Abstract
Due to potential adverse effects on animal wellbeing, the use of nonpharmaceutical-grade substances in animal research must be scientifically justified in cases where a pharmaceutical-grade version of the substance exists. This requirement applies to all substances, including vehicles used to solubilize experimental drugs. To date, no studies have evaluated the direct effect of the pharmaceutical classification of a compound on animal wellbeing. In this study, we evaluated intraperitoneal administration of pharmaceutical-grade corn oil, nonpharmaceutical-grade corn oil, and saline in female C57BL/6J mice. Compounds were administered every 48 h for a total of 4 injections. Mice were evaluated clinically by using body weight, body condition score, visual assessment score, CBC, and serum chemistries. Animals were euthanized at 24 h and 14 d after the final injection. Inflammation of the peritoneal wall and mesenteric fat was assessed microscopically by using a semiquantitative scoring system. Saline-dosed groups had lower pathology scores at both time points. At day 21, pharmaceutical-grade corn oil had a significantly higher pathology score compared with nonpharmaceutical-grade corn oil. No other significant differences between the corn oil groups were observed. The use of nonpharmaceutical grade corn oil did not result in adverse clinical consequences and is presumed safe to use for intraperitoneal injection in mice. Differences in inflammation between the 2 groups suggest that the use of either pharmaceutical-grade or nonpharmaceutical-grade corn oil should be consistent within a study.
Abbreviations: BCS, body condition score; PG, pharmaceutical grade; NPG, nonpharmaceutical grade; VAS, visual assessment score
Pharmaceutical grade (PG) compounds are substances that are either approved by the FDA or have an established chemical purity standard published by a recognized pharmacopeia.2 Approved PG products are defined by individual monographs, which stipulate their terms of production, storage, testing procedures, and acceptable test results. The use of PG products in animal research ensures both purity and sterility and promotes the likelihood of reproducible data between studies. However, several challenges exist regarding the use of PG products, including price, availability, and the need for compounding in small laboratory animals. Nonpharmaceutical grade (NPG) compounds are substances that are not FDA-approved and do not have an established chemical purity standard. These substances are not guaranteed to be sterile and may have increased variability between batches due to the lack of a formal purity standard.
The use of NPG compounds is specifically addressed in the Guide for the Care and Use of Laboratory Animals, 8th edition, and the USDA's Animal Care Policy Manual.5,12 Currently, NPG substances can be used only when there is scientific justification or when a PG alternative is not available. Approval generally occurs at the time of IACUC protocol review, where consideration is given to parameters that may affect animal wellbeing, such as product pH, sterility, and storage.
In an animal research setting, corn oil is commonly used as a feed additive or a delivery vehicle for lipophilic substances. The current study was initiated after a laboratory switched to PG corn oil as a delivery vehicle for tamoxifen and reported significantly decreased Cre activation compared with previous experiments using NPG corn oil. Although the scientific justification for switching back to NPG corn oil appeared to be present, no data regarding the effect on animal health that supported the use of one preparation over the other were available.
Corn oil production is largely driven by the food industry, such that virtually all corn oil undergoes a rigorous refinement process to meet industry standards and health regulations.3 The final step of this process requires steam distillation, with temperatures reaching between 400 to 500 °F, thus aiding in deodorization and sterilization of end products.3 This means that both PG and NPG oil are processed in the same manner and labeling is based on defined pharmacopeia testing standards and results rather than on the production process.
Given the limited data that address how the pharmaceutical classification of drug vehicles influences animal wellbeing, our objective was to determine differences in animal wellbeing associated with the use of PG and NPG corn oil. Due to the nature of corn oil production and the unlikelihood of bacterial growth, we hypothesized that animal wellbeing would not differ between PG and NPG corn oil groups. Measures of animal wellbeing in this study included body weight, body condition score (BCS), visual assessment score (VAS), CBC, serum chemistries, and gross and microscopic findings at necropsy. To our knowledge, this study is the first to address animal wellbeing directly related to the pharmaceutical classification of a vehicle used commonly in research.
Materials and Methods
Animals.
