Abstract
In guinea pigs, studies addressing the efficacy, safety, and pharmacokinetic profiles of different sustained-release buprenorphine (SRB) formulations are still in their infancy. Here we assessed the pharmacokinetic profiles of 3 SRB dosages (SR-LAB, ZooPharm; SRBLow, 0.15 mg/kg; SRBMedium, 0.3 mg/kg; and SRBHigh, 0.6 mg/kg) for 72 h after a single subcutaneous administration to 8 (4 male and 4 female) healthy guinea pigs. Body weight, fecal output, and cortisol levels were also monitored and the results compared with those of the sham group. Within the first h after administration, the maximal plasma concentration (Cmax) of the drug was 64.3 ± 9.2 ng/mL (males) and 71.3 ± 3.7 ng/mL (females) in the SRBHigh group; 11.5 ± 3.2 ng/mL (males) and 6.9 ± 0.9 ng/mL (females) in the SRBMedium group; and 2.3 ± 0.8 ng/mL (males) and 2.0 ± 0.5 ng/mL (females) in the SRBLow group. After 72 h, therapeutic levels of the drug (>1 ng/mL) were observed only in guinea pigs treated with SRBHigh (both sexes) and males treated with SRBMediu cm. Fecal output (quantity and distribution) and body weight were significantly lower in the SRB groups as compared with the sham group, and with the SRBHigh group showing larger reductions. Baseline levels of serum cortisol in healthy females (1440 ± 106 ng/mL) were significantly greater than in males (550 ± 66 ng/mL). But, independent of the sex, SRB administration significantly reduced those levels. In conclusion, the data indicate that all 3 SRB dosages can be safely used in guinea pigs. However, therapeutic levels of the drug were observed for at least 48 h only guinea pigs treated with SRBHigh and SRBMedium. Further investigation is needed to determine if these dosages can alleviate pain in guinea pigs.
Abbreviations: Cmax, maximal plasma concentration; Cmin, minimal plasma concentration; IRB, immediate release buprenorphine; SRB, sustained release buprenorphine; Tmax, time to maximal concentration; LC-MS, liquid chromatography mass spectrometry; OIBD, Opioid-induced bowel dysfunction; TTC, time-to-consumption
Guinea pigs are used as experimental subjects in a variety of research disciplines.33 However, compared with previous years, the USDA's 2016 Annual Report on Animal Usage indicated that the number of guinea pigs in biomedical research has decreased.36 Despite that, some behavioral traits1,33 of this USDA-covered species are still preferred in some immunologic studies24,31 and in some preclinical research involving the development of novel drugs29,41 or medical devices.19,39 As a consequence, current models still subject guinea pigs to different levels of pain that must be adequately managed and alleviated.3,6,17,20,28
Appropriate pain-relieving measures to counteract painful stimuli is a humane necessity and serves as the cornerstone of many animal regulatory policies.3,17,28 Among the different analgesic protocols, immediate-release buprenorphine (IRB) is a federally regulated opioid commonly used to manage pain in guinea pigs.6,20,33 Compared with other analgesics,2,5,6 IRB provides relatively long-lasting analgesia,6 can be easily administered, and is relatively safe.7,8,13,33 Despite advances, the use of IRB is limited by its pharmacokinetic profile.6,7,9,13,20 To maintain therapeutic levels, current protocols typically call for administration of IRB every 12 h, which can increase animal handling as well as compliance issues associated with missed or inaccurate redosing.3,6,17,20,28 Moreover, due to the lack of studies correlating the use of IRB with pharmacokinetic profiles and pain assessment methods in guinea pigs,15,38 an optimal plasma concentration of the drug required to alleviate clinically relevant pain remains unknown. Therefore, it is still unclear if the efficacy of IRB as extrapolated from other species 15,38 is equivalent or adequate for guinea pigs .5,6,8,13,20
Formulations of sustained-release buprenorphine (SRB) were recently introduced into the veterinary market to minimize redosing and restraint issues.5,8,13 As a consequence, studies addressing the efficacy, safety and pharmacokinetic profiles of SRB in laboratory species are becoming common within the laboratory animal community.5,8,13 Some of these efforts, particularly in mice and rats, have already led to FDA indexation of the first SRB formulation (Animalgesics) for both species.3 Among other SRB suppliers, an increasing number of studies describe the pharmacokinetic profiles of the Zoopharm formulation in different species.8,13,30 In mice and rats, a single dose of buprenorphine SR-LAB (Zoopharm, referred from now on as SRB) can sustain plasma concentrations of the drug within therapeutic levels (1 ng/ml) for up to 72 h with minimal adverse effects.8,13,30
However, similar studies in guinea pigs are still in their early stages. A recent study related pharmacokinetic profiles to the response to pressure in the left hindpaw of healthy female guinea pigs treated with SRB (0.3 mg/kg) or IRB (0.05 mg/kg) buprenorphine.32 According to the results, the plasma concentration of buprenorphine stayed above 1 ng/ml within the first 24 h after SRB administration.32 However, within this time frame, significant changes between the groups (SRB vs IRB) in the amount of pressure needed to elicit a withdrawal response were observed only within the first 12 h.32 Because guinea pigs are known to hide signs of pain,1,11,33 these findings could pose welfare concerns 3,17 as it is unclear if a single administration of an SRB dose of 0.3 mg/kg could alleviate significant pain for more than 12 h.6,11,20,32,38 Moreover, buprenorphine is known to cause anorexia and constipation in many species.6,19 Therefore, the extent of these and other adverse effects in guinea pigs treated with SRB remain to be determined.
Therefore, in this study, we sought to determine the pharmacokinetic properties and potential adverse effects of 3 SRB dosages in healthy guinea pigs over a 3-d period. We hypothesized that at least 1 of the SRB regimens would provide sustained levels of the drug equal to or greater than 1 ng/mL for 3 d with minimal or no adverse effects to the animals.
Materials and Methods
Animals.
All procedures using animals were approved by the IACUC of the University of Southern California. SPF Dunkin–Hartley guinea pigs (4 male, 4 female; age, 5 mo; weight [mean ± 1 SD], 580.00 ± 0.03 g) were obtained from Charles River Laboratories (Kingston, NY). On arrival to our AAALAC-accredited facility, the animals were grouped by sex and acclimated in their new enclosure (40 × 109 × 60 cm; Allentown Caging, Allentown, PA) for 1 wk, with unrestricted access to cage enrichment (for example, toys, shades), food (Teklad Global Guinea Pig Diet 2040, Envigo, Madison, WI; timothy hay cubes), and filtered water.
Experimental design.
In this study, all guinea pigs were monitored daily during all phases of the study. We reduced the number of animals needed by subjecting the same guinea pigs (n = 8) to all treatment groups. After the initial acclimation period, each guinea pig was single-housed in a wire-bottom cage (19 × 40 × 20 cm, Allentown Caging, Allentown, PA) for a total of 7 d to record daily changes in body weight and fecal output during the 3 d before (baseline) and 3 d after the administration of saline (Sham) or 1 of the 3 SRB dosages (SRBLow, SRBMedium, and SRBHigh). For each SRB dosage group, guinea pigs were transported to the procedure room, where blood and vital signs were collected at 0, 1, 3, 6, 12, 24, 48, and 72 h after administration.
For sequential blood collection,4 body weight was taken to calculate the following escalating SRB dosages (SRBLow, – 0.15 mg/kg; SRBMedium, – 0.3 mg/kg; SRBHigh, – 0.6 mg/kg; SRB-Lab, Zoopharm, Windsor, CO) using known concentrations of IRB in guinea pigs20,32,33 as references with the formula:
Between each treatment, guinea pigs were housed in same-sex groups in their primary enclosure (40 × 109 × 60 cm - Allentown Caging, Allentown, PA) for 4-6 wk to allow adequate recovery and minimize distress by encouraging cage socialization.
Single-housing effect on body weight and fecal output before and after SRB administration.
