Abstract
Memory responses seen after whole-cell pertussis (wP) and acellular pertussis (aP) vaccine priming are different and reflect better long-term protection against pertussis disease seen with the whole-cell vaccines. Although acellular vaccines generate higher levels of antigen-specific IgG to the antigens included in the aP vaccines, there are many more pertussis antigens included in whole-cell vaccines. Acellular vaccine priming is associated with skewing of the immune response to a more Th2-like response, whereas whole-cell priming is associated with a Th1/Th17 response. Repeated booster doses of acellular vaccine in children primed with acellular vaccine has been shown to result in progressively shorter duration of protection against disease. This may be explained by the generation of higher levels of antigen-specific IgG4, which does not bind complement and leads to a suboptimal inflammatory response and impaired phagocytosis and antimicrobial defense. In contrast, whole-cell priming followed by aP vaccine boosters results in better opsonization, phagocytosis, and complement mediated killing through the preferential induction of IgG1.
Children primed with acellular pertussis vaccines exhibit a Th2-dominant immune response and possess higher levels of antigen-specific IgG4, which leads to a suboptimal immune response and the progressive loss of protection.
Great Debates
What are the most interesting topics likely to come up over dinner or drinks with your colleagues? Or, more importantly, what are the topics that don't come up because they are a little too controversial? In Immune Memory and Vaccines: Great Debates, Editors Rafi Ahmed and Shane Crotty have put together a collection of articles on such questions, written by thought leaders in these fields, with the freedom to talk about the issues as they see fit. This short, innovative format aims to bring a fresh perspective by encouraging authors to be opinionated, focus on what is most interesting and current, and avoid restating introductory material covered in many other reviews.
The Editors posed 13 interesting questions critical for our understanding of vaccines and immune memory to a broad group of experts in the field. In each case, several different perspectives are provided. Note that while each author knew that there were additional scientists addressing the same question, they did not know who these authors were, which ensured the independence of the opinions and perspectives expressed in each article. Our hope is that readers enjoy these articles and that they trigger many more conversations on these important topics.
BACKGROUND
Pertussis outbreaks have been reported in various countries that have switched from whole-cell pertussis (wP) to acellular pertussis (aP) vaccines (Pillsbury et al. 2014; Tan et al. 2015). Many of these outbreaks have occurred in children who had received only acellular vaccines and reports of waning immunity with the acellular vaccines have appeared (Klein et al. 2012, 2016; Sheridan et al. 2012; Witt et al. 2013; Gambhir et al. 2015). In an attempt to understand why acellular vaccines provide less durable protection than whole-cell vaccines, several studies have investigated immunological differences between aP and wP vaccines. Although there are also important differences in the early innate immune response, this review will only focus on quantitative and functional differences in immunological memory induced by primary and booster vaccination with wP and aP vaccines.
DIFFERENCES IN ANTIGEN COMPOSITION
Acellular vaccines consist of a limited number of pertussis antigens. These vaccines are equally or more immunogenic for the included antigens than the whole-cell vaccines, which is likely attributable to the fact that acellular vaccines contain a higher content of each individual antigen (Edwards et al. 1995). Initial studies on the memory response following vaccination with acellular and whole-cell vaccines predominately focused on the antigens included in the acellular vaccines. Immune responses to additional antigens contained in the whole-cell vaccines have not been comprehensively examined. The inclusion of only a restricted number of antigens in the acellular vaccines has likely contributed to reduced protection, particularly because we have already seen that the acellular vaccine selects for pertactin-deficient strains that are increasingly prevalent in many areas where the acellular vaccines are used (Lam et al. 2014; Zeddeman et al. 2014; Martin et al. 2015). The impact of these pertactin-deficient strains on disease burden is actively being assessed (Breakwell et al. 2016).
