Abstract
The high incidence of pertussis in vaccinated adolescents suggests the failing of immune memory. We argue that acellular pertussis vaccines generate memory cells that are effectively reactivated by boosters better than by Bordetella pertussis exposure. We propose that there are two main causes. One is the induction of vaccine-specific immunity rather than pathogen-specific immunity. The second is that strictly mucosal infections such as B. pertussis poorly reactivate memory B and T cells residing deep in lymph nodes or tissues. Developing new vaccines for infants or adolescents will be immunologically and economically challenging. Let us hope that maternal and infant immunization, to date the most effective strategies against pertussis death, will remain so.
Acellular pertussis vaccines exhibit only short-term effectiveness. This may be due to (1) vaccine-specific (not pathogen-specific) immunity and (2) the failure of memory B and T cells to reactivate upon pathogen exposure.
Great Debates
What are the most interesting topics likely to come up over dinner or drinks with your colleagues? Or, more importantly, what are the topics that don't come up because they are a little too controversial? In Immune Memory and Vaccines: Great Debates, Editors Rafi Ahmed and Shane Crotty have put together a collection of articles on such questions, written by thought leaders in these fields, with the freedom to talk about the issues as they see fit. This short, innovative format aims to bring a fresh perspective by encouraging authors to be opinionated, focus on what is most interesting and current, and avoid restating introductory material covered in many other reviews.
The Editors posed 13 interesting questions critical for our understanding of vaccines and immune memory to a broad group of experts in the field. In each case, several different perspectives are provided. Note that while each author knew that there were additional scientists addressing the same question, they did not know who these authors were, which ensured the independence of the opinions and perspectives expressed in each article. Our hope is that readers enjoy these articles and that they trigger many more conversations on these important topics.
The resurgence of pertussis is difficult to define and quantitate but it is observed in most countries, which switched to acellular pertussis (aP) vaccines for infant immunization (World Health Organization 2015). Incidence rates vary greatly and are highest in adolescents (Klein et al. 2012, 2016; Acosta et al. 2015; Esposito et al. 2016), although an increase in infant mortality was recently reported for example in the United Kingdom (Amirthalingam et al. 2014; Health Protection Report 2016). The failure of current aP vaccines in adolescents being established, defining its cause would be the obvious way to pave the road toward new vaccines. Yet, because there is an absence of correlates of protection for adolescent pertussis, speculation is the rule.
Many potential epidemiological and immunological contributing factors are being discussed. The widespread availability of polymerase chain reaction (PCR) results in diagnoses being established after only a few days of cough, contrasting with the former use of clinical features including cough for at least 3 weeks and culture confirmation (World Health Organization 1991, 2003). The cyclic outbreak pattern of pertussis epidemics (Fine and Clarkson 1982) and the risks of antigenic shift and/or infection by other strains like Bordetella parapertussis or Bordetella holmesii (Mattoo and Cherry 2005) confuse the pattern. However, the rapid decay of antibodies and decline of vaccine efficacy in fully primed and recently boosted adolescents (Edelman et al. 2007; Acosta et al. 2015; Esposito et al. 2016; Klein et al. 2016) suggests that the main issue is the waning of immune memory to pertussis. Failures of humoral and cellular responses are being discussed, including the intrinsic role of T cells and/or their failing help to B cells (van Twillert et al. 2015).
We elaborate in this debate our hypothesis that aP vaccine failure already starts at priming. We focus as main causes on the concepts of the original “chemical” sin and the inability to activate mucosal defense and we then discuss future prospects.
FAILURE OF PRIMING BY aP VACCINES
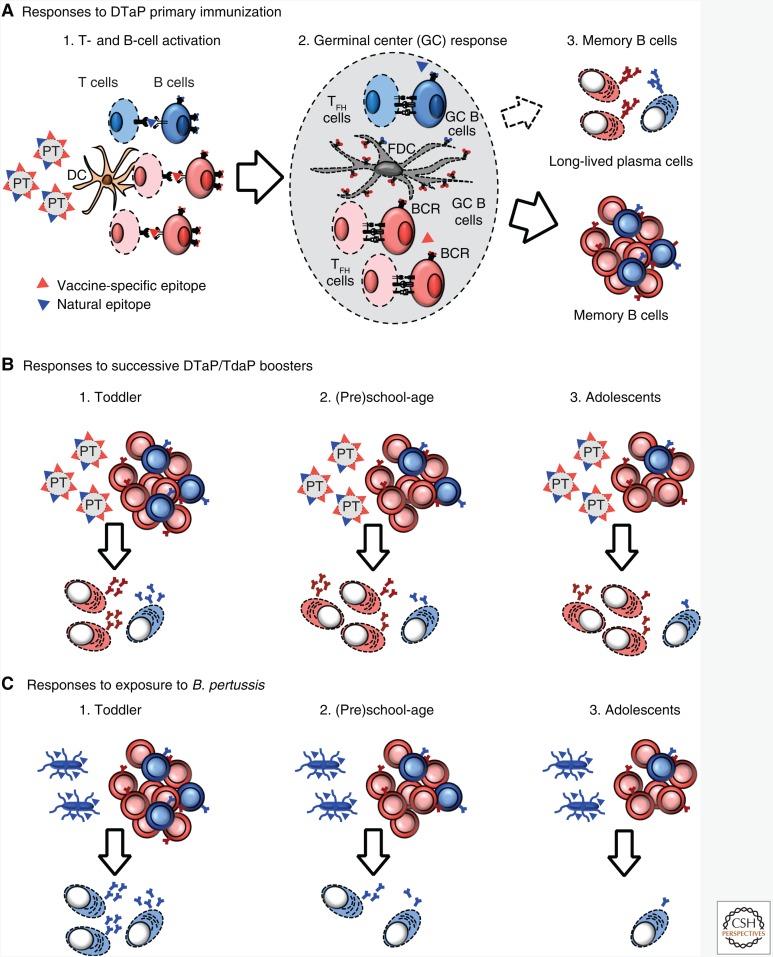
The short-term effectiveness of aP vaccines in aP-primed adolescents correlates with the rapid waning of vaccine-induced antibodies (Le et al. 2004; Lai et al. 2012; Aase et al. 2014). It contrasts with that observed and predicted in whole-cell (wP)-primed adolescents (Bailleux et al. 2008). When antibodies do not persist despite several vaccine doses, it implies that sufficiently strong germinal center reactions (reviewed in De Silva and Klein 2015) have not been generated to elicit high-avidity plasma cells capable of efficiently homing to the bone marrow for decades of survival (Fig. 1A) (Slifka et al. 1998; De Silva and Klein 2015). This implies that the germinal center–derived affinity maturation, selection, differentiation, survival and/or recall of B-cell clones with enhanced antigen affinity have not been successful.
