Abstract
We evaluated PCR testing of filter tops from cages maintained on an IVC system through which exhaust air is filtered at the cage level as a method for detecting parasite- infected and -infested cages. Cages containing 4 naïve Swiss Webster mice received 360 mL of uncontaminated aspen chip or α-cellulose bedding (n = 18 cages each) and 60 mL of the same type of bedding weekly from each of the following 4 groups of cages housing mice infected or infested with Syphacia obvelata (SO), Aspiculuris tetraptera (AT), Myocoptes musculinus (MC), or Myobia musculi (MB) and Radfordia affinis (RA; 240 mL bedding total). Detection rates were compared at 30, 60, and 90 d after initiating bedding exposure, by using PCR analysis of filter tops (media extract and swabs) and testing of mouse samples (fur swab [direct] PCR testing, fecal flotation, anal tape test, direct examination of intestinal contents, and skin scrape). PCR testing of filter media extract detected 100% of all parasites at 30 d (both bedding types) except for AT (α-cellulose bedding, 67% detection rate); identified more cages with fur mites (MB and MC) than direct PCR when cellulose bedding was used; and was better at detecting parasites than all nonmolecular methods evaluated. PCR analysis of filter media extract was superior to swab and direct PCR for all parasites cumulatively for each bedding type. Direct PCR more effectively detected MC and all parasites combined for aspen chip compared with cellulose bedding. PCR analysis of filter media extract for IVC systems in which exhaust air is filtered at the cage level was shown to be a highly effective environmental testing method.
Abbreviations: AT, Aspiculuris tetraptera; MB, Myobia musculi; MC, Myocoptes musculinus; RA, Radfordia affinis; SO, Syphacia obvelata
Endo- and ectoparasites remain a common contaminant of contemporary laboratory mouse colonies as demonstrated in a 2006 survey, which reported that pinworms and fur mites had been detected by up to 75% and 40%, respectively, of surveyed institutions.10
Syphacia obvelata (SO) and Aspiculuris tetraptera (AT) are the cosmopolitan murine pinworms58 and Myocoptes musculinus (MC), Myobia musculi (MB), and Radfordia affinis (RA) are the fur mites commonly excluded from and monitored in mouse colonies because of their effects on animal health and research, including behavior, gastrointestinal physiology, immunology, reproduction, growth, dermatology, and hematopoiesis.8,22,32,38,39,42,43,48,52,62,64 Furthermore, these infections and infestations may limit efficient sharing of unique mouse lines between institutions.15
First described in the early 1800s,49,53 the endoparasitic murine oxyurid nematodes have direct life cycles and transmit infections by means of ingestion of embryonated (infective) eggs.3,49 SO and AT can be distinguished through their morphologic as well as life-cycle differences, including the location in the host where the adult nematode resides, site of ova deposition, and time required for eggs to embryonate.1-3,5,11-13,28,49,58
The murine acarids from the families Myobidae (MB and RA) and Myocoptidae (MC) are nonburrowing, thermotactic, obligate parasites, spending their entire life cycle on the host. Infestations are transmitted through direct contact. Although these mite species preferentially inhabit different locations in the host, they reside on dorsal anterior regions of the mouse. MC is easy to distinguish microscopically; MB and RA are morphologically similar but can be differentiated due to the presence of a second tarsal claw in RA.64
The reported prevalence of murine pinworm infections and fur mite infestations varies markedly, but their apparent ubiquity and potential for environmental persistence underlie the importance of instituting a biosecurity program using optimal detection methods.6,10,16,49
Traditionally, pinworms have been diagnosed antemortem by using the anal tape test (SO)3,19,27,49,54,58 and fecal concentration (AT) methods.3,47,49,58 Antemortem pinworm detection can be challenging because false-negative results are common when testing is conducted during the prepatent period or because of intermittent egg shedding.9,14,15,47 In commonly used soiled bedding colony health monitoring programs, pinworm detection may be affected by the inability to transmit infections to soiled-bedding sentinels.15,18 Traditional antemortem testing methods are considered less sensitive than postmortem testing methods.15 Fecal concentration techniques, including flotation and sedimentation methods, improve the recovery and identification of parasites.57 Flotation with sodium nitrate solution (specific gravity, 1.20) has recently been shown to be the most practical and effective fecal concentration method for identifying AT infected feces.23 In addition, direct evaluation of cecal and colonic contents was demonstrated to be superior to Swiss roll histology for postmortem detection of pinworms, particularly AT.23 Direct evaluation of cecal and colonic contents at necropsy is considered to be the ‘gold standard’ for pinworm diagnosis.17,20,21,44 Even though SO infections have been identified by ELISA,41,55 serologic pinworm assays are currently unavailable commercially. Recently, real-time PCR analysis has been introduced as a sensitive and specific diagnostic method that reportedly can detect fewer than 10 copies of DNA.17,21,27,35,46 In a recent study, PCR analysis detected the greatest number of SO-infected mice among tested methods.23 Given these results, our laboratory uses a combination of PCR testing (fur swab and feces) and direct examination of intestinal contents to optimize pinworm detection and reduce the likelihood of a false-negative result in colony health monitoring programs.23
Traditionally, fur mites have been detected by means of skin scraping with tape testing and pelt examination.50,63 These parasites have also been identified on histopathology.64 IgE has been suggested as a novel serodiagnostic marker;52 however, contemporary testing now includes PCR testing of fur swabs.26,34,51 In addition, claims in the literature vary regarding the effectiveness of soiled bedding at transferring fur mites to sentinels.26,36,50
More recently, PCR testing for rodent pathogens has expanded to include testing of IVC system components. One group achieved a 94.1% detection rate by week 4 when PCR testing swabs applied to the exhaust manifolds of a intracage supply–perimeter capture IVC system,37 each of which housed a single cage of mice infested with either RA or MB.31 The authors noted that this method would likely be effective for IVC racks in which the exhaust air is not filtered prior to the sampling site. Similarly, another group detected AT in exhaust-air dust by PCR testing after AT-infected mice had been housed on a intracage supply–intracage exhaust (direct)37 IVC system for 1 wk.33 Another study recently showed that this method was ineffective for a rack in which exhaust air was filtered prior to the sampling site. In particular, the method failed to detect mouse hepatitis virus 1, mouse norovirus 4, mouse parvovirus 1e, Pasteurella pneumotropica, Helicobacter spp., fur mites (MC and RA), pinworms (AT and SO), and large intestinal protozoa (Tritrichomonas muris and Entamoeba muris) when PCR was performed on swabs collected from the exhaust plenum of an intracage supply–intracage exhaust (indirect) IVC rack.4,37 Therefore, we surmised that the filter top needs to be tested in an intracage supply–intracage exhaust (indirect) IVC system, in which air is filtered at the cage level before it is exhausted.
We first performed a pilot study in which nucleic acid was extracted from the filter tops of cages maintained on an intracage supply–intracage exhaust (indirect) IVC rack for approximately 30 d while housing mice infected with SO and AT (n = 1) or infested with MC and MB (n = 2) or MB and RA (n = 2). The extracts of the filter top media tested PCR-positive for all agents except AT. In addition, after approximately 3 mo of use, adhesive swabs of the IVC rack exhaust prefilter and prefilter manifold were positive for MB, MC, RA, and SO but negative for AT. We report here an expanded study conducted to determine whether PCR testing of filter top media extracts from soiled-bedding sentinel cages receiving bedding from cages housing fur-mite-infested and pinworm-infected mice is a reliable detection method as compared with PCR testing of filter top swabs, PCR analysis of samples collected directly from the animals, and traditional testing methods. Our hypothesis was that PCR testing of filter top media extracts from sentinel cages would yield superior detection rates.
Materials and Methods
Animals.
