golgi network or endosomes) to specific regions of the plasma membrane by motor proteins along the cytoskeleton tracks. After arriving at their destination, the transport carriers are physically attached to the plasma membrane by tether proteins. Finally, the soluble N-ethylmaleimide-sensitive factor (NSF) attachment protein receptor (SNARE) complex is assembled to drive membrane fusion. Throughout this process, signaling molecules function at various steps to regulate the activities of the trafficking machinery and cytoskeleton to ensure the spatial specificity.
Figure 1.
Regulation of exocytic trafficking from the Golgi to the plasma membrane. Diagram illustrates exocytic trafficking from the Golgi to the plasma membrane. Numbers specify each trafficking step. Components and regulators of individual steps are the following: (1) Rabs regulate vesicle exit and loading onto the cytoskeleton. Cdc42 regulates actin dynamics to promote vesicle/tubule formation; (2) Rho family of GTPases regulates actin cable assembly in yeasts; (3) Rab GTPases regulate vesicle movement along the cytoskeleton; (4) Cdc42 and components of the polarity complexes regulate microtubule orientation by directly or indirectly interacting with microtubule plus-end-binding proteins such as APC, CLIP170, and stathmin; (5) The phosphoinositides on the plasma membrane recruits guanine nucleotide exchange factors (GEFs) for Rho GTPases; (6) The exocyst complex tethers vesicles to the plasma membrane. Components of the exocyst directly interact with phosphoinositides and the Rho and Rab families of GTPases, which regulate the assembly and localization of the exocyst complex; (7) The assembly of the soluble N-ethylmaleimide-sensitive factor (NSF) attachment protein receptor (SNARE) complex drives membrane fusion, which can be regulated by accessory proteins, Rabs, and the exocyst.
In this review, we will first discuss the core machinery and major regulators that govern polarized exocytosis. We will then summarize the recent works toward a better understanding of polarized exocytosis in different cellular systems and processes.
MOLECULAR BASIS AND REGULATORY MECHANISMS OF POLARIZED EXOCYTOSIS
The Exocyst Complex and the SNAREs
The exocyst is an octameric protein complex consisting of Sec3, Sec5, Sec6, Sec8, Sec10, Sec15, Exo70, and Exo84. The exocyst is considered as a tether complex that mediates the initial contact between the vesicle and the plasma membrane, and promotes SNARE assembly (reviewed in Guo et al. 2000; Munson and Novick 2006; He and Guo 2009; Heider and Munson 2012; Wu and Guo 2015). It was shown in yeast and mammalian cells that two subunits of the exocyst complex, Sec3 and Exo70, bind to the phospholipid PI(4,5)P2 on the plasma membrane (He et al. 2007; Liu et al. 2007; Zhang et al. 2008; Baek et al. 2010). Similarly in plants, it was shown that Sec3 binds to PI(4,5)P2 and this interaction is critical for pollen tube growth (Bloch et al. 2016). Structural analyses revealed that the Sec3 amino-terminal PI(4,5)P2-binding region has a PH domain-like fold that is evolutionarily conserved among yeasts, plants, and mammals (Baek et al. 2010; Yamashita et al. 2010). In Exo70, the PI(4,5)P2 interaction is mediated through a patch of basic residues at its carboxyl terminus (Dong et al. 2005; Hamburger et al. 2006; He et al. 2007; Liu et al. 2007; Moore et al. 2007). Sec3 and Exo70 also interact with Rho GTPases localized to the plasma membrane (see below). The dual interaction of the exocyst with phospholipids and small GTPases suggests a “coincidence detection” mechanism in the targeting of the exocyst to a specific region of the plasma membrane. It was shown that ectopic targeting of Sec3 to mitochondria or peroxisomes led to mistargeting of secretory vesicles to these surrogate organelles (Luo et al. 2014). The data support the role of Sec3 in vesicle targeting.
While Sec3 and some of the Exo70 proteins associate with the plasma membrane via PI(4,5)P2, other members of the exocyst associate with the secretory vesicles (Boyd et al. 2004). Sec15 interacts with the Rab proteins in their GTP-bound state (Guo et al. 1999; Zhang et al. 2004; Wu et al. 2005). The interaction may mediate the recruitment of the rest of the exocyst subunits to the secretory vesicles and later control their interactions with Sec3 at the plasma membrane (Guo et al. 1999; Luo et al. 2014). Another exocyst subunit, Sec10, was shown to interact with the GTP-bound Arf6, which plays an important role in membrane recycling to the plasma membrane via endosomes (Prigent et al. 2003). It is also interesting to note that Arf6 is required for leading-edge localization of Cdc42, Rac1, and the Par6/aPKC complex, and inhibition of Arf6 causes depletion of PI(4,5)P2 from the plasma membrane (Aikawa et al. 2003; Osmani et al. 2010).
The assembly of the exocyst complex can be regulated by phosphorylation. Phosphorylation of Exo70 by ERK promotes exocyst complex assembly and exocytosis in mammalian cells in response to growth factor signaling (Ren and Guo 2012; Lu et al. 2016). In yeast, mitotic phosphorylation of Exo84 by cell-cycle-dependent kinase, CDK1, disrupts exocyst complex assembly and thus inhibits cell-surface expansion during mitosis, when cells are preparing for division (Luo et al. 2013) (also see below).
Subsequent to vesicle tethering, the v-SNARE proteins in the vesicle and the t-SNARE proteins on the plasma membrane interact with each other to catalyze membrane fusion (Rothman 1994; Baker and Hughson 2016). The interactions between the exocyst and the SNARE proteins have been reported. In yeast, Sec6 was shown to interact with both the v-SNARE protein Snc2 and the t-SNARE proteins Sec9 (Sivaram et al. 2005; Shen et al. 2013; Dubuke et al. 2015). Sec6 also interacts with the Sec1/Munc18 family protein Sec1 (Morgera et al. 2012). However, the role of Sec6 on SNARE assembly and membrane fusion remains unclear. Exo84 interacts with Sro7/77, which bind to Sec9 (Lehman et al. 1999; Zhang et al. 2005); disruption of the interaction leads to a block in exocytosis (Zhang et al. 2005). Most recently, it was shown in yeast that the exocyst subunit Sec3 directly binds to the t-SNARE protein Sso1/2, and promotes its interaction with the other t-SNARE protein Sec9. Thus, Sec3 catalyzes the first rate-limiting step of the SNARE assembly and membrane fusion (Yue et al. 2017).
The Cytoskeletons
During polarized exocytosis, the cytoskeletons serve as tracks for directional transport of secretory vesicles. While the microtubules are used for rapid movement over long distances, the actin microfilament transports vesicles locally with slower speed (Apodaca 2001).
Microtubules
The microtubules have been shown to mediate vesicular trafficking from cell center to the cell periphery (Burgo et al. 2012). Microtubules also regulate cell polarity by delivering positional information to cell cortex (Siegrist and Doe 2007). One classical example is the polarized cell growth in fission yeast, in which correct positioning of the new pole following unipolar cell growth at the old pole is needed for the formation of their rod-shaped morphology. It was suggested that microtubules deliver microtubule- and actin-binding proteins to the new pole, where the proteins are transferred and anchored to the membrane and further regulate actin dynamics locally to promote polarized cell growth (Sawin and Nurse 1998; Sawin and Snaith 2004; Martin et al. 2005). Other biological processes that involve cellular response to extracellular polarity cues, such as cell migration and expansion of neuronal growth cone, also utilize similar mechanisms to position the leading edge (Siegrist and Doe 2007; Dent et al. 2011).
Several polarity regulatory complexes control polarized traffic through modulating the orientation of the microtubules. The Par3/Par6/aPKC complex activates the microtubule plus-end-binding protein APC through inactivation of GSK3β, the kinase that phosphorylates and inactivates APC. Thus, activation of APC stabilizes and orients the microtubules toward specific plasma membrane domains, and thus guide the protein and vesicle traffic for cell polarization (Etienne-Manneville and Hall 2003; Iden and Collard 2008). Dlg1 can interact with APC at the microtubule plus end and together they regulate polarization of the microtubule network (Etienne-Manneville et al. 2005). Similarly, Patj was also found to regulate the orientation of the microtubule organization center (MTOC) in migrating epithelial cells (Shin et al. 2007) and Scribble controls the localization and activity of Cdc42 through interaction with βPIX, the guanine nucleotide exchange factor for Cdc42 (Osmani et al. 2006).