Figure 1.
Pertussis toxin B-cell responses to primary and booster acellular pertussis (aP) immunizations or following exposition to Bordetella pertussis. Immune response to priming with aP (A) leads to formation of anti-pertussis toxin (PT) memory B cells. These are directed either against the epitopes unique to denatured PT (red) or of wild-type PT (blue). Upon boosting (B), the proportion of anti-PT memory B cells specific to wild-type PT declines proportionally to the increasing number of doses (and thus age). Consequently, fewer and fewer wild-type PT-specific B cells are available for reactivation at time of exposure to B. pertussis (C).
Remarkably, secondary anamnestic antibody responses elicited in children or adolescents appear to not be stronger than primary infant responses (see Table 1; also discussed in Thierry-Carstensen et al. 2013). We found no prospective study directly comparing anti-pertussis toxin (PT) responses of aP-primed subjects following the 4th, 5th, or 6th dose of aP—which prevents evidence-based direct comparisons. However, reported studies that included at least infants and children or children and adolescents (Table 1) did not show any dose-dependent increase nor drastically reduced anti-PT titers with age and successive doses. This suggests that hyporesponsiveness secondary to the loss of memory B cells may not play a critical role, although it may contribute to the picture; in the absence of sufficient boosting (see below), antigen-specific memory may get lost (Posfay-Barbe et al. 2010).
Table 1.
Comparison of anti-pertussis toxin (PT) antibody concentrations in cohorts of acellular pertussis (aP)-primed children assessed after boosting at various ages
| Priming with aP vaccine (PT dose in µg; Nx = doses; age in months) | Age at last aP dose (PT dose in µg) | Anti-PT GMCs (EU/mL ; N = number of study subjects) | References |
|---|---|---|---|
| Italy | |||
| DTaP3, 25 µg; 2× (2,4) DTagdPT3, 5 µg; 2× (2,4) |
6 mo (25 µg) 6 mo (5 µg) |
51.3, CI 95%, 47.9–54.9 (total N = 1572) 94.4, CI 95%, 88.8–100.3 (total N = 1572) |
Greco et al. 1996 |
| DTaP3, 25 µg; 3× (2,4,6) DTagdPT3, 5 µg; 3× (2,4,6) |
5–6 yr (25 µg) 5–6 yr (5 µg) |
68.9, CI 95%, 61.00–77.78 (N = 277) 120.7, CI 95%, 108.32–134.38 (N = 281) |
Tozzi et al. 2001 |
| Sweden | |||
| DTaP2, 25 µg; 2× (2,4) | 6 mo (25 µg) | Median 50 (estimated from graph) (N = 186) | Gustafsson et al. 1996 |
| DTaP5, 10 µg; 2× (2,4) | 6 mo (10 µg) | Median 75 (estimated from graph) (N = 178) | |
| DTagdP, 5 µg; 2× (2,4) DTaP5, 20 µg; 2× (2,4) |
6 mo 6 mo |
Median 158 (N = 80) Median 50 (estimated from graph) (N = 80) |
Olin et al. 1997 |
| DTaP5, 20 µg; 3× (2,4,6) | 5 yr (2.5 µg) | 22, CI 95%, 20–25 (N = 440) | Carlsson et al. 2015 |
| DTaP5, 20 µg; 3× (2,4,6) followed by TdaP5, 2.5 µg; 1× (5 yr) |
15 yr (2.5 µg) 15 yr (20 µg) |
20, CI 95%, 17–24 (N = 114) 74, CI 95%, 61–90 (N = 113) |
Carlsson et al. 2015 |
| Germany | |||
| DTaP, 25µg; 2× (3,4) | 5 mo (25 µg) | 49, CI not reported (N = 571) | Schmitt et al. 1996 |
| DTaP, 25µg; 2× (3,4,5) | 15–19 mo (25 µg) | 109, CI not reported (N = 571(?)) | Zepp et al. 1996 |
| DTaP, 4× (3,4,5, 15–19 mo) TdaP, 1× (5.8 yr earlier) Or DTaP, 1× (5.8 yr earlier) |
10–12 yr (8 µg) | 51.8, CI 95%, 41.6–64.7 (N = 34) 52.0, CI 95%, 45.3–59.8 (N = 93) |
Zepp et al. 2007 |
| Canada | |||
| TdaP5, 2.5 µg, 12–55 yr | >5 yr | 144, CI 95%, 132–157 (N = 449) | Halperin et al. 2000a |
| TdaP5-IPV (2.5 µg) Td-IPV, aP alone |
12–18 yr Adults Adolescents |
172, CI 95%, 155–191 (N = 350) 115, CI 95%, 103–128 (N = 366) 365, CI 95%, 306–434 (N = 116) |
Halperin et al. 2000b |
| TdaP | >10 yr ago (2.5 µg) | 116, CI 95%, 105–129 (N = 318) | Halperin et al. 2012 |
| Taiwan | |||
| DTaP, 20 µg; 2× (2,4) | 6 mo (20 µg) | 131, CI 95%, 113–152 (N = 64) | Lee et al. 1999 |
| DTaP, 20 µg; 3× (2,4,6) | 18 mo (20 µg) | 216, CI 95%, 184–253 (N = 61) | Lee et al. 1999 |
| DTP, 4× | 6–8 yr (8 µg) 15–20 yr (8 µg) |
89.2, CI 95%, 68.7–115.9 (N = 59) 53.3, CI 95%, 43.4–65.4 (N = 119) |
Huang et al. 2005 |
Unless stated otherwise, anti-PT antibodies were detected by ELISA 4–12 wk after the booster dose. To minimize confounding factors, we selected a few studies in which children from various age groups were assessed in parallel—as compared to postprimary responses in similar/close cohorts.