Female C57BL/6J mice (n = 90; age, 6 to 7 wk) were purchased from The Jackson Laboratory (Bar Harbor, ME) and acclimated for at least 3 d prior to beginning the study. Throughout the experiment, mice were maintained on a 12:12-h light:dark cycle and group-housed in autoclaved IVC with unrestricted access to both autoclaved water and PicoLab Irradiated Diet 5053 (LabDiet, St Louis, MO). All animal procedures performed in this study were approved by the IACUC of Vanderbilt University Medical Center (Nashville, TN). Mice were housed in an AAALAC-accredited facility in accordance with the National Research Council's Guide for the Care and Use of Laboratory Animals and the Public Health Service Policy on Humane Care and Use of Laboratory Animals.5,8 Mice were maintained in a barrier facility free of mouse hepatitis virus, mouse parvovirus, minute virus of mice, lymphocytic choriomeningitis virus, Sendai virus, pneumonia virus of mice, epizootic diarrhea of infant mice, Theiler mouse encephalomyelitis virus, mouse poxvirus, mouse adenovirus, mouse reovirus, mouse norovirus, Mycoplasma pulmonis, Helicobacter spp., endoparasites (Syphacia spp. and Aspiculuris spp.), and ectoparasites (Myobia musculi, Radfordia affinis, Myocoptes musculinus, and Psorergates simplex).
Intraperitoneal dosing.
Treatments included either PG corn oil (Welch, Holme, and Clark, Newark, NJ), NPG corn oil labeled as a delivery vehicle for fat-soluble compounds (product no. C8267, Sigma Aldrich, St Louis, MO), or 0.9% normal saline (Patterson Veterinary Supply, Devens, MA). Each treatment group contained 30 mice. Compounds were administered by intraperitoneal injection every 48 h for a total of 4 injections over a 7-d period.
Intraperitoneal injections were performed by a single researcher (JSH). Briefly, mice were manually restrained, and the head was tilted downward. A needle was inserted into the right caudal abdominal quadrant at an angle of approximately 30°. A fresh 25-gauge, 5/8-in. needle was used for each mouse. The plunger was retracted to verify negative pressure within the peritoneal space and the absence of ingesta. The injected volume was standardized at 0.1 mL for each treatment group.
On day 8, 24 h after the final injection, half of the mice in each group (n = 15) were submitted for terminal blood collection and necropsy. The remaining mice were maintained for an additional 14 d before being similarly necropsied on day 21.
Clinical assessments.
All mice were weighed at baseline (day 1) and 24 h after the final injection (day 8). Animals surviving to 21 d were also weighed 1 wk (day 14) and 2 wk (day 21) after the final injection.
Health also was assessed according to a BCS system11 and VAS1 system at the same time points described earlier. Two examiners blinded to the treatment groups independently scored each mouse's BCS and VAS during the same session; examiner scores were averaged for each test prior to analysis.
The BCS system is a ranking of the mouse body condition on a scale of 1 to 5, with 3 representing a normal, well-conditioned mouse.11 The score requires both observation and handling of the mice to assess muscle and body fat coverage over the spinal vertebrae. At a BCS of 1, the skeletal structure is extremely prominent and vertebrae are clearly segmented. At the other end of the scale, a BCS of 5 represents a mouse that is smooth and bulky, with no sign of bone structure even with firm palpation.
The VAS system is an indicator of overall mouse health. It combines the score for 3 characteristics (hair coat, eyes and ambulation, and posture) to result in an overall condition score.1 The lowest score, 0, represents normal for each characteristic. Hair coat condition is ranked on a scale of 0 to 3, with 3 representing a very rough hair coat or hair loss. The eyes are ranked from 0 (open, alert) to 2 (closed). Finally, the coordination and posture of the mouse are evaluated on a scale of 0 to 5. At a score of 1, the mouse is walking awkwardly or is slightly hunched. With each increase in score, the mouse's coordination and posture are progressively worse; a score of 5 indicates that the mouse is hunched and not moving. Healthy mice have an overall VAS of 0.
Hematology.
