Abstract
Vaporized hydrogen peroxide (VHP) is used to decontaminate clinical, biocontainment, and research animal rooms and equipment. To assist with its implementation in a murine facility, we developed a safe and effective method of VHP sterilization of IVC racks and air handling units (AHU). Safety of VHP decontamination was assessed by ensuring VHP levels dissipated to less than 1 ppm in the room prior to personnel reentry and inside the primary enclosure prior to the return of mice; this condition occurred at least 18 h after the VHP cycle. Efficacy of VHP sterilization was assessed by using chemical indicators, biologic indicators, and PCR testing for Staphylococcus xylosus, a commensal organism of murine skin and an opportunistic pathogen, which was present in 160 of 172 (93%) of specimens from occupied IVC racks and the interior surfaces of in-use AHU. Neither mechanized washing nor hand-sanitizing eradicated S. xylosus from equipment airway interiors, with 17% to 24% of specimens remaining PCR-positive for S. xylosus. ‘Static–open’ VHP exposure of sanitized equipment did not ensure its sterilization. In contrast, ‘active–closed’ VHP exposure, in which IVC racks were assembled, sealed, and connected to AHU set to the VHP cycle, increased the proportion of chemical indicators that detected sterilizing levels of VHP inside the assembled equipment, and significantly decreased PCR-detectable S. xylosus inside the equipment. Supplementing bulk steam sterilization of the primary enclosure with VHP sterilization of the secondary housing equipment during room change-outs may help to mitigate opportunistic agents that jeopardize studies involving immunodeficient strains.
Abbreviations: AHU, air handling unit; CI, chemical indicator; BI, biological indicator; VHP, vaporized hydrogen peroxide
Given that immunodeficient mice comprise an ever-growing proportion of murine inventories, the list of agents to be excluded from, monitored and tolerated, or not tolerated in murine facilities has expanded well beyond established pathogens (that is, agents that cause disease in immunocompetent hosts) to include opportunistic microorganisms, including members of the skin and intestinal microbiome.7,8,22,24 For example, Enterococcus spp. and Klebsiella oxytoca, members of the murine gut microbiome, can cause nephritis,8 and Pasteurella pneumotropica, a prevalent opportunist,6 can lead to pleuropneumonia or bronchopneumonia22 in aged NOD/LtSz-scid/IL2Rγnull (NSG) mice. Corynebacterium bovis can cause hyperkeratotic acanthotic dermatitis in nude mice,7 contribute to skin disease in haired Pkrdcscid mice23 and hairless immunocompetent SKH1-Hrhr mice,9 and become disseminated by airborne and fomite transmission resulting in resilient facility contamination.5,16,17 Staphylococcus xylosus, a commensal organism of murine skin, can cause dermatitis1,4,21,26 and abscess formation in some immunodeficient strains.10 To avoid obtaining invalid data from studies involving immunodeficient strains because of infection by opportunistic bacteria in a murine facility that is otherwise SPF and free of viral antibodies, we now test the exhaust interiors of secondary housing equipment. To decrease the prevalence of opportunistic bacteria, we have developed a method for sterilizing housing rooms and secondary housing equipment by using vaporized hydrogen peroxide (VHP). Mechanized washing and a 180 °F final rinse of IVC racks is insufficient to remove these organisms.16 In addition, although washing followed by autoclaving effectively eliminates some opportunistic bacterial pathogens from particular IVC rack models,16 various components of IVC racks, the electronic air-handling unit (AHU), and the plastic and rubber hose connections of some systems cannot be steam-sterilized. The use of VHP decontamination to eliminate opportunistic pathogens in IVC racks, AHU, and other secondary housing equipment (for example, connecting hoses) has not been documented previously.
Although Pasteurella pneumotropica and Helicobacter spp. are commonly reported opportunistic bacteria in murine facilities,6 we exclude Helicobacter spp. from our facility and report here that PCR analysis frequently detects S. xylosus in the airway interiors of soiled secondary housing equipment. S. xylosus is a commensal of murine skin and a potential opportunistic pathogen in some immunodeficient strains.1,4,10,21 Reasoning that S. xylosus is not routinely monitored or excluded from murine research facilities or from the murine production colonies of commercial vendors, we document here the broad, facility-wide prevalence of S. xylosus on mice and its presence with murine dander as a contaminant inside IVC exhaust plenums, connecting hoses, and AHU prefilter chambers. In light of its widespread prevalence, we also evaluated the extent to which PCR evidence of S. xylosus can be used as an indication of sterilization of secondary housing equipment and whether PCR-positivity might diminish as soiled IVC racks and AHU are washed or hand-sanitized and then exposed to VHP. Our results demonstrate that S. xylosus PCR monitoring revealed the facility-wide prevalence of S. xylosus, and contributed to the validation of sterilization of secondary housing equipment by VHP.
VHP can be used to safely decontaminate an empty animal housing room; an isolated, actively blowing AHU in the same room;14 or a biocontainment facility.13 In addition, VHP decontamination is routinely used in topographically complex human clinical settings in the presence of electronic and computer-assisted patient diagnostic, monitoring, and support equipment to mitigate the environmental prevalence of agents responsible for nosocomial infections, including methicillin-resistant Staphylococcus aureus, vancomycin-resistant Enterococcus spp., norovirus, Acinetobacter spp., and Clostridium difficile.2,15,18-20,25 This established use in complex clinical settings and the threat of study invalidation due to infections by opportunistic agents5,7,16,17 that prompted our present evaluation to determine whether VHP can be used to decontaminate murine IVC racks and electronic AHU, thereby eliminating a broadly prevalent commensal and opportunistic agent while permitting the efficient and safe return of mice to the VHP-decontaminated equipment and housing room.
VHP involves the conversion of liquid, concentrated 35% hydrogen peroxide (H2O2) to a gaseous phase by flash vaporization.3,11,12 As reference, 1% liquid H2O2 is an approved direct food applicant, 3% liquid H2O2 is used as a topical antiseptic, 6% liquid H2O2 is a hair colorant, and 35% liquid H2O2 is used as a VHP source reagent for surface sterilization. During decontamination, VHP is dispersed in a dry air stream onto all exposed surfaces, including portable equipment, sealed within a room. In its gaseous phase, even low VHP concentrations of 0.1 mg/L (10 ppm) are rapidly, broadly antimicrobial and sporicidal through oxidation, with a 6-log reduction of microbes occurring in minutes at 150 to 400 ppm, after which VHP degrades to water and oxygen, with negligible contribution to sealed room atmospheric oxygen and no condensation, because component concentrations are held below saturation levels in gaseous form throughout the VHP cycle.3,11,12
Compared with other surface sterilants, such as chlorine dioxide and formaldehyde, VHP has a much more favorable safety profile, with a lower required use concentration (that is, 150 to 400 ppm compared with 350 to 1500 ppm and 8000 to 10,000 ppm, respectively), a higher permissible exposure limit (that is, 1 ppm compared with 0.1 ppm and 0.75 ppm, respectively), and a higher threshold defined as immediately dangerous to life or health (that is, 75 ppm compared with 5 ppm and 10 ppm, respectively).3,11,12 In part due to the dry nature of the VHP decontaminating cycle and high material compatibility, VHP is safe on many surfaces, including electronics, metals, plastics, and elastomers, resulting in no substantive change in physical (for example, strength, flexibility) or chemical properties.12,15,20 Variables that can decrease the antimicrobial efficacy of VHP include increased temperature, decreased humidity, and the presence of materials that contribute to VHP decomposition or absorption, including cellulosic material (for example, bedding, cardboard, paper), galvanized steel, and water in the sealed room.3,11,12
VHP generators operate as flow-through systems, with the air stream acting as a carrier to deliver the sterilant gaseous vapor to the decontamination site. Afterward, during the aeration phase, VHP is quickly catalytically degraded from peak decontaminating levels of 150 to 400 ppm to levels safe for personnel reentry (that is, less than 1 ppm) in approximately 1 h;3,11,12 this process is accelerated by using an aeration catalytic accelerator, which is positioned with the VHP generator in the sealed room prior to decontamination. Safe VHP dissipation is assessed by using a handheld H2O2 monitor to ensure that levels are below the long-term exposure limit 8-h time-weighted average of less than 1ppm in the VHP exposed room.