Actin
While mammalian cells use microtubules for trafficking, the budding yeast assemble short actin filaments into cables for polarized delivery of vesicles to the growing end of the daughter cells (the “bud tip”) for polarized growth. The polarized localization of formins and their activation by the Rho family of GTPases direct the actin cables to the bud tip or mother–daughter junction (Pruyne et al. 2002; Goode and Eck 2007). The yeast class V myosin Myo2 is responsible for the transport of post-Golgi secretory vesicles along actin cables (for review, see Pruyne et al. 2004). About 10 myosin motors associate with a single secretory vesicle en route to the bud tip (Donovan and Bretscher 2012). Myo2 is activated on binding to secretory vesicles, and the delivery cycle of the myosin is regulated by sequential interactions with different Rab proteins and the exocyst complex (Lipatova et al. 2008; Donovan and Bretscher 2012). Sec15, functioning as the downstream effector of the Rab protein Sec4 (Guo et al. 1999), interacts with myosin-V and may act as an adaptor to attach molecular motors to post-Golgi secretory vesicles (Jin et al. 2011).
The Small GTPases
Several members of the Ras superfamily of small GTPases regulate different aspects of polarized exocytosis (Wu et al. 2008; Wu and Guo 2015). The small GTPases function as molecular switches that cycle between GTP- and GDP-bound form. In the active GTP-bound form, these small GTPases interact with the secretory machinery and cytoskeleton to regulate polarized exocytosis. These small GTPases are activated by the guanine nucleotide exchange factors (GEFs), which promote GDP dissociation and GTP loading, and inactivated by the GTPase-activating proteins (GAPs), which facilitate GTP hydrolysis. Here we focus on the Rab, Rho, and Ral proteins in polarized exocytosis.
Rab GTPases
The Rab proteins constitute the largest family of small GTPases that includes more than 60 members in mammals. Different Rabs are localized to distinct membrane-bound organelles or vesicles, where they regulate each step of membrane traffic through their downstream effectors (reviewed in Stenmark 2009; Hutagalung and Novick 2011). The effectors of Rab GTPases are a diverse set of proteins that range from components of the trafficking machinery and cytoskeletal motors to kinases and adaptor proteins. As mentioned above, the exocyst subunit Sec15 is a direct downstream effector of the exocytotic Rab protein, which controls the recruitment of the exocyst to the vesicles (Guo et al. 1999; Zhang et al. 2004; Wu et al. 2005). In yeast, the Rab protein Sec4 also interacts with Myo2, a member of the class V myosin that mediates the transport of cargos along actin cables, therefore orchestrating vesicle transport and tethering (Lipatova et al. 2008; Jin et al. 2011; Donovan and Bretscher 2012). Sec4 also interacts with Sro7, which is a homolog of Lgl that interacts with both the exocyst and t-SNARE proteins (Lehman et al. 1999; Zhang et al. 2005; Grosshans et al. 2006).
The Rab GTPases coordinate with each other and are activated sequentially along the exocytic pathway (Mizuno-Yamasaki et al. 2012; Novick 2016). It was found in both yeast and mammalian cells that the GEF (e.g., Sec2 and Rabin8) for a downstream Rab protein (e.g., Sec4 and Rab8) can be a direct effector of an upstream Rab (Ypt31/32 and Rab11) (Ortiz et al. 2002; Knödler et al. 2010). In yeast, Ypt31/32 recruit Sec2 to the secretory vesicles (Ortiz et al. 2002). In mammalian cells, Rab11 is not only required for Rabin8 localization, but also stimulates the GEF activity of Rabin8 toward Rab8 (Knödler et al. 2010). The cascade may lead to an ordered series of transitions from one Rab protein to the next and ensure the directional flow of trafficking (Das and Guo 2011; Mizuno-Yamasaki et al. 2012; Novick 2016).
Rho GTPases
The Rho family of small GTPases regulates a wide range of cellular processes such as cytoskeleton organization, focal adhesion formation, cell polarity, and vesicle traffic (Tapon and Hall 1997; Jaffe and Hall 2005; Park and Bi 2007). Apart from regulating vesicle transport through remodeling cytoskeletons, Rho GTPases directly interact with the secretion machinery. In yeast, Cdc42, Rho1, and Rho3 interact with the exocyst subunits Sec3 and Exo70 (Adamo et al. 1999, 2001; Robinson et al. 1999; Guo et al. 2001; Zhang et al. 2001, 2005; Baek et al. 2010; Wu et al. 2010; Yamashita et al. 2010). The interaction of the Rho GTPases with the exocyst may mediate the polarized localization of the exocyst to the bud tip for polarized cell growth or kinetically regulate the activity of the exocyst in vesicle tethering and membrane fusion.
Cdc42 is a central player in the establishment of cell polarity (Etienne-Manneville 2004). In yeast, besides directly interacting with the exocyst subunits Sec3 and Exo70, Cdc42 regulates actin dynamics by activating actin nucleation-promoting factors such as N-WASP and formins (for review, see Pruyne et al. 2004; Park and Bi 2007). Moreover, it was proposed that Cdc42 regulate the activity of the Rab GTPase Sec4 by regulating its GAPs, Msb3, and Msb4 (Gao et al. 2003). In mammalian cells, Cdc42 also regulates the positioning of microtubule plus end through the Cdc42-Par6/aPKC-GSK3β-APC pathway (Etienne-Manneville and Hall 2003), the Cdc42-IQGAP-CLIP170/APC pathway (Fukata et al. 2002), and the Cdc42-PAK-stathmin pathway (Daub et al. 2001), which are important for establishing polarity axis and reorienting microtubule and Golgi for polarized vesicle traffic. Cdc42 was also shown to inhibit COPI-mediated retrograde traffic from Golgi to ER (Wu et al. 2000; Chen et al. 2005) and promote anterograde traffic within Golgi (Park et al. 2015).
Cdc42 itself can be regulated by positive feedback loops, which amplify the initial polarity cue and stabilize the polarity axis. Studies in yeast suggest that targeted vesicle traffic mediated by actin cables, the exocyst complex, and Rab GTPases is required for polarized transport of Cdc42 to the plasma membrane (for review, see Slaughter et al. 2009). In mammalian cells, Cdc42 can recruit the microtubule plus-end-binding proteins such as CLIP-170 and APC through several signaling pathways. The plus-end-binding proteins anchor microtubule plus ends to the cell cortex, where active Cdc42 further orients the MTOC and the Golgi apparatus. As such, polarized delivery of more cortical polarity proteins or GEFs for Rho GTPases leads to reinforcement of cell polarity (Watanabe et al. 2005; Siegrist and Doe 2007).
Ral GTPases
Ral GTPases were generally viewed as proto-oncogenes as they induce cellular transformation through Ras-dependent or -independent signaling pathways. Recent studies have implicated them in vesicle trafficking as the active RalA and RalB directly interact with the exocyst subunits Sec5 and Exo84 (Brymora et al. 2001; Moskalenko et al. 2002, 2003; Polzin et al. 2002; Sugihara et al. 2002; Feig 2003; Jin et al. 2005). Phosphorylation of the exocyst component Sec5 by PKC dissociates it from RalA and may facilitate the next round of regulation by RalA (Chen et al. 2011). The Ral-exocyst interaction has also been implicated in tumorigenesis and cell migration (Balakireva et al. 2006; Chen et al. 2006, 2007; Chien et al. 2006; Rossé et al. 2006; Lalli 2009; Bodemann et al. 2011; Hazelett et al. 2011).
Phosphoinositides
Intracellular compartments are marked by different phosphoinositides, which not only specify the organelles but also regulate membrane traffic and signaling. Different phosphoinositides recruit specific cytosolic proteins, act as allosteric regulators of effector proteins, or regulate vesicle fission and fusion by modulating membrane curvature (De Camilli et al. 1996; Corvera et al. 1999; De Matteis and Godi 2004; Roth 2004; Behnia and Munro 2005; Di Paolo and De Camilli 2006). The conversions of different phospholipids are mediated by lipid kinases and phosphatases that have been shown to regulate membrane traffic and are regulated by several small GTPases. For exocytic trafficking in yeast, the Golgi pool of PI(4)P controls both the recruitment and subsequent activation of Sec4 for exocytic traffic via its GEF, Sec2 (Mizuno-Yamasaki et al. 2010). The subsequent attenuation of PI(4)P along the exocytic trafficking to the plasma membrane switches Sec2 binding to the Sec4 effector Sec15 (Medkova et al. 2006). Coupled to the Rab and exocyst, PI(4)P transition also mediates the association of myosin-V to the secretory vesicles (Santiago-Tirado et al. 2011).