The study population was composed of 144 female (age, 3 to 5 wk), Swiss Webster (Tac:SW) mice (Taconic Biosciences, Germantown, NY). Mice were SPF for mouse hepatitis virus, mouse rotavirus, lymphocytic choriomeningitis virus, ectromelia virus, mouse parvovirus, minute virus of mice, murine norovirus, pneumonia virus of mice, reovirus type 3, Sendai virus, Theiler mouse encephalomyelitis virus, mouse adenovirus, K virus, polyoma virus, mouse cytomegalovirus, mouse thymic virus, Haantan virus, lactic dehydrogenase elevating virus, cilia-associated respiratory bacillus, and Mycoplasma pulmonis. Animals were also free of Helicobacter spp., Salmonella spp., Clostridium piliforme, Corynebacterium kutscheri, Citrobacter rodentium, endoparasites, and ectoparasites on arrival. All mice were housed in a quarantine facility under ABSL 2 conditions in solid-bottom, polysulfone cages (model no. 9, Thoren Caging Systems, Hazelton, PA; Figure 1) and housed on an IVC system (model no. 9-140-10-14-1-4-5TM, Mobile Maxi-Miser PIV System, Thoren Caging Systems; Figures 1 and 2) using intracage supply–intracage exhaust (indirect) with the supply and exhaust blowers located on the bottom of the rack and exhausted into the room (HEPA-filtered); the rate of intracage air exchange was previously determined to be 42 ± 32 changes hourly;60 other characteristics of this IVC system, including air flow dynamics, have been previously described;60 Animals were housed on autoclaved aspen chip bedding (PWI Industries Canada, Quebec, Canada) or autoclaved α-cellulose bedding (Alpha-Dri, Shepherd Specialty Papers, Watertown, TN); γ-irradiated feed (LabDiet 5058, PMI, St Louis, MO) and acidified water (pH, 2.5 to 2.8) was provided free-choice by using a 500-mL water bottle with a stainless steel cap with integrated sipper tube. Cages were changed weekly in a class II type 2A biologic safety cabinet (NU S602-500, series SP, Nuaire, Plymouth, MN). The holding room was ventilated with 95% filtered outside air at 15 air changes hourly. Room temperature was maintained at 72 ± 2 °F (21.5 ± 1 °C) and relative humidity at 30% and 70%. Light:dark photoperiod cycle was maintained at 12:12 h intervals.
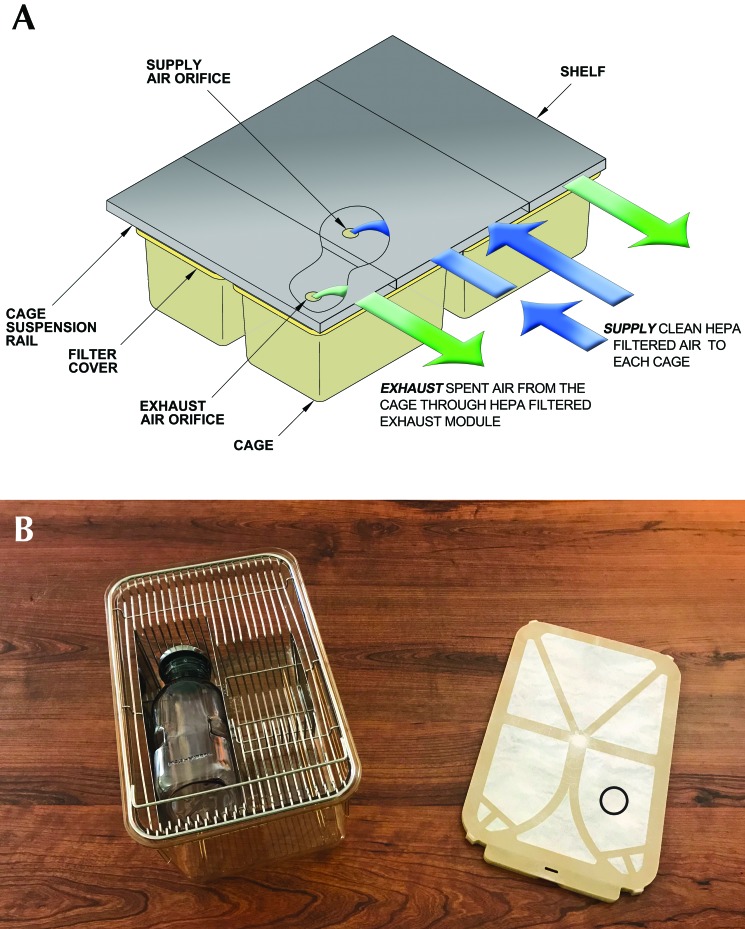
Figure 1.
IVC system used in the current study. (A) Cage air-flow diagram. HEPA-filtered air is supplied through sealed shelf plenums, directly through an air supply orifice located above the cage filter top. Air is exhausted through an exhaust-air orifice, also located above the filter top. (B) Mouse cage with filter top. Approximate exhaust site (circle) used for sample collection from the filter top. Reproduced with permission from the manufacturer (Thoren Caging Systems).
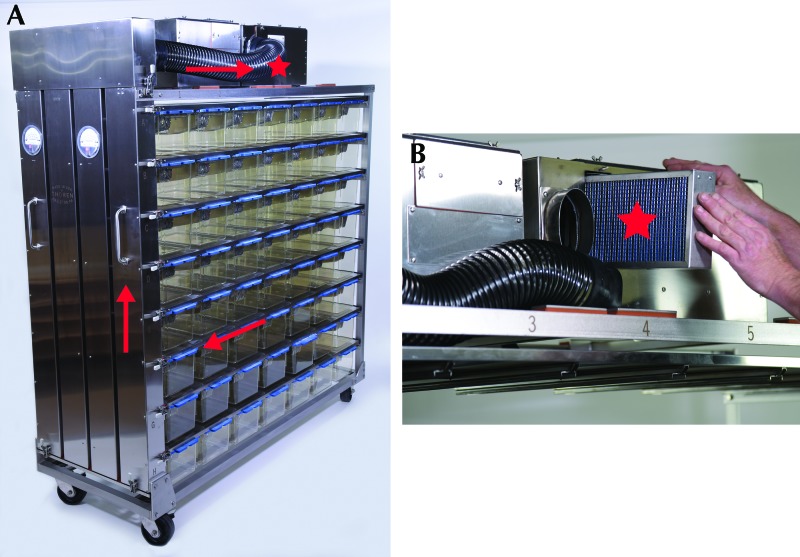
Figure 2.
IVC system used in the current study. (A) IVC caging system. Exhaust air (arrows) flow from the cage exhaust to the rack exhaust prefilter (asterisk). (B) The rack exhaust prefilter (asterisk) can be removed for sampling of the prefilter or exhaust fan box surfaces. Reproduced with permission from the manufacturer (Thoren Caging Systems).
Animal use was approved by the Memorial Sloan Kettering Cancer Center's IACUC. The animal care and use program is AAALAC-accredited, and all animals are maintained in accordance with the recommendations provided in the Guide for the Use and Care of Laboratory Animals, 8th edition.29
Experimental design.
A total of 36 IVC, each housing 4 naïve Swiss Webster mice and containing either aspen chip or α-cellulose bedding (18 each), received approximately 40% (240 mL) soiled ‘parasite-contaminated’ bedding (10% [60 mL] from each of the 4 groups of infected–infested cages described later) of the same bedding type during weekly cage change. The ‘parasite contaminated’ bedding was pooled from 2 cages each housing Swiss Webster and NOD scid γ mice (NOD.Cg-Prkdcscid IL2rgtm1Wjl/SzJ[NSG]; The Jackson Laboratory, Bar Harbor, ME) infected with either SO or AT or infested with MC or with MB and RA maintained at our institution. NSG mice were used to perpetuate the endo- and ectoparasitic infections and infestations in the contaminated source cages because their immunocompromised status likely increased the parasite burden. The remaining bedding was obtained from cages housing parasite-negative mice (60%; approximately 360 mL). The mice housed in the cages used to provide parasite-contaminated bedding were tested at the start and end of the study by using traditional methods (anal tape, fecal flotation, skin scrape) and direct PCR analysis (MB- and RA-infested cages only at start of study, all at study end) to confirm their infected or infested status.