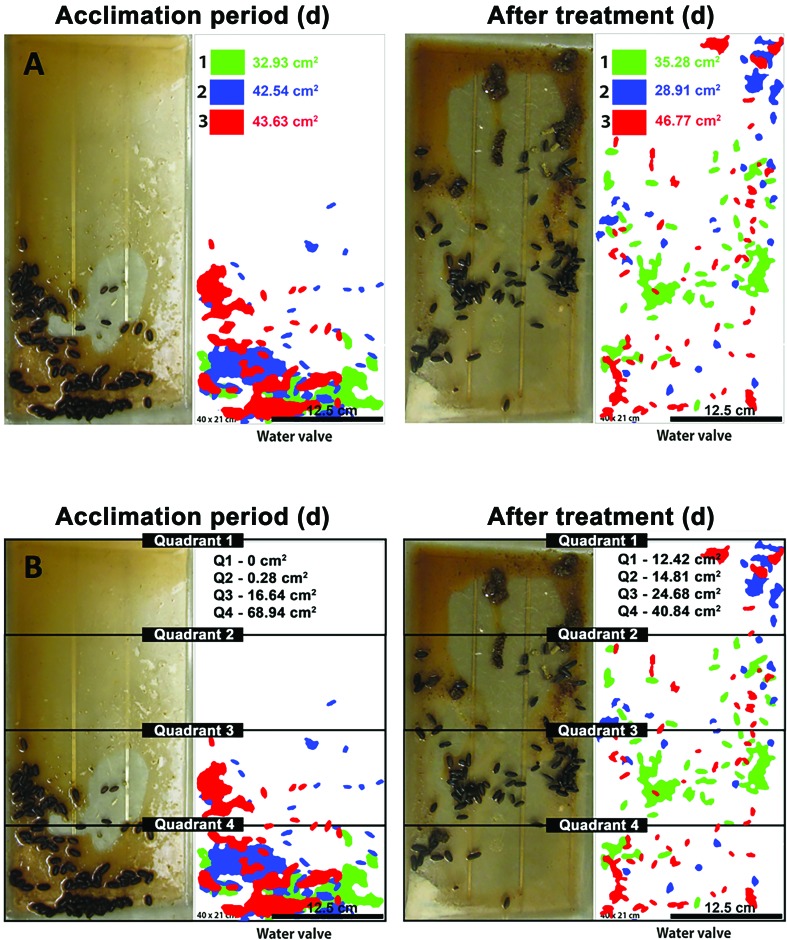
Once guinea pigs were single-housed, cages were changed daily for 6 d. During each cage change, the husbandry staff was instructed to move the cages away from the automatic water valve to prevent water accumulation inside the cages. At each cage change, body weight was measured. Likewise, images of the cage bottoms were taken to assess fecal output (quantity and distribution; (Figure 1) 3 d before (acclimation period [baseline]) and 3 d after each treatment (Sham, SRBLow, SRBMedium, and SRBHigh). Photoshop CC (Adobe, San Jose, CA) was used to determine the total area of the fecal pellets observed within each day before and after SRB administration (Figure 1). This information was further used to calculate changes in the fecal output area (expressed as a percentage) after SRB administration. The changes were compared with the sham group and with the data obtained during the acclimation period (baseline).
Figure 1.
Schematic representation of the quantitative analysis of fecal output. Images of the cage bottoms of single-housed guinea pigs were recorded at 1 d (green), 2 d (blue), and 3 d (red) before treatment (acclimation period) and 3 d (red), 2 d (blue), and 1 d (green) after the administration of SRB (Sham, SRBLow, SRBMedium and SRBHigh). (A) Photoshop CC was used to determine the total area (in cm2) of fecal pellet distribution on each day; this information was used to calculate changes in the fecal output area (expressed as a percentage) after treatment compared with results obtained during the acclimation period (baseline). (B) The same images were used to determine the effect of SRB on the distribution of the fecal pellets inside the cages. In particular, each image was divided into 4 quadrants, and the cumulative fecal output area in each quadrant during each period (acclimation period and after SRB administration) was calculated. This information was used to find changes (compared with baseline) in the total fecal output area during the first 3 d after administration in each quadrant. The orientation of each cage relative to the automated water supply in the rack is indicated. Cage dimensions, 40 × 20 cm; scale bar, 12.5 cm2.
To determine the effect of SRB on the distribution of the fecal pellets inside the cages, the same images of the bottom of the cages were divided into 4 identical quadrants (Q1-4). The cumulative fecal output areas for each quadrant were then obtained within each period (acclimation [baseline]; and post-SRB administration). This information was finally used to determine significant changes per quadrant in the total fecal output area in all tested groups (Sham, SRBLow, SRBMedium and SRBHigh) within the first 72 h after SRB administration.
Sequential blood collection.
For blood collection, animals were anesthetized with 2-4% isoflurane in an induction chamber and then transferred to the surgical table, where anesthesia was maintained via nose cone. Briefly, each guinea pig was placed in dorsal recumbence over a water recirculating blanket. Rectal temperature, heart rate and respiration were continuously measured using a rodent anesthesia monitoring suite (PhysioSuite, Kent Scientific, Torrington, CT).
On the first day (day 0), the cervical ventral region of each guinea pig was shaved and properly prepared for aseptic collection of less than 1 mL of blood from the jugular vein. After the first blood sample was collected (time point 0), anesthetized guinea pigs were then placed in lateral recumbence and 1 injection of saline or SRB was subcutaneously administered with a 20-gauge needle to a shaved area (2 x 2 cm) located in the right scapular region. Subsequent blood collections were performed at 1, 3, 6, 12, 24, 48 and 72 h after saline or SRB administration. At the end of each blood collection, animals were allowed to fully recover. Finally, between blood draws, we monitored the general status of the animals as well as the development of injection site reaction, anemia, anorexia, dehydration, local phlebitis and constipation.
Pharmacokinetics, serum cortisol, and biochemistry analysis.
Blood samples were transferred to EDTA-coated (pharmacokinetic and cortisol studies) or serum separator (biochemistry) tubes. Plasma and serum were immediately separated at 1700 x g for 5 min in a refrigerated centrifuge, and samples were stored at –80 °C until analyzed in our validated buprenorphine liquid chromatography–mass spectrometry (LC-MS) assay.40 Plasma SRB(Low, Medium, High) buprenorphine levels were analyzed using noncompartmental pharmacokinetic modeling. Serial blood quantification was used to calculate pharmacokinetic parameters such as minimum and maximum drug concentration (Cmin, Cmax), time to reach maximum concentration in plasma (Tmax), half-life and area under the curve (AUC0-72h). Serum was also used to quantitate cortisol levels as previously described.34 As a consequence, blood samples from each treatment group were also collected at 0 and 72 h before submission to Antech Diagnostics (Irvine, CA) for serum biochemistry analysis.
Statistical analysis.
A randomized block design was used in this study. The data were normally distributed and were analyzed by an individual blinded to the treatment groups. Two-way ANOVA with Bonferroni correction was used to assess significant changes between and within treatment groups (baseline, sham, SRBLow, SRBMedium, and SRBHigh) at various time points (0, 1, 3, 6, 12, 24, 48 and 72 h). The Tukey test was used to identify significant differences in the fecal output distribution. All data were analyzed with Prism (GraphPad, La Jolla, Ca) and are reported as mean ± 1 SD. Statistical significance was set at a P value of less than 0.05.
Results
Effect of SRB on the fecal output of healthy guinea pigs.
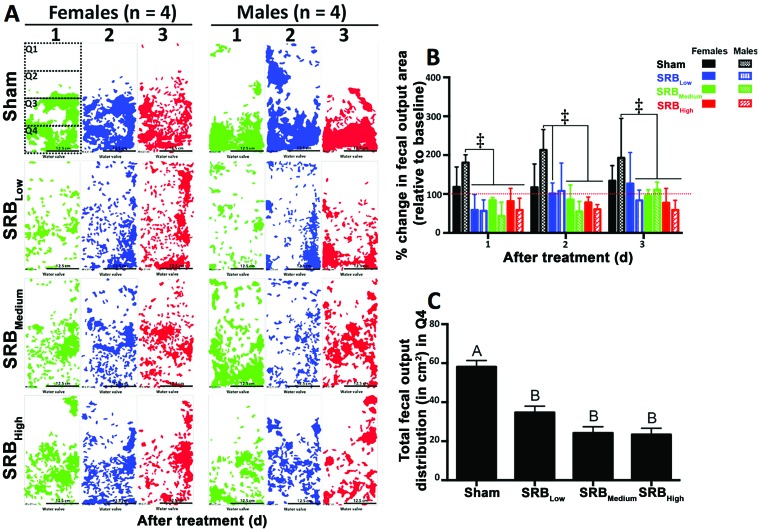
Independent of sex and time point, the fecal output area of the sham group remained consistently above baseline values (P < 0.05) throughout the study period (Figure 2). During the first day, the fecal output (percentage of total area of cage bottom) in the sham group was 18% ± 26% and 80% ± 10% above the baseline values reported for both female and male guinea pigs, respectively (Figure 2 B). During the second day, fecal output of the sham groups was 17% ± 30% (females) and 113% ± 26% (males) above baseline values (Figure 2 B). On the third day, female and male guinea pigs in the sham group had, respectively, 33% ± 20% and 92% ± 51% more fecal pellets on the bottoms of the cages than had been measured during the acclimation (baseline) phase of the study (Figure 2 B).