Despite these relatively similar antibody responses, protective efficacy is lower and shorter in adolescents (Witt et al. 2012) than in preschool children (Lambert 2014), suggesting the failure of vaccine memory. Yet, a single dose of aP vaccine effectively reactivates immune memory in individuals primed with wP vaccines (see Huang et al. 2005). Furthermore, a single dose of wP vaccine at priming is sufficient to reduce the risk of adolescent pertussis, and the more wP doses at priming, the better (Sheridan et al. 2012; Baxter et al. 2013; Klein et al. 2013; Witt et al. 2013). Thus, we believe the major problem is not the waning of memory in adolescence, but the failure of inducing optimal pertussis immunity at time of priming.
The Original “Chemical” Sin
Few antigens are included in current aP vaccines, a key one being PT. PT antibodies are sufficient to protect infants from severe pertussis (Cherry et al. 1998; Storsaeter et al. 1998) and are better markers of vaccine responses as their recall responses are not confounded by exposure to cross-reacting bacteria. In contrast, antibody responses to other antigens such as filamentous hemagglutinin (FHA) can be confounded by cross-reactive bacteria. PT is by nature a moderately immunogenic antigen, which further partly loses its antigenicity when chemically inactivated; it induces low antibody titers even after four doses in infancy and responses to a sixth dose of PT are, remarkably, not stronger than in infants (see Table 1).
That protective efficacy conferred by aP vaccines is lower in immunologically mature, primed, and boosted adolescents than in infants and toddlers is counterintuitive. One potential explanation is that distinct definitions of pertussis are used in various age groups in which awareness, surveillance, and detection systems differ. However, a plausible immunological hypothesis is that of the “original antigenic sin,” referred to by Cherry as “linked epitope suppression” (Cherry et al. 2004; Cherry 2013). Initially defined to explain the modest efficacy of repeat influenza vaccines (Kim et al. 2009), this concept implies that the repeated exposure to vaccine-specific antigens progressively generates more and more vaccine-specific effector and memory B cells. These cells increasingly compete with naïve B cells and, thus, the host loses the capacity to adapt and react to the slightly different bacterial epitopes (Cherry et al. 2004, 2010).
During the development of aP vaccines, PT was determined as the main antigen. Its preparation required detoxification for which two options were available in the 1980s: the conventional chemical detoxification of PT after its production or the genetic modification of Bordetella pertussis strains producing genetically detoxified PT (Podda et al. 1995; Ibsen 1996). For nonscientific reasons, currently licensed aP vaccines include chemically detoxified PT. Chemical detoxification, however, means protein denaturation and thus potential alterations of the tridimensional structure. A study using competing monoclonal antibodies showed that this denaturation process indeed generates distinct epitopes from the ones present on naïve PT (Sutherland et al. 2011). Thus, infant priming followed by repeated boosting by chemically detoxified PT likely generates effector and memory B cells that are more and more vaccine-specific and less proficient to bind to structurally distinct native PT (Fig. 1B). Differences between natural and vaccine antigens can also arise from a change in the phenotype of pertussis strains (Poolman and Hallander 2007; Bart et al. 2014). However, the showed lack of expression of pertactin (PRN) (Hegerle and Guiso 2014; Lam et al. 2014; Martin et al. 2015) did not affect vaccine effectiveness (Breakwell et al. 2016). We thus see the chemical denaturation of PT, which reduces its modest immunogenicity (e.g., the original “chemical” sin), as mainly responsible for the progressive loss of responses to PT boosters. The direct demonstration of this hypothesis would require substituting chemically for genetically detoxified PT in infant vaccines and following them up to adolescence, a daunting task.