New filter tops (spunbonded polyester filters, Reemay, Old Hickory, TN) were used and confirmed to be free of parasite nucleic acid by PCR-testing the filter media by using adhesive swabs. In addition, the naïve Swiss Webster mice were confirmed to be free of parasite nucleic acid by PCR-testing adhesive swabs and feces, as described later, prior to initiating the study. One animal was mite-positive on this initial screen but negative during speciation testing; therefore this likely was a false-positive result. Filter tops remained on the cages for the duration of the study. All cages were autoclaved prior to exiting the quarantine area where this study was conducted. Cages were subsequently sanitized in a tunnel washer using water heated to a final rinse of 180 °F. Water temperature was monitored daily. At 30, 60, and 90 d after initiating transfer of parasite-contaminated bedding, PCR analysis for each parasite was performed on a section of filter top media, adhesive swabs applied to the filter top media, and pooled adhesive swabs and feces (by cage) from each mouse (1 fecal pellet collected directly from each mouse) housed within the cage. In addition, at the same time points, an anal tape test, skin scraping, and examination of cecal and colonic contents were performed on each mouse within the cage; fecal flotation was performed on pooled feces (by cage) collected from each mouse. PCR analysis for each parasite was also performed on pooled adhesive swabs collected from the exhaust prefilter (1 swab) and exhaust prefilter manifold (1 swab) at each time point.
Parasite detection methods.
PCR analysis of swabs of filter tops.
Adhesive swabs (Pigeon Corporation, Tokyo, Japan) were wiped over the entire inside surface of a 2×2-in. section of the filter media centered on the exhaust site; the head of the swab was rotated as it passed through the filter.
PCR analysis of extracts of filter tops.
After swabbing, the same 2×2-in. section of filter (Figure 1) was removed from the lid by using a sterile scalpel blade and placed into a 50-mL conical tube (Corning Falcon 50-mL Conical Centrifuge Tubes, Fisher Scientific, Waltham, MA). Filters, as well as swabs, were washed with a lysis buffer, and nucleic acid was obtained through magnetic isolation as previously described.26
PCR analysis of exhaust prefilter and exhaust manifold.
An adhesive swab was wiped over the entire surface of the filter media on the rack-facing side of the rack exhaust prefilter (Figure 2); the head of the swab was rotated as it passed through the filter. All reachable surfaces of the exhaust prefilter manifold were wiped with an adhesive swab as described for the filter. Both swabs were pooled and analyzed by PCR for each parasite at each time point.
PCR analysis of animal feces and swabs.
For each mouse, adhesive swabs were wiped systematically over the fur of the dorsum and ventrum prior to swabbing the perianal region. Swabs and feces were tested as pooled samples for each cage.
PCR analysis.
PCR analysis was performed by using validated and established TaqMan PCR assays for SO, AT, MC, MB, and RA (Charles River Research Animal Diagnostic Services, Wilmington, MA). Reactions were conducted on lysates obtained from filter top media, swabs of filter top media, or pooled swabs and fecal pellets as previously described, by using proprietary real-time fluorogenic 5′ nuclease PCR assays.26 All PCR primers and probes targeted 28S rRNA sequences. The testing and interpretation algorithm used by the testing laboratory is as follows. Isolated DNA first was screened with 2 primer and probe sets that target sequences common to a subset of nematodes within the superfamily Oxuroidea. If either of the screening assays was positive, DNA was reisolated from retained sample lysate and retested by the screening assays as well as 3 species-specific assays that target unique sequences for AT, SO, and Syphacia muris. A positive result was reported when the repeated screening assay or species-specific assays were positive (real-time PCR cycle threshold values equivalent to or greater than approximately 1 template copy per PCR reaction). A similar approach was taken for the fur mite assays. Isolated DNA was screened by using 2 assays, one targeting sequences common to MB- and RA-related species and a second targeting sequences common to MC-related species. When either screening assay was positive, DNA was reisolated from the retained sample lysate and retested by screening assays as well as by each species specific assay. To monitor for successful DNA recovery after extraction and to assess whether PCR inhibitors were present, a nucleic acid recovery control assay was performed. Exogenous algae DNA was added to the sample lysis prior to extraction to yield approximately 200 copies of isolated nucleic acid per reaction well (approximately 40 copies/µL; 5 µL nucleic acid total added to PCR), which then underwent real-time PCR analysis targeting the algae sequence. Nucleic acid recovery control assays for samples that demonstrated greater than a log10 loss of template copies compared with control wells were diluted 1:4 and retested or reextracted or both.
Sodium nitrate flotation.
Between 7 and 16 (mean, 13) fecal pellets were mixed with sodium nitrate solution (Fecasol, Vetoquinol ISA, Fort Worth, TX; specific gravity, 1.2) in a fecal flotation device (Fecatector, Henry Schein, Dublin, OH), and flotation solution was added until a meniscus formed above the rim. A coverslip was placed on top of the meniscus. After 15 min, the coverslip was transferred to a microscope slide, and the entire coverslip was examined under 40× to 100× magnification. A positive result was reported when at least one egg was detected.
Anal tape test.
Approximately 2.5 cm of clear cellophane tape (Scotch Transparent Tape 600, 3M, St Paul, MN) was applied to the perianal region of each mouse for approximately 1 to 2 s, adhered to a microscope slide, and systematically examined under 100× magnification.
Examination of cecal and colonic contents (intestinal contents).
The cecum and colon were harvested from each mouse after euthanasia, separately macerated (that is, sliced) and maintained in warm tap water for approximately 15 min in a culture dish, and examined under 4× magnification.
Skin scrape.
Skin scrapings were collected by using a no. 20 scalpel blade (Bard-Parker Carbon Rib-Back Blades Size 20, Aspen Surgical, Caledonia, MI). The blade was scraped at a 90° angle to the surface of the skin over approximately a 1-cm2 area of skin of the head and neck (caudal scalp and neck cranial to scapulae), back (midline dorsal thoracolumbar junction), and caudoventral abdomen. Loose hair and debris from each site were transferred by using 3 separate pieces (approximately 2×2.5 cm) of cellophane tape and affixed to a single glass slide. Slides were systematically examined by using a 4× objective until the entire piece of tape, including hair fibers extending beyond its borders, was evaluated.
Statistics.
The detection rate was calculated on the basis of the number of cages with positive test results relative to the number of cages exposed. The McNemar test was used for paired comparisons. For fur mites, PCR analysis of filter top media analysis was compared with PCR analysis of filter top swabs, direct PCR testing, and skin scrape assays. For pinworms, PCR analysis of filter top media was compared with PCR testing of filter top swabs, direct PCR assay, fecal flotation, and direct examination of intestinal contents. For all parasites combined, PCR analysis of filter top media was compared with PCR testing of filter top swabs and direct PCR assay. The Fisher exact test was used for unpaired comparisons. PCR assays were compared between bedding types for each parasite and for all parasites combined. Values were considered to differ when the P value was less than 0.05. All computations were performed by using SAS version 9.4 (SAS Institute, Cary, NC).
Results
Comparison of parasite detection methods.