In the present report, to ensure murine safety, we assessed the VHP levels inside hoses connected to the AHU and inside murine primary enclosures docked on the VHP-exposed IVC rack once the VHP levels in the unsealed room were safe for personnel reentry (that is, less than 1 ppm). In this way, we developed an understanding of the potential for residual H2O2 off-gassing by VHP-exposed IVC racks, connecting hoses, and AHU and established an appropriate postVHP exposure interval that ensured the safe return of mice to the treated equipment and housing room. Efficacy of VHP decontamination was assessed by using biological indicators (BI) and chemical indicators (CI) and by using PCR testing of secondary housing equipment for the presence of S. xylosus before and after VHP exposure. Consequently, we here describe the facility-wide prevalence of S. xylosus and a S. xylosus PCR-validated safe and effective ‘active–closed’ VHP exposure method of IVC and AHU sterilization. This active–closed VHP exposure offers murine facilities the opportunity to supplement bulk steam-sterilization of the primary enclosure with VHP sterilization of the secondary supporting equipment during room change-outs, thereby improving the microbial security of studies involving immunodeficient mice.
Materials and Methods
Setting.
Evaluations were conducted in the 29,931 ft2 (net) murine facility of the H Lee Moffitt Cancer Center and Research Institute, Florida's only National Cancer Institute-designated Comprehensive Cancer Center. Occupied in 2005, this facility accommodates, at capacity, 62,496 mice in 15,624 IVC in 19 housing rooms, including 8064 immunodeficient mice in 2016 IVC in 4 isolation rooms separate from housing for immunocompetent strains, and excludes murine norovirus, Helicobacter spp., Syphacia spp., Aspiculuris tetraptera, parainfluenza virus type 1 (Sendai virus), mouse coronavirus (mouse hepatitis virus), Mycoplasma pulmonis, paramyxovirus (pneumonia virus of mice), parvoviruses (minute virus of mice and mouse parvovirus type 1), poliovirus (Theiler murine encephalomyelitis virus), reovirus type 3, Lymphocytic choriomeningitis virus, mouse adenovirus type 1 and 2, poxvirus (Ectromelia), rotavirus (epizootic diarrhea of infant mice virus), papovavirus (K virus), Hantaan virus, cilia-associated respiratory bacillus, Clostridium piliforme (Tyzzer disease), Encephalitozoon cuniculi, and Corynebacterium bovis.
Housing rooms wherein safety and efficacy evaluations were made were either 95 or 105 m3 in volume. Diffuser grills of building HVAC supply or exhaust air were covered, thimble connections for AHU exhaust were sealed, and the VHP generator and aeration catalytic accelerator were positioned in the room, sealed inside, and controlled from outside the room by using an electronic console.
IVC racks and AHU.
IVC racks (Blueline, Tecniplast, Buguggiate, Italy) were used in a double-sided rack configuration, with each rack holding 126 IVC (that is, 63 cages per rack per side). Each pair of racks was ventilated and exhausted by a separate AHU. As described later, 4 models of AHU (SlimLine, TouchSlim, Touchslim-Plus, and SmartFlow; Tecniplast) were assessed in a static–open’ setting. Each AHU model is a separate, mobile, low-energy-consuming (that is, 0.45 W per cage), HEPA-filtered air supply and exhaust unit. In addition, one of the tested AHU (that is, SmartFlow) offers a programmable VHP decontaminating cycle, which operates AHU fans at a slower airspeed to ensure sufficient VHP contact time of interior duct and prefilter chamber surfaces. Therefore, only the fourth-generation SmartFlow AHU was assessed in the active–closed configuration, as detailed later.
IVC racks and plenums were washed in a mechanized rack washer (Basil 9500 model, Steris Lifesciences, Mentor, OH) ensuring 180 °F final rinse temperature. Connecting hoses were hand-washed in peroxyacetic–hydrogen peroxide solution (6 mL per gal.; Decon-Spore 200 Plus, Veltek Associates, Malvern, PA). The exterior and accessible interior surfaces, prefilter chambers, and the hose connecting ports of electronic AHU were hand-sanitized (Oxivir Tb, Diversey, Sturtevant, WI), an accelerated hydrogen peroxide formulation typically used in healthcare facilities2 as a broad-spectrum virucide, bactericide, tuberculocide, and fungicide surface cleaner. This product disinfects in 1 min, kills methicillin-i.e., resistant Staphylococcus aureus and norovirus, meets bloodborne pathogen standards for blood and body fluid decontamination, and is tuberculocidal in 5 min. All mechanically washed IVC racks, hand-sanitized connecting hoses, and AHU were exposed to VHP in an otherwise empty, sealed, housing room.
VHP production and aeration system.
The mobile VHP generator (model Z2, Bioquell, Horsham, PA) that we used converts 35% concentrate liquid H2O2 to VHP, which is delivered into a sealed room during a timed exposure cycle lasting approximately 4.5 h, depending on room volume. At the completion of each VHP cycle, the aeration system (model R-30, Bioquell) aids in the catalytic conversion of VHP to water and oxygen to levels less than 1 ppm, which are safe for reentry of personnel. The VHP exposure cycle consists of 5 phases: conditioning, gassing G1, gassing G2, dwell, and aeration. During the initial conditioning phase, the internal temperature of the VHP generator so that the 35% concentrate liquid H2O2 is flash-vaporized into gaseous VHP. Sensors within the VHP generator stabilize to ambient temperature, and humidity and pumps prime, a process requiring 5 to 10 min, depending on voltage. Once an acceptable internal generator temperature threshold for H2O2 flash vaporization has been achieved, the gassing G1 phase begins with a rapid increase in room VHP concentration, followed by the gassing G2 phase and a further increase in VHP concentration, which results in rapid, broad antimicrobial and sporicidal activity through oxidation. After the G1 and G2 gassing phases, the dwell phase ensures that peak VHP decontaminating concentrations of 200 to 400 ppm are maintained in the sealed room for 20 to 30 min. Aeration begins when the sealed room air is drawn in through the base of the VHP generator unit and is blown from its top, converting VHP to water and oxygen and resulting in a rapid decline in VHP levels, a process accelerated by the use of the aeration catalytic accelerator.