On either day 8 or 21 of the study, mice were euthanized by carbon dioxide overdose in accordance with the AVMA Guidelines for the Euthanasia of Animals: 2013 Edition.7 Whole blood was collected by cardiocentesis at necropsy for CBC (Forcyte Hematology Analyzer, Oxford Science, Oxford, CT) and serum chemistry analysis. Serum chemistry analysis included triglyceride, ALT, AST, BUN, creatinine, and glucose levels (Vet Ace, Alfa-Wassermann, West Caldwell, NJ). All blood samples were obtained between 0700 and 1400.
Necropsy and histopathology.
A full necropsy was performed on all mice in the study. Tissues were collected and fixed in 10% neutral buffered formalin for at least 48 h, processed routinely, embedded in paraffin, sectioned at 4 μm, and stained with hematoxylin and eosin. Sections of lung, liver, spleen, pancreas, kidney, intestine, mesenteric fat, and body wall underwent routine microscopic evaluation by a board-certified veterinary pathologist (KLB), who was blinded to the composition of the study groups.
To assess the presence and degree of inflammation caused by the intraperitoneal injections, the body wall and mesenteric fat were collected for pathology scoring. Inflammation was scored by using a scale of 0 to 4. For the body wall, a score of 0 indicated no inflammation; 1, minimal infiltration of the peritoneal surface with mononuclear cells and minimal mesothelial response; 2, mild inflammation with mild mesothelial response; 3, moderate inflammation with moderate mesothelial response; and 4, marked inflammatory infiltrates with marked mesothelial reaction. For the mesenteric fat, a score of 0 indicated no inflammation; 1, mild infiltration of the fat with macrophages and lymphocytes; 2, mild infiltration of the fat with macrophages and lymphocytes, with few poorly organized granulomas present; 3, moderate infiltration of by macrophages and lymphocytes, with organized granulomas but few areas of necrosis; and 4, marked inflammation by macrophages and lymphocytes, organized granulomas, and extensive areas of fat necrosis. The 2 inflammation scores (body wall and mesenteric fat) were averaged for each mouse to generate a single pathology score per animal.
Statistical analysis.
To compare the results of BCS and VAS over time in the same mouse, we used nonparametric repeated-measures ANOVA followed by a Friedman posthoc test. Linear regression was used to compare mouse body weights over time. To compare hematology and serum chemistry results, we used nonparametric nonrepeated measures ANOVA followed by a paired Student t test. Pathology scores were evaluated by using an unpaired t test followed by a Mann–Whitney test. All statistics were calculated by using Prism software (version 6.07, GraphPad, La Jolla, CA). A P value of less than 0.05 was considered statistically significant.
Results
Animals.
Five mice (all of which received PG corn oil) were excluded from part or all of the study. Two of these 5 mice arrived from the vendor with malocclusion and were excluded from body weight measurements and CBC and serum chemistry analysis. The malocclusion was managed during the study, and VAS, BCS, and pathology scoring results from these 2 mice were included in the final analyses. The decision to include these mice in part of the study was based on the fact that both animals had normal BCS and VAS at all time points. They were included in pathology scoring due to their normal clinical assessments and the belief that any abdominal inflammation observed in these animals would be a result of the intraperitoneal injections and not due to malocclusion. The remaining 3 mice were included in baseline data and were excluded from the rest of the study. Of these remaining 3 mice, 2 were dosed with the wrong compound at the second injection, and the last one went into respiratory distress after the fourth injection and was immediately euthanized. Hemoabdomen was identified at necropsy.
Clinical assessments.
Body weight.
Body weight increased significantly (P < 0.001), consistent with a normal growth curve, across all treatments groups. There was no significant difference between treatment groups at any point in time (P = 0.6202; Figure 1).
Figure 1.