For mice exposed to aspen chip bedding, PCR analysis of filter top media had the highest detection rate, detecting 100% of all parasites on days 30, 60, and 90 and overall (Table 1). PCR testing of filter top swabs also detected a high percentage of parasites, including 100% of endoparasites on day 60 and 100% of ectoparasites on day 90, but this method did not consistently detect 100% of either. Direct PCR assay detected lower percentages of parasites on day 30, with increasing detection rates over the course of the study, culminating in 100% detection rates on day 90, except for the SO assay in which 83% of parasite-positive cages were detected. Direct PCR assay detected RA in 100% of the cages. For the traditional test methods, examination of intestinal contents had higher detection rates for AT and SO than did the other traditional methods for their respective parasites. Examination of intestinal contents detected AT in 100% of the cages on days 60 and 90 but identified SO in only 83% and 67% at the same time points, respectively. Fecal floatation detected AT in 17% of the cages at each time point and overall. The anal tape test never detected SO in more than 50% of the cages (39% overall). The anal tape test also detected the occasional MC infestation (33% overall). Skin scrape did not detect MB in any cage until day 90 (67%; 22% overall); detected MC in 50% of the cages on day 30, 67% on day 90, and 50% overall; and did not detect RA in cages until day 90 (50%; 17% overall).
Table 1.
Results for aspen chip bedding
| Day | Test | MB | MC | RA | AT | SO |
| 30 | PCR testing of filter top media | 100 | 100 | 100 | 100 | 100 |
| PCR testing of filter top swabs | 83 | 67 | 100 | 50 | 100 | |
| Direct PCR testing | 50 | 83 | 100 | 67 | 67 | |
| Fecal float | NA | NA | NA | 17 | 0 | |
| Anal tape | NA | 17 | NA | 0 | 50 | |
| Intestinal contents | NA | NA | NA | 83 | 50 | |
| Skin scrape | 0 | 50 | 0 | NA | 17 | |
| 60 | PCR testing of filter top media | 100 | 100 | 100 | 100 | 100 |
| PCR testing of filter top swabs | 100 | 83 | 100 | 100 | 100 | |
| Direct PCR testing | 67 | 100 | 100 | 100 | 83 | |
| Fecal float | NA | NA | NA | 17 | 0 | |
| Anal tape | NA | 50 | NA | 0 | 17 | |
| Intestinal contents | NA | NA | NA | 83 | 83 | |
| Skin scrape | 0 | 33 | 0 | NA | 17 | |
| 90 | PCR testing of filter top media | 100 | 100 | 100 | 100 | 100 |
| PCR testing of filter top swabs | 100 | 100 | 100 | 67 | 83 | |
| Direct PCR testing | 100 | 100 | 100 | 100 | 83 | |
| Fecal float | 33 | NA | NA | 17 | 0 | |
| Anal tape | NA | 33 | NA | 0 | 50 | |
| Intestinal contents | 0 | 0 | 0 | 100 | 67 | |
| Skin scrape | 67 | 67 | 50 | NA | NA | |
| All | PCR testing of filter top media | 100 | 100 | 100 | 100 | 100 |
| PCR testing of filter top swabs | 94 | 83 | 100 | 72 | 94 | |
| Direct PCR testing | 72 | 94 | 100 | 89 | 78 | |
| Fecal float | 11 | NA | NA | 17 | 0 | |
| Anal tape | NA | 33 | NA | 0 | 39 | |
| Intestinal contents | NA | NA | NA | 89 | 67 | |
| Skin scrape | 22 | 50 | 17 | NA | 11 |
NA, not applicable
Data are given as the percentage of positive cages (n = 6 per time point; 18 total).
For mice exposed to α-cellulose bedding, PCR analysis of filter top media had the highest detection rate, detecting 100% of all parasites on days 30, 60, and 90 and overall, except for AT, for which it had a detection rate of 67% on days 30 and 90 (78% overall; Table 2). PCR testing of filter top swabs detected a high percentage of some parasites, including 100% of RA and SO throughout the study and 100% for MB (except for 83% on day 90). However, PCR testing of filter top swabs detected MC in 67% of the cages at all time points. AT detection varied from 50% of the cages on day 30 to 67% of the cages on day 90 (50% overall).
Table 2.
Results for α-cellulose bedding
| Day | Test | MB | MC | RA | AT | SO |
| 30 | PCR testing of filter top media | 100 | 100 | 100 | 67 | 100 |
| PCR testing of filter top swabs | 100 | 67 | 100 | 50 | 100 | |
| Direct PCR testing | 33 | 33 | 83 | 83 | 83 | |
| Fecal float | NA | NA | NA | 0 | 0 | |
| Anal tape | NA | NA | NA | 0 | 33 | |
| Intestinal contents | NA | NA | NA | 67 | 17 | |
| Skin scrape | 0 | 0 | 0 | NA | NA | |
| 60 | PCR testing of filter top media | 100 | 100 | 100 | 100 | 100 |
| PCR testing of filter top swabs | 100 | 67 | 100 | 33 | 100 | |
| Direct PCR testing | 67 | 33 | 100 | 100 | 100 | |
| Fecal float | NA | NA | NA | 17 | 0 | |
| Anal tape | NA | NA | NA | 0 | 50 | |
| Intestinal contents | NA | NA | NA | 83 | 83 | |
| Skin scrape | 0 | 0 | 0 | NA | NA | |
| 90 | PCR testing of filter top media | 100 | 100 | 100 | 67 | 100 |
| PCR testing of filter top swabs | 83 | 67 | 100 | 67 | 100 | |
| Direct PCR testing | 50 | 67 | 83 | 83 | 100 | |
| Fecal float | 17 | NA | NA | 0 | 17 | |
| Anal tape | NA | NA | NA | 0 | 33 | |
| Intestinal contents | NA | NA | NA | 100 | 83 | |
| Skin scrape | 17 | 33 | 17 | NA | 17 | |
| All | PCR testing of filter top media | 100 | 100 | 100 | 78 | 100 |
| PCR testing of filter top swabs | 94 | 67 | 100 | 50 | 100 | |
| Direct PCR testing | 50 | 44 | 89 | 89 | 94 | |
| Fecal float | 6 | NA | NA | 6 | 6 | |
| Anal tape | NA | NA | NA | NA | 39 | |
| Intestinal contents | NA | NA | NA | 83 | 61 | |
| Skin scrape | 6 | 11 | 6 | NA | 6 |
NA, not applicable
Data are given as the percentage of positive cages (n = 6 per time point; 18 total).
Direct PCR assay generally had lower detection rates than the other 2 PCR methods. Direct PCR testing detected 83% of the cages with SO on day 30 and all of the cages for the remainder of the study. Direct PCR analysis had the same detection rates for RA and AT cages, detecting 100% on day 60 and 83% on days 30 and 90, respectively (89% overall). Direct PCR detection rates were lower in detecting MB and MC cages, detecting 33% on day 30, and 50% and 67% on day 90, respectively (50% and 44% overall, respectively).
For the traditional test methods, examination of intestinal contents detected greater numbers of cages positive for AT and SO than the other traditional methods detected their respective parasites. Examination of intestinal contents detected AT in 83% of the cages on day 60 and 100% on day 90 (83% overall), whereas it identified SO in 83% of the cages on days 60 and 90 (61% overall). Fecal floatation did not detect AT in any cage on days 30 and 90 and only 17% of the cages on day 60 (6% overall). The anal tape test detected SO in 33% of the cages on days 30 and 90 and 50% on day 60 (39% overall). Skin scrape did not detect ectoparasites in any cage on days 30 and 60 and identified MB in only 17%, MC in 33%, and RA in 17% of the cages on day 90 (6%, 11%, 6% overall, respectively). Note that for both bedding studies, SO was occasionally detected on skin scrape.