Safety and efficacy of VHP decontamination.
We verified the efficacy of VHP decontamination of IVC and AHU equipment by using BI and CI and by PCR testing of equipment surfaces for S. xylosus. Biological indicators (Bioquell) detect VHP levels capable of a 6-log reduction in microorganismal growth from Geobacillus stearothermophilus spores sealed inside pouches that were incubated in trypticase soy broth at 60 °C for 7 d. Chemical indicators (Bioquell) comprised a semiquantitative visual indication of 2-log, 4-log, 6-log, or greater than 6-log microbial deactivating levels of VHP. Bioquell CI are visual indicator cards which immediately document the effectiveness of the VHP decontamination cycle at its completion. Each CI card has a series of symbols that change from blue toward white when exposed to VHP. After VHP cycle completion, the color of the CI card symbols are compared with preprinted reference colors shown as halos on the card, as a visual indication of the progressive reduction in bioburden caused by the VHP level achieved during the decontamination cycle and detected by the card. This immediate indication of efficacy was confirmed by assessing BI after their 7-d incubation. In addition, CI document the distribution of VHP levels within a room or within assembled IVC and AHU equipment interiors.
VHP decontamination efficacy was evaluated after equipment were used in either a static–open or active–closed setting. For static–open VHP exposures, the electronic AHU was turned off, its hose connections left unsealed, and its prefilters were in place; IVC racks were unassembled, with vertical and horizontal plenums left unsealed. For active–closed VHP exposures, IVC rack plenums were fully assembled and sealed, air supply and exhaust hoses connected IVC racks to AHU, prefilters were in place, and the AHU set to a VHP cycle. Before a VHP cycle was initiated, indicators were placed on the AHU prefilter, in the supply and exhaust plenums of each IVC rack, and throughout the room on the ceiling and walls. We assessed 7 static–open VHP cycles, in which a total of 28 IVC racks and 17 AHU were exposed, for efficacy. We also assessed the efficacy in 5 VHP active–closed cycles, in which a total of 20 IVC racks and 11 AHU were exposed to VHP. To document the VHP levels achieved in the room air, 3 CI and 1 BI were placed in the room during each of the 12 VHP cycles assessed. The safety of VHP decontamination was assessed by using a handheld H2O2 monitor (Drager Safety, Lubeck, Germany), to ensure levels in the room were below the long-term exposure limit 8-h time-weighted average of less than 1 ppm prior to personnel reentry. In addition, we checked the VHP levels inside the hoses connecting the AHU to IVC racks that had been VHP-exposed in an active–closed setting and inside the cages (Blueline 1284L ventilated cages, 14.5 in. × 8.75 in. × 5.25 in., Tecniplast) that were docked on the treated IVC racks after levels in the room were less than 1 ppm.
PCR surveillance for S. xylosus.
Sterile swabs (FLOQ, Copan Flock Technologies, Brescia, Italy) were used to collect specimens from soiled, washed, or VHP-exposed equipment surfaces by tracing a circular pattern for 3 circumferences as the swab tip was rolled. To determine the facility prevalence of S. xylosus, 34 AHU and 61 IVC racks (that is, all of the secondary housing support equipment in active use in the facility) were surveyed over a 7-mo period. In addition, feces from mice housed on the IVC racks were collected and assessed for PCR evidence of S. xylosus. Murine feces and swab samples of IVC rack plenums, AHU exhaust prefilter chambers, connecting hoses, and other secondary housing equipment surfaces were submitted (IDEXX BioResearch, Columbia, MO) for S. xylosus real-time PCR testing, an assay that targets a region of the 16S rRNA gene that is conserved among all S. xylosus genomic sequences deposited in GenBank, by using a FAM–TAMRA-labeled hydrolysis probe. We chose this PCR assay to ensure sufficient DNA recovery and the absence of PCR inhibitors in extracted nucleic acids. Real-time PCR analysis was performed by using standard primer and probe concentrations and a mastermix (LC480 ProbesMaster, Roche Applied Science, Indianapolis, IN) on a real-time PCR platform (LightCycler 480, Roche). The copy number estimate of S. xylosus DNA for each PCR test was calculated by plotting the real-time crossing point values from the S. xylosus PCR assays on a standard curve of log-fold dilutions of a positive control containing a known copy number.
Statistics.
An unconditional exact test using R 3.4 statistical software (R Foundation for Statistical Computing, Vienna, Austria) was performed to determine the proportion of CI detecting a VHP level capable of a greater than 6-log reduction in bioburden in equipment exposed in either the static–open compared with active–closed setting. We also used an unconditional exact test to determine the proportion of S. xylosus-positive PCR samples obtained from VHP-exposed equipment in either the static–open compared with active–closed setting. A P value less than 0.05 was considered to define a statistically significant difference.
Results
We assessed efficacy in 7 static–open VHP cycles during which 28 IVC racks and 17 AHU were treated and in 5 active–closed cycles involving 20 IVC racks and 11 AHU (Figure 1). VHP cycles routinely achieved required concentrations of greater than 150 ppm during exposure of secondary housing equipment, with peak VHP levels (mean ± 1 SD) of 312.0 ± 26.4 ppm throughout the 20-min dwell phase. Indicators were placed in the room and in the airways of equipment to be decontaminated, to enable us to assess the efficacy of each VHP cycle (Figure 2). The total time required for completion of a VHP cycle was approximately 4.5 h, depending on room volume. VHP cycles did not substantively alter the room's ambient relative humidity, which was 47.9% ± 4.9% at the initiation of the VHP cycle and 51.9% ± 5.2% at its completion, or ambient temperature, which was 25.2 ± 1.1 °C at the initiation of the VHP cycle and 26.1 ± 0.4 °C at its completion (Table 1). When the electronic console outside the room indicated that the ambient VHP level inside the sealed room was less than 1 ppm, the door was unsealed and VHP levels were reassessed by using a handheld H2O2 monitor. VHP levels throughout the exposed room varied from 2 to 13 ppm, with a mean of 2.0 ± 0.2 ppm detected at the unsealed door's threshold (Figure 3). Consequently, we allowed additional time for VHP dissipation after the console indicated a VHP level of less than 1 ppm inside the room prior to room reentry, with personnel entering when the handheld H2O2 monitor also indicated levels as less than 1 ppm at the door's threshold (typically 90 min after VHP cycle completion).
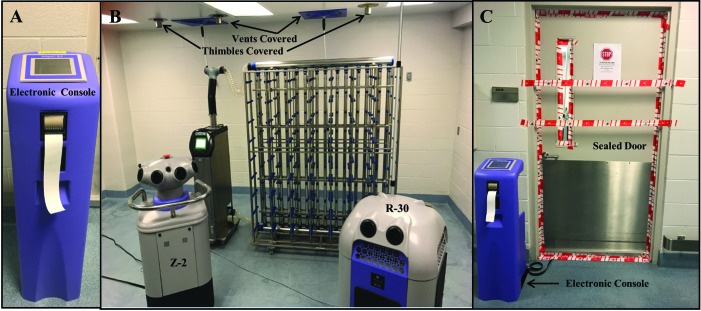
Figure 1.