At the plasma membrane, phosphoinositides PI(4,5)P2 and PI(3,4,5)P3 function as important signaling molecules for cell polarity. The levels of PI(4,5)P2 and PI(3,4,5)P3 on the plasma membrane at a given site are regulated by PI4P5-kinases, PI3-kinase, and phosphatase and tensin homolog (PTEN) phosphatase, respectively (Leevers et al. 1999). In polarized Madin–Darby canine kidney (MDCK) cells, PI(3,4,5)P3 is localized to the basolateral membrane (Gassama-Diagne et al. 2006; Martin-Belmonte et al. 2007). In a 3D cystogenesis model, it was shown that PTEN localizes to the apical membrane during epithelial morphogenesis, where it excludes PI(3,4,5)P3 from this domain (Martin-Belmonte et al. 2007). Also, PI(4,5)P2 recruits the adaptor protein annexin2 to the apical domain, which in turn recruits Cdc42 and the aPKC/Par6 complex. Cdc42 activation of the aPKC/Par3/Par6 complex has also been shown to play a role in directional cell migration (Etienne-Manneville et al. 2005). As mentioned above, the exocyst interacts directly with PI(4,5)P2 (He et al. 2007; Liu et al. 2007; Zhang et al. 2008). It was shown that the exocyst complex is recruited for the establishment or maintenance of epithelial polarity (Grindstaff et al. 1998).
POLARIZED EXOCYTOSIS IN DIFFERENT TYPES OF CELLS
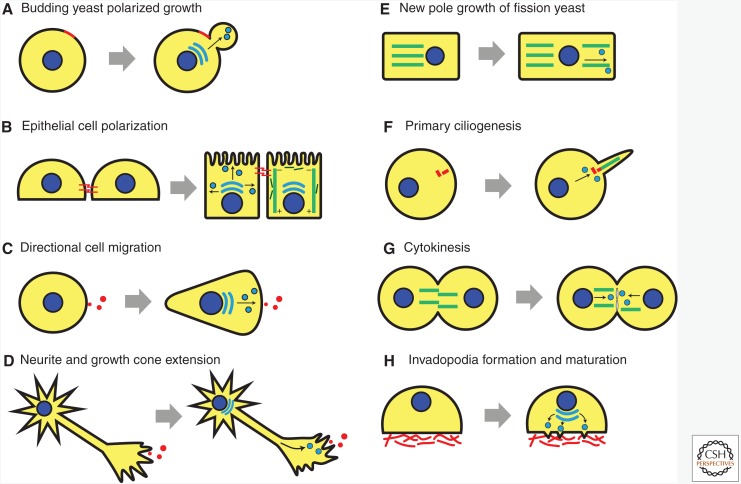
Polarized exocytosis is an evolutionarily conserved process observed in many different types of eukaryotic cells (Fig. 2). While cells need polarized exocytosis for their diverse growth properties and physiological functions, they also offer excellent model systems that help understand polarized exocytosis. The variations on the theme confer the unique morphologies and functions that different types of cells display.
Figure 2.
Polarized exocytosis functions to establish and maintain polarity in different cell types and biological processes. (A) Asymmetric growth of daughter cells in budding yeast involves polarized exocytosis. Red: bud scar. (B) The establishment and maintenance of apicobasolateral polarity of epithelial cells requires polarized traffic to specific plasma membrane domains. Red: E-cadherin. (C) The front–rear polarity of migrating cells along the chemoattractant gradient involves active endo-exocytosis at the leading edge. Red: chemoattractant. (D) Neurite and growth cone extension requires polarized membrane addition. (E) Polarized growth at the new pole of fission yeasts. Green: microtubules. (F) Primary ciliogenesis requires polarized traffic at the base of the cilium. Red: γ-tubulin. (G) Cytokinesis requires polarized traffic for membrane abscission. (H) Generation and maturation of invadopodia requires polarized exocytosis for the formation of membrane protrusion and secretion of matrix metalloproteinases (MMPs). Red: extracellular matrix (ECM).
Polarized Cell Growth in Budding Yeast
The budding yeast Saccharomyces cerevisiae undergoes asymmetric growth, and thus provides an excellent model system for the study of cell polarity. After defining a site for bud emergence based on the spatial cue from the previous cell cycle (e.g., “bud scar”) or a stochastic process, a yeast cell build actin cables, along which post-Golgi secretory vesicles are transported to the daughter cell plasma membrane. The class V myosin, Myo2, mediates the polarized transport process (for review, see Pruyne et al. 2004). Cdc42 is a master regulator in polarity establishment as it orchestrates actin reorganization and vesicle traffic (for review, see Bi and Park 2012). First, it regulates the polarized organization of the actin cables through the formin protein Bni1 (Pruyne et al. 2004; Park and Bi 2007; Breitsprecher and Goode 2013). Second, Cdc42 regulates the localization and/or activation of the exocyst complex (Wu et al. 2008; Wu and Guo 2015). In cdc42 mutants, exocytosis defects were detected (Adamo et al. 2001). In addition, disruption of exocyst-Cdc42 interactions leads to secretion and polarity defects (Zhang et al. 2001, 2008). While Cdc42 is a key regulator of exocytosis, exocytosis in turn regulates the polarized localization of Cdc42 (Wedlich-Soldner et al. 2003). This positive feedback loop is important for the establishment or reenforcement of cell polarity.
Apicobasolateral Polarity of Epithelial Cells
The establishment of epithelial polarity is initiated by the formation of cell–cell contact, transactivation of E-cadherin at the cell–cell contact, and a positive feedback loop between E-cadherin and Cdc42 to strengthen the adhesion (Kim et al. 2000; Chu et al. 2004). As discussed previously, Cdc42 is able to orient the microtubules, further amplifying the polarity cue by directing polarized transport of other proteins.
The sorting of apical and basolateral proteins has long been recognized to be required to establish, reinforce, and maintain epithelial polarity (Apodaca et al. 2012). The biosynthetic sorting machinery and the polarized trafficking routes in epithelial cells have been intensively studied for decades (reviewed in Mostov et al. 2000; Ellis et al. 2006; Carmosino et al. 2010). It has been reviewed in other articles and will not be discussed here. For exocytic trafficking, the Rab GTPases Rab8 and Rab11 have been shown to regulate basolateral trafficking via Golgi and sorting endosomes (Ang et al. 2003; Lock and Stow 2005). Rab11 was also reported to be involved in apical trafficking (Satoh et al. 2005). A possible explanation might be that Rab11 resides on apical recycling endosomes to regulate apical targeting and also on common recycling endosomes to mediate basolateral sorting (Ellis et al. 2006). Similarly, the involvement of Rab8 in apical trafficking in intestinal cells was also reported (Sato et al. 2007). The exocyst complex, acting downstream of Rab8 and Rab11 or Ral GTPases, has been shown to be localized to the apex of the basolateral domain and regulates the basolateral, but not apical, targeting of vesicles (Grindstaff et al. 1998; Moskalenko et al. 2002; Ang et al. 2003; Lock et al. 2005). Studies have also shown that the exocyst functions together with the Par3/6 complex in apical domain trafficking (Blankenship et al. 2007; Oztan et al. 2007; Bryant et al. 2010). During de novo lumen generation, proper targeting of Par3 and Cdc42 and the delivery of apical membrane components are governed by Rab11, Rab8, and the exocyst (Bryant et al. 2010; Datta et al. 2011).
Front–Rear Polarity of Migrating Cells
The migrating cells generate a front–rear polarity axis in response to extracellular stimuli. It has been shown that active exocytosis occurs at the leading edge that involves polarized organization of microtubules (Schmoranzer et al. 2003). Cdc42 and components of the polarity complexes have been shown to be localized to the leading edge, which may orchestrate the rearrangement of microtubules and polarized distribution of effector proteins. This may, in turn, form positive feedback loops and promote polarized vesicle traffic toward the leading edge (Osmani et al. 2006; Shin et al. 2007; Nelson 2009). Besides Cdc42, the other Rho family of GTPases also regulates directional migration by regulating actin dynamics and adhesion organization (Ridley et al. 2003). RalB also regulates polarized traffic to the leading edge through promoting the exocyst complex assembly and localization (Rossé et al. 2006). The exocyst subunits, including Exo70, interact with the Arp2/3 complex and WAVE complexes to facilitate actin polymerization for membrane protrusion (Zuo et al. 2006; Liu et al. 2012; Biondini et al. 2016). The role of Exo70 in regulating actin dynamics may be coordinated with its capability of inducing membrane deformation at the leading edge for directional cell migration (Zhao et al. 2013). It was also found that the Par3/6 complex interacts with the exocyst under the control of RalA, and this interaction is important for directional migration of the neuronal progenitor cells (Lalli 2009; Zuo et al. 2009; Das et al. 2014).
The epithelial–mesenchymal transition (EMT) is thought to play important roles during embryonic development and tumor metastasis. EMT can be regulated at the mRNA level by alternative splicing by proteins such as ESRP1/2 (Warzecha and Carstens 2012). The exocyst subunit Exo70 exists as different splicing isoforms in epithelial cells and mesenchymal cells. ESRP1/2 mediate the isoform switching of Exo70 during EMT, and this switch is important for the transition between the apicobasolateral polarity of epithelial cells and the front–rear polarity of the migrating cells (Lu et al. 2013).