PCR analysis of filter top media was compared for each parasite to each relevant test for aspen chip bedding-exposed and α cellulose bedding-exposed cages. For aspen chip bedding, PCR analysis of filter top media detected MB more effectively than did skin scrape at days 30, 60, and overall (P = 0.03, P = 0.03, and P = 0.0001, respectively). For α-cellulose bedding, PCR analysis of filter top media was more effective at detecting MB than direct PCR overall (P = 0.004) and skin scrape at day 30, 60, and overall (P = 0.03, P = 0.03, and P < 0.0001, respectively). For aspen chip bedding, PCR analysis of filter top media was more effective at detecting MC than skin scrape overall (P = 0.004). For α-cellulose bedding, PCR analysis of filter top media more effectively detected MC than did PCR testing of filter top swabs overall (P = 0.03); direct PCR overall (P = 0.002); and skin scrape at days 30 and 60 and overall (P = 0.03, P = 0.03, and P < 0.0001, respectively). For aspen chip bedding, tests did not differ significantly regarding identification of RA. For α-cellulose bedding, PCR analysis of filter top media detected RA more frequently than did skin scrape at days 30 and 60 and overall (P = 0.03, P = 0.03, and P < 0.0001, respectively).
For aspen chip bedding and α cellulose bedding, PCR analysis of filter top media was more effective at detecting AT than fecal floatation overall (P < 0.0001 and P = 0.0002, respectively). For aspen chip bedding, PCR analysis of filter top media was more effective at detecting SO than were tape testing and examination of intestinal contents overall (P = 0.001 and P = 0.03, respectively). For α-cellulose bedding, PCR analysis of filter top media was more effective at detecting SO than were tape testing and examination of intestinal contents overall (P = 0.001 and P = 0.02, respectively).
We also compared PCR analysis of filter top media with the other PCR tests cumulatively across all parasites for each bedding type. For aspen chip bedding, PCR analysis of filter top media detected parasites more effectively than did PCR testing of filter top swabs at day 30 and overall (P = 0.03 and P = 0.002, respectively) and direct PCR testing at day 30 and overall (P = 0.008 and P = 0.0005, respectively). For α-cellulose bedding, PCR analysis of filter top media was more effective at detecting parasites than PCR testing of filter top swabs at day 60 and overall (P = 0.03 and P = 0.008, respectively) as well as direct PCR testing at days 30 and 60 and overall (P = 0.02, P = 0.03, and P = 0.0002, respectively).
We then compared PCR methods between bedding types. Direct PCR assay detected MC overall more often when aspen chip bedding was used (P = 0.003). When PCR tests were compared cumulatively across all parasites, direct PCR assay was more effective at detecting parasites by using aspen bedding at day 90 and overall (P = 0.03 and P = 0.02, respectively). In addition, PCR analysis of filter top media with aspen chip bedding had numerically higher detection rates for all parasites combined at day 30 and day 90 (100% compared with 93%) and for all time points (100% compared with 96%), but these comparisons were not statistically significant.
Mice had no clinical signs associated with ectoparasitic infestation or endoparasitic infection throughout the study.
PCR analysis of rack exhaust prefilter.
Swabs collected from the rack exhaust prefilter and manifold were negative for all parasites on day 0, positive for RA on day 30; positive for SO and mites on day 60; and positive for MC, RA, and SO on day 90.
Discussion
Testing for rodent pathogens has recently expanded to include PCR testing of environmental samples, including IVC system components. This method may be effective for IVC systems in which cage exhaust is not filtered prior to the sampling site.27 We hypothesized that PCR testing of cage lid filters would be a viable alternative for IVC systems in which air is filtered at the cage level. In this study, we compared parasite detection methods in sentinel cages exposed to soiled bedding collected from cages housing mice infested with fur mites and infected with pinworms using 2 contact bedding types, aspen chip and α cellulose. We hypothesized that differences in the amounts of particulates (that is, dust) would affect the ability to detect parasite nucleic acid on the filter tops. Bedding containing a greater amount of dust would increase the ability to detect parasite nucleic acid. Specifically, we compared detection rates of nucleic acid extracts from a section of filter media collected from the filter top, nucleic acid eluted from adhesive swabs applied to the filter top, and samples collected directly from the animals and tested by using both PCR analysis and traditional testing methods, including fecal floatation, the anal tape test, direct examination of intestinal contents, and skin scraping.
When cages were bedded with aspen chips, PCR analysis of filter top media was positive for all parasites at all time points. Although PCR testing of filter top swabs detected a high percentage of parasites, including all endoparasites on day 60 and all ectoparasites on day 90, it did not consistently detect all parasites at all time points throughout the study. Not surprisingly, except for detecting RA in all cages throughout the study, direct PCR assays detected low percentages of parasites on day 30, with increasing detection rates over the course of the study, culminating in 100% detection rates on day 90, except for detecting SO in 83% of the cages. These findings likely reflect differences in prepatent periods and increasing endo- and ectoparasite burdens in and on the mice over time.
For the traditional test methods conducted on cages bedded with aspen chip, examination of intestinal contents had higher detection rates for AT (100% on days 60 and 90) and SO (83% and 67% on days 60 and 90, respectively) than the other nonmolecular methods for their corresponding parasites. Fecal flotation detected AT in only 17% of the cages, and the anal tape test never detected SO in more than 50% of the cages at any time point (39% overall). We found similarly low detection rates in our previous study.23 Interestingly, the anal tape test detected the occasional MC infestation, likely due to the presence of mites on hair near the perineum. Not surprisingly, skin scrape detected a low percentage of ectoparasites on days 30 and 60, detecting neither MB nor RA until day 90. Skin scrape detection rates were highest on day 90, detecting MB and MC in approximately 2/3 and RA in half of the cages. Similar to the increasing detection rates for direct PCR assays, this pattern likely was related to increasing ectoparasite burdens over time.
For cages containing α-cellulose, results for PCR analysis of filter top media were similar to those for aspen chip bedding, and PCR of filter media extract detected all parasites, except for AT, by day 30. Even though AT was detected in only 2/3 of the cages on days 30 and 90, the parasite was detected in all cages on day 60. Perhaps the longer prepatent period for AT and deposition of its eggs within feces, as compared with perianally for SO, contributed to these results, in that more SO nucleic acid was available to be aerosolized. Although PCR testing of filter top swabs detected a high percentage of most parasites at all time points, this method detected MC and AT in low numbers of cages at most time points. Direct PCR generally had lower detection rates than the other PCR methods evaluated, and it was best at detecting RA. Similar to the results for aspen chip bedding, intestinal content examination yielded higher detection rates for AT and SO than the other traditional methods, but, in general, the traditional test methods had low detection rates. Surprisingly with both bedding types, SO was occasionally detected in skin scrape samples. Again, perianal deposition of SO eggs increases the likelihood of contamination with SO eggs of adjacent areas of the fur when skin scraping was performed and within the cage environment.
For each parasite, PCR analysis of filter top media was compared with the other tests for each bedding type. For cages bedded with α-cellulose, PCR analysis of filter top media was more effective than PCR testing of filter top swabs at detecting MC (overall) and than direct PCR assays at detecting MB and MC (overall). In the context of MB and MC, it is unclear why PCR analysis of filter top media was superior to other PCR methods when tested on α-cellulose-bedded cages but not when using aspen chip bedding. However, not surprisingly for both bedding types, PCR analysis of filter top media was more effective than the skin scrape at detecting MB (days 30 and 60 and overall), MC (aspen chip bedding: overall; α-cellulose: days 30 and 60 and overall), and RA (α cellulose: days 30 and 60 and overall). Aspen-chip-bedded cages yielded no significant differences between tests for RA. For both bedding types, PCR analysis of filter top media was more effective than fecal flotation at detecting AT (overall) and better than the tape test and intestinal contents at detecting SO (overall). Not surprisingly, PCR analysis of filter top media was superior to traditional antemortem tests for the endo- and ectoparasites considered for both bedding types. This finding was consistent with previous studies that have shown PCR assay, albeit direct, to generally have higher detection rates than the tape test,21 fecal flotation,23 and skin scrape.34,51
PCR analysis of filter top media was superior to the other PCR methods evaluated at detecting all parasites, when considered cumulatively, for each bedding type overall. For aspen-chip-bedded cages, PCR analysis of filter top media was more effective at detecting parasites than PCR testing of filter top swabs (day 30 and overall) and when compared with direct PCR (day 30 and overall). For α-cellulose-bedded cages, PCR analysis of filter top media was more effective at detecting parasites as compared with PCR testing of filter top swabs (day 60 and overall) and direct PCR assay (days 30 and 60 and overall).