Room preparation prior to VHP exposure. Room volume was calculated and programmed via the (A) electronic console outside of the room which controlled the (B) VHP generator and aeration catalytic accelerator, which were positioned inside the room with housing equipment to be decontaminated. HVAC supply and exhaust vents and ceiling thimble connections were covered and the (C) room door sealed with tape.
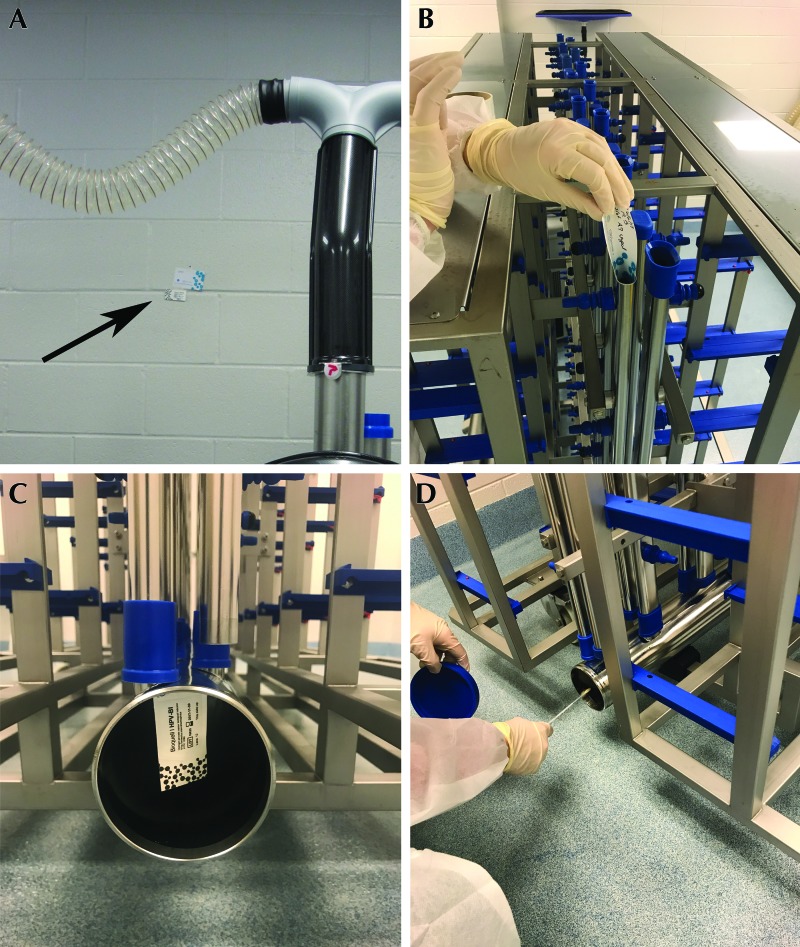
Figure 2.
To assess the efficacy of each VHP cycle, indicators were placed in the room before VHP exposure, including (A) CI (arrow) on the room wall, and inside equipment airways, including (B) CI inside vertical manifolds and (C) BI inside exhaust plenums of IVC racks. In addition, specimens were collected (D) from soiled, washed, or VHP-exposed equipment surfaces and then PCR-analyzed for the presence of S. xylosus.
Table 1.
Phases of VHP cycles
| Phase (duration) |
||||||
| Trial | Conditioning (12 min) | G1 gassing (12 to 25 min) | G2 gassing (2 h 15 min) | Dwell (20 min) | Aeration (25 to 45 min) | Completion (30 to 45 min) |
| Vaporizer temperature (°C) | ||||||
| 1 | 30.0 | 119.7 | 120.2 | 120.3 | 123.7 | 28.5 |
| 2 | 30.3 | 119.6 | 120.1 | 120.1 | 122.7 | 26.4 |
| 3 | 26.3 | 119.7 | 119.9 | 120.2 | 121.3 | 27.5 |
| 4 | 23.4 | 119.7 | 120.2 | 117.1 | 122.0 | 28.0 |
| Mean | 27.5 | 119.7 | 120.1 | 119.4 | 122.4 | 27.6 |
| VHP concentration (ppm) | ||||||
| 1 | 148 | 280 | 212 | 10 | ||
| 2 | 199 | 343 | 248 | 10 | ||
| 3 | 126 | 304 | 219 | 10 | ||
| 4 | 127 | 319 | 207 | 10 | ||
| Mean | 151 | 315 | 315 | 10 | ||
| Airflow (m3/h) | ||||||
| 1 | 17.7 | 43.7 | 42.6 | 42.8 | 44.4 | 49.3 |
| 2 | 17.8 | 43.1 | 41.7 | 41.9 | 42.9 | 48.2 |
| 3 | 16.4 | 44.2 | 43.4 | 44.0 | 44.5 | 46.1 |
| 4 | 16.4 | 44.5 | 43.6 | 43.6 | 45.4 | 46.2 |
| Mean | 17.1 | 43.9 | 42.8 | 43.1 | 44.3 | 47.5 |
| Relative humidity (%) | ||||||
| 1 | 46.7 | 50.2 | 72.7 | 87.2 | 79.4 | 49.1 |
| 2 | 41.6 | 46.3 | 75.0 | 93.5 | 84.2 | 46.6 |
| 3 | 50.4 | 52.7 | 75.0 | 95.8 | 86.1 | 53.4 |
| 4 | 52.8 | 53.2 | 75.0 | 99.4 | 89.4 | 58.4 |
| Mean | 47.9 | 50.6 | 74.4 | 94.0 | 84.8 | 51.9 |
| Ambient temperature (°C) | ||||||
| 1 | 25.6 | 23.9 | 24.5 | 25.3 | 25.2 | 26.4 |
| 2 | 26.6 | 24.1 | 24.6 | 25.2 | 25.0 | 26.4 |
| 3 | 24.5 | 24.0 | 24.7 | 24.5 | 25.2 | 25.6 |
| 4 | 24.1 | 23.8 | 24.2 | 25.3 | 25.1 | 25.8 |
| Mean | 25.2 | 24.0 | 24.5 | 25.1 | 25.1 | 26.1 |
As cycle phases completed, the vaporizer temperature emitted increased from the beginning of gassing until the end of aeration. The initial conditioning phase increases the temperature of the VHP vaporizer. Once optimal temperature is generated, the first gassing (G1 phase) is initiated followed by an increase of hydrogen peroxide vapor which is then detected in parts per million (ppm). Mean peak VHP concentration occurred during the dwell phase. Relative humidity and ambient temperature increased and decreased relative to cycle phases.
Figure 3.
Completion of VHP exposure was assessed first from outside the room by using (A) the electronic console, ensuring that it reported less than 1 ppm inside the sealed room and then after unsealing the door (B) leading into the room containing equipment after VHP exposure and assessing H2O2 levels at (C) the door threshold and (D) various locations throughout the room by using a handheld H2O2 monitor.