Neurite and Growth Cone Extension in Neuronal Cells
The neuronal growth cone guidance shares several key features with directional cell migration (Siegrist and Doe 2007). The growth cone contains a peripheral actin network and a central microtubule domain. While the cortical actin networks generate membrane protrusions similar to lamellipodia, the microtubules are required for polarized positioning of the protrusions. It was speculated that a constant flow of vesicles moves toward the extending membrane domains of the growth cone (Bretscher 1996). Later studies showed that polarized exocytosis is indeed required for attractive axon guidance (Tojima et al. 2007). The exocyst complex was found to associate with microtubules and mediate polarized vesicle traffic to promote neurite outgrowth (Vega and Hsu 2001; Dupraz et al. 2009). The Par3/6 complex interacts with the exocyst, and this interaction is involved in directional migration of neural progenitor cells (Lalli and Hall 2005; Lalli 2009; Zuo et al. 2009; Das et al. 2014). A component in the Scrib polarity complex, Lgl1, was shown to be localized to neuronal growth cone and act upstream of Rab10 to promote axon growth, probably through polarized vesicle traffic (Wang et al. 2011).
CELLULAR PROCESSES THAT REQUIRE POLARIZED EXOCYTOSIS
As polarized exocytosis mediates the deposition of proteins and membranes at specific domains of the cell surface, it is needed for the accomplishment of many essential cellular processes (Fig. 2). Below we will discuss a few examples.
Primary Ciliogenesis
In the prevalent model for primary ciliogenesis, the delivery of ciliary membranes and proteins requires polarized exocytosis at the base of cilia (reviewed in Nachury et al. 2010). Several studies have suggested that polarized trafficking machinery has a role in the growth and function of primary cilia. A Rab cascade involving Rab11-Rabin8-Rab8 mediates polarized exocytosis at the periciliary base and promotes ciliogenesis (Knödler et al. 2010). Rabin8 and Rab8 also interact with the coat complex BBSome and the tethering complex TRAPPII to promote ciliogenesis (Nachury et al. 2007; Westlake et al. 2011). Moreover, the exocyst complex is localized to the base of the cilia and regulates ciliogenesis together with Par3/6 and Rab10 in MDCK cells (Rogers et al. 2004; Zuo et al. 2009; Babbey et al. 2010). Recently, the exocyst was shown to physically and genetically interact with Arl13b, one of ARF family GTPases known to regulate ciliogenesis (Seixas et al. 2016).
Cytokinesis
During cytokinesis, cells need to rearrange membrane phospholipids and cytoskeletons, and redirect intracellular membrane traffic pathways (reviewed in Hehnly and Doxsey 2012). It was shown more than 50 years ago that cell growth dramatically decreases during mitosis (Prescott and Bender 1962). The mitotic growth arrest is thought to help cells to reorganize their structure and adapt to the increased energy demands for subsequent cell division or regeneration (Goranov and Amon 2010). Cell-surface expansion decreases as cells approach metaphase and recovers after the metaphase–anaphase transition as a result of SNARE-mediated exocytosis (Boucrot and Kirchhausen 2007, 2008). As elaborated above, phosphorylation of Exo84 by mitotic Cdk1 inhibits exocyst assembly and blocks membrane expansion before mitosis (Luo et al. 2013). Also, the Rab protein Sec4 is phosphorylated during mitosis, which leads to its dissociation from Sec15 (Lepore et al. 2016). The recycling endosomes are associated with γ–tubulin ring complex and depletion of Rab11 delays mitosis (Hehnly et al. 2014).
At the late stage of cytokinesis, membrane deposition is needed at the junction of two daughter cells for separation, a process termed abscission. Exocytic trafficking and cytoskeleton are coordinated during this process (for reviews, see Chen et al. 2012; Schiel et al. 2013). Time-lapse imaging shows the trafficking of Golgi-derived vesicles from daughter cells to the cleavage furrow during mammalian cytokinesis (Goss and Toomre 2008). In fission yeast, the exocyst is implicated in membrane deposition (Fig. 2) (Wang et al. 2012, 2016). During ingression of the cleavage furrow, the central spindle microtubules are compacted to form the structure known as the midbody. It was shown that centriolin, a component of the midbody, interacts with the exocyst and the SNARE- binding protein Snapin (Gromley et al. 2005). RalA and RalB play distinct roles in regulating the exocyst complex to promote cytokinesis (Cascone et al. 2008).
Invadopodia
Invadopodia are actin-based cell-surface protrusions that degrade the extracellular matrix (ECM) (reviewed in Murphy and Courtneidge 2011). It was reported that the secretion of the ECM degrading enzyme matrix metalloproteinases (MMPs) at invadopodia is mediated by the exocyst complex (Sakurai-Yageta et al. 2008; Liu et al. 2009; Ren and Guo 2012; Monteiro et al. 2013; Lu et al. 2016). Besides the exocyst, SNARE proteins including TI-VAMP/VAMP7 also mediate MMP secretion (Steffen et al. 2008). Recently, it was found that invadopodia function as docking and secretion sites for MMP-bearing exosomes to promote the invasive behavior of cancer cells (Hoshino et al. 2013).
CONCLUDING REMARKS
Although polarized exocytosis takes many forms and is involved in different biological processes, the principle trafficking pathways and key regulators are mostly conserved. It is likely that more cellular processes will be linked to polarized exocytosis in the future. Moreover, new studies may reveal additional factors and add more complexity to the already complicated regulatory mechanisms. Now the field is poised to offer molecular insights to the pathology of diseases ranging from tumorigenesis to neurodegeneration, in which several key regulators of polarity have already been implicated. The challenge now is to cross the divide between basic cell biology and medicine.
ACKNOWLEDGMENTS
Due to the broad nature of this subject, we were unable to provide a complete survey of the field of polarized exocytosis, but instead chose to highlight a few key processes. We apologize for any references we may have left out. Research in Wei Guo’s laboratory is supported by grants from the National Institutes of Health.
Footnotes
Editor: Keith E. Mostov
Additional Perspectives on Cell Polarity available at www.cshperspectives.org
REFERENCES
- Adamo JE, Rossi G, Brennwald P. 1999. The Rho GTPase Rho3 has a direct role in exocytosis that is distinct from its role in actin polarity. Mol Biol Cell 10: 4121–4133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamo JE, Moskow JJ, Gladfelter AS, Viterbo D, Lew DJ, Brennwald PJ. 2001. Yeast Cdc42 functions at a late step in exocytosis, specifically during polarized growth of the emerging bud. J Cell Biol 155: 581–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aikawa Y, Martin TFJ. 2003. ARF6 regulates a plasma membrane pool of phosphatidylinositol (4,5) bisphophate required for regulated exocytosis. J Cell Biol 162: 647–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ang AL, Fölsch H, Koivisto UM, Pypaert M, Mellman I. 2003. The Rab8 GTPase selectively regulates AP-1B-dependent basolateral transport in polarized Madin-Darby canine kidney cells. J Cell Biol 163: 339–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apodaca G. 2001. Endocytic traffic in polarized epithelial cells: role of the actin and microtubule cytoskeleton. Traffic 2: 149–159. [DOI] [PubMed] [Google Scholar]
- Apodaca G, Gallo LI, Bryant DM. 2012. Role of membrane traffic in the generation of epithelial cell asymmetry. Nat Cell Biol 14: 1235–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babbey CM, Bacallao RL, Dunn KW. 2010. Rab10 associates with primary cilia and the exocyst complex in renal epithelial cells. Am J Physiol Renal Physiol 299: F495–F506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baek K, Knödler A, Lee SH, Zhang X, Orlando K, Zhang J, Foskett TJ, Guo W, Dominguez R. 2010. Structure-function study of the N-terminal domain of exocyst subunit Sec3. J Biol Chem 285: 10424–10433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker RW, Hughson FM. 2016. Chaperoning SNARE assembly and disassembly. Nat Rev Mol Cell Biol 17: 465–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balakireva M, Rossé C, Langevin J, Chien YC, Gho M, Gonzy-Treboul G, Voegeling-Lemaire S, Aresta S, Lepesant JA, Bellaiche Y, et al. 2006. The Ral/exocyst effector complex counters c-Jun N-terminal kinase-dependent apoptosis in Drosophila melanogaster. Mol Cell Biol 26: 8953–8963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behnia R, Munro S. 2005. Organelle identity and the signposts for membrane traffic. Nature 438: 597–604. [DOI] [PubMed] [Google Scholar]