Failure of Postexposure Immune Memory Reactivation
By definition, anamnestic responses reactivate affinity-matured memory B (and T) cells and therefore are faster than primary responses. The contribution of immune memory to protection depends on a number of factors, including the comparative kinetics of immune reactivation and disease; infections with short incubation time, such as Haemophilus influenzae b infections, classically occur in primed children (Pichichero 2009). It only takes a few days for antibody titers to increase to significantly higher levels following the boosting of aP-primed children or adolescents (Schure et al. 2013) and pertussis does not follow a rapid course; its average incubation time of 7–10 days should allow reactivation of vaccine-induced immunity. Yet, 2–3 weeks are required for anti-PT antibodies to peak in the serum in aP- or wP-primed children or adolescents with PCR-proven pertussis (Hallander et al. 2009)!
Why does pertussis infection fail to rapidly reactivate immunity? B. pertussis infection is strictly a mucosal pathogen, with no bacteremia. Therefore, there is likely limited antigen dissemination to the draining lymph nodes where memory B cells essentially reside, expecting to eventually be reactivated by antigen. In addition, B. pertussis exerts local immunomodulatory effects, which limit the migration of (antigen-loaded) dendritic cells to the draining lymph nodes (de Gouw et al. 2011; Adkins et al. 2014). Thus, aP vaccines readily reactivate pertussis immunity at time of boosting—but rapid reactivation of pertussis immunity does not occur at time of B. pertussis exposure, resulting in infection and symptoms.
Current aP vaccines were designed to induce serum antibodies, and the role of T cells and that of their specific polarization is much discussed (van Twillert et al. 2015). The development of the baboon challenge model (Warfel et al. 2012, 2014) has clearly showed that only wP vaccines reduce the intensity and duration of bacterial colonization (Warfel et al. 2014). Whether this results from the inclusion of too few antigens, the lack of optimized fimbriae (Gorringe and Vaughan 2014), or the preferential induction of Th2 rather than of Th1/Th17 (Warfel and Merkel 2013; Ross et al. 2013) responses by aP vaccines, the limited set of baboon-derived data concurs to the epidemiological evidence. The continued use of wP vaccines in the United Kingdom, providing an average of 15 years of protection contrasting to 5 years for aP vaccines, would have prevented the resurgence of pertussis, and the associated loss of infant lives (Choi et al. 2016).
WHAT LIES AHEAD?
To date, maternal immunization is highly effective against infant death (Amirthalingam et al. 2014; Dabrera et al. 2015), especially if performed in the second trimester (Eberhardt et al. 2016). Beneficial for term infants, second trimester maternal immunization may even contribute to the protection of the most vulnerable preterm infants (Eberhardt et al., in press). As the switch to aP vaccines occurred in the late 1990s or early 2000s, current mothers were primed with wP vaccines. However, the cohort of aP-primed subjects will soon reach childbearing age and be confronted with the same issue of limited protective efficacy as adolescents currently are. It is thus urgent to define whether improved booster vaccines might be developed for future aP-primed pregnant women. Existing options include the use of genetically detoxified PT, shown to be more immunogenic than chemically detoxified PT, inducing higher neutralizing antibody titers and a Th1/Th17 response that may improve mucosal responses (Seubert et al. 2014). To reduce immune escape, additional antigens such as improved fimbriae antigens (Gorringe and Vaughan 2014), adenylate cyclase toxin (ACT), or the autotransporter BrkA could be considered (Brummelman et al. 2015). As the current alum-based adjuvantation does not induce Th1 responses, different toll-like receptor (TLR) agonists are being tested (reviewed in Rumbo and Hozbor 2014) and the use of nanoparticles seems promising in animal models (Gaillard et al. 2014). Finally, nasal live attenuated vaccines inducing dendritic cell (DC) maturation and Th1/Th17 responses are in clinical trials (reviewed in Brummelman et al. 2015).
Will it be sufficient to introduce novel vaccines at the time of adolescent or adult boosting? How many doses of aP vaccines (3, 4, 5, 6, or more?) will be compatible with the effective recall of immune memory by novel adolescent/adult vaccines? These questions are crucial. Indeed, the development of new infant vaccines will be most challenging: first, new vaccines will have to be tested against combination penta- or hexavalent vaccines in current use, requiring very large sample sizes. Next, assessing antibody responses will not be sufficient and infant pertussis efficacy trials will likely be required. This challenging adventure would become logistically and financially impossible should the follow-up period include the demonstration of the proper induction and reactivation of memory throughout adolescence and adulthood! Should the development of novel booster vaccines fail to properly reactivate aP-primed immunity, the key to the control of pertussis may be to return to its primary objective: to prevent pertussis deaths through maternal and early infant immunization, and to learn again how to live with pertussis outbreaks affecting (and efficiently boosting) older children and adolescents.
CONCLUDING REMARKS
The failure of aP vaccines to induce sufficient immune memory responses and to prevent pertussis in the adolescent is multifactorial and already begins with priming. Improved vaccines that induce potent mucosal defense would be needed. Potential options are to include more antigens, which are more similar to the native ones, to improve adjuvantation or change the route of immunization to improve mucosal defense and reduce colonization. Pending their availability, or should these approaches fail, implementing high levels of maternal and early infant immunization with available pertussis vaccines is crucial and needs to be promoted.