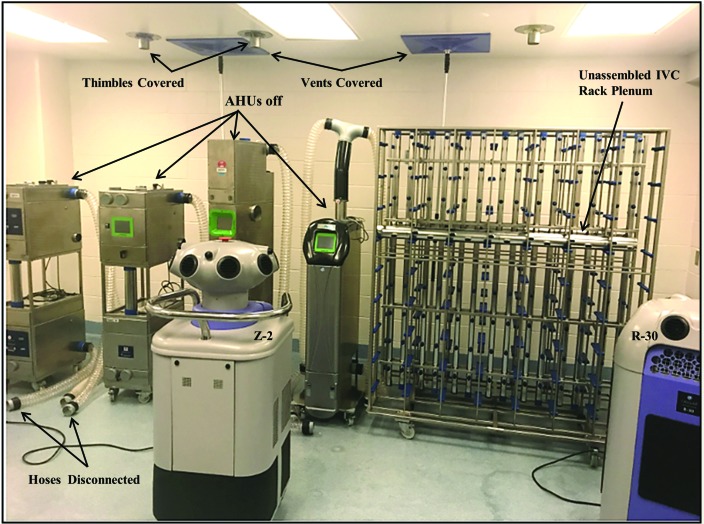
To determine whether the most time-efficient process of equipment VHP sterilization could be used and relied on to eliminate opportunistic bacteria from interior and exterior surfaces of secondary housing equipment, VHP exposure of washed equipment was first assessed with equipment in a static–open setting, with the AHU off, hose connections open and unsealed, and IVC racks unassembled and with vertical and horizontal plenums unsealed at one end (Figure 4). This process was tested first to determine whether VHP effectively decontaminated the surfaces of plenums, hoses, and prefilter chambers immediately after washing or hand-sanitizing, without reassembly. All 21 CI placed inside the sealed room documented that the achieved VHP levels were capable of inducing at least a 6-log reduction in microbial counts and all 7 BI in the room documented growth inhibition of G. stearothermophilus spores. In addition, all 4 CI and all 3 BI placed inside the open end of hoses, plenums, or manifolds of IVC racks and in an AHU prefilter chamber left open similarly detected sterilizing levels of VHP. However, in marked contrast, only 1 of 26 (4%) of the indicators placed deep inside plenums or manifolds near the capped end or inside an AHU prefilter chamber left closed detected sterilizing levels of VHP (Table 2). Of the indicators placed inside the static–open plenums, manifolds, hoses, and AHU prefilter chambers, only 5 of 22 CI (23%) recorded at least 6-log microbial reduction VHP levels, and only 3 of 11 BI (27%) demonstrated growth inhibition of G. stearothermophilus spores.
Figure 4.
Static–open VHP exposure, with ceiling thimble connections and HVAC supply and exhaust vents covered, all AHU off, hoses left disconnected, and the IVC rack left unassembled and unconnected to an AHU.
Table 2.
PCR detection of S. xylosusand results of chemical indicators (CI) and biological indicators (BI) during static–open compared with active–closed VHP exposure
| Static–open VHP exposure (7 cycles, involving a total of 28 IVC racks and 17 AHU) | |||
| S xylosus PCR analysis of processing equipment | Soiled | Washed | VHP exposed |
| No. positive/no. tested (%) | 23/31 (94%) | 6/25 (24%) | 17/48 (35%)# |
| No. of CI with ≥6 log kill/ no. tested | No. of BI with no growth/ no. tested | ||
| Room VHP indicators | 21/21 (100%) | 7/7 (100%) | |
| Equipment VHP indicators (open end)a | 4/4 (100%) | 3/3 (100%) | |
| Equipment VHP Indicators (closed end)b | 1/18 (6%) | 0/8 (0%) | |
| Active–closed VHP exposure (5 cycles, involving 20 IVC racks and 11 AHU) | |||
| S xylosus PCR analysis of processing equipment | Soiled | Washed | VHP exposed |
| No. positive/no. tested (%) | 10/10 (100%) | 2/12 (17%) | 0/12 (0%)d |
| No. of CI with ≥6 log kill/ no. tested | No. of BI with no growth/ no. tested | ||
| Room VHP indicators | 15/15 (100%) | 5/5 (100%) | |
| Equipment VHP indicators (sealed)c | 18/18 (100%) | 3/4 (75%) | |
Indicators placed inside an unsealed hose, or the plenum or manifold's open, uncapped end, or the AHU exhaust prefilter chamber left open.
Indicators were placed inside the plenum or manifold's capped end, or the AHU exhaust prefilter chamber left closed.
Indicators were placed inside the sealed and connected plenum, manifold, or AHU exhaust prefilter chamber, with the AHU set to the VHP cycle.
P = 0.008
To determine whether S. xylosus, a commensal organism of the murine skin and potential opportunistic pathogen in some immunodeficient strains, is shed from the skin of healthy mice and detectable by PCR analysis of murine feces or inside IVC exhaust plenums, connecting hoses, or AHU prefilter chambers, we evaluated murine feces and environmental specimens collected over 7 mo from all of the secondary housing equipment that was in active use, specifically from 34 AHU and 61 IVC racks. Environmental PCR surveillance for S. xylosus inside equipment revealed that 160 of 172 specimens (93%) from soiled IVC components and AHU exhaust prefilter chambers were PCR-positive for S. xylosus, at a mean copy number of 3135 ± 9270. S. xylosus was detected by PCR analysis in 66 of 98 of murine fecal specimens (67%), at a mean copy number of 2292 ± 8731.
In light of this marked murine and environmental prevalence of S. xylosus inside IVC and the airways of secondary housing equipment, we assessed the efficacy of VHP sterilization of equipment by PCR monitoring for S. xylosus as an indicator species when equipment was soiled, after it had been washed (that is, IVC racks) or hand-sanitized (that is, AHU, connecting hoses), and after it had been VHP-exposed through the static–open method. Consequently, 23 of 31 (94%) specimens obtained from soiled equipment were PCR-positive for S. xylosus, 6 of 25 (24%) samples were S. xylosus PCR-positive after washing or hand-sanitizing, and 17 of 48 (35%) specimens were S. xylosus PCR-positive after static–open VHP exposure.
Some models of AHU (for example, SmartFlow) offer a VHP decontaminating cycle, which operates AHU fans at a slower airspeed to ensure sufficient VHP contact time of interior components and surfaces. We therefore used the VHP-programmable AHU to perform subsequent VHP exposures in the active–closed configuration, in which the IVC rack plenums were fully assembled and sealed, air supply and exhaust hoses were connected IVC racks to AHU, prefilters were in place, and the AHU was set to the VHP cycle (Figure 5). The AHU discharged into the room. All 15 CI placed inside the sealed room documented that VHP levels capable of at least 6-log microbial reduction were achieved, and all 5 BI confirmed growth inhibition of G. stearothermophilus spores. In addition, all 18 CI placed inside the active–closed plenums, manifolds, hoses, and AHU prefilter chambers recorded 6-log or greater microbial reduction, and 3 of the 4 BI produced no growth of G. stearothermophilus spores after 7 d of incubation. The BI that yielded positive growth of G. stearothermophilus had been placed inside the last vertical manifold, furthest from the air supply. Furthermore, all 10 samples from soiled IVC components were PCR-positive for S. xylosus, 2 of 12 (17%) remained S. xylosus PCR-positive after washing or hand-sanitizing, and 0 of 12 were S. xylosus PCR-positive after active–closed VHP exposure. The proportion of CI that confirmed 6-log reduction in bioburden after equipment was VHP-exposed in the active–closed setting (that is, 18 of 18 [100%]) was significantly (P = 0.036) greater than that (that is, 5 of 22 [23%]) after static–open VHP exposure. Similarly, the proportion of S. xylosus PCR-positive specimens obtained from equipment that was VHP-exposed in the active–closed setting (that is, 0 of 12 [0%]) was significantly lower(P = 0.008) than that (that is, 17 of 48 [35%]) after static–open VHP exposure.