Figure 2.
Effect of SRB on the quantity and distribution of fecal output from healthy guinea pigs. (A) The cumulative (n = 4) fecal output diagram for each day (1 d [green], 2 d [blue] and 3 d [red] )in both females and males in all groups. In the day-1 diagram for the females in the sham group, dashed boxes were added to indicate the 4 equal quadrants inside the cages. The cumulative total areas from both sexes and all time points were used to compare the total fecal output areas between treatment groups and quadrants. (B) With the exception of females in the sham group (all time points) and SRBLow group (day 2) and males in the SRBLow group (day 3), the total fecal output areas were lower than those for males in the sham group, independent of the dose of SRB tested (‡, P < 0.001, 2-way ANOVA). The red dotted line represents the fecal output quantity during the acclimation period (baseline). (C) Changes in the fecal output distribution after administration in the sham and SRB groups were significant only in quadrant 4 (Q4). Different uppercase letters represent significant differences between Sham and SRB groups (P < 0.001, Tukey test). Cage dimensions, 40 × 20 cm; Scale bar, 12.5 cm.
Compared with baseline values, fecal output fluctuated in a sex- and time-dependent manner independent of the SRB tested dosage (Figure 2). During day 1, the fecal output in the SRBLow (41% ± 20% and 43% ± 14%), SRBMedium (16% ± 3% and 57% ± 18%), and SRBHigh (18% ± 16% and 41% ± 15%) groups were, respectively, below baseline values reported in both males and females (* P < 0.05, Figure 2 B). On day 2, the fecal output in females and males treated with SRBLow were, respectively, 1% ± 14% and 7% ± 36% above baseline values. Within the same time period, the fecal output of female and male guinea pigs treated with the SRBMedium (14% ± 19% and 45% ± 13%) and SRBHigh (22% ± 7% and 39% ± 6%) doses remained, respectively, below baseline values (Figure 2 B). After 3 d, fecal output below baseline values was reported only in males treated with SRBLow (16% ± 13%), females treated with SRBMedium (4% ± 8%), and both females and males guinea pigs treated with SRBHigh (22% ± 19% and 41% ± 12%) (Figure 2 B).
When compared to sham, guinea pigs treated with SRB had significantly decreased fecal output concentrations independent of the sex, time point or dose (Figure 2AB). However, throughout the study, significant differences between groups were observed only between males in the sham group and guinea pigs (both sexes) treated with SRBMedium and SRBHigh (P < 0.001, Figure 2 B). No differences in fecal output were observed over time within the SRB groups. During the first day after SRB administration, animals treated with SRBLow had fecal output concentrations significantly lower than those of the Sham group (males). However, within the following days, significant differences between sham and SRBLow were observed only in females (day 2) and males (day 3), respectively (P < 0.001, Figure 2 B).
Effect of SRB on the fecal output distribution in cages of single-housed guinea pigs.
Independent of sex, overall fecal output distribution was significantly different only for quadrant 4 (P < 0.001, Figure 2 C – located close to the water valve). The total posttreatment fecal output in this quadrant was higher in the sham group (58.1 ± 3.2 cm2) compared with all other SRB groups tested (P < 0.001, Figure 2 C). No other differences were observed between treatment groups.
Effect of SRB on the body weight of healthy guinea pigs.
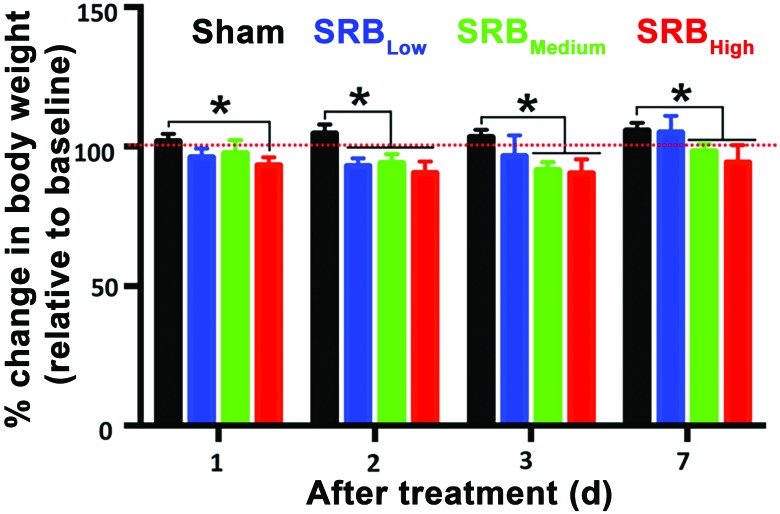
Independent of dose and time point, sex had no effect on body weight. Therefore, the data in Figure 3 represent average weights among all tested subjects (n = 8 per group). Compared with baselines collected during the acclimation period, body weights in the sham group remained consistently above baseline values throughout the study period (Figure 3). Specifically, body weight in the Sham group was, respectively, 1.0% ± 0.9% (day 1), 4.7% ± 1.1% (day 2) and 3.4% ± 0.9% (day 3) higher (P < 0.05) than baseline levels. After 7 d, the overall average weight in all sham-treated guinea pigs was 5.8% ± 0.9% higher (P < 0.05) than that measured during the acclimation (baseline) phase.
Figure 3.
Effect of SRB on the body weight of healthy guinea pigs. The red dotted line indicates the baseline body weights collected during the acclimation period. Body weight did not differ between sexes. *, P < 0.05 (2-way ANOVA).
Guinea pigs treated with any of the 3 SRB dosages lost less than 10% of their baseline body weight during the first 3 d after drug administration. Specifically, on day 1, the average body weight lost was 3.7% ± 1.1%, 2.5% ± 1.7% and 6.6% ± 1.0%, respectively, in the SRBLow, SRBMedium, and SRBHigh groups (P < 0.05, Figure 3). On day 2, guinea pigs had lost 6.9% ± 1.0% (SRBLow), 5.6% ± 1.1% (SRBMedium), and 9.4% ± 1.4% (SRBHigh) of body weight when compared with baselines (P < 0.05, Figure 3). Weight loss on day 3 averaged 3.5% ± 2.7% (SRBLow), 8.5% ± 1.0% (SRBMedium), and 9.5% ± 1.8% (SRBHigh) relative to baseline (P < 0.05, Figure 3). By day 7, guinea pigs treated with SRBLow had body weight 5.0 ± 2.09 % above baseline values (Figure 3). Even though no significant differences were reported within the same time point, the average body weight in guinea pigs treated with SRBMedium (1.7 ± 0.9 %) and SRBHigh (5.7 ± 2.2 %) were still below values collected during the acclimation (baseline) phase of this study (P < 0.05, Figure 3).
In this study, no significant differences were observed between SRB groups. Comparisons between all time points and tested dosages revealed that the average weight after SRB administration was lower than observed in the sham group (P < 0.05, Figure 3). At day 1, the average body weight in the sham group was statistically greater only when compared to SRBHigh (P < 0.05, Figure 3). Two days after SRB administration, the average body weight in the sham group was statistically greater than that observed in all SRB groups (P < 0.05, Figure 3). At days 3 and 7, significant differences in body weight were observed only between the sham animals and those treated with SRBMedium and SRBHigh (P < 0.05, Figure 3).
Physiologic and serum biochemical changes after SRB administration.
Throughout this study, body temperature, heart rate, and respiratory rate did not differ between groups or sexes. The overall averages for temperature (39.2 ± 0.1 °C; reference, 34 to 40 °C), heart rate (261 ± 2 bpm; reference, 240 to 350 bpm), and respiration (56 ± 1 breaths per minute; reference, 40 to 150 breaths per minute) were within normal published values18,22,33 for healthy guinea pigs. In addition, no skin reactions to any of the tested doses were observed in any of the animals throughout the study period. Although all biochemistry markers were within normal limits for healthy guinea pigs,7,22 the serum AST concentration in samples collected before drug administration (baseline) and 3 d after SRBLow treatment were significantly (P < 0.05) lower than those observed during the same time period in guinea pigs treated with SRBMedium or SRBHigh (Tables 1 and 2).