HUMORAL RESPONSE DIFFERENCES BETWEEN ACELLULAR AND WHOLE-CELL VACCINES
There are also differences in the humoral response between the two vaccines. Because the antigens in the acellular vaccine are also present in the whole-cell vaccine, many studies have compared the magnitude of the antibody response to these antigens. Antigen-specific serum IgG levels were also used as an immunogenicity marker during the large randomized efficacy trials in the 1980s–1990s. Results from these studies indicated that acellular vaccination induced much higher antibody levels against each individual antigen than whole-cell vaccination (Edwards et al. 1995). However, serum IgG levels following primary vaccination wane rapidly. Of note, waning of antibody levels is not restricted to acellular vaccines, as antibody levels also decline rapidly following whole-cell vaccine priming (Huang et al. 1996; Giuliano et al. 1998; Hendrikx et al. 2009). Despite the waning levels of circulating antibodies, memory B cells specific to the acellular vaccine antigens have been found in peripheral blood years after vaccination (Buisman et al. 2009; Hendrikx et al. 2011a; Carollo et al. 2014). As disease incidence was increasing in older vaccinated children, acellular booster vaccination was introduced, initially in children primed with whole-cell vaccine, but later also in children primed with acellular vaccines. This was found to induce a very robust humoral response in both groups. Despite this response, evidence is accumulating that the duration of protection after repetitive boosting with acellular vaccines is progressively shortening (Tartof et al. 2013; Acosta et al. 2015).
ACELLULAR BOOSTER VACCINATION AND AFFINITY MATURATION
During the process of antibody affinity maturation, germinal center IgG+ memory B cells acquire increased antibody avidity for their cognate antigen through somatic hypermutation in the variable domains of the IgG-encoding genes. Thus, comparison of antibody avidity following repeated antigen exposure provides insight into the maturation stage of the B-cell response. The avidity of IgG antibodies against pertussis toxin (PT), pertactin (Prn), and filamentous hemagglutinin (FHA) is significantly higher in acellular versus whole-cell-primed children (Hendrikx et al. 2009; Prelog et al. 2013; Schure et al. 2013). Importantly, affinity maturation seems to be further accelerated by repeated aP vaccination. For instance, a significant increase in avidity index was seen in the IgG response to PT and Prn following a sixth dose of acellular vaccine (Hendrikx et al. 2011b). Although the avidity of acellular vaccine antigen-specific IgG also increases over time in wP-primed children, this process seems to occur in a slower fashion than in aP-primed children.
SKEWING TOWARD IgG4
Another important difference can be found in the subtype distribution of the IgG response between acellular versus whole-cell-primed children (Hendrikx et al. 2011b). Notably, IgG4 was found to represent a higher proportion of all PT, FHA, and Prn-specific IgG antibodies in acellular-primed children than in those primed with whole-cell vaccines. Booster doses of acellular vaccine augmented this process, as the postacellular booster IgG subtype distribution was even further skewed toward IgG4. Giammanco and colleagues reported a similar phenomenon when comparing the anti-PT IgG response between convalescent and aP-vaccinated children (Giammanco et al. 2003). In their study, unvaccinated children who were convalescent from laboratory-confirmed pertussis were found to produce mainly IgG1/IgG3 antibodies against PT, whereas IgG1, IgG2 but also IgG4 were most prevalent in both healthy and convalescent children primed with acellular vaccines.
IMMUNOGLOBULIN CLASS-SWITCHING DURING THE COURSE OF PERTUSSIS VACCINATION
Together, these observations point to essential differences in the antigen-specific IgG responses between wP and aP vaccination, both in the avidity as well as in IgG subtype distribution. How should these findings be interpreted? Recent studies suggest that B cells preferentially follow a programmed order of Ig class switching over the course of an immune response, a process thought to be coupled to affinity maturation (Jackson et al. 2013, 2014). In this model, when naïve IgM+ antigen-specific B cells first come into contact with their cognate antigen, they first switch to IgG3, then to IgG1 and to IgG2, and finally to IgG4. This order reflects the genomic ordering of the IgG subclass genes within the constant domain locus. Of note, this model is also in line with allergen immunotherapy strategies in which increasing doses of allergens are administered followed by repetitive maintenance dosing. Such treatment frequently induces a relative increase in allergen-specific IgG4 (Francis et al. 2008; Burks et al. 2012; Freiberger et al. 2016; Virchow et al. 2016). The later-formed IgG4 isotype is unable to bind C1q and activate the complement cascade (Schumaker et al. 1976; Bindon et al. 1988). Because IgG4 also binds to the inhibitory IgG receptor FcγRIIb, an increase in allergen-specific IgG4 is thought to restrict the hyperinflammatory response in allergic patients (James and Till 2016). Interestingly, this immune response pattern to allergen immunotherapy closely mimics the IgG response that is observed over the course of repetitive aP vaccinations. However, for pertussis vaccination this is likely to have unwanted consequences, as an increase in IgG4 is expected to lead to a reduction in bactericidal activity (Bruggemann et al. 1987; Irani et al. 2015). Considering that memory B cells can also serve as antigen-presenting cells, this may also have consequences in further skewing of cellular immunity. This is particularly relevant in the context of booster vaccination (i.e., when high-affinity IgG+ memory B cells are present).