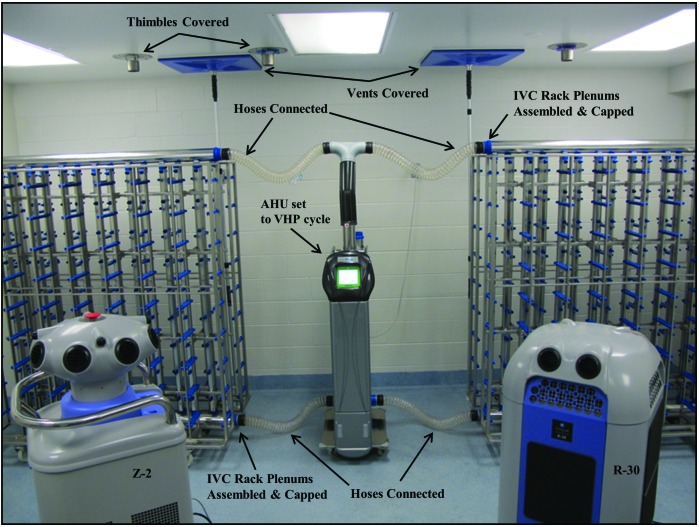
Figure 5.
Active–closed VHP exposure, with ceiling thimble connections and HVAC supply and exhaust vents covered, the IVC rack fully assembled, plenums capped, and hoses connecting each IVC rack to an AHU set to the VHP cycle.
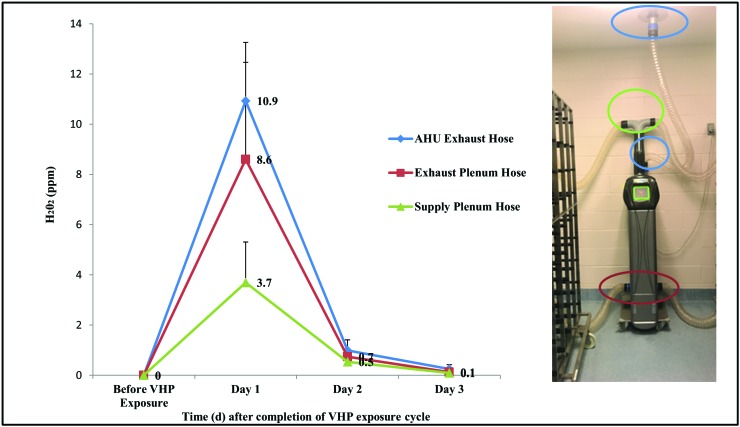
To establish an appropriate post exposure interval after which mice could be returned safely to equipment that had been decontaminated through active–closed VHP exposure, we used a handheld H2O2 monitor after H2O2 levels inside the room were less than 1 ppm. To facilitate VHP dissipation, house HVAC diffusers and thimble connections were uncovered, and the AHU was hose-connected to a ceiling thimble exhaust and switched from the VHP cycle to a standard cycle of 75 air changes hourly. Approximately 2.5 h after VHP cycle completion, mean VHP levels inside hoses were still modestly elevated, at a mean VHP level of 3.7 ± 1.6 ppm inside the IVC supply plenum hose, 8.6 ± 3.9 ppm inside the IVC exhaust plenum hose, and 10.9 ± 2.3 ppm inside the AHU exhaust hose that was thimble-connected (Figure 6). VHP levels were then sampled over the course of 3 d until minimally detectable within the connected hosing. By day 2, approximately 18 to 20 h after VHP cycle completion, mean VHP levels were 0.7 ± 0.3 ppm inside the IVC supply plenum hose, 1.0 ± 0.2 ppm inside the IVC exhaust plenum hose, and 1.5 ± 0.1 ppm inside the AHU exhaust hose.
Figure 6.
VHP levels detected inside hoses that connected the AHU to either the IVC rack supply (green) or exhaust (red) plenum or to the ceiling thimble (blue) before and after active–closed VHP exposure.
To determine whether VHP levels accumulate within a recently docked unit added to the IVC rack after active–closed VHP exposure, we placed assembled units containing bedding and a cotton square into docking locations 1, 4, and 7 on the top, 4th, and 9th rows of the IVC rack and measured VHP levels inside each box. The handheld H2O2 monitor was placed inside, in the middle of the microisolation, during each assessment. VHP levels did not differ substantively between docking locations. VHP levels were elevated 90 min after completion of an active–closed VHP cycle, at a mean level of 2.0 ± 0.5 ppm inside the bedded box. VHP levels had safely dissipated approximately 18 h after VHP cycle completion to a mean of 0.3 ± 0.1 ppm, which is below the long-term exposure limit 8-h time-weighted average of less than 1 ppm. We then measured the VHP levels in empty boxes after active–closed exposure; mean VHP levels of 0.9 ± 0.2 ppm were detected approximately 5 h after VHP cycle completion. Given these findings regarding residual H2O2 off-gassing by IVC and AHU equipment after active–closed VHP exposure, we returned the mice to the decontaminated equipment and housing rooms only after sufficient aeration, which is more than 18 h after completion of the VHP cycle completion, or the following morning.
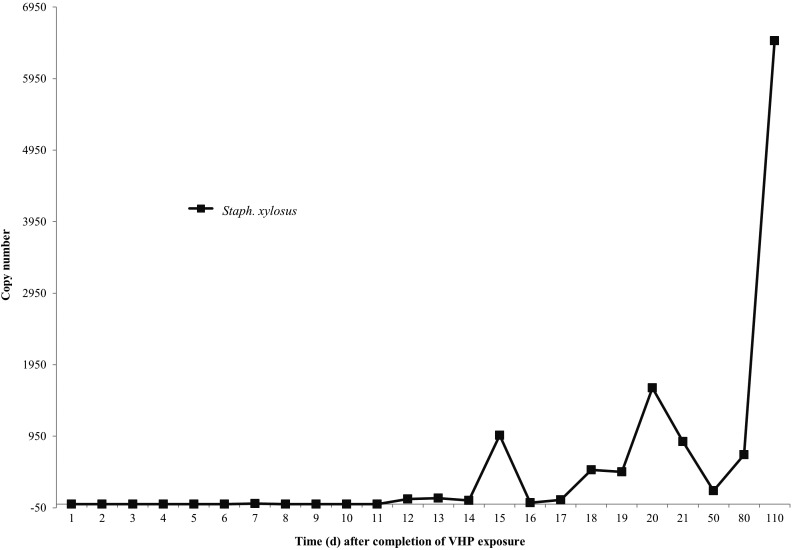
To determine when S. xylosus is reliably PCR-detectable in the IVC exhaust plenum after mice are returned to active–closed VHP-sterilized equipment, we pooled fecal specimens from 68 cages of immunodeficient mice, including NOD.Cg-PrkdcscidIl2rgtm1Wjl/SzJ (NSG), NOD.Cg-PrkdcscidIl2rgtm1WjlTg(CMV-IL3,CSF2,KITLG)1Eav/MloySzJ (NSGS), NOD.CB17-Prkdcscid/J (SCID), and NU/J (nude) strains; the cages occupied 54% of the docking slots on the IVC rack. Specimens were collected daily from the IVC exhaust plenum and PCR-tested for S. xylosus. Overall 7 of the 10 pooled murine fecal specimens (70%) were PCR-positive for S. xylosus at a mean copy number of 5497 ± 14,382. S. xylosus was not detected in the IVC exhaust plenum of the rack housing these mice on days 1 through 6; specimens became PCR-detectable (copy number, 8) on day 7 after VHP exposure and was routinely PCR-detectable daily on days 12 through 21 after active–closed VHP exposure, with a mean copy number of 468 ± 537, and remained positive during the 7-mo PCR surveillance period at a mean copy number of 2453 ± 3496 (Figure 7).