- Bi E, Park HO. 2012. Cell polarization and cytokinesis in budding yeast. Genetics 191: 347–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biondini M, Sadou-Dubourgnoux A, Paul-Gilloteaux P, Zago G, Arslanhan MD, Waharte F, Formstecher E, Hertzog M, Yu J, Guerois R, et al. 2016. Direct interaction between Exocyst and Wave complexes promotes cell protrusions and motility. J Cell Sci 129: 3756–3769. [DOI] [PubMed] [Google Scholar]
- Blankenship JT, Fuller MT, Zallen JA. 2007. The Drosophila homolog of the Exo84 exocyst subunit promotes apical epithelial identity. J Cell Sci 120: 3099–3110. [DOI] [PubMed] [Google Scholar]
- Bloch D, Pleskot R, Pejchar P, Potocky M, Trpkosova P, Cwiklik L, Vukasinovic N, Sternberg H, Yalovsky S, Zarsky V. 2016. Exocyst SEC3 and phosphoinositides define sites of exocytosis in pollen tube initiation and growth. Plant Physiol 10.1104/pp.16.00690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodemann BO, Orvedahl A, Cheng T, Ram RR, Ou YH, Formstecher E, Maiti M, Hazelett CC, Wauson EM, Balakireva M, et al. 2011. RalB and the exocyst mediate the cellular starvation response by direct activation of autophagosome assembly. Cell 144: 253–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucrot E, Kirchhausen T. 2007. Endosomal recycling controls plasma membrane area during mitosis. Proc Natl Acad Sci 104: 7939–7944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucrot E, Kirchhausen T. 2008. Mammalian cells change volume during mitosis. PLoS ONE 3: e1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd C, Hughes T, Pypaert M, Novick P. 2004. Vesicles carry most exocyst subunits to exocytic sites marked by the remaining two subunits, Sec3p and Exo70p. J Cell Biol 167: 889–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitsprecher D, Goode BL. 2013. Formins at a glance. J Cell Sci 126: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretscher M. 1996. Moving membrane up to the front of migrating cell. Cell 85: 465–467. [DOI] [PubMed] [Google Scholar]
- Bryant DM, Datta A, Rodríguez-Fraticelli AE, Peränen J, Martín-Belmonte F, Mostov KE. 2010. A molecular network for de novo generation of the apical surface and lumen. Nat Cell Biol 12: 1035–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brymora A, Valova VA, Larsen MR, Roufogalis BD, Robinson PJ. 2001. The brain exocyst complex interacts with RalA in a GTP-dependent manner: Identification of a novel mammalian Sec3 gene and a second Sec15 gene. J Biol Chem 276: 29792–29797. [DOI] [PubMed] [Google Scholar]
- Burgo A, Proux-Gillardeaux V, Sotirakis E, Bun P, Casano A, Verraes A, Liem RK, Formstecher E, Coppey-Moisan M, Galli T. 2012. A molecular network for the transport of the TI-VAMP/VAMP7 vesicles from cell center to periphery. Dev Cell 23: 166–180. [DOI] [PubMed] [Google Scholar]
- Carmosino M, Valenti G, Caplan M, Svelto M. 2010. Polarized traffic towards the cell surface: How to find the route. Biol Cell 102: 75–91. [DOI] [PubMed] [Google Scholar]
- Cascone I, Selimoglu R, Ozdemir C, Del Nery E, Yeaman C, White M, Camonis J. 2008. Distinct roles of RalA and RalB in the progression of cytokinesis are supported by distinct RalGEFs. EMBO J 27: 2375–2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JL, Fucini RV, Lacomis L, Erdjument-Bromage H, Tempst P, Stamnes M. 2005. Coatomer-bound Cdc42 regulates dynein recruitment to COPI vesicles. J Cell Biol 169: 383–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen XW, Inoue M, Hsu SC, Saltiel AR. 2006. RalA-exocyst-dependent recycling endosome trafficking is required for the completion of cytokinesis. J Biol Chem 281: 38609–38616. [DOI] [PubMed] [Google Scholar]
- Chen XW, Leto D, Chiang SH, Wang Q, Saltiel AR. 2007. Activation of RalA is required for insulin-stimulated Glut4 trafficking to the plasma membrane via the exocyst and the motor protein Myo1c. Dev Cell 13: 391–404. [DOI] [PubMed] [Google Scholar]
- Chen XW, Leto D, Xiao J, Goss J, Wang Q, Shavit JA, Xiong T, Yu G, Ginsburg D, Toomre D, et al. 2011. Exocyst function is regulated by effector phosphorylation. Nat Cell Biol 13: 580–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CT, Hehnly H, Doxsey SJ. 2012. Orchestrating vesicle transport, ESCRTs and kinase surveillance during abscission. Nat Rev Mol Cell Biol 13: 483–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien Y, Kim S, Bumeister R, Loo YM, Kwon SW, Johnson CL, Balakireva MG, Romeo Y, Kopelovich L, Gale M Jr, et al. 2006. RalB GTPase-mediated activation of the IκB family kinase TBK1 couples innate immune signaling to tumor cell survival. Cell 127: 157–170. [DOI] [PubMed] [Google Scholar]
- Chu YS, Thomas WA, Eder O, Pincet F, Perez E, Thiery JP, Dufour S. 2004. Force measurements in E-cadherin-mediated cell doublets reveal rapid adhesion strengthened by actin cytoskeleton remodeling through Rac and Cdc42. J Cell Biol 167: 1183–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corvera S, D'Arrigo A, Stenmark H. 1999. Phosphoinositides in membrane traffic. Curr Biol 11: 460–465. [DOI] [PubMed] [Google Scholar]
- Das A, Guo W. 2011. Rabs and the exocyst in ciliogenesis, tubulogenesis and beyond. Trends Cell Biol 21: 383–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das A, Gajendra S, Falenta K, Oudin MJ, Peschard P, Feng S, Wu B, Marshall CJ, Doherty P, Guo W. 2014. RalA promotes a direct exocyst-Par6 interaction to regulate polarity in neuronal development. J Cell Sci 127: 686–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta A, Bryant DM, Mostov KE. 2011. Molecular regulation of lumen morphogenesis. Curr Biol 21: R126–R136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daub H, Gevaert K, Vandekerckhove J, Sobel A, Hall A. 2001. Rac/Cdc42 and p65PAK regulate the microtubule-destabilizing protein stathmin through phosphorylation at serine 16. J Biol Chem 276: 1677–1680. [DOI] [PubMed] [Google Scholar]
- De Camilli P, Emr SD, McPherson PS, Novick P. 1996. Phosphoinositides as regulators in membrane traffic. Science 271: 153–1539. [DOI] [PubMed] [Google Scholar]
- De Matteis MA, Godi A. 2004. PI-loting membrane traffic. Nat Cell Biol 6: 487–492. [DOI] [PubMed] [Google Scholar]
- Dent EW, Gupton SL, Gertler FB. 2011. The growth cone cytoskeleton in axon outgrowth and guidance. Cold Spring Harb Perspect Biol 3: a001800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Paolo G, De Camilli P. 2006. Phosphoinositides in cell regulation and membrane dynamics. Nature 443: 651–657. [DOI] [PubMed] [Google Scholar]
- Dong G, Hutagalung AH, Fu C, Novick P, Reinisch KM. 2005. The structures of exocyst subunit Exo70p and the Exo84p C-terminal domains reveal a common motif. Nat Struct Mol Biol 12: 1094–1100. [DOI] [PubMed] [Google Scholar]
- Donovan KW, Bretscher A. 2012. Myosin-V is activated by binding secretory cargo and released in coordination with Rab/Exocyst. Dev Cell 23: 769–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubuke ML, Maniatis S, Shaffer SA, Munson M. 2015. The exocyst subunit Sec6 interacts with assembled exocytic SNARE complexes. J Biol Chem 290: 28245–28256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupraz S, Grassi D, Bernis ME, Sosa L, Bisbal M, Gastaldi L, Jausoro I, Caceres A, Pfenninger KH, Quiroga S. 2009. The TC10-Exo70 complex is essential for membrane expansion and axonal specification in developing neurons. J Neurosci 29: 13292–13301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis MA, Potter BA, Cresawn KO, Weisz OA. 2006. Polarized biosynthetic traffic in renal epithelial cells: Sorting, sorting, everywhere. Am J Physiol Renal Physiol 291: F707–F713. [DOI] [PubMed] [Google Scholar]