ACKNOWLEDGMENTS
This work is supported by funds from the Center for Vaccinology of the University of Geneva.
Footnotes
Editors: Shane Crotty and Rafi Ahmed
Additional Perspectives on Immune Memory and Vaccines: Great Debates available at www.cshperspectives.org
REFERENCES
- Aase A, Herstad TK, Jørgensen SB, Leegaard TM, Berbers G, Steinbakk M, Aaberge I. 2014. Anti-pertussis antibody kinetics following DTaP-IPV booster vaccination in Norwegian children 7–8 years of age. Vaccine 32: 5931–5936. [DOI] [PubMed] [Google Scholar]
- Acosta AM, DeBolt C, Tasslimi A, Lewis M, Stewart LK, Misegades LK, Messonnier NE, Clark TA, Martin SW, Patel M. 2015. Tdap vaccine effectiveness in adolescents during the 2012 Washington State pertussis epidemic. Pediatrics 135: 981–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adkins I, Kamanova J, Kocourkova A, Svedova M, Tomala J, Janova H, Masin J, Chladkova B, Bumba L, Kovar M, et al. 2014. Bordetella adenylate cyclase toxin differentially modulates toll-like receptor-stimulated activation, migration and T cell stimulatory capacity of dendritic cells. PLoS ONE 9: e104064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amirthalingam G, Andrews N, Campbell H, Ribeiro S, Kara E, Donegan K, Fry NK, Miller E, Ramsay M. 2014. Effectiveness of maternal pertussis vaccination in England: An observational study. Lancet 384: 1521–1528. [DOI] [PubMed] [Google Scholar]
- Bailleux F, Coudeville L, Kolenc-Saban A, Bevilacqua J, Barreto L, André P. 2008. Predicted long-term persistence of pertussis antibodies in adolescents after an adolescent and adult formulation combined tetanus, diphtheria, and 5-component acellular pertussis vaccine, based on mathematical modeling and 5-year observed data. Vaccine 26: 3903–3908. [DOI] [PubMed] [Google Scholar]
- Bart MJ, Harris SR, Advani A, Arakawa Y, Bottero D, Bouchez V, Cassiday PK, Chiang CS, Dalby T, Fry NK, et al. 2014. Global population structure and evolution of Bordetella pertussis and their relationship with vaccination. MBio 5: e01074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter R, Bartlett J, Rowhani-Rahbar A, Fireman B, Klein NP. 2013. Effectiveness of pertussis vaccines for adolescents and adults: Case-control study. BMJ 347: f4249. [DOI] [PubMed] [Google Scholar]
- Breakwell L, Kelso P, Finley C, Schoenfeld S, Goode B, Misegades LK, Martin SW, Acosta AM. 2016. Pertussis vaccine effectiveness in the setting of pertactin-deficient pertussis. Pediatrics 137: e20153973. [DOI] [PubMed] [Google Scholar]
- Brummelman J, Wilk MM, Han WG, van Els CA, Mills KH. 2015. Roads to the development of improved pertussis vaccines paved by immunology. Pathog Dis 73: ftv067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson RM, Gustafsson L, Hallander HO, Ljungman M, Olin P, Gothefors L, Nilsson L, Netterlid E. 2015. Two consecutive randomized controlled pertussis booster trials in children initially vaccinated in infancy with an acellular vaccine: The first with a five-component Tdap vaccine to 5-year olds and the second with five- or monocomponent Tdap vaccines at age 14–15 years. Vaccine 33: 3717–3725. [DOI] [PubMed] [Google Scholar]
- Cherry JD. 2013. Pertussis: Challenges today and for the future. PLoS Pathog 9: e1003418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherry JD, Gornbein J, Heininger U, Stehr K. 1998. A search for serologic correlates of immunity to Bordetella pertussis cough illnesses. Vaccine 16: 1901–1906. [DOI] [PubMed] [Google Scholar]
- Cherry JD, Xing DX, Newland P, Patel K, Heininger U, Corbel MJ. 2004. Determination of serum antibody to Bordetella pertussis adenylate cyclase toxin in vaccinated and unvaccinated children and in children and adults with pertussis. Clin Infect Dis 38: 502–507. [DOI] [PubMed] [Google Scholar]
- Cherry JD, Heininger U, Richards DM, Storsaeter J, Gustafsson L, Ljungman M, Hallander HO. 2010. Antibody response patterns to Bordetella pertussis antigens in vaccinated (primed) and unvaccinated (unprimed) young children with pertussis. Clin Vaccine Immunol 17: 741–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi YH, Campbell H, Amirthalingam G, van Hoek AJ, Miller E. 2016. Investigating the pertussis resurgence in England and Wales, and options for future control. BMC Med 14: 121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabrera G, Amirthalingam G, Andrews N, Campbell H, Ribeiro S, Kara E, Fry NK, Ramsay M. 2015. A case-control study to estimate the effectiveness of maternal pertussis vaccination in protecting newborn infants in England and Wales, 2012–2013. Clin Infect Dis 60: 333–337. [DOI] [PubMed] [Google Scholar]