It is not surprising that PCR analysis of filter top media was either equivalent or more effective than PCR testing of filter top swabs at detecting all parasites. The spunbonded polyester filter (Reemay) would be expected to accumulate greater amounts of particulate within the filter matrix as well on its inner surface than what resides solely on the inner surface, which is likely only partially picked up by the swab. From a practical standpoint, this situation necessitates maintaining the lid on the sentinel cage during cage changes and sacrificing the filter top for testing; however, the costs of the top are modest (approximately $6) when contrasted to the cost of performing PCR analysis and the potential programmatic costs of a false-negative result. Perhaps more important was the finding that PCR analysis of filter top media was superior to directly testing the animals within the cage, which is a commonly used method. Furthermore, accumulation of nucleic acid within and on the filter media over time circumvents the challenges associated with directly testing mice for pinworms, given that Syphacia spp., for example, are known to exhibit egg shedding periodicity40,61 and that endoparasites, in general, have lower burdens over time due to increased immunity.14,24,30,33,41,45,55 Future studies are warranted for determining whether the presence of sentinel mice is necessary to stir up dust and debris that can become lodged in the filter for testing. In addition, it should be noted that one cannot eliminate the possibility of false-positive PCR results due to sample contamination, either at the diagnostic lab or during cage change or sample collection.
In addition, some institutions house different numbers of mice in their sentinel cages and may change cages less frequently than our institution (4 per cage and once weekly, respectively), thus potentially affecting the parasite burden within the cage as well as the amount of dust and debris generated. Likewise, because endoparasitic burden decreases with age,7,14,25,30,41,45,49,55 the 3- to 5-wk-old female Swiss Webster mice used in the current study, which we selected to represent the sentinel mice used at our institution, initially may have been more susceptible to parasitic infection. Furthermore, the parasite burden within the sentinel cage is dependent on the prevalence within the facility, thus affecting the positive predictive value of a diagnostic test.56 We selected a 1:10 ratio (10%) of parasite-contaminated:uncontaminated bedding to model the level of infestation or infection that might reasonably be detected. In our facility, a sample of soiled bedding is collected from each of 40 cages; samples are combined and placed into each sentinel cage during weekly cage change. PCR analysis of filter top media reliably detected endo- and ectoparasites from soiled-bedding sentinel cages after only 1 mo of receiving the contaminated bedding.
Comparing PCR methods between bedding types revealed that direct PCR was more effective at detecting MC (overall) as well as all parasites combined (day 90 and overall) when aspen chip bedding was used as compared with α-cellulose bedding. In addition, although the difference did not achieve statistical significance, PCR analysis of filter top media in cages bedded with aspen chip detected more positive cages as compared with α-cellulose bedding for all parasites combined on days 30 and 90 (100% compared with 93%), and for all time points (100% compared with 96%). Although aspen chip bedding appeared to better transmit endo- and ectoparasites via dirty bedding for PCR detection, whether the difference can be attributed to the bedding itself or whether this pattern was due to differing parasite burdens within the source cages is unclear. However, hardwood bedding chips have the highest dust content (0.13%); more so than corncob, corncob-paper mixed, and paper bedding.65 Therefore, a difference in dust generation may have contributed to the higher detection rates in cages bedded with aspen chip.
Fur mite nucleic acid clearly can be transmitted to sentinel cages by means of dirty bedding. Historically, this method of transmission has been reported to be variably successful.26,36,50,59 Likewise, skin scrape results from this study demonstrated low levels of apparently active infestations. However, the historically variable success of transferring of fur mites was likely due to the need for active infestations as well as less sensitive diagnostic methods. Endo- and ectoparasite burdens within the dirty bedding, stage of infestation, and technical experience could also have affected the results from previous reports. PCR testing of environmental samples—in this case, samples collected from filter tops—enabled detection of fur mites as their nucleic acid is aerosolized and accumulates over time in and on the filter adjacent to the exhaust plenum orifice.
The limited testing of the rack exhaust prefilters and exhaust manifold showed promise for their use as an alternative site for collecting environmental PCR samples, even though the cage exhaust was filtered at the filter top. Swabs of the study rack exhaust prefilter were positive for 3 of the 5 parasites (MC, RA, and SO) on day 90. However, the study rack exhaust prefilter was reusable, and therefore media was not removed for extraction and testing on day 90. Disposable filters are available and may be advantageous for the same reasons as indicated for the filter lid media. Our current results are in contrast to recent work, in which rack plenum testing was negative for all parasites tested.4 This difference may be due, in part, to difficulties in sampling a site with ample dust accumulation.
In conclusion, PCR analysis of filter top media was, in general, superior to all the other PCR methods evaluated (filter top swab and direct testing of mice) as well as traditional methods for detecting murine endo- and ectoparasites and reliably detected the presence of parasites after only 1 mo in cages receiving as little as a 1:10 ratio of contaminated:uncontaminated bedding weekly. Furthermore, all other testing methods evaluated may generate an unacceptable number of false-negative results. PCR analysis of filter top media resulted in detection rates that were higher (albeit nonsignificantly) for all parasites combined when cages were bedded with aspen chips as compared with α-cellulose, presumably because of the higher dust content of aspen chips. Therefore, bedding type should be considered when using environmental PCR testing to detect parasites housed on this type, and perhaps other types, of IVC systems. Finally, although preliminary testing showed promise, further studies are warranted to evaluate the effectiveness of rack exhaust prefilter PCR testing or sampling of other exhaust components in this type of IVC system. Because PCR testing of cage lid filters for IVC systems in which air is filtered at the cage level was highly effective, lid filter PCR testing of sentinel cages at 2- to 3-mo intervals could augment or even replace direct testing of sentinels for common murine parasites.
Acknowledgments
We thank the staff of the Laboratory of Comparative Pathology (especially Jacqueline Candelier and Desiree Powell) and the postdoctoral research fellows (Andrew Gorman, Melissa Nashat, Nicholas Tataryn, Samantha Peneyra, Miranda Gallo, and Christopher Chellieutte-Nieves) for their support. We also thank Charles River Research Animal Diagnostic Services for their generous technical and financial support of PCR testing.
This study was funded, in part, through the NIH/NCI Cancer Center Support Grant P30-CA008748 and the NIH Research Education Grant R25OD010447-02.