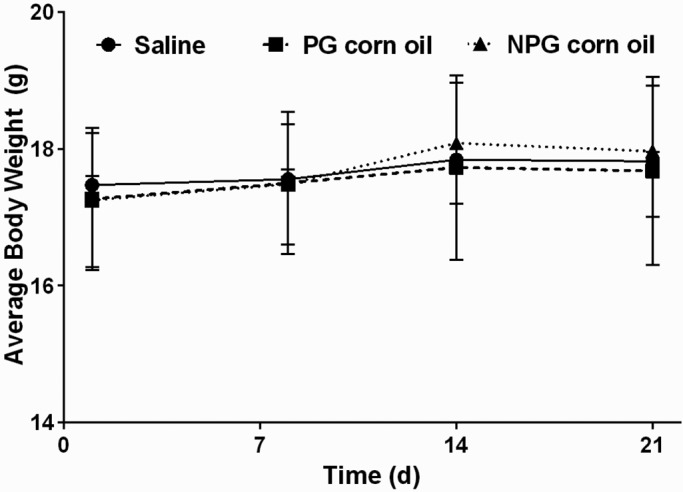
Body weight (mean ± SEM [error bars]) over time. Neither the rate of weight gain nor final weight differed between groups (P = 0.4842 and P = 0.6539, respectively).
BCS.
BCS did not differ between treatment groups at any of the measured time points during this study (P = 0.33). Average BCS ranged from 2.75 to 3.0 throughout the study. No mouse had more than one BSC lower than 3.0, and no mouse was ever marked below 3.0 by both observers.
VAS.
VAS did not differ between treatment groups at any of the measured time points during this study (P = 0.33). Average VAS ranged between 0.0 and 0.5 throughout the study. No mouse received more than one score above 0.0, and no mouse was ever marked above 0.0 by both observers.
Hematology.
CBC.
Hct did not differ between treatment groups at either time point, according to nonparametric nonrepeated-measures ANOVA followed by paired Student t testing (Table 1).
Table 1.
Hematologic parameters (mean ± SEM) for mice in saline, PG corn oil, and NPG corn oil treatment groups at days 8 and 21
| Saline |
PG corn oil |
NPG corn oil |
||||
| Day 8 | Day 21 | Day 8 | Day 21 | Day 8 | Day 21 | |
| Hematocrit (%) | 42.99 ± 3.11 | 39.49 ± 2.85 | 43.42 ± 3.98 | 40.15 ± 2.99 | 43.0 ± 3.63 | 40.46 ± 1.94 |
| White blood cells (no. per µL) | 5.03 ± 1.57 | 4.92 ± 1.29a | 6.01 ± 1.89 | 6.41 ± 1.45a | 6.23 ± 2.36 | 5.67 ± 1.10 |
| Neutrophils (no. per µL) | 0.81 ± 0.34b,d | 0.79 ± 0.23c | 1.40 ± 0.55b | 1.04 ± 0.24c | 1.48 ± 0.83d | 1.00 ± 0.43 |
| Lymphocytes (no. per µL) | 3.72 ± 1.12 | 3.71 ± 1.00 | 4.08 ± 1.39 | 4.82 ± 1.27 | 4.19 ± 1.61 | 4.15 ± 0.98 |
Data are given as mean ± SD (n = 11 – 13 per group)
Significant (P = 0.00796) difference between saline and PG corn oil WBC counts on day 21
Significant (P = 0.00239) difference between saline and PG corn oil neutrophil counts on day 8
Significant (P = 0.00762) difference between saline and PG corn oil neutrophil counts on day 21
Significant (P = 0.00954) difference between saline and NPG corn oil neutrophil counts on day 8
WBC count did not differ between saline and PG corn oil treatment groups at day 8 (P = 0.152), but there was a significant difference at day 21 (P = 0.008; Table 1). WBC count did not differ between saline and NPG corn oil treatment groups at day 8 (P = 0.124) or day 21 (P = 0.098; Table 1) nor between PG and NPG corn oil groups at day 8 (P = 0.793) or day 21 (P = 0.139; Table 1). One of the mice that was treated with NPG corn oil and euthanized on day 8 had a WBC count of 11.42 cells/µL, which exceeded our inhouse reference range (1.8 to 10.7 cells/µL). In addition, this animal's total neutrophil count was elevated (3.76 cells/µL; reference range, 0.1 to 2.4 cells/µL), but the lymphocyte count and neutrophil:lymphocyte ratio were within normal limits.