It should be noted that not all of the antigens included in the acellular vaccines show identical patterns of changes in the IgG subtype distribution following vaccination. For example, the PT-specific IgG response following whole-cell priming seems to be skewed more toward IgG2. In this context, it should be noted that the PT content in the Dutch wP vaccine and in one of the whole-cell vaccines used in the European efficacy trials was very low and consequently induced very low titers of PT antibodies (Hendrikx et al. 2009). In this scenario, it is possible that the de novo B-cell response to the higher PT content in the aP vaccine may outcompete the relatively few wP-induced PT-specific memory B cells. Evidence in favor of this explanation can be found in the relatively high proportion of PT-specific “early” IgG3 in the wP-primed group following the first acellular booster dose. We hypothesize that high-affinity, noninflammatory antibodies that adequately neutralize the toxic and immunomodulatory effects of soluble PT would normally be highly beneficial during infection. Unfortunately, the chemical treatment of PT is known to destroy many of the protective epitopes in the acellular vaccine (Ibsen 1996) and, in this case, high avidity may thus not equal neutralization. Replacing the chemically inactivated PT with a genetically inactivated PT would thus be a logical step to improve vaccination efficacy. Head-to-head immunogenicity comparison studies of the chemically and genetically detoxified PT antigens have clearly shown the enhanced immunogenicity and functional activity of the genetically detoxified PT (Edwards et al. 1995).
The IgG response toward FHA also shows progressive switching to IgG4 following acellular booster vaccination, similar to PT. For Prn, this is not the case as the acellular vaccination response is dominated by IgG1, even after the 4y boost, with very little IgG4 produced. As IgG1 is much more effective in binding complement, these antibodies should lead to effective direct and indirect killing of Bordetella pertussis. As Prn is also chemically inactivated, similar to PT and FHA, it is unclear why the humoral response toward this antigen is different from that against FHA and PT. However, what is clear is that Prn represents the most effective antigen of all aP antigens to induce functional antibodies (Hellwig et al. 2003). Unfortunately, because expression of Prn is apparently not essential for bacterial survival, this may also explain the rapid emergence of Prn-negative mutant strains in countries that have switched to acellular vaccines.
DIFFERENCES IN CELLULAR RESPONSES
Another major reason that the acellular vaccines are less effective than the whole-cell vaccines, is that the two vaccines stimulate very different T-cell responses. Mills and colleagues were the first to show that cellular immunity, in particular interferon γ (IFN-γ), played an important role in protection against infection with B. pertussis (Mahon et al. 1997). Whole-cell vaccines were shown to induce predominately Th1 and Th17 cell immunity, whereas the acellular vaccines induced a mixed Th2 and Th17 response (Ross et al. 2013). Interleukin (IL)-17 has been shown to play an important role in the defense against mucosal infections with extracellular bacteria (Kolls and Khader 2010). Further studies in mice showed that CD4+ T cells from acellular vaccine-primed animals secreted IL-4, IL-5, and IL-17 (Th2/Th17), but relatively lower concentrations of IFN-γ (Ross et al. 2013; Brummelman et al. 2015). In contrast, the whole-cell vaccines induced a mixed IFN-γ/IL-17A (Th1/Th17) response (Ross et al. 2013). These studies were extended to the baboon model, wherein immunization with acellular vaccines, conferred protection against disease but not infection or transmission, and was associated with a Th1/Th2 type CD4+ T-cell response. In contrast, whole-cell vaccinated baboons were better protected against both colonization and transmission, which was associated with the induction of a Th1/Th17 memory response (Warfel et al. 2014).
T-CELL RESPONSES TO PRIMARY VACCINATION
So how do these findings extend to cellular responses in humans? Although it is difficult to formally compare the T-cell response across different clinical studies because of significant differences in how cellular responses are measured, human studies have generally confirmed the same observations with acellular vaccines inducing a Th2-dominated, yet mixed Th2/Th1/Th17 type of CD4+ T-cell response in young children (Ryan et al. 1998; Ausiello et al. 1999; Mascart et al. 2007; Schure et al. 2012). In contrast, the whole-cell vaccines induced a Th1/Th17-type CD4+ T-cell response, similar to that seen after natural infection (Ryan et al. 1998, 2000; Mascart et al. 2003, 2007; Rowe et al. 2005; Vermeulen et al. 2010; Ross et al. 2013). In summary, comparisons of the acellular and whole-cell vaccines in humans are largely consistent with the data from animal models, suggesting that Th2 dominance is associated with the acellular vaccine and that a Th1/Th17 profile is associated with the whole-cell vaccine.