Table 1.
Biochemistry profile of healthy female guinea pigs (n = 4) after SRB administration
| After administration |
||||||||||
| Baseline |
SRBLow |
SRBMedium |
SRBHigh |
|||||||
| Units | Mean ± 1 SD | Range | Mean± 1 SD | Range | Mean ± 1 SD | Range | Mean ± 1 SD | Range | Reference range5,21 | |
| Total protein | g/dL | 5.36 ± 0.46 | 4.8–6 | 5.55 ± 0.53 | 5–6 | 5.1 ± 1.41 | 3.6–6 | 5.1 ± 0.35 | 4.8–5.4 | 4.5–7.1 |
| Albumin | g/dL | 3.14 ± 0.30 | 2.5–3.6 | 3.13 ± 0.46 | 2.5–3.6 | 2.7 ± 0.65 | 1.8–3.3 | 2.7 ± 0.35 | 2.4–3 | 2.3–4.8 |
| Globulin | g/dL | 2.22 ± 0.31 | 1.8–2.8 | 2.43 ± 0.33 | 2–2.8 | 2.4 ± 0.42 | 1.8–2.7 | 2.4 ± 0.00 | 2.4 | 1.7–2.6 |
| Albumin: globulin | — | 1.44 ± 0.23 | 1–1.7 | 1.3 ± 0.29 | 1–1.6 | 1.13 ± 0.15 | 1–1.3 | 1.15 ± 0.17 | 1–1.3 | — |
| AST | IU/L | 52.83 ± 10.04a | 39–69 | 49.5 ± 11.7a | 40–64 | 100.5 ± 59.32b | 42–183 | 86.25 ± 25.38b | 54–108 | 38–643 |
| ALT | IU/L | 34.17 ± 5.22 | 33–36 | 38 ± 2.94 | 35–41 | 38.5 ± 7.94 | 35–43 | 40.5 ± 7.14 | 37–45 | 32–138 |
| ALP | IU/L | 91.25 ± 19.17 | 69–120 | 106.5 ± 15.61 | 84–120 | 79.5 ± 13.3 | 71–81 | 72.5 ± 6.24 | 71–90 | 68–399 |
| GGT | IU/L | 8.33 ± 5.26 | 7–12 | 11.5 ± 0.58 | 8–13 | 9 ± 2.45 | 6–12 | 12.25 ± 6.65 | 9–14 | 3–14 |
| Total bilirubin | mg/dL | 0.10 ± 0.00 | 0.1 | 0.1 ± 0.00 | 0.1 | 0.1 ± 0.00 | 0.1 | 0.1 ± 0.00 | 0.1 | 0.1–0.8 |
| BUN | mg/dL | 22.00 ± 5.19 | 16–30 | 18.75 ± 7.54 | 15–30 | 21.75 ± 3.77 | 18–27 | 20.25 ± 1.5 | 18–21 | 15.7–31.5 |
| Creatinine | mg/dL | 0.48 ± 0.12 | 0.4–0.5 | 0.45 ± 0.13 | 0.4–0.5 | 0.55 ± 0.1 | 0.5–0.7 | 0.5 ± 0.00 | 0.5 | 0.4–1.8 |
| BUN:creatinine | — | 65.50 ± 28.27 | 30–120 | 60 ± 31.62 | 30–100 | 40.5 ± 10.25 | 30–54 | 40.5 ± 3 | 36–42 | — |
| Phosphorus | mg/dL | 6.04 ± 1.78 | 4.9–9.3 | 7.2 ± 1.21 | 5.4–8 | 5.63 ± 0.93 | 4.8–6.9 | 5.33 ± 1.28 | 4.9–6.9 | 4.2–15 |
| Glucose | mg/dL | 152.25 ± 59.19 | 129–192 | 95.75 ± 34.3 | 88–126 | 96.50±23.41 | 81–126 | 110.25 ± 9.91 | 96–117 | 80–546 |
| Calcium | mg/dL | 10.55 ± 0.60 | 9–11.4 | 10.38 ± 0.94 | 9–11.1 | 10.5 ± 0.88 | 9.3–11.4 | 10.43 ± 0.86 | 9.3–11.4 | 9.0–14.6 |
| Magnesium | mEq/L | 2.31 ± 0.75 | 2.1–2.5 | 2.13 ± 0.25 | 2–2.5 | 2.55 ± 0.52 | 2.3–2.7 | 2.4 ± 0.24 | 2.1–2.7 | 2.1–2.7 |
| Sodium | mEq/L | 136.58 ± 6.57 | 128–148 | 136.25 ± 5.25 | 133–144 | 143 ± 5.35 | 137–150 | 144.5 ± 9.98 | 130–152 | 121–159.5 |
| Potassium | mEq/L | 5.18 ± 0.54 | 4.5–6.2 | 5.15 ± 0.51 | 4.5–5.6 | 4.95 ± 0.3 | 4.8–5.4 | 4.58 ± 0.38 | 4.2–5.1 | 4–23.11 |
| Na:K | — | 26.67 ± 1.87 | 24–30 | 26.75 ± 2.5 | 24–30 | 29 ± 2.71 | 25–31 | 31.75 ± 4.79 | 25–36 | — |
| Chloride | mEq/L | 101.58 ± 4.89 | 94–112 | 98.25 ± 3.3 | 94–102 | 110 ± 2.83 | 106–112 | 111.5 ± 9.26 | 98–119 | 96–130.7 |
| Cholesterol | mg/dL | 59.17 ± 19.57 | 34–90 | 59.5 ± 23.81 | 34–84 | 69.75 ± 36.94 | 42–123 | 78 ± 20.05 | 57–105 | 20–133 |
| Triglycerides | mg/dL | 125.92 ± 53.35 | 45–213 | 105.5 ± 54.06 | 45–168 | 128.25 ± 40.28 | 75–165 | 156.75 ± 59.31 | 96–225 | 28–284 |
Different lowercase letters indicate significantly (P < 0.05) different values.
Table 2.