- de Gouw D, Diavatopoulos DA, Bootsma HJ, Hermans PW, Mooi FR. 2011. Pertussis: A matter of immune modulation. FEMS Microbiol Rev 35: 441–474. [DOI] [PubMed] [Google Scholar]
- De Silva NS, Klein U. 2015. Dynamics of B cells in germinal centres. Nat Rev Immunol 15: 137–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberhardt CS, Blanchard-Rohner G, Lemaître B, Boukrid M, Combescure C, Othenin-Girard V, Chilin A, Petre J, de Tejada BM, Siegrist CA. 2016. Maternal immunization earlier in pregnancy maximizes antibody transfer and expected infant seropositivity against pertussis. Clin Infect Dis 62: 829–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman K, He Q, Mäkinen J, Sahlberg A, Haanperä M, Schuerman L, Wolter J, Mertsola J. 2007. Immunity to pertussis 5 years after booster immunization during adolescence. Clin Infect Dis 44: 1271–1277. [DOI] [PubMed] [Google Scholar]
- Esposito S, Principi N, European Society of Clinical Microbiology Infectious Diseases (ESCMID) Vaccine Study. 2016. Immunization against pertussis in adolescents and adults. Clin Microbiol Infect 10.1016/j.cmi.2016.01.003. [DOI] [PubMed] [Google Scholar]
- Fine PE, Clarkson JA. 1982. The recurrence of whooping cough: Possible implications for assessment of vaccine efficacy. Lancet 1: 666–669. [DOI] [PubMed] [Google Scholar]
- Gaillard ME, Bottero D, Errea A, Ormazábal M, Zurita ME, Moreno G, Rumbo M, Castuma C, Bartel E, Flores D, et al. 2014. Acellular pertussis vaccine based on outer membrane vesicles capable of conferring both long-lasting immunity and protection against different strain genotypes. Vaccine 32: 931–937. [DOI] [PubMed] [Google Scholar]
- Gorringe AR, Vaughan TE. 2014. Bordetella pertussis fimbriae (Fim): Relevance for vaccines. Expert Rev Vaccines 13: 1205–1214. [DOI] [PubMed] [Google Scholar]
- Greco D, Salmaso S, Mastrantonio P, Giuliano M, Tozzi AE, Anemona A, Ciofi degli Atti ML, Giammanco A, Panei P, Blackwelder WC, et al. 1996. A controlled trial of two acellular vaccines and one whole-cell vaccine against pertussis. Progetto Pertosse Working Group. N Engl J Med 334: 341–348. [DOI] [PubMed] [Google Scholar]
- Gustafsson L, Hallander HO, Olin P, Reizenstein E, Storsaeter J. 1996. A controlled trial of a two-component acellular, a five-component acellular, and a whole-cell pertussis vaccine. N Engl J Med 334: 349–355. [DOI] [PubMed] [Google Scholar]
- Hallander HO, Ljungman M, Storsaeter J, Gustafsson L. 2009. Kinetics and sensitivity of ELISA IgG pertussis antitoxin after infection and vaccination with Bordetella pertussis in young children. APMIS 117: 797–807. [DOI] [PubMed] [Google Scholar]
- Halperin SA, Smith B, Russell M, Hasselback P, Guasparini R, Skowronski D, Meekison W, Parker R, Lavigne P, Barreto L. 2000a. An adult formulation of a five-component acellular pertussis vaccine combined with diphtheria and tetanus toxoids is safe and immunogenic in adolescents and adults. Vaccine 18: 1312–1319. [DOI] [PubMed] [Google Scholar]
- Halperin SA, Smith B, Russell M, Scheifele D, Mills E, Hasselback P, Pim C, Meekison W, Parker R, Lavigne P, et al. 2000b. Adult formulation of a five component acellular pertussis vaccine combined with diphtheria and tetanus toxoids and inactivated poliovirus vaccine is safe and immunogenic in adolescents and adults. Pediatr Infect Dis J 19: 276–283. [DOI] [PubMed] [Google Scholar]
- Halperin SA, Scheifele D, De Serres G, Noya F, Meekison W, Zickler P, Larrivée L, Langley JM, McNeil SA, Dobson S, et al. 2012. Immune responses in adults to revaccination with a tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccine 10 years after a previous dose. Vaccine 30: 974–982. [DOI] [PubMed] [Google Scholar]
- Health Protection Report. 2016. Laboratory confirmed pertussis in England: Data to end-May 2016. Vol. 10, No. 23, July 15. [Google Scholar]
- Hegerle N, Guiso N. 2014. Bordetella pertussis and pertactin-deficient clinical isolates: Lessons for pertussis vaccines. Expert Rev Vaccines 13: 1135–1146. [DOI] [PubMed] [Google Scholar]
- Huang LM, Chang LY, Tang H, Bock HL, Lu CY, Huang FY, Lin TY, Lee CY. 2005. Immunogenicity and reactogenicity of a reduced-antigen-content diphtheria-tetanus-acellular pertussis vaccine in healthy Taiwanese children and adolescents. J Adolesc Health 37: 517. [DOI] [PubMed] [Google Scholar]
- Ibsen PH. 1996. The effect of formaldehyde, hydrogen peroxide and genetic detoxification of pertussis toxin on epitope recognition by murine monoclonal antibodies. Vaccine 14: 359–368. [DOI] [PubMed] [Google Scholar]
- Kim JH, Skountzou I, Compans R, Jacob J. 2009. Original antigenic sin responses to influenza viruses. J Immunol 183: 3294–3301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein NP, Bartlett J, Rowhani-Rahbar A, Fireman B, Baxter R. 2012. Waning protection after fifth dose of acellular pertussis vaccine in children. N Engl J Med 367: 1012–1019. [DOI] [PubMed] [Google Scholar]