Serum chemistry.
According to nonparametric nonrepeated-measures ANOVA followed by paired Student t testing, none of the serum chemistry analytes varied significantly between groups at either of the time points tested.
Necropsy and histopathology.
Necropsy.
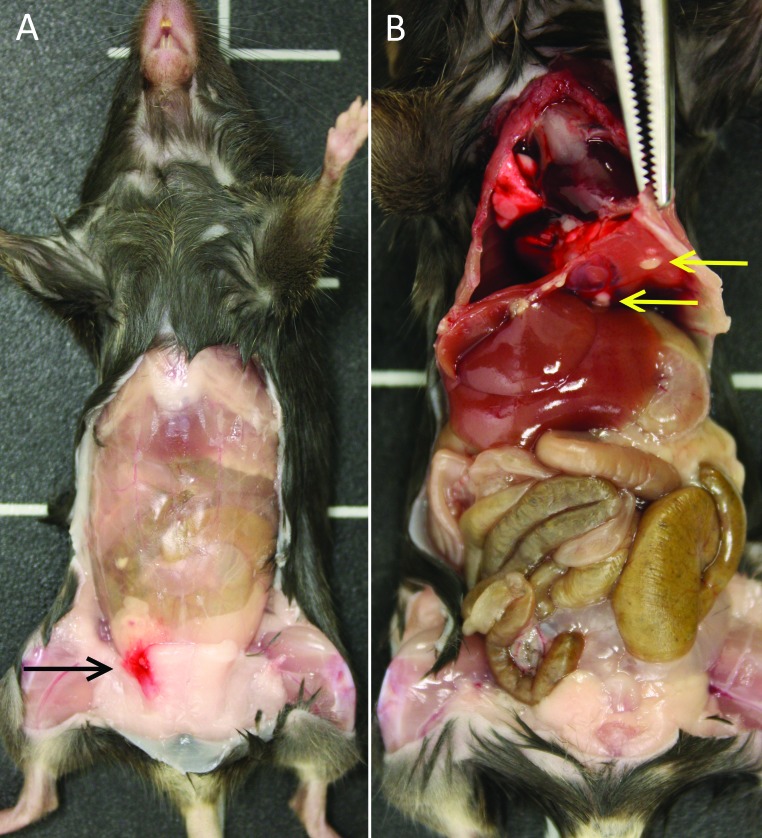
The most common gross necropsy finding at day 8 was focal body-wall hemorrhage at the injection site (Figure 2 A), which was present in all treatment groups. The most common gross necropsy finding at day 21 was small, nodular inflammatory foci on the peritoneal surface of the diaphragm (Figure 2 B), which occurred in the PG and NPG groups only. In addition, one mouse in the NPG group that was necropsied on day 21 had a focal (approximately 5 mm in diameter) mesenteric abscess. Heavy growth of Burkholderia spp. was isolated on aerobic culture of the abscess. This Burkholderia isolate was resistant to all antibiotics tested except trimethoprim–sulfamethoxazole.
Figure 2.
Representative gross images at necropsy on days 8 and 21. (A) Focal hemorrhage at the injection site (black arrow) in a mouse treated with NPG corn oil and necropsied on day 8. (B) Inflammatory foci along the diaphragm (yellow arrows) in a mouse that received PG corn oil and was necropsied on day 21.
Histopathology.
Sections of lung, liver, spleen, pancreas, kidney, intestine, mesenteric fat, and body wall were evaluated microscopically for inflammation. There were no abnormalities identified in sections of lung, liver, spleen, pancreas, kidney, or intestine. Inflammation was present in the mesenteric fat and on peritoneal surfaces of the body wall. Inflammation was compared between the groups using a semiquantitative method described above.
Pathology score.