Biochemistry profile of healthy male guinea pigs (n = 4) after SRB administration
| After administration |
||||||||||
| Baseline |
SRBLow |
SRBMedium |
SRBHigh |
|||||||
| Units | Mean ± 1 SD | Range | Mean ± 1 SD | Range | Mean ± 1 SD | Range | Mean ± 1 SD | Range | Reference range5,21 | |
| Total protein | g/dL | 5.13 ± 0.56 | 4.5–6.6 | 4.98 ± 0.21 | 4.8–5.2 | 4.88 ± 0.45 | 4.5–5.4 | 5.03 ± 0.38 | 4.5–5.4 | 4.5–6.9 |
| Albumin | g/dL | 3.03 ± 0.37 | 2.7–3.9 | 2.95 ± 0.26 | 2.7–3.3 | 2.85 ± 0.57 | 2.4–3.6 | 2.63 ± 0.38 | 2.2–3 | 2.3–4.9 |
| Globulin | g/dL | 2.1 ± 0.29 | 1.8–2.6 | 2.03 ± 0.29 | 1.8–2.4 | 2.03 ± 0.15 | 1.8–2.1 | 2.4 ± 0.24 | 2.1–2.6 | 1.7–2.6 |
| Albumin:globulin | — | 1.47 ± 0.18 | 1.2–1.8 | 1.5 ± 0.29 | 1.2–1.8 | 1.4 ± 0.42 | 1.1–2 | 1.1 ± 0.22 | 0.9–1.4 | — |
| AST | IU/L | 59.75 ± 26.54a | 39–129 | 51.75 ± 22.37a | 38–84 | 131.25 ± 95.13b | 39–261 | 286.5 ± 222.3b | 90–498 | 38–541 |
| ALT | IU/L | 38.83 ± 9.07 | 35–45 | 32.75 ± 6.02 | 31–38 | 36 ± 11.22 | 34–51 | 108 ± 78.73 | 39–189 | 32–175 |
| ALP | IU/L | 82.5 ± 13.08a | 72–99 | 77 ± 4.9a | 71–83 | 120.17 ± 25.97b | 72–162 | 136.25 ± 26.39b | 104–162 | 68–368 |
| GGT | IU/L | 11.08 ± 4.27 | 10–14 | 12.5 ± 4.73 | 12–14 | 7.5 ± 1.73 | 7–9 | 13 ± 4.24 | 12–14 | 4–14 |
| Total bilirubin | mg/dL | 0.1 ± 0.00 | 0.1 | 0.1 ± 0.00 | 0.1 | 0.1 ± 0.00 | 0.1 | 0.2 ± 0.12 | 0.1–0.3 | 0.1–0.8 |
| BUN | mg/dL | 22.17 ± 4.17 | 15–30 | 20 ± 2.83 | 18–24 | 18.75 ± 1.5 | 18–21 | 22.5 ± 3 | 21–27 | 15.7–33 |
| Creatinine | mg/dL | 0.48 ± 0.1 | 0.4–0.5 | 0.43 ± 0.05 | 0.4–0.5 | 0.55 ± 0.1 | 0.5–0.7 | 0.5 ± 0.00 | 0.5 | 0.4–1.8 |
| BUN:creatinine | — | 63.5 ± 22.04 | 30–100 | 62.5 ± 12.58 | 50–80 | 34.5 ± 3 | 30–36 | 45 ± 6.00 | 42–54 | — |
| Phosphorus | mg/dL | 5.56 ± 1.37 | 4.9–6.4 | 6.3 ± 1.05 | 6–6.5 | 4.88 ± 0.45 | 4.5–5.4 | 4.88 ± 0.57 | 4.5–5.7 | 4.2–6.5 |
| Glucose | mg/dL | 131.25 ± 31.27 | 102–153 | 113.25 ± 3.77 | 108–117 | 85.83 ± 35.99 | 81–138 | 93.25 ± 32.53 | 80–138 | 80–533 |
| Calcium | mg/dL | 10.37 ± 0.72 | 9.6–12.3 | 10.33 ± 0.29 | 9.9–10.5 | 10.13 ± 0.45 | 9.6–10.5 | 10.65 ± 0.17 | 10.5–10.8 | 9.0–14.4 |
| Magnesium | mEq/L | 2.36 ± 0.65 | 2.2–2.6 | 2.4 ± 0.14 | 2.2–2.7 | 2.18 ± 0.29 | 2.1–2.3 | 2.18 ± 0.15 | 2.1–2.4 | 2.1–2.7 |
| Sodium | mEq/L | 137.58 ± 8.8 | 127–158 | 138.75 ± 5.19 | 131–142 | 146.25 ± 5.38 | 141–153 | 147.75 ± 1.71 | 146–150 | 121–160.7 |
| Potassium | mEq/L | 5.38 ± 1.15 | 4.2–7.5 | 5.13 ± 0.93 | 4.2–6.4 | 4.58 ± 0.29 | 4.2–4.8 | 5.25 ± 0.71 | 4.5–6 | 4–22.31 |
| Na:K | — | 26.58 ± 4.52 | 19–34 | 28 ± 5.16 | 22–34 | 32 ± 2.45 | 29–35 | 28.75 ± 3.86 | 25–33 | — |
| Chloride | mEq/L | 101.92 ± 6.02 | 96–118 | 100.25 ± 3.5 | 96–104 | 111 ± 4.69 | 106–117 | 111.5 ± 3.42 | 107–115 | 96–128.9 |
| Cholesterol | mg/dL | 33 ± 11.22 | 21–57 | 28.5 ± 866 | 21–36 | 37.5 ± 7.14 | 33–48 | 42.75 ± 11.32 | 33–54 | 20–128 |
| Triglycerides | mg/dL | 65.33 ± 18.25 | 39–105 | 56.5 ±11.966 | 42–69 | 62.25 ± 17.73 | 45–84 | 119.25 ± 44.72 | 69–177 | 28–245 |
Different lowercase letters indicate significantly (P < 0.05) different value
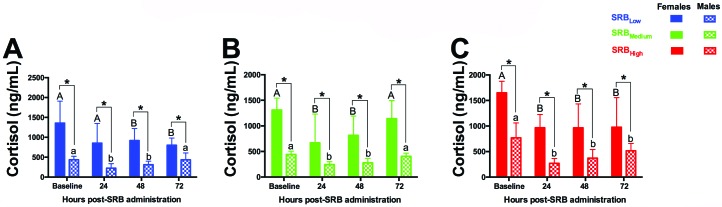
Baseline levels of serum cortisol in healthy female guinea pigs (1440 ± 106 ng/mL) were significantly (P < 0.05) higher than those observed in males (550 ± 66 ng/mL) throughout the study period (Figure 4). With the exception of female guinea pigs in the first 24 h after SRBLow administration (Figure 4 A), all SRB groups had significant decreases (P < 0.05) in serum cortisol in both sexes in a time-dependent manner for at least 48 h. At the end of this study, cortisol levels in females (all SRB groups) and males treated with SRHigh remained significantly lower than baseline values (P < 0.05, Figure 4).
Figure 4.
Serum cortisol dynamics in healthy guinea pigs treated with 3 SRB dosages. (A) SRBHigh. (B) SRBMedium. (C) SRBLow. Within each time point, different uppercase letters represent significant differences in cortisol levels in female guinea pigs before (baseline) and after administration. Within each time point, different lowercase letters represent significant differences in cortisol levels in male guinea pigs before (baseline) and after SRB administration. *, Significant (P < 0.05, 2-way ANOVA) difference between sexes at the same time point.
Pharmacokinetics of 3 SRB dosages in healthy guinea pigs using a LC-MS quantitative assay.
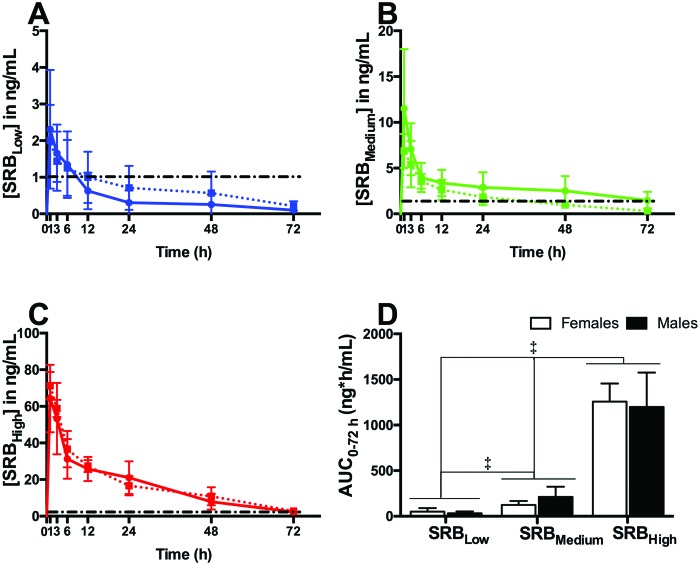
For all drug-treated groups, the detection limit of the assay (Cmin) was 0.1 ng/mL. Within all SRB groups, no significant differences in the pharmacokinetic profiles or AUC(0-72 h) were observed between sexes. All significant differences in plasma concentrations of the drug were dose-dependent only (Figure 5). For all SRB groups, Cmax occurred at 1 h after administration (Tmax). At this time point, guinea pigs treated with the SRBHigh had the highest plasma concentration (males, 64.3 ± 9.2 ng/mL; females, 71.3 ± 3.7 ng/mL), followed by SRBMedium (males, 11.5 ± 3.2 ng/mL; females, 6.9 ± 0.9 ng/mL) and SRBLow (males, 2.3 ± 0.8 ng/mL; females, 2.0 ± 0.5 ng/mL). Therapeutic levels of the drug (>1 ng/mL, Figure 5) were observed throughout this study in guinea pigs treated with SRBHigh (both sexes) and SRBMedium (males only). In females treated with SRBMedium, therapeutic levels of the drug were observed only within the first 24 h of this study (Figure 5 B). Guinea pigs treated with SRBLow maintained plasma concentration of the drug above 1 ng/ml during the first 6 h after SRBLow administration (Figure 5 A). At the end of the study (72 h), the SRBHigh plasma concentration averaged 2.3 ng/mL (Figure 5 C), whereas the concentration in guinea pigs treated with SRBMedium averaged 0.9 ng/mL (Figure 5 B). Within the same time point, the plasma concentration of the drug in guinea pigs treated with SRBLow was below the detection limit (Figure 5 A).
Figure 5.
Pharmacokinetics of 3 concentrations of SRB in healthy guinea pigs (females, dotted lines; males, solid lines). In A through C, black dashed lines represent target therapeutic levels (1 ng/mL). Overall plasma concentrations are expressed as AUC0-72 h. (D) AUC0-72 h of the SRBHigh group was greater than those for SRBMedium and SRBLow. ‡, P < 0.001 (one-way ANOVA).