References
- 1.Anya AO. 1966. Studies on the biology of some oxyurid nematodes. I. Factors in the development of eggs of Aspiculuris tetraptera Schulz. J Helminthol 40:253–260. [DOI] [PubMed] [Google Scholar]
- 2.Anya AO. 1966. Studies on the biology of some oxyurid nematodes. II. The hatching of eggs and development of Aspiculuris tetraptera Schulz, within the host. J Helminthol 40:261–268. [DOI] [PubMed] [Google Scholar]
- 3.Baker DG. 2007. Parasites of rats and mice, p 303–397. In: Baker DG. Flynn's parasites of laboratory animals, 2nd ed. Ames (IA): Blackwell Publishing. [Google Scholar]
- 4.Bauer BA, Besch-Williford C, Livingston RS, Crim MJ, Riley LK, Myles MH. 2016. Influence of rack design and disease prevalence on detection of rodent pathogens in exhaust debris samples from individually ventilated caging systems. J Am Assoc Lab Anim Sci 55:782–788. [PMC free article] [PubMed] [Google Scholar]
- 5.Behnke JM. 1974. The distribution of larval Aspiculuris tetraptera Schulz during a primary infection in Mus musculus, Rattus norvegicus and Apodemus sylvaticus. Parasitology 69:391–402. [DOI] [PubMed] [Google Scholar]
- 6.Behnke JM. 1975. Aspiculuris tetraptera in wild Mus musculus. The prevalence of infection in male and female mice. J Helminthol 49:85–90. [DOI] [PubMed] [Google Scholar]
- 7.Behnke JM. 1976. Aspiculuris tetraptera in wild Mus musculus. Age resistance and acquired immunity. J Helminthol 50:197–202. [PubMed] [Google Scholar]
- 8.Bugarski D, Jovcic G, Katic-Radivojevic S, Petakov M, Krstic A, Stojanovic N, Milenkovic P. 2006. Hematopoietic changes and altered reactivity to IL17 in Syphacia obvelata-infected mice. Parasitol Int 55:91–97. [DOI] [PubMed] [Google Scholar]
- 9.Bunte R, Nolan T. 2006. Searching for Aspiculuris tetraptera: lessons learned. J Am Assoc Lab Anim Sci 45:86. [Google Scholar]
- 10.Carty AJ. 2008. Opportunistic infections of mice and rats: Jacoby and Lindsey revisited. ILAR J 49:272–276. [DOI] [PubMed] [Google Scholar]
- 11.Chan KF. 1952. Life-cycle studies on the nematode Syphacia obvelata. Am J Hyg 56:14–21. [DOI] [PubMed] [Google Scholar]
- 12.Chan KF. 1953. The effect of storage at low temperatures on the infectivity of Aspiculuris tetraptera eggs. J Parasitol 39:42. [Google Scholar]
- 13.Chan KF. 1955. The distribution of larval stages of Aspiculuris tetraptera in the intestine of mice. J Parasitol 41:529–532. [PubMed] [Google Scholar]
- 14.Clarke CL, Perdue KA. 2004. Detection and clearance of Syphacia obvelata infection in Swiss Webster and athymic nude mice. Contemp Top Lab Anim Sci 43:9–13. [PubMed] [Google Scholar]
- 15.Clifford CB, Watson J. 2008. Old enemies, still with us after all these years. ILAR J 49:291–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dix J, Astill J, Whelan G. 2004. Assessment of methods of destruction of Syphacia muris eggs. Lab Anim 38:11–16. [DOI] [PubMed] [Google Scholar]
- 17.Dole VS, Zaias J, Kyricopoulos-Cleasby DM, Banu LA, Waterman LL, Sanders K, Henderson KS. 2011. Comparison of traditional and PCR methods during screening for and confirmation of Aspiculuris tetraptera in a mouse facility. J Am Assoc Lab Anim Sci 50:904–909. [PMC free article] [PubMed] [Google Scholar]
- 18.Effler JC, Hickman-Davis JM, Erwin JG, Cartner SC, Schoeb TR. 2008. Comparison of methods for detection of pinworms in mice and rats. Lab Anim (NY) 37:210–215. [DOI] [PubMed] [Google Scholar]
- 19.Eguíluz C, Viguera E, Perez J. 2001. Modification of the anal tape method for detection of pinworms in rodents. Lab Anim (NY) 30:54–55. [DOI] [PubMed] [Google Scholar]
- 20.Farrar PL, Wagner JE, Kagiyama N. 1994Syphacia spp., p 219–224. In: Waggie K, Kagiyam N, Allen A, Nomura T. Manual of microbiological monitoring of laboratory animals. 2nd ed. Bethesda (MD): US Department of Health and Human Services, National Institutes of Health (publication no. 94-2498). [Google Scholar]
- 21.Feldman SH, Bowman SG. 2007. Molecular phylogeny of the pinworms of mice, rats, and rabbits and its use to develop molecular beacon assays for the detection of pinworms in mice. Lab Anim (NY) 36:43–50. [DOI] [PubMed] [Google Scholar]
- 22.Galton M. 1963. Myobic mange in the mouse leading to skin ulceration and amyloidosis. Am J Pathol 43:855–865. [PMC free article] [PubMed] [Google Scholar]
- 23.Gerwin PM, Arbona RJR, Riedel ER, Lepherd ML, Henderson KS, Lipman NS. 2016. Evaluation of traditional and contemporary methods for detecting Syphacia obvelata and Aspiculuris tetraptera in laboratory mice. J Am Assoc Lab Anim Sci 56:32–41. [PMC free article] [PubMed] [Google Scholar]
- 24.Goodroe AE, Baxter VK, Watson J. 2016. Guidance regarding sample collection and refinement of fecal flotation exam for the isolation of Aspiculuris tetraptera. J Am Assoc Lab Anim Sci 55:541–547. [PMC free article] [PubMed] [Google Scholar]
- 25.Grove KA, Smith PC, Booth CJ, Compton SR. 2012. Age-associated variability in susceptibility of Swiss Webster mice to MPV and other excluded murine pathogens. J Am Assoc Lab Anim Sci 51:789–796. [PMC free article] [PubMed] [Google Scholar]
- 26.Henderson KS, Perkins CL, Havens RB, Kelly MJ, Francis BC, Dole VS, Shek WR. 2013. Efficacy of direct detection of pathogens in naturally infected mice by using a high-density PCR array. J Am Assoc Lab Anim Sci 52:763–772. [PMC free article] [PubMed] [Google Scholar]
- 27.Hill WA, Randolph MM, Mandrell TD.2009. Sensitivity of perianal tape impressions to diagnose pinworm (Syphacia spp.) infections in rats (Rattus norvegicus) and mice (Mus musculus). J Am Assoc Lab Anim Sci 48:378–380. [PMC free article] [PubMed] [Google Scholar]
- 28.Hsieh KY. 1952. The effect of the standard pinworm chemotherapeutic agents on the mouse pinworm Aspiculuris tetraptera. Am J Hyg 56:287–293. [PubMed] [Google Scholar]
- 29.Institute for Laboratory Animal Research 2011. Guide for the care and use of laboratory animals. 8th ed. Washington (DC): National Academies Press. [Google Scholar]
- 30.Jacobson RH, Reed ND. 1974. The thymus dependency of resistance to pinworm infection in mice. J Parasitol 60:976–979. [PubMed] [Google Scholar]
- 31.Jensen ES, Allen KP, Henderson KS, Szabo A, Thulin JD. 2013. PCR testing of a ventilated caging system to detect murine fur mites. J Am Assoc Lab Anim Sci 52:28–33. [PMC free article] [PubMed] [Google Scholar]
- 32.Jungmann P, Freitas A, Bandeira A, Nobrega A, Coutinho A, Marcos MA, Minoprio P. 1996. Murine acariasis. II. Immunological dysfunction and evidence for chronic activation of Th2 lymphocytes. Scand J Immunol 43:604–612. [DOI] [PubMed] [Google Scholar]
- 33.Kapoor P, Hayes YO, Jarrell LT, Bellinger DA, Thomas RD, Lawson GW, Arkema JD, Fletcher CA, Nielsen JN. 2017. Evaluation of anthelmintic resistance and exhaust air dust PCR as a diagnostic tool in mice enzootically infected with Aspiculuris tetraptera. J Am Assoc Lab Anim Sci 56:273–289. [PMC free article] [PubMed] [Google Scholar]