Compared with the saline group, the PG corn oil treatment group had higher pathology scores at both day 8 (P = 0.0004) and day 21 (P < 0.0001; Figure 3). In addition, pathology scores differed between saline and NPG corn oil groups at day 8 (P = 0.0002) and day 21 (P < 0.0001), with higher average pathology scores in the NPG corn oil group at both time points (Figure 3). There was no significant difference between PG and NPG oils at day 8 (P = 0.4367), but there was a significant difference at day 21 (P < 0.0008). On day 21, the PG corn oil group had a higher average pathology score than the NPG corn oil group (Figure 3). Representative histology from all groups is included in Figure 4.
Figure 3.
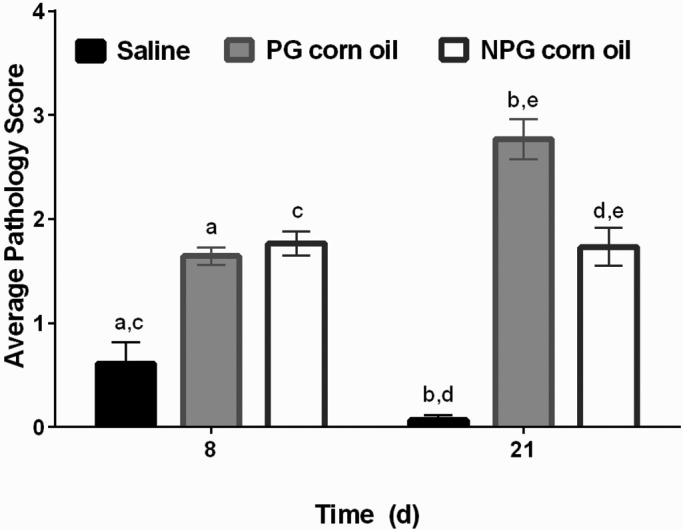
Pathology score (mean ± SEM [error bars]) at days 8 and 21 of the study. There were significant differences between saline and PG corn oil groups at day 8 (a, P = 0.0004) and day 21 (b, P = less than 0.0001). There were also significant differences between saline and NPG corn oil groups at day 8 (c, P = 0.0002) and day 21 (d, P = less than 0.0001). PG and NPG groups did not differ at day 8 (P = 0.4367), but there was a significant difference at day 21 (e, P = 0.0008).
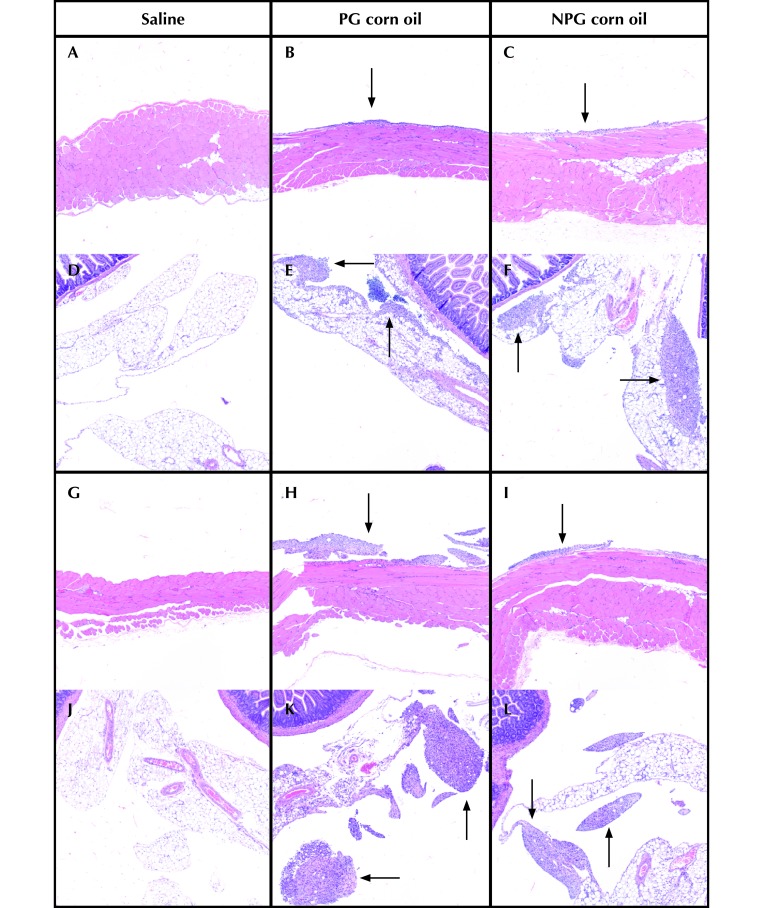
Figure 4.