Finally, the AUC0 to 72 h was significantly (P < 0.001) greater in the SRBHigh group (males, 1198 ± 188 ng*hour/mL; females, 1257 ± 99 ng*hour/mL) than in the SRBMedium group (males, 213 ± 56 ng*hour/mL; females, 127 ± 21 ng*hour/mL) and SRBLow group (males, 32 ± 10 ng*hour/mL; females, 50 ± 19 ng*hour/mL; Figure 5 D).
Discussion
In this study, we characterized the pharmacokinetic profiles and adverse effects of 3 SRB dosages (SRBLow – 0.15 mg/kg, SRBMedium – 0.3 mg/kg, SRBHigh – 0.6 mg/kg) in healthy guinea pigs for 72 h after a single subcutaneous administration.
In guinea pigs, the recommended dose of IRB is usually 0.05 mg/kg SC given twice daily.6,20,32 A recent study indicated that at this dose, the Cmax of the drug (2.33 ng/mL) is reached 1 h after administration.32 In our study, we also used the recommended IRB dose6,20,32 to extrapolate the equivalent SRB dose required (0.3 mg/kg) to provide sustained plasma levels of buprenorphine for 72 h. However, unlike the previous work,32 we used the recommended IRB dose6,20,32 in our escalating design to determine the effects of both SRB extremes (low, 0.15 mg/kg; high, 0.6 mg/kg) in healthy guinea pigs. As a result, within the tested range, our approach will allow safe and target customization of different SRB dosages in future pain-specific models.
In the laboratory animal community, therapeutic levels of buprenorphine of 1 ng/mL or greater were originally derived from humans5,21,26 and have been carried over as an acceptable target for clinically significant pain relief without in-depth validation in other species.5-10,13,16,21,25-27,32,35,37,38 Buprenorphine is mainly metabolized in the liver at different rates as norbuprenorphine, buprenorphine-3-glucuronide, and norbuprenorphine-3-glucuronide,21,26 and different species also have different metabolic rates.2,21,26 Therefore, depending on the target species and its metabolic activity, the concentration of buprenorphine needed for therapeutic efficacy might also differ from the established 1 ng/mL.
According to our results, guinea pigs treated with SRBHigh (both sexes) and SRBMedium (males only) displayed therapeutic levels of buprenorphine above >1 ng/mL throughout the study period. However, female guinea pigs treated with SRBMedium (0.3 mg/kg) sustained plasma concentration of the drug above 1 ng/mL during the first 24 h only, as previously described.32 As a result, it is unclear if this concentration of SRB would be adequate to alleviate pain in female guinea pigs undergoing a surgical procedure, given that recent comparisons between SRB at 0.3 mg/kg (that is, SRBMedium) and the IRB formulation revealed that the amount of pressure needed to elicit a withdrawal response in the paw of female guinea pigs differed only during the first 12 h of the study.32 Therefore, it is critical that administration of any of the SRB dosages tested here are closely monitored for the development of adverse effects or signs of pain that would prompt intervention until an optimal plasma concentration of the drug is found.
In the literature, adverse effects related to the use of SRB have been described in many laboratory animal species.5,8,13,16,32,38 Of those, injection site reactions are by far the most cited.13,25-27,32,35 In particular for the formulation we used in the current study (SRB-LAB), these reactions were associated with the biodegradable vehicle used to deliver buprenorphine.13,26,32 Due to the local inflammation caused by this vehicle and the infection associated with repeated self-trauma of the injection site,26,35 the skin lesions have consistently been characterized as mild ulcerative dermatitis or full-thickness necrosis with concurrent cellulitis, inflammation, and hemorrhage.13,25-27,35 In an attempt to counteract this problem, the manufacturer replaced the original vehicle with a new polymer that is believed to be more biocompatible and thus less likely to elicit a skin reaction.32 Consequently, in our current study, none of the single subcutaneous injections of any of the tested SRB doses resulted in injection site reactions during the first 72 h after administration. As previously observed,16,32,37 the current formulation of SRB was designed to elicit little or undetectable reactivity at the injection site in the skin after single administration in most species. Further investigation is needed to determine whether similar results are achieved when multiple administrations of the drug are needed, particularly during prolonged treatment regimens, in guinea pigs or any other laboratory animal species.
Opioid-induced bowel dysfunction (OIBD) is a common adverse effect associated with the use of opioid analgesics.20,21 The pathophysiology of this syndrome is complex and not fully characterized.21 However, most of the effects of peripherally-administered opioids on μ-receptor occurs in the gut wall or CNS.6,8,13,16,21,32,38 In the literature, OIBD comprises several symptoms, but anorexia and constipation are commonly associated with buprenorphine.5-10,13,16,21,25-27,32,35,37,38 Significant changes in body weight are subtle.5-8,13,16,25-27,32,35,37,38 In small laboratory animals (mice, rats, and rabbits) undergoing surgery, weight loss after SRB administration is usually transient (averaging less than 10% of body weight) and is highly dose-dependent.5,8,10,13,16,30,32 In reports involving large-animal species (i.e. dogs, macaques, sheep, swine),26,27,35,37 the use of SRB to manage pain appears to be associated with mild decreases in appetite, particularly in dogs.27 Overall, in large animals, body weight after administration of the recommended SRB dose is described in the literature to fluctuate within normal values for the corresponding species.26,27,35,37
In our study, significant changes in body weight were observed between time points and the tested SRB dose independent of the sex. As previously reported in small laboratory species,5,8,10,13,16,30 our guinea pigs lost less than 10% of their body weight. Similar results have recently been reported in healthy female guinea pigs after single administration of IRB (0.05 mg/kg) or SRB (0.3 mg/kg).32 In that study, weight loss during the first 3 d was comparable between the 2 drug formulations; however, the authors did not describe the time needed to recover the lost weight.32 In our study, all animals regained the lost weight 7 d after SRB administration. Moreover, at this time point, their body weights were comparable to that measured during the acclimation phase of this study (baselines). The transient weight loss in our study might be explained in part by OIBD, given that anorexia is a common symptom.5,8,13,16,25-27,35,37,38 However, the fast weight recovery might also be due to this species’ need to frequently eat to maintain its high metabolic demands.11,32,33 In fact, a common observation in this study was that all guinea pigs started to eat immediately after anesthesia recovery independent of the SRB tested group.
The so called ‘time-to-consumption’ (TTC) was recently introduced as a potential proxy to assess pain in guinea pigs.11 Even though a carprofen-based analgesia protocol was used in that study and no significant differences in the TTC were observed after surgery, the guinea pigs were highly food-motivated.11 As described in our current study and elsewhere,32 SRB caused weight loss in healthy guinea pigs within the first 72 h after administration. However, how TTC11 could change postoperatively in the context of SRB-based regimens remains unknown. Therefore, future investigations should establish correlations between changes in body weight immediately after surgery and TTC to better predict appropriate intervention in SRB pain-specific protocols.
Constipation is another OIBD symptom commonly associated with buprenorphine.5-10,13,16,21,25-27,32,35,37,38 The myenteric and submucaosal plexus in the gut wall are, respectively, responsible for gastrointestinal motility and secretion.21 Opioids modify gastrointestinal function by inhibiting the excitatory and inhibitory neural pathways within the enteric nervous system coordinating motility.1,21,26 Inhibition of excitatory neural pathways depresses peristaltic contractions, ultimately leading to delayed gastric emptying and slowing of intestinal transit.5-10,13,16,25-27,32,35,37,38 This decreased transit has recently been used to monitor gastrointestinal hypomotility after buprenorphine administration in rabbits.23 In guinea pigs, however, similar work remains to be performed regarding the administration of either IRB or SRB formulations . In our study, we used daily fecal output measurements in single-housed guinea pigs to assess the degree of constipation after administration of the tested SRB dosages. Consequently, we developed a quantitative method that provides an accurate and real-time assessment of the fecal output after SRB administration. This method can be easily adopted to quantitate changes in fecal output whenever constipation related to OIBD is a concern.5-10,13,16,21,23,25-27,32,35,37,38 Moreover, this method could be used further to similarly assess the daily fecal output effects of SRB in pain-specific studies.