T-CELL RESPONSES TO ACELLULAR BOOSTING
The response to one or more booster doses of acellular vaccine in both acellular and whole-cell-primed children has been studied over time to determine whether cellular memory wanes more rapidly after acellular than whole-cell vaccine. Buisman and colleagues examined acellular-primed children, 3 years after priming (Buisman et al. 2009). They found a higher T-cell response in acellular-primed children than in whole-cell-primed children. This response was not boosted after a fifth dose of acellular vaccine in the acellular-primed children, but was boosted in the whole-cell-primed children (Schure et al. 2012). It should be noted though that the cellular responses before booster doses in the acellular-primed children were already quite high. At age 6 years, 2 years after the booster dose, peripheral blood cells from acellular-primed children produced lower levels of pertussis-specific IL-17 when compared with those from whole-cell-primed children (Schure et al. 2013). A recent study by Bancroft et al. (2016) showed that the initial Th1 versus Th2 programs that are induced by primary vaccination with whole-cell and acellular vaccines, respectively, are maintained on boosting with acellular vaccines, even decades after the primary dose. They also found stronger T-cell responses in acellular-primed individuals than in those primed with whole-cell vaccines. This further confirms that the differences in the cellular response between the two vaccines are not necessarily the result of a difference in the magnitude of the vaccine response, but more reflect differences in effector function of the activated T cells. A recent study by de Rond and colleagues (2015) found that CCR7 and CD45RA expression on CD4+ T cells was lower in the acellular-primed children, which may suggest that the CD4+ T-cell responses in the acellular-primed children are more terminally differentiated than those seen in the whole-cell-primed children. Although further research is needed to fully understand the differences in the human cellular response to pertussis vaccination, these findings suggest that (repetitive) acellular vaccination may lead to early exhaustion of cellular immunity.
SKEWING OF IMMUNE MEMORY BY PRIMARY AND BOOSTER VACCINATION
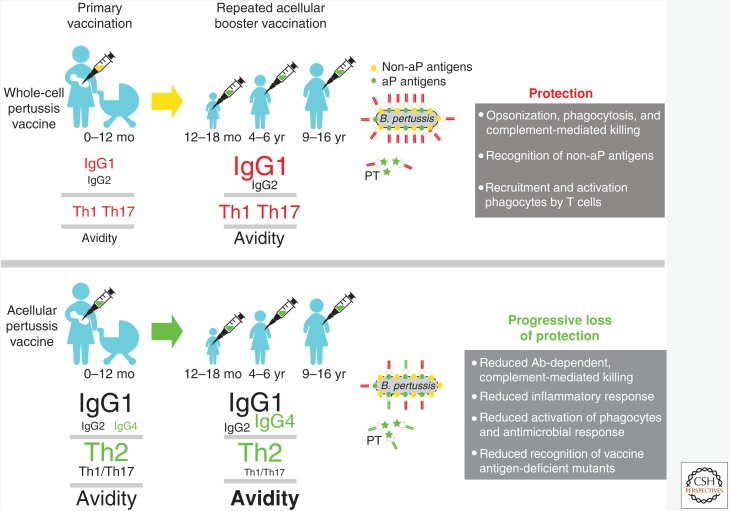
In summary, as illustrated in Figure 1, the memory responses seen after wP and aP priming are quite different and are likely reflective of the better long-term protection against pertussis seen with the whole-cell vaccines. Acellular vaccine priming generates higher levels of antigen-specific IgG1 to the antigens included in the aP vaccine, but not to other antigens included in wP vaccines. Acellular vaccine priming is associated with skewing of the immune response to a more Th2-like response, than that seen with whole-cell priming. Repeated booster doses of acellular vaccine in children primed with acellular vaccine also have a profound impact on the memory responses, generating high levels of antigen-specific IgG4. IgG4 does not bind complement, resulting in suboptimal antibody-dependent, complement-mediated killing (Weiss et al. 2004). Moreover, IgG4 may also contribute to a suboptimal inflammatory response and impaired phagocytosis and antimicrobial defense. Progressive skewing toward a Th2 response is also seen in these children. In contrast, whole-cell priming followed by aP vaccine boosters may result in better opsonization, phagocytosis, and antibody-dependent, complement-mediated killing through the preferential induction of IgG1. In addition, memory responses to antigens included in the whole-cell but not in the acellular vaccines have been detected. Finally, the recruitment and engagement of Th1/Th17 T cells is preferentially seen in children primed with whole-cell vaccines.