- Etienne-Manneville S. 2004. Cdc42-the center of polarity. J Cell Sci 117: 1291–1300. [DOI] [PubMed] [Google Scholar]
- Etienne-Manneville S, Hall A. 2003. Cdc42 regulates GSK3β and adenomatous polyposis coli to control cell polarity. Nature 421: 753–756. [DOI] [PubMed] [Google Scholar]
- Etienne-Manneville S, Manneville JB, Nicholls S, Ferenczi MA, Hall A. 2005. Cdc42 and Par6-PKCζ regulate the spatially localized association of Dlg1 and APC to control cell polarization. J Cell Biol 170: 895–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feig LA. 2003. Ral-GTPases: Approaching their 15 minutes of fame. Trends Cell Biol 13: 419–425. [DOI] [PubMed] [Google Scholar]
- Fukata M, Watanabe T, Noritake J, Nakagawa M, Yamaga M, Kuroda S, Matsuura Y, Iwamatsu A, Perez F, Kaibuchi K. 2002. Rac1 and Cdc42 capture microtubules through IQGAP and CLIP-170. Cell 109: 873–885. [DOI] [PubMed] [Google Scholar]
- Gao XD, Albert S, Tcheperegine SE, Burd CG, Gallwitz D, Bi E. 2003. The GAP activity of Msb3p and Msb4p for the Rab GTPase Sec4p is required for efficient exocytosis and actin organization. J Cell Biol 162: 635–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gassama-Diagne A, Yu W, ter Beest M, Martin-Belmonte F, Kierbel A, Engel J, Mostov K. 2006. Phosphatidylinositol-3,4,5-trisphosphate regulates the formation of the basolateral plasma membrane in epithelial cells. Nat Cell Biol 8: 963–970. [DOI] [PubMed] [Google Scholar]
- Goode BL, Eck MJ. 2007. Mechanism and function of formins in the control of actin assembly. Annu Rev Biochem 76: 593–627. [DOI] [PubMed] [Google Scholar]
- Goranov AI, Amon A. 2010. Growth and division—Not a one-way road. Curr Opin Cell Biol 22: 795–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goss JW, Toomre DK. 2008. Both daughter cells traffic and exocytose membrane at the cleavage furrow during mammalian cytokinesis. J Cell Biol 181: 1047–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grindstaff KK, Yeaman C, Anandasabapathy N, Hsu SC, Rodriguez-Boulan E, Scheller RH, Nelson WJ. 1998. Sec6/8 complex is recruited to cell–cell contacts and specifies transport vesicle delivery to the basal–lateral membrane in epithelial cells. Cell 93: 731–740. [DOI] [PubMed] [Google Scholar]
- Gromley A, Yeaman C, Rosa J, Redick S, Chen CT, Mirabelle S, Guha M, Sillibourne J, Doxsey SJ. 2005. Centriolin anchoring of exocyst and SNARE complexes at the midbody is required for secretory-vesicle-mediated abscission. Cell 123: 75–87. [DOI] [PubMed] [Google Scholar]
- Grosshans BL, Andreeva A, Gangar A, Niessen S, Yates JR, Brennwald P, Novick P. 2006. The yeast lgl family member Sro7p is an effector of the secretory Rab GTPase Sec4p. J Cell Biol 172: 55–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W, Roth D, Walch-Solimena C, Novick P. 1999. The exocyst is an effector for Sec4p, targeting secretory vesicles to sites of exocytosis. EMBO J 18: 1071–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W, Sacher M, Barrowman J, Ferro-Novick S, Novick P. 2000. Protein complexes in transport vesicle targeting. Trends Cell Biol 10: 251–255. [DOI] [PubMed] [Google Scholar]
- Guo W, Tamanoi F, Novick P. 2001. Spatial regulation of the exocyst complex by Rho1 GTPase. Nat Cell Biol 3: 353–360. [DOI] [PubMed] [Google Scholar]
- Hamburger ZA, Hamburger AE, West AP Jr, Weis WI. 2006. Crystal structure of the S. cerevisiae exocyst component Exo70p. J Mol Biol 356: 9–21. [DOI] [PubMed] [Google Scholar]
- Hazelett CC, Sheff D, Yeaman C. 2011. RalA and RalB differentially regulate development of epithelial tight junctions. Mol Biol Cell 22: 4787–4800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He B, Guo W. 2009. The exocyst complex in polarized exocytosis. Curr Opin Cell Biol 21: 537–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He B, Xi F, Zhang X, Zhang J, Guo W. 2007. Exo70 interacts with phospholipids and mediates the targeting of the exocyst to the plasma membrane. EMBO J 26: 4053–4065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hehnly H, Doxsey S. 2012. Polarity sets the stage for cytokinesis. Mol Biol Cell 23: 7–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hehnly H, Doxsey S. 2014. Rab11 endosomes contribute to mitotic spindle organization and orientation. Dev Cell 28: 497–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heider MR, Munson M. 2012. Exorcising the exocyst complex. Traffic 13: 898–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino D, Kirkbride KC, Costello K, Clark ES, Sinha S, Grega-Larson N, Tyska MJ, Weaver AM. 2013. Exosome secretion is enhanced by invadopodia and drives invasive behavior. Cell Rep 5: 1159–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutagalung AH, Novick PJ. 2011. Role of Rab GTPases in membrane traffic and cell physiology. Physiol Rev 91: 119–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iden S, Collard JG. 2008. Crosstalk between small GTPases and polarity proteins in cell polarization. Nat Rev Mol Cell Biol 9: 846–859. [DOI] [PubMed] [Google Scholar]
- Jaffe AB, Hall A. 2005. Rho GTPases: Biochemistry and biology. Annu Rev Cell Dev Biol 21: 247–269. [DOI] [PubMed] [Google Scholar]
- Jin R, Junutula JR, Matern HT, Ervin KE, Scheller RH, Brunger AT. 2005. Exo84 and Sec5 are competitive regulatory Sec6/8 effectors to the RalA GTPase. EMBO J 24: 2064–2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y, Sultana A, Gandhi P, Franklin E, Hamamoto S, Khan AR, Munson M, Schekman R, Weisman LS. 2011. Myosin V transports secretory vesicles via a Rab GTPase cascade and interaction with the exocyst complex. Dev Cell 21: 1156–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Li Z, Sacks DB. 2000. E-cadherin-mediated cell–cell attachment activates Cdc42. J Biol Chem 275: 36999–37005. [DOI] [PubMed] [Google Scholar]
- Knödler A, Feng S, Zhang J, Zhang X, Das A, Peränen J, Guo W. 2010. Coordination of Rab8 and Rab11 in primary ciliogenesis. Proc Natl Acad Sci 107: 6346–6351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalli G. 2009. RalA and the exocyst complex influence neuronal polarity through PAR-3 and aPKC. J Cell Sci 122: 1499–1506. [DOI] [PubMed] [Google Scholar]
- Lalli G, Hall A. 2005. Ral GTPases regulate neurite branching through GAP-43 and the exocyst complex. J Cell Biol 171: 857–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leevers SJ, Vanhaesebroeck B, Waterfield MD. 1999. Signalling through phosphoinositide 3-kinases: The lipids take centre stage. Curr Opin Cell Biol 11: 219–225. [DOI] [PubMed] [Google Scholar]
- Lehman K, Rossi G, Adamo JE, Brennwald P. 1999. Yeast homologues of tomosyn and lethal giant larvae function in exocytosis and are associated with the plasma membrane SNARE, Sec9. J Cell Biol 146: 125–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepore D, Spassibojko O, Pinto G, Collins RN. 2016. Cell cycle-dependent phosphorylation of Sec4p controls membrane deposition during cytokinesis. J Cell Biol 214: 691–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipatova Z, Tokarev AA, Jin Y, Mulholland J, Weisman LS, Segev N. 2008. Direct interaction between a myosin V motor and the Rab GTPases Ypt31/32 is required for polarized secretion. Mol Biol Cell 19: 4177–4187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Zuo X, Yue P, Guo W. 2007. Phosphatidylinositol 4,5-bisphosphate mediates the targeting of the exocyst to the plasma membrane for exocytosis in mammalian cells. Mol Biol Cell 18: 4483–4492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Yue P, Artym VV, Mueller SC, Guo W. 2009. The role of the exocyst in matrix metalloproteinase secretion and actin dynamics during tumor cell invadopodia formation. Mol Biol Cell 20: 3763–3771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Zhao Y, Sun Y, He B, Yang C, Svitkina T, Goldman YE, Guo W. 2012. Exo70 stimulates the Arp2/3 complex for lamellipodia formation and directional cell migration. Curr Biol 22: 1510–1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lock JG, Stow JL. 2005. Rab11 in recycling endosomes regulates the sorting and basolateral transport of E-cadherin. Mol Biol Cell 16: 1744–1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H, Liu J, Liu S, Zeng J, Ding D, Carstens RP, Cong Y, Xu X, Guo W. 2013. Exo70 isoform switching upon epithelial–mesenchymal transition mediates cancer cell invasion. Dev Cell 27: 560–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H, Liu S, Zhang G, Kwong LN, Zhu Y, Miller JP, Hu Y, Zhong W, Zeng J, Wu L, et al. 2016. Oncogenic BRAF-mediated melanoma cell invasion. Cell Rep 15: 2012–2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo G, Zhang J, Luca FC, Guo W. 2013. Mitotic phosphorylation of Exo84 disrupts exocyst assembly and arrests cell growth. J Cell Biol 202: 97–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo G, Zhang J, Guo W. 2014. The role of Sec3p in secretory vesicle targeting and exocyst complex assembly. Mol Biol Cell 25: 3813–3822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin SG, McDonald WH, Yates JR III, Chang F. 2005. Tea4p links microtubule plus ends with the formin For3p in the establishment of cell polarity. Dev Cell 8: 479–491. [DOI] [PubMed] [Google Scholar]