- Klein NP, Bartlett J, Fireman B, Rowhani-Rahbar A, Baxter R. 2013. Comparative effectiveness of acellular versus whole-cell pertussis vaccines in teenagers. Pediatrics 131: e1716–e1722. [DOI] [PubMed] [Google Scholar]
- Klein NP, Bartlett J, Fireman B, Baxter R. 2016. Waning Tdap effectiveness in adolescents. Pediatrics 137: e20153326. [DOI] [PubMed] [Google Scholar]
- Lai FY, Thoon KC, Ang LW, Tey SH, Heng D, Cutter JL, Phoon MC, Chow VT. 2012. Comparative seroepidemiology of pertussis, diphtheria and poliovirus antibodies in Singapore: Waning pertussis immunity in a highly immunized population and the need for adolescent booster doses. Vaccine 30: 3566–3571. [DOI] [PubMed] [Google Scholar]
- Lam C, Octavia S, Ricafort L, Sintchenko V, Gilbert GL, Wood N, McIntyre P, Marshall H, Guiso N, Keil AD, et al. 2014. Rapid increase in pertactin-deficient Bordetella pertussis isolates, Australia. Emerg Infect Dis 20: 626–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert LC. 2014. Pertussis vaccine trials in the 1990s. J Infect Dis 209: S4–S9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le T, Cherry JD, Chang SJ, Knoll MD, Lee ML, Barenkamp S, Bernstein D, Edelman R, Edwards KM, Greenberg D, et al. 2004. Immune responses and antibody decay after immunization of adolescents and adults with an acellular pertussis vaccine: The APERT Study. J Infect Dis 190: 535–544. [DOI] [PubMed] [Google Scholar]
- Lee CY, Thipphawong J, Huang LM, Lee PI, Chiu HH, Lin W, Debois H, Harrison D, Xie F, Barreto L. 1999. An evaluation of the safety and immunogenicity of a five-component acellular pertussis, diphtheria, and tetanus toxoid vaccine (DTaP) when combined with a Haemophilus influenzae type b-tetanus toxoid conjugate vaccine (PRP-T) in Taiwanese infants. Pediatrics 103: 25–30. [DOI] [PubMed] [Google Scholar]
- Martin SW, Pawloski L, Williams M, Weening K, DeBolt C, Qin X, Reynolds L, Kenyon C, Giambrone G, Kudish K, et al. 2015. Pertactin-negative Bordetella pertussis strains: Evidence for a possible selective advantage. Clin Infect Dis 60: 223–227. [DOI] [PubMed] [Google Scholar]
- Mattoo S, Cherry JD. 2005. Molecular pathogenesis, epidemiology, and clinical manifestations of respiratory infections due to Bordetella pertussis and other Bordetella subspecies. Clin Microbiol Rev 18: 326–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olin P, Rasmussen F, Gustafsson L, Hallander HO, Heijbel H. 1997. Randomised controlled trial of two-component, three-component, and five-component acellular pertussis vaccines compared with whole-cell pertussis vaccine. Ad Hoc Group for the Study of Pertussis Vaccines. Lancet 350: 1569–1577. [DOI] [PubMed] [Google Scholar]
- Pichichero ME. 2009. Booster vaccinations: Can immunologic memory outpace disease pathogenesis? Pediatrics 124: 1633–1641. [DOI] [PubMed] [Google Scholar]
- Podda A, Bona G, Canciani G, Pistilli AM, Contu B, Furlan R, Meloni T, Stramare D, Titone L, Rappuoli R, et al. 1995. Effect of priming with diphtheria and tetanus toxoids combined with whole-cell pertussis vaccine or with acellular pertussis vaccine on the safety and immunogenicity of a booster dose of an acellular pertussis vaccine containing a genetically inactivated pertussis toxin in fifteen- to twenty-one-month-old children. Italian Multicenter Group for the Study of Recombinant Acellular Pertussis Vaccine. J Pediatr 127: 238–243. [DOI] [PubMed] [Google Scholar]
- Poolman JT, Hallander HO. 2007. Acellular pertussis vaccines and the role of pertactin and fimbriae. Expert Rev Vaccines 6: 47–56. [DOI] [PubMed] [Google Scholar]
- Posfay-Barbe KM, Kobela M, Sottas C, Grillet S, Taguebue J, Ekoe T, Lambert PH, Lecoultre C, Siegrist CA. 2010. Frequent failure of adolescent booster responses to tetanus toxoid despite infant immunization: Waning of infancy-induced immune memory? Vaccine 28: 4356–4361. [DOI] [PubMed] [Google Scholar]
- Ross PJ, Sutton CE, Higgins S, Allen AC, Walsh K, Misiak A, Lavelle EC, McLoughlin RM, Mills KH. 2013. Relative contribution of Th1 and Th17 cells in adaptive immunity to Bordetella pertussis: Towards the rational design of an improved acellular pertussis vaccine. PLoS Pathog 9: e1003264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumbo M, Hozbor D. 2014. Development of improved pertussis vaccine. Hum Vaccin Immunother 10: 2450–2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt HJ, Schuind A, Knuf M, Beutel K, Schulte-Wissermann H, Gahr M, Schult R, Folkens J, Rauh W, Bogaerts H, et al. 1996. Clinical experience of a tricomponent acellular pertussis vaccine combined with diphtheria and tetanus toxoids for primary vaccination in 22,505 infants. J Pediatr 129: 695–701. [DOI] [PubMed] [Google Scholar]