Figure 1.
Impact of primary and booster vaccination on protection against pertussis. Primary vaccination with several doses of whole-cell or acellular vaccine occurs during the first year of life. Acellular vaccine priming generates higher levels of antigen-specific IgG1, greater antibody avidity, and a more Th2-directed response than whole-cell priming. Repeated booster doses of acellular vaccine in acellular-primed children generate more antigen-specific IgG4, greater Th2 skewing, and much higher antibody avidity than acellular boosters in whole-cell-primed children. Further, antibodies generated in whole-cell-primed children are functionally more active in opsonization and killing of Bordetella pertussis organisms. Whole-cell-primed children retain protection against pertussis organisms while acellular-primed children have a progressive loss of protection, also because of the emergence of vaccine antigen-deficient mutants. The size of the text in the figure is proportional to the relative magnitude of the response. (Larger text responses are greater than smaller text responses.) Green- or red-colored text reflects the major immunological differences between the loss of protective responses with acellular priming or the protection afforded with whole-cell priming, respectively.
Efforts directed to improve pertussis vaccines need to address the limitations of acellular vaccine priming. Potential options to improve the acellular vaccines include the addition of other antigens, modification with adjuvants to drive more Th1/Th17/IgG1 responses, administering live attenuated whole pertussis organisms as a priming dose before the administration of the acellular vaccines, reducing the antigen content of the acellular vaccines, and including pertussis antigens with more native structures without chemical modifications. It is hoped that a better understanding of the immunologic differences between the immune responses after acellular and whole-cell priming will assist in this effort.
Footnotes
Editors: Shane Crotty and Rafi Ahmed
Additional Perspectives on Immune Memory and Vaccines: Great Debates available at www.cshperspectives.org
REFERENCES
- Acosta AM, DeBolt C, Tasslimi A, Lewis M, Stewart LK, Misegades LK, Messonnier NE, Clark TA, Martin SW, Patel M. 2015. Tdap vaccine effectiveness in adolescents during the 2012 Washington State pertussis epidemic. Pediatrics 135: 981–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ausiello CM, Lande R, Urbani F, la Sala A, Stefanelli P, Salmaso S, Mastrantonio P, Cassone A. 1999. Cell-mediated immune responses in four-year-old children after primary immunization with acellular pertussis vaccines. Infect Immun 67: 4064–4071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bancroft T, Dillon MB, da Silva Antunes R, Paul S, Peters B, Crotty S, Lindestam Arlehamn CS, Sette A. 2016. Th1 versus Th2 T cell polarization by whole-cell and acellular childhood pertussis vaccines persists upon re-immunization in adolescence and adulthood. Cell Immunol 304–305: 35–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bindon CI, Hale G, Bruggemann M, Waldmann H. 1988. Human monoclonal IgG isotypes differ in complement activating function at the level of C4 as well as C1q. J Exp Med 168: 127–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breakwell L, Kelso P, Finley C, Schoenfeld S, Goode B, Misegades LK, Martin SW, Acosta AM. 2016. Pertussis vaccine effectiveness in the setting of pertactin-deficient pertussis. Pediatrics 10.1542/peds.2015-3973. [DOI] [PubMed] [Google Scholar]
- Bruggemann M, Williams GT, Bindon CI, Clark MR, Walker MR, Jefferis R, Waldmann H, Neuberger MS. 1987. Comparison of the effector functions of human immunoglobulins using a matched set of chimeric antibodies. J Exp Med 166: 1351–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brummelman J, Helm K, Hamstra HJ, van der Ley P, Boog CJ, Han WG, van Els CA. 2015. Modulation of the CD4+ T cell response after acellular pertussis vaccination in the presence of TLR4 ligation. Vaccine 33: 1483–1491. [DOI] [PubMed] [Google Scholar]