- Martin-Belmonte F, Gassama A, Datta A, Yu W, Rescher U, Gerke V, Mostov K. 2007. PTEN-mediated apical segregation of phosphoinositides controls epithelial morphogenesis through Cdc42. Cell 128: 383–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medkova M, France YE, Coleman J, Novick P. 2006. The rab exchange factor Sec2p reversibly associates with the exocyst. Mol Biol Cell 17: 2757–2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno-Yamasaki E, Medkova M, Coleman J, Novick P. 2010. Phosphatidylinositol 4-phosphate controls both membrane recruitment and a regulatory switch of the Rab GEF Sec2p. Dev Cell 18: 828–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno-Yamasaki E, Rivera-Molina F, Novick P. 2012. GTPase networks in membrane traffic. Ann Rev Biochem 81: 637–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteiro P, Rossé C, Castro-Castro A, Irondelle M, Lagoutte E, Paul-Gilloteaux P, Desnos C, Formstecher E, Darchen F, Perrais D, et al. 2013. Endosomal WASH and exocyst complexes control exocytosis of MT1-MMP at invadopodia. J Cell Biol 203: 1063–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore BA, Robinson HH, Xu Z. 2007. The crystal structure of mouse Exo70 reveals unique features of the mammalian exocyst. J Mol Biol 371: 410–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgera F, Sallah MR, Dubuke ML, Gandhi P, Brewer DN, Carr CM, Munson M. 2012. Regulation of exocytosis by the exocyst subunit Sec6 and the SM protein Sec1. Mol Biol Cell 23: 337–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moskalenko S, Henry DO, Rosse C, Mirey G, Camonis JH, White MA. 2002. The exocyst is a Ral effector complex. Nat Cell Biol 4: 66–72. [DOI] [PubMed] [Google Scholar]
- Moskalenko S, Tong C, Rosse C, Mirey G, Formstecher E, Daviet L, Camonis J, White MA. 2003. Ral GTPases regulate exocyst assembly through dual subunit interactions. J Biol Chem 278: 51743–51748. [DOI] [PubMed] [Google Scholar]
- Mostov KE, Verges M, Altschuler Y. 2000. Membrane traffic in polarized epithelial cells. Curr Opin Cell Biol 12: 483–490. [DOI] [PubMed] [Google Scholar]
- Munson M, Novick P. 2006. The exocyst defrocked, a framework of rods revealed. Nat Struct Mol Biol 13: 577–581. [DOI] [PubMed] [Google Scholar]
- Murphy DA, Courtneidge SA. 2011. The “ins” and “outs” of podosomes and invadopodia: Characteristics, formation and function. Nat Rev Mol Cell Biol 12: 413–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachury MV, Loktev AV, Zhang Q, Westlake CJ, Peränen J, Merdes A, Slusarski DC, Scheller RH, Bazan JF, Sheffield VC, et al. 2007. A core complex of BBS proteins cooperates with the GTPase Rab8 to promote ciliary membrane biogenesis. Cell 129: 1201–1213. [DOI] [PubMed] [Google Scholar]
- Nachury MV, Seeley ES, Jin H. 2010. Trafficking to the ciliary membrane: How to get across the periciliary diffusion barrier? Annu Rev Cell Dev Biol 26: 59–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson WJ. 2009. Remodeling epithelial cell organization: Transitions between front–rear and apical-basal polarity. Cold Spring Harb Perspect Biol 1: a000513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick P. 2016. Regulation of membrane traffic by Rab GEF and GAP cascades. Small GTPases 18: 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz D, Medkova M, Walch-Solimena C, Novick P. 2002. Ypt32 recruits the Sec4p guanine nucleotide exchange factor, Sec2p, to secretory vesicles; evidence for a Rab cascade in yeast. J Cell Biol 157: 1005–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osmani N, Vitale N, Borg J-P, Etienne-Manneville S. 2006. Scrib controls Cdc42 localization and activity to promote cell polarization during astrocyte migration. Curr Biol 16: 2395–2405. [DOI] [PubMed] [Google Scholar]
- Osmani N, Peglion F, Chavrier P, Etienne-Manneville S. 2010. Cdc42 localization and cell polarity depend on membrane traffic. J Cell Biol 191: 1261–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oztan A, Silvis M, Weisz OA, Bradbury NA, Hsu SC, Goldenring JR, Yeaman C, Apodaca G. 2007. Exocyst requirement for endocytic traffic directed toward the apical and basolateral poles of polarized MDCK cells. Mol Biol Cell 18: 3978–3992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park HO, Bi E. 2007. Central roles of small GTPases in the development of cell polarity in yeast and beyond. Microbiol Mol Biol Rev 71: 48–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SY, Yang JS, Schmider AB, Soberman RJ, Hsu VW. 2015. Coordinated regulation of bidirectional COPI transport at the Golgi by CDC42. Nature 521: 529–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polzin A, Shipitsin M, Goi T, Feig LA, Turner TJ. 2002. Ral-GTPase influences the regulation of the readily releasable pool of synaptic vesicles. Mol Cell Biol 22: 1714–1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescott DM, Bender MA. 1962. Synthesis of RNA and protein during mitosis in mammalian tissue culture cells. Exp Cell Res 26: 260–268. [DOI] [PubMed] [Google Scholar]
- Prigent M, Dubois T, Raposo G, Derrien V, Tenza D, Rossé C, Camonis J, Chavrier P. 2003. ARF6 controls post-endocytic recycling through its downstream exocyst complex effector. J Cell Biol 163: 1111–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruyne D, Evangelista M, Yang C, Bi E, Zigmond S, Bretscher A, Boone C. 2002. Role of formins in actin assembly: Nucleation and barbed-end association. Science 297: 612–615. [DOI] [PubMed] [Google Scholar]
- Pruyne D, Legesse-Miller A, Gao L, Dong Y, Bretscher A. 2004. Mechanisms of polarized growth and organelle segregation in yeast. Annu Rev Cell Dev Biol 20: 559–591. [DOI] [PubMed] [Google Scholar]
- Ren J, Guo W. 2012. ERK1/2 regulates exocytosis through direct phosphorylation of the exocyst component Exo70. Dev Cell 22: 967–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley AJ, Schwartz MA, Burridge K, Firtel RA, Ginsberg MH, Borisy G, Parsons JT, Horwitz AR. 2003. Cell migration: Integrating signals from front to back. Science 302: 1704–1709. [DOI] [PubMed] [Google Scholar]
- Robinson NG, Guo L, Imai J, Toh EA, Matsui Y, Tamanoi F. 1999. Rho3 of Saccharomyces cerevisiae, which regulates the actin cytoskeleton and exocytosis, is a GTPase which interacts with Myo2 and Exo70. Mol Cell Biol 19: 3580–3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers KK, Wilson PD, Snyder RW, Zhang X, Guo W, Burrow CR, Lipschutz JH. 2004. The exocyst localizes to the primary cilium in MDCK cells. Biochem Biophys Res Commun 319: 138–143. [DOI] [PubMed] [Google Scholar]
- Rossé C, Hatzoglou A, Parrini MC, White MA, Chavrier P, Camonis J. 2006. RalB mobilizes the exocyst to drive cell migration. Mol Cell Biol 26: 727–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth M. 2004. Phosphoinositides in constitutive membrane traffic. Physiol Rev 84: 699–730. [DOI] [PubMed] [Google Scholar]
- Rothman JE. 1994. Mechanisms of intracellular protein transport. Nature 372: 55–63. [DOI] [PubMed] [Google Scholar]