Our quantitative method for assessing daily fecal output revealed interesting information related to the distribution of the fecal pellets in the bottom of the cages. According to our data, independent of the SRB group test, sex, and time point, single-housed guinea pigs during the acclimation phase of this study (baseline) had increased fecal output deposition at the lower quadrant of the cage (quadrant 4) compared with other quadrants. The automated water valve was located in this same quadrant. However, this pattern significantly changed after SRB administration. Hallucinations are known adverse effects of buprenorphine in humans.21,26,30 Perhaps the loss of environmental awareness related to the use of this opioid explains the significant changes in fecal output distribution in the cages.21,26,30 One could also suggest that the disturbance in fecal output distribution observed in this study was a reflection of distress related to procedures performed in this study;11,32,33,36 however, we controlled for such effects with our sham group. Animals in this group were exposed to the same experimental stressors that SRB-based groups were. Yet, the fecal output distribution in this group (Sham) was significantly higher in Q4 and remained unchanged throughout the study period. As a conclusion, the reasons why single-housed guinea pigs during the acclimation period ‘preferred’ to defecate close to the water supply remain to be determined. Although SRB affected this pattern, further work must be performed to determine whether other analgesics and clinically relevant painful stimuli can also alter this pattern.11,32,36 Because TTC recently failed as a proxy indicator for pain or distress in guinea pigs undergoing survival surgery,11 our data support the hypothesis that changes in fecal output distribution in single-housed guinea pigs should be further explored for that purpose.
In terms of physiologic changes, buprenorphine causes limited respiratory depression, with a ceiling effect at higher dosages.20,21,32,33 We saw similar trends in guinea pigs treated with SRBHigh. In general, the respiratory rate was lower in animals belonging to this group compared with the other groups. Prior to blood collection at each time point, we initially anesthetized the guinea pigs with isoflurane in an induction chamber and then transferred them to the stage where monitoring of vital signs took place. As a result, each data point for temperature, heart rate, and respiratory rate was collected within 10 min from start to full anesthesia recovery. Therefore, one could expect to find significant changes in the respiratory rate after SRBHigh administration in procedures requiring prolonged anesthesia monitoring.20,21,32,33
Other important physiological changes observed in this study were related to serum concentrations of AST and cortisol. Independent of sex, data collected 3 d after SRB administration revealed that serum AST concentrations in guinea pigs treated with SRBMedium and SRBHigh were at least twice as high as baseline values. Even though the AST values were within the normal range for the strain,7,22 users must carefully consider the use of SRB (0.3 to 0.6 mg/kg) in animals with known liver disease. Likewise, especially in toxicology studies, users must also be aware of potential confounding changes in AST values after SRB administration.
Transportation of the animals to the location where anesthesia and blood draws were performed might have influenced the cortisol baseline values reported at the beginning of this study. Moreover, blood samples collected after SRB administration revealed significant reduction in the cortisol values. Gender had a significant effect on cortisol concentrations. Independent of the SRB dose or time point, female guinea pigs had cortisol levels that were significantly greater than those observed in males undergoing the same procedures. Because this is the first report to compare cortisol levels in healthy guinea pigs and all animals were acclimated to the procedures described, we do not know if other procedures would result in similar cortisol dynamics. Likewise, the cortisol levels we report here in guinea pigs cannot be compared with published data from different species.2,14,34 Guinea pigs have a very high metabolic rate1,11 and are known to be extremely sensitive to stressful stimuli.1,11,20,32,33,38 Making such comparisons without further data could result in inaccurate conclusions. Overall, future studies on the use of SRB should be conducted to fully understand the effect of this formulation on animal wellbeing and research data.
One limitation of the current study was that all assessments involved the same 8 guinea pigs. The goals of this study was to provide an unbiased description from both extremes (low to high) of the pharmacokinetic profiles and potential adverse effects of SRB in healthy guinea pigs. We used the same animals in each treatment group in order to adhere to the ‘3 Rs’ of laboratory animal science. This approach is deemed acceptable in pharmacology studies40 and allowed us to better describe interindividual differences across doses. Consequently, this study now provides in-depth considerations for the use of SRB in more predictable pain-specific analgesic protocols.
In conclusion, this study was designed to describe the pharmacokinetic profiles and adverse effects of 3 dosages of SRB in healthy male and female guinea pigs. Single, subcutaneous administration of any of the tested doses (SRBLow, 0.15 mg/kg; SRBMedium, 0.3 mg/kg; SRBHigh, 0.6 mg/kg) resulted in no significant physiologic changes during anesthesia. Likewise, independent of the dose tested, no injection site reactions were observed within the study period. Potential adverse effects related to the use of any of the tested doses in single-housed animals was dose-dependent and included significant changes in AST, fecal output quantity and distribution, and body weight. In addition, the plasma concentrations after administration of the SRBMedium (males) and SRBHigh (both sexes) dosages remained above established therapeutic levels (1 ng/mL) for at least 72 h. Within the tested period, guinea pigs receiving SRBHigh (0.6 mg/kg) had the highest overall plasma concentration of the drug. These results provide guidelines for appropriate SRB dosages in guinea pigs. Further investigation is needed to determine whether the doses described in this study effectively alleviate pain in guinea pigs using established models.
Acknowledgments
We thank the Department of Animal Resources (USC) husbandry staff for invaluable assistance during all phases of this study and the research staff in Dr. Louie's analytical laboratory for assisting with the pharmacokinetic studies. This work was supported by Grants for Laboratory Animal Science (GLAS) from the American Association for Laboratory Animal Science.
References
- 1.Agterberg MJ, van den Broek M, Philippens IH. 2010. A less stressful animal model: a conditional avoidance behavior task for guinea pigs. Lab Anim 44:206–210. [DOI] [PubMed] [Google Scholar]
- 2.Allison SO, Halliday LC, French JA, Novikov DD, Fortman JD. 2007. Assessment of buprenorphine, carprofen, and their combination for postoperative analgesia in olive baboons (Papio anubis). J Am Assoc Lab Anim Sci 46:24–31. [PMC free article] [PubMed] [Google Scholar]
- 3.Animal Welfare Regulations 2008. 9 CFR § 3.129. [Google Scholar]
- 4.Birck MM, Tveden-Nyborg P, Lindblad MM, Lykkesfeldt J. 2014. Nonterminal blood sampling techniques in guinea pigs. J Vis Exp 92:e51982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carbone ET, Lindstrom KE, Diep S, Carbone L. 2012. Duration of action of sustained-release buprenorphine in 2 strains of mice. J Am Assoc Lab Anim Sci 51:815–819. [PMC free article] [PubMed] [Google Scholar]
- 6.Carbone L. 2012. Pain management standard in the 8th ed. of the guide for the care and use of laboratory animals. J Am Assoc Lab Anim Sci 51:322–328. [PMC free article] [PubMed] [Google Scholar]
- 7.Charles River. [Internet] 2012. Technical sheet. Hartley guinea pig biochemistry, North American colonies January 2008 to December 2008. [Cited 05 September 2017]. Available at: http://www.criver.com/files/pdfs/rms/guinea-pigs/hartley/rm_rm_r_hartley_guinea_pig_clinical_pathology_data.aspx