Figure 7.
Mean quantitative PCR copy numbers of S.xylosus detected in the exhaust plenum following VHP active–closed exposure and the return of murine inventory.
Discussion
Here we describe a safe and effective method of VHP sterilization of murine IVC racks, AHU, and other secondary housing equipment; we validated the method's efficacy by demonstrating the eradication of S. xylosus, a commensal organism of murine skin and an opportunistic pathogen.1,4,10,21,26 Although VHP decontamination of complex clinical settings is a safe and effective means of mitigating the prevalence of nosocomial infectious agents,15,18-20,25 our current report demonstrates that VHP is also applicable to the topographically complex research animal setting, where it can be used to sterilize murine IVC racks, electronic AHU, and other secondary housing equipment and permits the efficient and safe return of mice to the decontaminated equipment within 20 h of VHP cycle completion.
We tested 2 methods of equipment VHP exposure. We first tested a static–open VHP exposure procedure, which we evaluated largely because of its efficiency, given that by using this method a group of IVC racks can be VHP decontaminated in bulk, unassembled, and without any associated AHU. However, although rapid, the static–open VHP exposure method proved ineffective, with 77% of CI placed inside the plenums, hoses, and AHU prefilter chambers indicating a less than 6-log microbial reduction and 73% of BI yielding growth of G. stearothermophilus spores. This absence of reliable VHP sterilization of IVC and AHU airway interior surfaces was supported by PCR surveillance for S. xylosus, in which 35% of specimens collected from equipment airway interiors remained S. xylosus PCR-positive after static–open VHP exposure. Although at least one end of plenums, manifolds, hoses, and prefilter chambers were left unsealed during static–open VHP exposure, the depth and topographical complexity of these airways apparently prevented sufficient penetration of VHP to achieve sterility.
As a result, our facility invested in additional IVC racks and AHU with a VHP programmable cycle. In addition, during subsequent VHP exposures, the secondary housing equipment was fully assembled, sealed, and set to the VHP cycle. This active–closed VHP protocol routinely achieved sterilizing levels of VHP, with all CIs placed inside sealed equipment airways recording VHP levels capable of a 6-log microbial reduction and most BI (that is, 3 of 4 [75%]) showing growth inhibition of G. stearothermophilus spores. The sole BI that permitted spore growth had been placed in the most remote interior sealed corner of the IVC exhaust manifold. This encouraging result was supported by the absence of PCR-detectable S. xylosus on the interior surfaces of IVC and AHU airway interiors after active–closed VHP exposure, despite detection of the organism in 17% of specimens collected from IVC plenum surfaces after mechanized washing with 180 °F final rinse. The active–closed assembly ensured that VHP was effectively drawn through the AHU chambers and IVC manifolds and plenums, because the proportion of CI in airway interiors that detected effective VHP levels was greater (P = 0.036) for the active–closed protocol than for the static–open procedure. In addition, the proportion of S. xylosus PCR-positive specimens obtained in the active–closed setting was significantly (P = 0.008) decreased compared with that from the static–open configuration.
Although personnel could safely reenter the unsealed VHP-exposed room approximately 90 min after VHP levels at the room threshold were less than 1ppm, we found that residual H2O2 off-gassing by the active–closed VHP-exposed equipment delayed the return of mice to the decontaminated equipment until approximately 20 h later, or the next morning. We have found that this delay does not impede routines but contributes to an efficient room change-out process, which occurs every 6 mo, with VHP decontamination of the housing room and all of its IVC racks, AHU, and changing stations while the displaced mouse populations are housed in autoclaved IVC microisolation units left static’ for approximately 24 h in a neighboring room.
Here we also determined that S. xylosus is PCR-detectable in 67% of murine fecal specimens and 93% of specimens from secondary housing equipment in active use. By monitoring the prevalence of this skin commensal, the presence of which is tolerated by commercial vendors and in many murine facilities, we were able to validate the VHP sterilization of secondary housing equipment and not rely solely on BI and CI as indirect indicators of effective equipment sterilization. These methods are especially relevant when developing effective decontamination routines to mitigate opportunistic agents that compromise research and that are not tolerated, such as C. bovis, which can broadly contaminate murine facilities and equipment and remains resilient to traditional decontamination efforts.5,7,16,17 Documenting the prevalence of S. xylosus and demonstrating that it repopulates the interior surfaces of IVC exhaust plenums in approximately 7 d after mice are returned to the VHP-exposed equipment contributes to the appropriate interpretation of murine health surveillance results detecting this agent. In addition, our findings identify S. xylosus as a useful indicator species for monitoring secondary housing equipment VHP sterilization routines. We are in the process of extending these findings and assessing the prevalence of a broad range of opportunistic bacteria in our murine facility. Furthermore, we are testing whether this S. xylosus PCR-validated active–closed VHP exposure protocol mitigates other opportunistic agents detected in IVC rack exhaust airways that can compromise research (for example, P. pneumotropica, C. bovis). Having developed an effective VHP sterilizing routine of secondary housing equipment, we are extending these findings by testing the applicability of VHP decontaminating exposure in common procedural and imaging areas frequented by immunodeficient mouse strains. Immunodeficient mouse strains comprise a large portion of murine inventories, and the agents needing to be monitored and excluded from murine facilities have increased to include opportunistic microorganisms and members of the microbiome. Consequently, an improved method of secondary housing equipment sterilization using VHP may help prevent the invalidation of studies involving immunodeficient mice. Here we describe a S. xylosus PCR-validated safe and effective active–closed VHP exposure method for sterilization of IVC and AHU. With this method, murine facilities are now able to supplement bulk steam-sterilization of the primary enclosure by using VHP sterilization of the secondary supporting equipment during room change-outs, thereby contributing to improved microbial security of studies involving immunodeficient mice, because their likelihood of exposure to opportunistic agents residually present in washed IVC plenums is decreased.
Acknowledgments
We acknowledge the animal care staff of the Division of Comparative Medicine (H Lee Moffitt Cancer Center and Research Institute, University of South Florida) for their contribution to this study. We also thank Dr Robert Livingston for his assistance.