- Sakurai-Yageta M, Recchi C, Le Dez G, Sibarita JB, Daviet L, Camonis J, D'Souza-Schorey C, Chavrier P. 2008. The interaction of IQGAP1 with the exocyst complex is required for tumor cell invasion downstream of Cdc42 and RhoA. J Cell Biol 181: 985–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santiago-Tirado FH, Legesse-Miller A, Schott D, Bretscher A. 2011. PI4P and Rab inputs collaborate in myosin-V-dependent transport of secretory compartments in yeast. Dev Cell 20: 47–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T, Mushiake S, Kato Y, Sato K, Sato M, Takeda N, Ozono K, Miki K, Kubo Y, Tsuji A, et al. 2007. The Rab8 GTPase regulates apical protein localization in intestinal cells. Nature 448: 366–369. [DOI] [PubMed] [Google Scholar]
- Satoh AK, O’Tousa JE, Ozaki K, Ready DF. 2005. Rab11 mediates post-Golgi trafficking of rhodopsin to the photosensitive apical membrane of Drosophila photoreceptors. Development 132: 1487–1497. [DOI] [PubMed] [Google Scholar]
- Sawin KE, Nurse P. 1998. Regulation of cell polarity by microtubules in fission yeast. J Cell Biol 142: 457–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawin KE, Snaith HA. 2004. Role of microtubules and tea1p in establishment and maintenance of fission yeast cell polarity. J Cell Sci 117: 689–700. [DOI] [PubMed] [Google Scholar]
- Schiel JA, Childs C, Prekeris R. 2013. Endocytic transport and cytokinesis: From regulation of the cytoskeleton to midbody inheritance. Trends Cell Biol 23: 319–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmoranzer J, Kreitzer G, Simon SM. 2003. Migrating fibroblasts perform polarized, microtubule-dependent exocytosis towards the leading edge. J Cell Sci 116: 4513–4519. [DOI] [PubMed] [Google Scholar]
- Seixas C, Choi SY, Polgar N, Umberger NL, East MP, Zuo X, Moreiras H, Ghossoub R, Benmerah A, Kahn RA, et al. 2016. Arl13b and the exocyst interact synergistically in ciliogenesis. Mol Biol Cell 27: 308–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen D, Yuan H, Hutagalung A, Verma A, Kümmel D, Wu X, Reinisch K, McNew JA, Novick P. 2013. The synaptobrevin homologue Snc2p recruits the exocyst to secretory vesicles by binding to Sec6p. J Cell Biol 202: 509–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin K, Wang Q, Margolis B. 2007. PATJ regulates directional migration of mammalian epithelial cells. EMBO Rep 8: 158–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegrist SE, Doe CQ. 2007. Microtubule-induced cortical cell polarity. Genes Dev 21: 483–496. [DOI] [PubMed] [Google Scholar]
- Sivaram MV, Saporita JA, Furgason ML, Boettcher AJ, Munson M. 2005. Dimerization of the exocyst protein Sec6p and its interaction with the t-SNARE Sec9p. Biochemistry 44: 6302–6311. [DOI] [PubMed] [Google Scholar]
- Slaughter BD, Smith SE, Li R. 2009. Symmetry breaking in the life cycle of the budding yeast. Cold Spring Harb Perspect Biol 1: a003384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffen A, Le Dez G, Poincloux R, Recchi C, Nassoy P, Rottner K, Galli T, Chavrier P. 2008. MT1-MMP-dependent invasion is regulated by TI-VAMP/VAMP7. Curr Biol 18: 926–931. [DOI] [PubMed] [Google Scholar]
- Stenmark H. 2009. Rab GTPases as coordinators of vesicle traffic. Nat Rev Mol Cell Biol 10: 513–525. [DOI] [PubMed] [Google Scholar]
- Sugihara K, Asano S, Tanaka K, Iwamatsu A, Okawa K, Ohta Y. 2002. The exocyst complex binds the small GTPase RalA to mediate filopodia formation. Nat Cell Biol 4: 73–78. [DOI] [PubMed] [Google Scholar]
- Tapon N, Hall A. 1997. Rho, Rac and Cdc42 GTPases regulate the organization of the actin cytoskeleton. Curr Opin in Cell Biol 9: 86–92. [DOI] [PubMed] [Google Scholar]
- Tojima T, Akiyama H, Itofusa R, Li Y, Katayama H, Miyawaki A, Kamiguchi H. 2007. Attractive axin guidance involves asymmetric membrane transport and exocytosis in the growth cone. Nat Neurosci 10: 58–66. [DOI] [PubMed] [Google Scholar]
- Vega IE, Hsu SC. 2001. The exocyst complex associates with microtubules to mediate vesicle targeting and neurite outgrowth. J Neurosci 21: 3839–3848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Tang X, Liu J, Trautmann S, Balasundaram D, McCollum D, Balasubramanian MK. 2002. The multiprotein exocyst complex is essential for cell separation in Schizosaccharomyces pombe. Mol Biol Cell 13: 515–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Liu Y, Xu XH, Deng CY, Wu KY, Zhu J, Fu XQ, He M, Luo ZG. 2011. Lgl1 activation of Rab10 promotes axonal membrane trafficking underlying neuronal polarization. Dev Cell 21: 431–444. [DOI] [PubMed] [Google Scholar]
- Wang N, Lee IJ, Rask G, Wu JQ. 2016. Roles of the TRAPP-II complex and the exocyst in membrane deposition during fission yeast cytokinesis. PLoS Biol 14: e1002437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warzecha CC, Carstens RP. 2012. Complex changes in alternative pre-mRNA splicing play a central role in the epithelial-to-mesenchymal transition (EMT). Semin Cancer Biol 22: 417–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T, Noritake J, Kaibuchi K. 2005. Regulation of microtubules in cell migration. Trends Cell Biol 15: 76–83. [DOI] [PubMed] [Google Scholar]
- Wedlich-Soldner R, Altschuler S, Wu L, Li R. 2003. Spontaneous cell polarization through actomyosin-based delivery of the Cdc42 GTPase. Science 299: 1231–1235. [DOI] [PubMed] [Google Scholar]
- Westlake CJ, Baye LM, Nachury MV, Wright KJ, Ervin KE, Phu L, Chalouni C, Beck JS, Kirkpatrick DS, Slusarski DC, et al. 2011. Primary cilia membrane assembly is initiated by Rab11 and transport protein particle II (TRAPPII) complex dependent trafficking of Rabin8 to the centrosome. Proc Natl Acad Sci 108: 2759–2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu B, Guo W. 2015. The exocyst at a glance. J Cell Sci 128: 2957–2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu WJ, Erickson JW, Lin R, Cerione RA. 2000. The γ-subunit of the coatomer complex binds Cdc42 to mediate transformation. Nature 405: 800–804. [DOI] [PubMed] [Google Scholar]
- Wu S, Mehta SQ, Pichaud F, Bellen HJ, Quiocho FA. 2005. Sec15 interacts with Rab11 via a novel domain and affects Rab11 localization in vivo. Nat Struct Mol Biol 12: 879–885. [DOI] [PubMed] [Google Scholar]
- Wu H, Rossi G, Brennwald P. 2008. The ghost in the machine: Small GTPases as spatial regulators of exocytosis. Trends Cell Biol 18: 397–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Turner C, Gardner J, Temple B, Brennwald P. 2010. The Exo70 subunit of the exocyst is an effector for both Cdc42 and Rho3 function in polarized exocytosis. Mol Biol Cell 21: 430–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita M, Kurokawa K, Sato Y, Yamagata A, Mimura H, Yoshikawa A, Sato K, Nakano A, Fukai S. 2010. Structural basis for the Rho- and phosphoinositide-dependent localization of the exocyst subunit Sec3. Nat Struct Mol Biol 17: 180–186. [DOI] [PubMed] [Google Scholar]
- Yue P, Zhang Y, Mei K, Zhu Y, Lesigang J, Wang S, Zhu Y, Dong G, Guo W. 2017. Sec3 promotes the binary t-SNARE complex assembly and membrane fusion. Nat Commun (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Bi E, Novick P, Du L, Kozminski KG, Lipschutz JH, Guo W. 2001. Cdc42 interacts with the exocyst and regulates polarized secretion. J Biol Chem 276: 46745–46750. [DOI] [PubMed] [Google Scholar]
- Zhang XM, Ellis S, Sriratana A, Mitchell CA, Rowe T. 2004. Sec15 is an effector for the Rab11 GTPase in mammalian cells. J Biol Chem 279: 43027–43034. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Su Z, Zhang F, Chen Y, Shin YK. 2005. A partially zipped SNARE complex stabilized by the membrane. J Biol Chem 280: 15595–15600. [DOI] [PubMed] [Google Scholar]
- Zhang X, Orlando K, He B, Xi F, Zhang J, Zajac A, Guo W. 2008. Membrane association and functional regulation of Sec3 by phospholipids and Cdc42. J Cell Biol 180: 145–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Liu J, Yang C, Capraro BR, Baumgart T, Bradley RP, Ramakrishnan N, Xu X, Radhakrishnan R, Svitkina T, et al. 2013. Exo70 generates membrane curvature for morphogenesis and cell migration. Dev Cell 26: 266–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo X, Zhang J, Zhang Y, Hsu SC, Zhou D, Guo W. 2006. Exo70 interacts with the Arp2/3 complex and regulates cell migration. Nat Cell Biol 8: 1383–1388. [DOI] [PubMed] [Google Scholar]
- Zuo X, Guo W, Lipschutz JH. 2009. The exocyst protein Sec10 is necessary for primary ciliogenesis and cystogenesis in vitro. Mol Biol Cell 20: 2522–2529. [DOI] [PMC free article] [PubMed] [Google Scholar]