- 8.Chum HH, Jampachairsri K, McKeon GP, Yeomans DC, Pacharinsak C, Felt SA. 2014. Antinociceptive effects of sustained-release buprenorphine in a model of incisional pain in rats (Rattus norvegicus). J Am Assoc Lab Anim Sci 53:193–197. [PMC free article] [PubMed] [Google Scholar]
- 9.Clark TS, Clark DD, Hoyt RF., Jr 2014. Pharmacokinetic comparison of sustained-release and standard buprenorphine in mice. J Am Assoc Lab Anim Sci 53:387–391. [PMC free article] [PubMed] [Google Scholar]
- 10.DiVincenti L, Jr, Meirelles LA, Westcott RA. 2016. Safety and clinical effectiveness of a compounded sustained-release formulation of buprenorphine for postoperative analgesia in New Zealand White rabbits. J Am Vet Med Assoc 248:795–801. [DOI] [PubMed] [Google Scholar]
- 11.Dunbar ML, David EM, Aline MR, Lofgren JL. 2016. Validation of a behavioral ethogram for assessing postoperative pain in guinea pigs (Cavia porcellus). J Am Assoc Lab Anim Sci 55:29–34. [PMC free article] [PubMed] [Google Scholar]
- 12.FDA. [Internet] 2014. Animalgesics for rodents. [Cited 05 September 2017]. Available at: http://www.fda.gov/downloads/AnimalVeterinary/DevelopmentApprovalProcess/MinorUseMinorSpecies/UCM373294.pdf
- 13.Foley PL, Liang H, Crichlow AR. 2011. Evaluation of a sustained-release formulation of buprenorphine for analgesia in rats. J Am Assoc Lab Anim Sci 50:198–204. [PMC free article] [PubMed] [Google Scholar]
- 14.Gong S, Miao YL, Jiao GZ, Sun MJ, Li H, Lin J, Luo MJ, Tan JH. 2015. Dynamics and correlation of serum cortisol and corticosterone under different physiologicl or stressful conditions in mice. PLoS One 10:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hanson CE, Ruble GR, Essiet I, Hartman AB. 2001. Effects of buprenorphine on immunogenicity and protective efficacy in the guinea pig keratoconjunctivitis model (sereny test). Comp Med 51:224–229. [PubMed] [Google Scholar]
- 16.Healy JR, Tonkin JL, Kamarec SR, Saludes MA, Ibrahim SY, Matsumoto RR, Wimsatt JH. 2014. Evaluation of an improved sustained-release buprenorphine formulation for use in mice. Am J Vet Res 75:619–625. [DOI] [PubMed] [Google Scholar]
- 17.Institute for Laboratory Animal Research 2011. Guide for the care and use of laboratory animals, 8th ed. Washington (DC): National Academies Press. [Google Scholar]
- 18.Kleven GA, Joshi P. 2016. Temperature preference in IAF hairless and Hartley Guinea pigs (Cavia porcellus). J Am Assoc Lab Anim Sci 55:161–167. [PMC free article] [PubMed] [Google Scholar]
- 19.Kinoshita M, Kikkawa YS, Sakamoto T, Kondo K, Ishihara K, Konno T, Pawsey N, Yamasoba T. 2015. Safety, reliability, and operability of cochlear implant electrode arrays coated with biocompatible polymer. Acta Otolaryngol 135:320–327. [DOI] [PubMed] [Google Scholar]
- 20.Kohn DF, Martin TE, Foley PL, Morris TH, Swindle MM, Vogler GA, Wixson SK, [Internet] 2006. [Cited 05 September 2017]. Guidelines for the assessment and management of pain in rodents and rabbits. Available at: https://www.aclam.org/Content/files/files/Public/Active/position_pain-rodent-rabbit.pdf. [PubMed]
- 21.Leppert W. 2012. The impact of opioid analgesics on the gastrointestinal tract function and the current management possibilities. Contemp Oncol (Pozn) 16:125–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Loeb WF, Quimby F. 1999. The clinical chemistry of laboratory animal, 2nd ed., Philadelphia (PA):Taylor and Francis. [Google Scholar]
- 23.Martin-Flores M, Singh B, Walsh CA, Brooks EP, Taylor LC, Mitchell LM. 2017. Effects of buprenorphine, methylnaltrexone, and their combination on gastrointestinal transit in healthy new zealand white rabbits. J Am Assoc Lab Anim Sci 56:155–159. [PMC free article] [PubMed] [Google Scholar]
- 24.Mnich E, Gajewski A, Rudnicka K, Gonciarz W, Stawerski P, Hinc K, Obuchowski M, Chmiela M. 2015. Immunoregulation of antigen presenting and secretory functions of monocytic cells by helicobacter pylori antigens in relation to impairment of lymphocyte expansion. Acta Biochim Pol 62:641–650. [DOI] [PubMed] [Google Scholar]
- 25.Molter CM, Barbosa L, Johnson S, Knych HK, Chinnadurai SK, Wack RF. 2015. Pharmacokinetics of a single subcutaneous dose of sustained release buprenorphine in northern elephant seals (Mirounga angustirostris). J Zoo Wildl Med 46:52–61. [DOI] [PubMed] [Google Scholar]
- 26.Nunamaker EA, Halliday LC, Moody DE, Fang WB, Lindeblad M, Fortman JD. 2013. Pharmacokinetics of 2 formulations of buprenorphine in macaques (Macaca mulatta and Macaca fascicularis). J Am Assoc Lab Anim Sci 52:48–56. [PMC free article] [PubMed] [Google Scholar]
- 27.Nunamaker EA, Stolarik DF, Ma J, Wilsey AS, Jenkins GJ, Medina CL. 2014. Clinical efficacy of sustained-release buprenorphine with meloxicam for postoperative analgesia in beagle dogs undergoing ovariohysterectomy. J Am Assoc Lab Anim Sci 53:494–501. [PMC free article] [PubMed] [Google Scholar]
- 28.Office of Laboratory Animal Welfare. [Internet] 2002. Public health service policy on humane care and use of laboratory animals. [Cited 05 September 2017]. Available at: https://grants.nih.gov/grants/olaw/references/PHSPolicyLabAnimals.pdf.
- 29.Reno FE, Edwards CN, Bendix JM, Torok-Batho M, Esdaile DJ, Piche C, Triest M, Carballo D. 2016. Needle-free nasal delivery of glucagon for treatment of diabetes-related severe hypoglycemia: toxicology of polypropylene resin used in delivery device. Cutan Ocul Toxicol 35:242–247. [DOI] [PubMed] [Google Scholar]
- 30.Roughan JV, Flecknell PA. 2002. Buprenorphine: a reappraisal of its antinociceptive effects and therapeutic use in alleviating postoperative pain in animals. Lab Anim 36:322–343. [DOI] [PubMed] [Google Scholar]
- 31.Sakthi S, Palaniyandi K, Gupta UD, Narayanan S. 2016. Lipoprotein LpqS deficient M. tuberculosis mutant is attenuated for virulence in vivo and shows protective efficacy better than BCG in guinea pigs. Vaccine 34:735–743. [DOI] [PubMed] [Google Scholar]
- 32.Smith BJ, Wegenast DJ, Hansen RJ, Hess AM, Kendall LV. 2016. Pharmacokinetcis and paw withdrawal pressure in female guinea pigs (Cavia porcellus) treated with sustained-release buprenorphine and buprenorphine hydrochloride. J Am Assoc Lab Anim Sci 55:789–793. [PMC free article] [PubMed] [Google Scholar]
- 33.Suckow MK, Stevens KA, Wilson RP. 2012. The laboratory rabbit, guinea pig, hamster, and other rodents, San Diego (CA): Academic Press. [Google Scholar]
- 34.Sundbom R, Jacobsen KR, Kalliokoski O, Hau J, Abelson KS. 2011. Postoperative corticosterone levels in plasma and feces of mice subjected to permanent catheterization and automated blood sampling. In Vivo 25:335–342. [PubMed] [Google Scholar]
- 35.Thiede AJ, Garcia KD, Stolarik DF, Ma J, Jenkins GJ, Nunamaker EA. 2014. Pharmacokinetics of sustained-release and transdermal buprenorphine in Gottingen minipigs (Sus scrofa domestica). J Am Assoc Lab Anim Sci 53:692–699. [PMC free article] [PubMed] [Google Scholar]
- 36.United States Department of Agriculture. [Internet] 2016. Research facility annual report. [Cited 05 September 2017]. Available at: https://www.aphis.usda.gov/aphis/ourfocus/animalwelfare/SA_Obtain_Research_Facility_Annual_Report.
- 37.Walkowiak KJ, Graham ML. 2015. Pharmacokinetics and antinociceptive activity of sustained-release buprenorphine in sheep. J Am Assoc Lab Anim Sci 54:763–768. [PMC free article] [PubMed] [Google Scholar]
- 38.Wallisch M, Subban CV, Nettleton RT, Olsen GD. 2010. Chronic in utero buprenorphine exposure causes prolonged respiratory effects in the guinea pig neonate. Neurotoxicol Teratol 32:398–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wancket LM. 2015. Animal models for evaluation of bone implants and devices: comparative bone structure and common model uses. Vet Pathol 52:842–850. [DOI] [PubMed] [Google Scholar]
- 40.Yardley MM, Neely M, Huynh N, Asatryan L, Louie SG, Alkana RL, Davies DL. 2014. Multiday administration of ivermectin is effective in reducing alcohol intake in mice at doses shown to be safe in humans. Neuroreport 25:1018–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhao M, Feng R, Shao D, Liu S, Lei M, Wang H, Sun X, Guo F, Hu H, Kameyama M, Hao L. 2015. Mg2+-dependent facilitation and inactivation of L-type ca2+ channels in guinea pig ventricular myocytes. J Pharmacol Sci 129:143–149. [DOI] [PubMed] [Google Scholar]