Representative images from the (A through C and G through I) peritoneal wall and (D through F and J through L) mesenteric fat collected on days 8 (A through F) and 21 (G through L) of the study. Both corn oil groups had increased inflammation (arrows) compared with the saline group at both time points. Inflammation did not differ between the PG and NPG groups at day 8. At day 21, granulomatous inflammation was increased in the PG compared with the NPG group. Hematoxylin and eosin stain; magnification, 100×.
Discussion
This study is the first to compare the difference between PG and NPG corn oil on animal health and wellbeing as evaluated according to body weight, BCS, VAS, clinical pathology, and inflammatory changes seen on histology.
We hypothesized that there would be no significant differences between the 2 corn oil groups and were surprised to find that PG corn oil resulted in a significantly higher pathology score at day 21 compared with both NPG corn oil and saline. In addition, the PG corn oil group had the highest WBC count at day 21, although the difference was not significant compared with the NPG corn oil group. All WBC counts were within normal reference range except for 1 NPG mouse necropsied on day 8, which had mild leukocytosis with mature neutrophilia. This animal did not exhibit any abnormal BCS, VAS, or body weight data, and its pathology score was 2.0. The apparent mild leukocytosis and mature neutrophilia in this mouse may represent a normal variation. The absence of an elevated WBC count in corn oil–treated mice with peritoneal inflammation suggests that the local response in the abdominal space has minimal generalized effects in mice.
Despite the differences in WBC counts and pathology scores among corn oil and saline groups, there was no evidence of discomfort or disease according to the body weight, BCS, and VAS findings. The measurements we used in this study reflect a realistic approach that research personnel likely would use to detect pain in mice. Alternatively, these results might suggest that these physical assessments lack sufficient sensitivity to detect clinical signs associated with mild inflammation in mice. Further studies with additional behavioral and pain testing, such as by using the Mouse Grimace Scale or nest quality testing, may detect subtle changes in animal wellbeing associated with the pharmaceutical-grade classification of a substance.4,6
The only unexpected outcome at gross necropsy was the focal mesenteric abscess that grew Burkholderia spp. This abscess occurred in a mouse treated with NPG corn oil and euthanized on day 21; this animal was otherwise clinically normal and had no evidence of intestinal perforation on either necropsy or histology. Burkholderia spp. are lipophilic, gram-negative bacteria that occupy diverse environmental niches including soil, water, plants, animals, and humans. These bacteria are known to grow opportunistically in oil due to their robust ability to produce lipase. In fact, Burkholderia has been studied as a natural agent to aid in biodegradation of crude oil for biocontainment purposes (that is, oil spills).9,10 The free oil in the abdomen of this mouse may have provided an environment that allowed for opportunistic infection by Burkholderia. The infection is unlikely related to the oil itself in light of production standards and the fact that none of the other 30 mice injected with NPG corn oil developed abscesses.3 Potential sources of infection include contamination from the skin during intraperitoneal injection.
A limitation of this study is that the corn oils were purchased from different vendors. This difference was unavoidable because no vendors sold both PG and NPG corn oils. The products were chosen according to the most commonly used substances at our institution, in an effort to generate results that mimic common practices. In addition, this study assessed only one product (corn oil) and one sex (female) and strain (C57BL/6J) of mice. Readers should proceed with caution before extrapolating these results to other products, sexes, and strains.
To our knowledge, this study is the first to evaluate the effects of PG compared with NPG corn oil on animal wellbeing. Unexpectedly, the mice treated with PG corn oil had higher pathology scores than those given NPG corn oil. However, the BCS and VAS did not indicate that the inflammation induced by either corn oil product resulted in pain or distress. Given these findings, there is no benefit to using PG corn oil compared with NPG corn oil. However, the mild differences in inflammation between the 2 groups suggest that the use of either PG or NPG corn oil should be consistent within a study. These findings cannot be extrapolated to other NPG compounds, and the use of NPG products in animals should still be justified and approved on a case-by-case basis.