References
- 1.Acuff NV, LaGatta M, Nagy T, Watford WT. 2017. Severe dermatitis associated with spontaneous Staphylococcus xylosus infection in Rag−/−Tpl2−/− mice. Comp Med 67:344–349. [PMC free article] [PubMed] [Google Scholar]
- 2.Alfa MJ, Lo E, Wald A, Dueck C, DeGagne P, Harding GK. 2010. Improved eradication of Clostridium difficile spores from toilets of hospitalized patients using an accelerated hydrogen peroxide as the cleaning agent. BMC Infect Dis 10:268–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bier ME. 1987. Method of vaporizing multicomponent liquids. US Patent no. RE.4642165 [Cited 15 June 2017]. Available at: http://patft.uspto.gov/netacgi/nph-Parser?Sect1=PTO1&Sect2=HITOFF&d=PALL&p= 1&u=/netahtml/PTO/srchnum.htm&r=1&f=G&l=50&s1=4642165.PN.&OS=PN/4642165&RS=PN/4642165
- 4.Bradfield JF, Wagner JE, Boivin GP, Steffen EK, Russell RJ. 1993. Epizootic fatal dermatitis in athymic nude mice due to Staphylococcus xylosus. Lab Anim Sci 43:111–113. [PubMed] [Google Scholar]
- 5.Burr HN, Wolf FR, Lipman NS. 2012. Corynebacterium bovis: epizootiologic features and environmental contamination in an enzootically infected rodent room. J Am Assoc Lab Anim Sci 51:189–198. [PMC free article] [PubMed] [Google Scholar]
- 6.Carty AJ. 2008. Opportunistic infections of mice and rats: Jacoby and Lindsey revisited. ILAR J 49:272–276. [DOI] [PubMed] [Google Scholar]
- 7.Clifford CB, Walton BJ, Reed TH, Coyle MB, White WJ, Amyx HL. 1995. Hyperkeratosis in athymic nude mice caused by a coryneform bacterium: microbiology, transmission, clinical signs, and pathology. Lab Anim Sci 45:131–139. [PubMed] [Google Scholar]
- 8.Foreman O, Kavirayani AM, Griffey SM, Reader R, Shultz LD. 2010. Opportunistic bacterial infections in breeding colonies of the NSG mouse strain. Vet Pathol 48:495–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gobbi A, Crippa L, Scanziani E. 1999. Corynebacterium bovis infection in immunocompetent hirsute mice. Lab Anim Sci 49:209–211. [PubMed] [Google Scholar]
- 10.Gozalo AS, Hoffman VJ, Brinster LR, Elkins WR, Ding L, Holland SM. 2010. Spontaneous Staphylococcus xylosus infection in mice deficient in NADPH oxidase and comparison with other laboratory mouse strains. J Am Assoc Lab Anim Sci 49:480–486. [PMC free article] [PubMed] [Google Scholar]
- 11.Grignol G, Eddington D, Karle D, Rickloff J. 2000. Chemical and biological aspects of hydrogen peroxide gas, Proceedings of the ISPE Barrier Isolation Technology Conference. Arlington, Virginia, 5–6 June 2000. [Cited 15 June 2017]. Available at: http://www.isolationinfo.com/pubs.asp. [Google Scholar]
- 12.Hultman C, Hill A, McDonnell G. 2007. The physical chemistry of decontamination with gaseous hydrogen peroxide. Pharm Engin 27:22–32. [Google Scholar]
- 13.Kaspari O, Lemmer K, Becker S, Lochau P, Howaldt S, Nattermann H, Grunow R. 2014. Decontamination of a BSL3 laboratory by hydrogen peroxide fumigation using 3 different surrogates for Bacillus anthracis spores. J Appl Microbiol 117:1095–1103. [DOI] [PubMed] [Google Scholar]
- 14.Krause J, McDonnell G, Riedesel H. 2001. Biodecontamination of animal rooms and heat-sensitive equipment with vaporized hydrogen peroxide. Contemp Top Lab Anim Sci 40:18–21. [PubMed] [Google Scholar]
- 15.Lemmen S, Scheithauer S, Hafner H, Yezli S, Mohr M, Otter JA. 2015. Evaluation of hydrogen peroxide vapor for the inactivation of nosocomial pathogens on porous and nonporous surfaces. Am J Infect Control 43:82–85. [DOI] [PubMed] [Google Scholar]
- 16.Manuel CA, Pugazhenthi U, Leszczynski JK. 2016. Surveillance of a ventilated rack system for Corynebacterium bovis by sampling exhaust-air manifolds. J Am Assoc Lab Anim Sci 55:58–65. [PMC free article] [PubMed] [Google Scholar]
- 17.Manuel CA, Pugazhenthi U, Spiegel SP, Leszczynski JK. 2017. Detection and elimination of Corynebacterium bovis from barrier rooms by using an environmental sampling surveillance program. J Am Assoc Lab Anim Sci 56:202–209. [PMC free article] [PubMed] [Google Scholar]
- 18.Otter JA, Mepham S, Athan B, Mack D, Smith R, Jacobs M, Hopkins S. 2016. Terminal decontamination of the Royal Free London's high-level isolation unit after a case of Ebola virus disease using hydrogen peroxide vapor. Am J Infect Control 44:233–235. [DOI] [PubMed] [Google Scholar]
- 19.Otter JA, Yezli S, Schouten MA, van Zanten AR, Houmes-Zielman G, Nohlmans-Paulssen M. 2010. Hydrogen peroxide vapor decontamination of an intensive care unit to remove environmental reservoirs of multidrug-resistant gram-negative rods during an outbreak. Am J Infect Control 38:754–756. [DOI] [PubMed] [Google Scholar]
- 20.Otter JA, Cummins M, Ahmad F, van Tonder C, Drabu YJ. 2007. Assessing the biological efficacy and rate of recontamination following hydrogen peroxide vapor decontamination. J Hosp Infect 67:182–188. [DOI] [PubMed] [Google Scholar]
- 21.Russo M, Invernizzi A, Gobbi A, Radaelli E. 2012. Diffuse scaling dermatitis in an athymic mude mouse. Vet Pathol 50:722–726. [DOI] [PubMed] [Google Scholar]
- 22.Santagostino SF, Arbona RJ, Nashat MA, White JR, Monette S. 2017. Pathology of aging in NOD scid γ female mice. Vet Pathol 54:855–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scanziani E, Gobbi A, Crippa L, Giusti AM, Pesenti E, Cavalletti E, Luini M. 1998. Hyperkeratosis-associated coryneform infection in severe combined immunodeficient mice. Lab Anim 32:330–336. [DOI] [PubMed] [Google Scholar]
- 24.Treuting PM, Clifford CB, Sellers RS, Brayton CF. 2012. Of mice and microflora: considerations for genetically engineered mice. Vet Pathol 49:44 –63. [DOI] [PubMed] [Google Scholar]
- 25.Weber DJ, Rutala WA, Miller MB, Huslage K, Sickbert-Bennett E. 2010. Role of hospital surfaces in the transmission of emerging health care-associated pathogens: norovirus, Clostridium difficile, and Acinetobacter species. Am J Infect Control 38 Suppl 1:S25–S33. [DOI] [PubMed] [Google Scholar]
- 26.Won YS, Kwon HJ, Oh GT, Kim BH, Lee CH, Park YH, Hyun BH, Choi YK.2002. Identification of Staphylococcus xylosus isolated from C57BL/6J-Nos2(tm1Lau) mice with dermatitis. Microbiol Immunol 46:629–632. [DOI] [PubMed] [Google Scholar]