- Schure RM, Hendrikx LH, de Rond LG, Oztürk K, Sanders EA, Berbers GA, Buisman AM. 2013. Differential T- and B-cell responses to pertussis in acellular vaccine-primed versus whole-cell vaccine-primed children 2 years after preschool acellular booster vaccination. Clin Vaccine Immunol 20: 1388–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seubert A, D’Oro U, Scarselli M, Pizza M. 2014. Genetically detoxified pertussis toxin (PT-9K/129G): Implications for immunization and vaccines. Expert Rev Vaccines 13: 1191–1204. [DOI] [PubMed] [Google Scholar]
- Sheridan SL, Ware RS, Grimwood K, Lambert SB. 2012. Number and order of whole cell pertussis vaccines in infancy and disease protection. JAMA 308: 454–456. [DOI] [PubMed] [Google Scholar]
- Slifka MK, Antia R, Whitmire JK, Ahmed R. 1998. Humoral immunity due to long-lived plasma cells. Immunity 8: 363–372. [DOI] [PubMed] [Google Scholar]
- Storsaeter J, Hallander HO, Gustafsson L, Olin P. 1998. Levels of anti-pertussis antibodies related to protection after household exposure to Bordetella pertussis. Vaccine 16: 1907–1916. [DOI] [PubMed] [Google Scholar]
- Sutherland JN, Chang C, Yoder SM, Rock MT, Maynard JA. 2011. Antibodies recognizing protective pertussis toxin epitopes are preferentially elicited by natural infection versus acellular immunization. Clin Vaccine Immunol 18: 954–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thierry-Carstensen B, Dalby T, Stevner MA, Robbins JB, Schneerson R, Trollfors B. 2013. Experience with monocomponent acellular pertussis combination vaccines for infants, children, adolescents and adults–A review of safety, immunogenicity, efficacy and effectiveness studies and 15 years of field experience. Vaccine 31: 5178–5191. [DOI] [PubMed] [Google Scholar]
- Tozzi AE, Anemona A, Stefanelli P, Salmaso S, Ciofi degli Atti ML, Mastrantonio P, Giammanco A; Progetto Pertosse Study Group. 2001. Reactogenicity and immunogenicity at preschool age of a booster dose of two three-component diphtheria-tetanus-acellular pertussis vaccines in children primed in infancy with acellular vaccines. Pediatrics 107: E25. [DOI] [PubMed] [Google Scholar]
- van Twillert I, Han WG, van Els CA. 2015. Waning and aging of cellular immunity to Bordetella pertussis. Pathog Dis 73: ftv071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warfel JM, Merkel TJ. 2013. Bordetella pertussis infection induces a mucosal IL-17 response and long-lived Th17 and Th1 immune memory cells in nonhuman primates. Mucosal Immunol 6: 787–796. [DOI] [PubMed] [Google Scholar]
- Warfel JM, Beren J, Kelly VK, Lee G, Merkel TJ. 2012. Nonhuman primate model of pertussis. Infect Immun 80: 1530–1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warfel JM, Zimmerman LI, Merkel TJ. 2014. Acellular pertussis vaccines protect against disease but fail to prevent infection and transmission in a nonhuman primate model. Proc Natl Acad Sci 111: 787–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witt MA, Katz PH, Witt DJ. 2012. Unexpectedly limited durability of immunity following acellular pertussis vaccination in preadolescents in a North American outbreak. Clin Infect Dis 54: 1730–1735. [DOI] [PubMed] [Google Scholar]
- Witt MA, Arias L, Katz PH, Truong ET, Witt DJ. 2013. Reduced risk of pertussis among persons ever vaccinated with whole cell pertussis vaccine compared to recipients of acellular pertussis vaccines in a large US cohort. Clin Infect Dis 56: 1248–1254. [DOI] [PubMed] [Google Scholar]
- World Health Organization. 1991. WHO meeting on case definitions of pertussis. pp. 4–5.
- World Health Organization. 2003. WHO–recommended standards for surveillance of selected vaccine-preventable diseases. pp. 28–30.
- World Health Organization. 2015. Pertussis vaccines: WHO position paper. Weekly Epidemiological Record, Vol. 90, September, pp. 433–458.26320265 [Google Scholar]
- Zepp F, Knuf M, Habermehl P, Schmitt JH, Rebsch C, Schmidtke P, Clemens R, Slaoui M. 1996. Pertussis-specific cell-mediated immunity in infants after vaccination with a tricomponent acellular pertussis vaccine. Infect Immun 64: 4078–4084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zepp F, Habermehl P, Knuf M, Mannhardt-Laakman W, Howe B, Friedland LR. 2007. Immunogenicity of reduced antigen content tetanus-diphtheria-acellular pertussis vaccine in adolescents as a sixth consecutive dose of acellular pertussis-containing vaccine. Vaccine 25: 5248–5252. [DOI] [PubMed] [Google Scholar]