- Buisman AM, de Rond CG, Ozturk K, Ten Hulscher HI, van Binnendijk RS. 2009. Long-term presence of memory B-cells specific for different vaccine components. Vaccine 28: 179–186. [DOI] [PubMed] [Google Scholar]
- Burks AW, Jones SM, Wood RA, Fleischer DM, Sicherer SH, Lindblad RW, Stablein D, Henning AK, Vickery BP, Liu AH, et al. 2012. Oral immunotherapy for treatment of egg allergy in children. N Engl J Med 367: 233–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carollo M, Pandolfi E, Tozzi AE, Buisman AM, Mascart F, Ausiello CM. 2014. Humoral and B-cell memory responses in children five years after pertussis acellular vaccine priming. Vaccine 32: 2093–2099. [DOI] [PubMed] [Google Scholar]
- de Rond L, Schure RM, Ozturk K, Berbers G, Sanders E, van Twillert I, Carollo M, Mascart F, Ausiello CM, van Els CA, et al. 2015. Identification of pertussis-specific effector memory T cells in preschool children. Clin Vaccine Immunol 22: 561–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards KM, Meade BD, Decker MD, Reed GF, Rennels MB, Steinhoff MC, Anderson EL, Englund JA, Pichichero ME, Deloria MA. 1995. Comparison of 13 acellular pertussis vaccines: Overview and serologic response. Pediatrics 96: 548–557. [PubMed] [Google Scholar]
- Francis JN, James LK, Paraskevopoulos G, Wong C, Calderon MA, Durham SR, Till SJ. 2008. Grass pollen immunotherapy: IL-10 induction and suppression of late responses precedes IgG4 inhibitory antibody activity. J Allergy Clin Immunol 121: 1120–1125.e2. [DOI] [PubMed] [Google Scholar]
- Freiberger SN, Zehnder M, Gafvelin G, Gronlund H, Kundig TM, Johansen P. 2016. IgG4 but no IgG1 antibody production after intralymphatic immunotherapy with recombinant MAT-Feld1 in human. Allergy 71: 1366–1370. [DOI] [PubMed] [Google Scholar]
- Gambhir M, Clark TA, Cauchemez S, Tartof SY, Swerdlow DL, Ferguson NM. 2015. A change in vaccine efficacy and duration of protection explains recent rises in pertussis incidence in the United States. PLoS Comput Biol 11: e1004138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giammanco A, Taormina S, Chiarini A, Dardanoni G, Stefanelli P, Salmaso S, Mastrantonio P. 2003. Analogous IgG subclass response to pertussis toxin in vaccinated children, healthy or affected by whooping cough. Vaccine 21: 1924–1931. [DOI] [PubMed] [Google Scholar]
- Giuliano M, Mastrantonio P, Giammanco A, Piscitelli A, Salmaso S, Wassilak SG. 1998. Antibody responses and persistence in the two years after immunization with two acellular vaccines and one whole-cell vaccine against pertussis. J Pediatr 132: 983–988. [DOI] [PubMed] [Google Scholar]
- Hellwig SM, Rodriguez ME, Berbers GA, van de Winkel JG, Mooi FR. 2003. Crucial role of antibodies to pertactin in Bordetella pertussis immunity. J Infect Dis 188: 738–742. [DOI] [PubMed] [Google Scholar]
- Hendrikx LH, Berbers GA, Veenhoven RH, Sanders EA, Buisman AM. 2009. IgG responses after booster vaccination with different pertussis vaccines in Dutch children 4 years of age: Effect of vaccine antigen content. Vaccine 27: 6530–6536. [DOI] [PubMed] [Google Scholar]
- Hendrikx LH, Ozturk K, de Rond LG, Veenhoven RH, Sanders EA, Berbers GA, Buisman AM. 2011a. Identifying long-term memory B-cells in vaccinated children despite waning antibody levels specific for Bordetella pertussis proteins. Vaccine 29: 1431–1437. [DOI] [PubMed] [Google Scholar]
- Hendrikx LH, Schure RM, Ozturk K, de Rond LG, de Greeff SC, Sanders EA, Berbers GA, Buisman AM. 2011b. Different IgG-subclass distributions after whole-cell and acellular pertussis infant primary vaccinations in healthy and pertussis infected children. Vaccine 29: 6874–6880. [DOI] [PubMed] [Google Scholar]
- Huang LM, Lee CY, Lin TY, Chen JM, Lee PI, Hsu CY. 1996. Responses to primary and a booster dose of acellular, component, and whole-cell pertussis vaccines initiated at 2 months of age. Vaccine 14: 916–922. [DOI] [PubMed] [Google Scholar]