- 34.Karlsson EM, Pearson LM, Kuzma KM, Burkholder TH. 2014. Combined evaluation of commonly used techniques, including PCR, for diagnosis of mouse fur mites. J Am Assoc Lab Anim Sci 53:69–73. [PMC free article] [PubMed] [Google Scholar]
- 35.Leutenneger CM. 2001. The real-time TaqMan PCR and applications in veterinary medicine. Veterinary Sciences Tomorrow 1:1–15. [Google Scholar]
- 36.Lindstrom KE, Carbone LG, Kellar DE, Mayorga MS, Wilkerson JD. 2011. Soiled bedding sentinels for the detection of fur mites in mice. J Am Assoc Lab Anim Sci 50:54–60. [PMC free article] [PubMed] [Google Scholar]
- 37.Lipman NS. 1999. Isolator rodent caging systems (state of the art): a critical view. Contemp Top Lab Anim Sci 38:9–17. [PubMed] [Google Scholar]
- 38.Lübcke R, Hutcheson FA, Barbezat GO. 1992. Impaired intestinal electrolyte transport in rats infested with the common parasite Syphacia muris. Dig Dis Sci 37:60–64. [DOI] [PubMed] [Google Scholar]
- 39.McNair DM, Timmons EH. 1977. Effects of Aspiculuris tetraptera and Syphacia obvelata on exploratory behavior of an inbred mouse strain. Lab Anim Sci 27:38–42. [PubMed] [Google Scholar]
- 40.Meade TM, Watson J. 2014. Characterization of rat pinworm (Syphacia muris) epidemiology as a means to increase detection and elimination. J Am Assoc Lab Anim Sci 53:661–667. [PMC free article] [PubMed] [Google Scholar]
- 41.Michels C, Goyal P, Nieuwenhuizen N, Brombacher F. 2006. Infection with Syphacia obvelata (pinworm) induces protective Th2 immune responses and influences ovalbumin-induced allergic reactions. Infect Immun 74:5926–5932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mohn G, Philipp EM. 1981. Effects of Syphacia muris and the anthelmintic fenbendazole on the microsomal monooxygenase system in mouse liver. Lab Anim 15:89–95. [DOI] [PubMed] [Google Scholar]
- 43.Morita E, Kaneko S, Hiragun T, Shindo H, Tanaka T, Furukawa T, Nobukiyo A, Yamamoto S. 1999. Fur mites induce dermatitis associated with IgE hyperproduction in an inbred strain of mice, NC/Kuj. J Dermatol Sci 19:37–43. [DOI] [PubMed] [Google Scholar]
- 44.Ooi HK, Oku Y, Kamiya M. 1994. Aspiculuris tetraptera, p 173–175. In: Waggie K, Kagiyam N, Allen A, Nomura T. Manual of microbiological monitoring of laboratory animals, 2nd ed. Bethesda (MD): National Institutes of Health (publication no. 94-2498). [Google Scholar]
- 45.Panter HC. 1969. Studies on host-parasite relationships. Syphacia obvelata in the mouse. J Parasitol 55:74–78. [PubMed] [Google Scholar]
- 46.Parel JD, Galula JU, Ooi HK. 2008. Characterization of rDNA sequences from Syphacia obvelata, Syphacia muris, and Aspiculuris tetraptera and development of a PCR-based method for identification. Vet Parasitol 153:379–383. [DOI] [PubMed] [Google Scholar]
- 47.Phillipson RF. 1974. Intermittent egg release by Aspiculuris tetraptera in mice. Parasitology 69:207–213. [DOI] [PubMed] [Google Scholar]
- 48.Pochanke V, Hatak S, Hengartner H, Zinkernagel RM, McCoy KD. 2006. Induction of IgE and allergic-type responses in fur mite-infested mice. Eur J Immunol 36:2434–2445. [DOI] [PubMed] [Google Scholar]
- 49.Pritchett KR. 2007. Helminth parasites of laboratory mice, p 551–564, chapter 22.In: Fox JG, Davisson MT, Quimby FW, Barthold SW, Newcomer CE, Smith AL. The mouse in biomedical research, vol 2, 2nd ed. New York (NY): Academic Press. [Google Scholar]
- 50.Ricart Arbona RJ, Lipman NS, Wolf FR. 2010. Treatment and eradication of murine fur mites: II. Diagnostic considerations. J Am Assoc Lab Anim Sci 49:583–587. [PMC free article] [PubMed] [Google Scholar]
- 51.Rice KA, Albacarys LK, Metcalf Pate KA, Perkins C, Henderson KS, Watson J. 2013. Evaluation of diagnostic methods for Myocoptes musculinus according to age and treatment status of mice (Mus musculus). J Am Assoc Lab Anim Sci 52:773–781. [PMC free article] [PubMed] [Google Scholar]
- 52.Roble GS, Boteler W, Riedel E, Lipman NS. 2012. Total IgE as a serodiagnostic marker to aid murine fur mite detection. J Am Assoc Lab Anim Sci 51:199–208. [PMC free article] [PubMed] [Google Scholar]
- 53.Rudolphi KA. 1801. [[Beobachtungen über die Eingeweidewurmer.]] Arch f Zool u Zoot 2:1–65 [[Article in German]]. [Google Scholar]
- 54.Sasa M, Tanaka H, Fukui M, Takata A. 1962. Internal parasites of laboratory animals, p 195–214. In: Harris RJC. The problems of laboratory animal disease. New York (NY): Academic Press. [Google Scholar]
- 55.Sato Y, Ooi HK, Nonaka N, Oku Y, Kamiya M. 1995. Antibody production in Syphacia obvelata -infected mice. J Parasitol 81:559–562. [PubMed] [Google Scholar]
- 56.Shek WR, Smith AL, Pritchett-Corning KR. 2015. Microbiological quality control for laboratory rodents and lagomorphs, p 463–510, chapter 11. In: Fox JG, Anderson LC, Otto G, Pritchett-Corning KR, Whary MT. Laboratory animal medicine, 3rd ed. New York (NY): Academic Press. [Google Scholar]
- 57.Smith PH, Wiles SE, Malone JB, Monahan CM. 2007. Collection, preservation, and diagnostic methods, p 1–13. In: Baker DG. Flynn's parasites of laboratory animals. Ames (IA): Blackwell Publishing. [Google Scholar]
- 58.Taffs LF. 1976. Pinworm infections in laboratory rodents: a review. Lab Anim 10:1–13. [DOI] [PubMed] [Google Scholar]
- 59.Thigpen JE, Lebetkin EH, Dawes ML, Amyx HL, Caviness GF, Sawyer BA, Blackmore DE. 1989. The use of dirty bedding for detection of murine pathogens in sentinel mice. Lab Anim Sci 39:324–327. [PubMed] [Google Scholar]
- 60.Tu H, Diberadinis LJ, Lipman NS. 1997. Determination of air distribution, exchange, velocity, and leakage in 3 individually ventilated rodent caging systems. Contemp Top Lab Anim Sci 36:69–73. [PubMed] [Google Scholar]
- 61.van der Gulden WJ. 1967. Diurnal rhythm in egg production by Syphacia muris. Exp Parasitol 21:344–347. [DOI] [PubMed] [Google Scholar]
- 62.Wagner M. 1988. The effect of infection with the pinworm (Syphacia muris) on rat growth. Lab Anim Sci 38:476–478. [PubMed] [Google Scholar]
- 63.West WL, Schofield JC, Bennett BT. 1992. Efficacy of the ‘micro-dot’ technique for administering topical 1% ivermectin for the control of pinworms and fur mites in mice. Contemp Top Lab Anim Sci 31:7–10. [Google Scholar]
- 64.Whary MT, Baumgarth N, Fox JG, Barthold SW. 2015Biology and diseases of mice, p 43–149. In: Fox JG, Anderson LC, Otto G, Pritchett-Corning KR, Whary MT. Laboratory animal medicine, 3rd ed. New York (NY): Academic Press. [Google Scholar]
- 65.Whiteside TE, Thigpen JE, Kissling GE, Grant MG, Forsythe D. 2010. Endotoxin, coliform, and dust levels in various types of rodent bedding. J Am Assoc Lab Anim Sci 49:184–189. [PMC free article] [PubMed] [Google Scholar]