Acknowledgments
We thank Dr William Dupont (Department of Biostatistics, Vanderbilt University) for consultation on sample size and study design; the Translational Pathology Shared Resource for their assistance with tissue processing; Philip Sullivan for his assistance in clinical assessment scoring; and Drs Atef Khalil and Carissa Jones for their assistance with necropsy and blood collection.
References
- 1.Adamson TW, Kendall LV, Goss S, Grayson K, Touma C, Palme R, Chen JQ, Borowsky AD. 2010. Assessment of carprofen and buprenorphine on recovery of mice after surgical removal of the mammary fat pad. J Am Assoc Lab Anim Sci 49:610–616. [PMC free article] [PubMed] [Google Scholar]
- 2.Brown P, Clarke C, Newcomer C, [Internet] 2012. OLAW online seminar: Use of nonpharmaceutical-grade chemicals and other substances in research with animals. May, 2012. [Cited 31 March 2017]. Available at: https://grants.nih.gov/grants/olaw/120301_NPG_slides.pdf
- 3.Corn Refiners Association. [Internet] 2006. Corn oil. [Cited 1 April 2017]. Available at: http://corn.org/wp-content/uploads/2009/12/CornOil.pdf
- 4.Gaskill BN, Karas AZ, Garner JP, Pritchet-Corning KR. 2013. Nest building as an indicator of health and welfare in laboratory mice. J Vis Exp 82:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Institute for Laboratory Animal Research 2011. Guide for the care and use of laboratory animals, 8th ed. Washington (DC):National Academies Press. [Google Scholar]
- 6.Langford DJ, Bailey AL, Chanda ML, Clarke SE, Drummond TE, Echols S, Glick S, Ingrao J, Klassen-Ross T, LaCroix-Fralish ML, Matsumiya L, Sorge RE, Sotocinal SG, Tabaka JM, Wong D, van den Maagdenberg AM, Ferrari MD, Craig KD, Mogil JS. 2010. Coding of facial expressions of pain in the laboratory mouse. Nat Methods 7:447–449. [DOI] [PubMed] [Google Scholar]
- 7.Leary S, Underwood W, Anthony R, Cartner S, Corey D, Grandin T, Greenacre CB, Gwaltney-Bran S, McCrackin MA, Meyer R, Miller D, Shearer J, Yanong R, [Internet] 2013. AVMA guidelines for the euthanasia of animals: 2013 edition. [Cited 31 March 2017]. Available at: https://www.avma.org/KB/Policies/Documents/euthanasia.pdf
- 8.National Institutes of Health. [Internet] 2002. PHS policy on humane care and use of laboratory animals. [Cited 31 March, 2017]. Available at: https://grants.nih.gov/grants/olaw/references/phspolicylabanimals.pdf
- 9.Okoh A, Ajisebutu S, Babalola G, Trejo-Hernandez M. 2001. Potential of burkholderia cepacia RQ1 in the biodegradation of heavy crude oil. Int Microbiol 4:83–87. [DOI] [PubMed] [Google Scholar]
- 10.Oliveira BH, Santos RÉ, Loiola LEA, Nascimento VMG. 2015. Overproduction and properties of lipase by a wild strain of burkholderia lata LBBIO-BL02 using chicken fat. Ann Microbiol 65:865–877. [Google Scholar]
- 11.Ullman-Culleré MH, Foltz CJ. 1999. Body condition scoring: a rapid and accurate method for assessing health status in mice. Lab Anim Sci 49:319–323. [PubMed] [Google Scholar]
- 12.United States Department of Agriculture (USDA). [Internet] 2016. Animal care policy manual: USDA animal care. Animal care resource guide: veterinary care, policy 3. [Cited 1 April 2017]. Available at: https://www.aphis.usda.gov/animal_welfare/downloads/Animal%20Care%20Policy%20Manual.pdf