- Ibsen PH. 1996. The effect of formaldehyde, hydrogen peroxide and genetic detoxification of pertussis toxin on epitope recognition by murine monoclonal antibodies. Vaccine 14: 359–368. [DOI] [PubMed] [Google Scholar]
- Irani V, Guy AJ, Andrew D, Beeson JG, Ramsland PA, Richards JS. 2015. Molecular properties of human IgG subclasses and their implications for designing therapeutic monoclonal antibodies against infectious diseases. Mol Immunol 67: 171–182. [DOI] [PubMed] [Google Scholar]
- Jackson KJ, Kidd MJ, Wang Y, Collins AM. 2013. The shape of the lymphocyte receptor repertoire: Lessons from the B cell receptor. Front Immunol 4: 263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson KJ, Wang Y, Collins AM. 2014. Human immunoglobulin classes and subclasses show variability in VDJ gene mutation levels. Immunol Cell Biol 92: 729–733. [DOI] [PubMed] [Google Scholar]
- James LK, Till SJ. 2016. Potential mechanisms for IgG4 inhibition of immediate hypersensitivity reactions. Curr Allergy Asthma Rep 16: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein NP, Bartlett J, Rowhani-Rahbar A, Fireman B, Baxter R. 2012. Waning protection after fifth dose of acellular pertussis vaccine in children. N Engl J Med 367: 1012–1019. [DOI] [PubMed] [Google Scholar]
- Klein NP, Bartlett J, Fireman B, Baxter R. 2016. Waning Tdap effectiveness in adolescents. Pediatrics 137: e20153326. [DOI] [PubMed] [Google Scholar]
- Kolls JK, Khader SA. 2010. The role of Th17 cytokines in primary mucosal immunity. Cytokine Growth Factor Rev 21: 443–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam C, Octavia S, Ricafort L, Sintchenko V, Gilbert GL, Wood N, McIntyre P, Marshall H, Guiso N, Keil AD, et al. 2014. Rapid increase in pertactin-deficient Bordetella pertussis isolates, Australia. Emerg Infect Dis 20: 626–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahon BP, Sheahan BJ, Griffin F, Murphy G, Mills KH. 1997. Atypical disease after Bordetella pertussis respiratory infection of mice with targeted disruptions of interferon-γ receptor or immunoglobulin μ chain genes. J Exp Med 186: 1843–1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin SW, Pawloski L, Williams M, Weening K, DeBolt C, Qin X, Reynolds L, Kenyon C, Giambrone G, Kudish K, et al. 2015. Pertactin-negative Bordetella pertussis strains: Evidence for a possible selective advantage. Clin Infect Dis 60: 223–227. [DOI] [PubMed] [Google Scholar]
- Mascart F, Verscheure V, Malfroot A, Hainaut M, Pierard D, Temerman S, Peltier A, Debrie AS, Levy J, Del Giudice G, et al. 2003. Bordetella pertussis infection in 2-month-old infants promotes type 1 T cell responses. J Immunol 170: 1504–1509. [DOI] [PubMed] [Google Scholar]
- Mascart F, Hainaut M, Peltier A, Verscheure V, Levy J, Locht C. 2007. Modulation of the infant immune responses by the first pertussis vaccine administrations. Vaccine 25: 391–398. [DOI] [PubMed] [Google Scholar]
- Pillsbury A, Quinn HE, McIntyre PB. 2014. Australian vaccine preventable disease epidemiological review series: Pertussis, 2006–2012. Commun Dis Intell Q Rep 38: E179–E194. [PubMed] [Google Scholar]
- Prelog M, Almanzar G, Rieber N, Ottensmeier B, Zlamy M, Liese J. 2013. Differences of IgG antibody avidity after an acellular pertussis (aP) booster in adolescents after a whole cell (wcP) or aP primary vaccination. Vaccine 31: 387–393. [DOI] [PubMed] [Google Scholar]
- Ross PJ, Sutton CE, Higgins S, Allen AC, Walsh K, Misiak A, Lavelle EC, McLoughlin RM, Mills KH. 2013. Relative contribution of Th1 and Th17 cells in adaptive immunity to Bordetella pertussis: Towards the rational design of an improved acellular pertussis vaccine. PLoS Pathog 9: e1003264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe J, Yerkovich ST, Richmond P, Suriyaarachchi D, Fisher E, Feddema L, Loh R, Sly PD, Holt PG. 2005. Th2-associated local reactions to the acellular diphtheria-tetanus-pertussis vaccine in 4- to 6-year-old children. Infect Immun 73: 8130–8135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan M, Murphy G, Ryan E, Nilsson L, Shackley F, Gothefors L, Oymar K, Miller E, Storsaeter J, Mills KH. 1998. Distinct T-cell subtypes induced with whole cell and acellular pertussis vaccines in children. Immunology 93: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan EJ, Nilsson L, Kjellman N, Gothefors L, Mills KH. 2000. Booster immunization of children with an acellular pertussis vaccine enhances Th2 cytokine production and serum IgE responses against pertussis toxin but not against common allergens. Clin Exp Immunol 121: 193–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumaker VN, Calcott MA, Spiegelberg HL, Muller-Eberhard HJ. 1976. Ultracentifuge studies of the binding of IgG of different subclasses to the Clq subunit of the first component of complement. Biochemistry 15: 5175–5181. [DOI] [PubMed] [Google Scholar]
- Schure RM, Hendrikx LH, de Rond LG, Ozturk K, Sanders EA, Berbers GA, Buisman AM. 2012. T-cell responses before and after the fifth consecutive acellular pertussis vaccination in 4-year-old Dutch children. Clin Vaccine Immunol 19: 1879–1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schure RM, Hendrikx LH, de Rond LG, Ozturk K, Sanders EA, Berbers GA, Buisman AM. 2013. Differential T- and B-cell responses to pertussis in acellular vaccine-primed versus whole-cell vaccine-primed children 2 years after preschool acellular booster vaccination. Clin Vaccine Immunol 20: 1388–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheridan SL, Ware RS, Grimwood K, Lambert SB. 2012. Number and order of whole cell pertussis vaccines in infancy and disease protection. JAMA 308: 454–456. [DOI] [PubMed] [Google Scholar]
- Tan T, Dalby T, Forsyth K, Halperin SA, Heininger U, Hozbor D, Plotkin S, Ulloa-Gutierrez R, Wirsing von Konig CH. 2015. Pertussis across the globe: Recent epidemiologic trends from 2000 to 2013. Pediatr Infect Dis J 34: e222–e232. [DOI] [PubMed] [Google Scholar]
- Tartof SY, Lewis M, Kenyon C, White K, Osborn A, Liko J, Zell E, Martin S, Messonnier NE, Clark TA, et al. 2013. Waning immunity to pertussis following 5 doses of DTaP. Pediatrics 131: e1047–e1052. [DOI] [PubMed] [Google Scholar]
- Vermeulen F, Verscheure V, Damis E, Vermeylen D, Leloux G, Dirix V, Locht C, Mascart F. 2010. Cellular immune responses of preterm infants after vaccination with whole-cell or acellular pertussis vaccines. Clin Vaccine Immunol 17: 258–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virchow JC, Backer V, Kuna P, Prieto L, Nolte H, Villesen HH, Ljorring C, Riis B, de Blay F. 2016. Efficacy of a house dust mite sublingual allergen immunotherapy tablet in adults with allergic asthma: A randomized clinical trial. JAMA 315: 1715–1725. [DOI] [PubMed] [Google Scholar]
- Warfel JM, Zimmerman LI, Merkel TJ. 2014. Acellular pertussis vaccines protect against disease but fail to prevent infection and transmission in a nonhuman primate model. Proc Natl Acad Sci 111: 787–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss AA, Patton AK, Millen SH, Chang SJ, Ward JI, Bernstein DI. 2004. Acellular pertussis vaccines and complement killing of Bordetella pertussis. Infect Immun 72: 7346–7351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witt MA, Arias L, Katz PH, Truong ET, Witt DJ. 2013. Reduced risk of pertussis among persons ever vaccinated with whole cell pertussis vaccine compared to recipients of acellular pertussis vaccines in a large US cohort. Clin Infect Dis 56: 1248–1254. [DOI] [PubMed] [Google Scholar]
- Zeddeman A, van Gent M, Heuvelman CJ, van der Heide HG, Bart MJ, Advani A, Hallander HO, Wirsing von Konig CH, Riffelman M, Storsaeter J, et al. 2014. Investigations into the emergence of pertactin-deficient Bordetella pertussis isolates in six European countries, 1996 to 2012. Euro Surveill 19: 20881. [DOI] [PubMed] [Google Scholar]