This simulation model study estimates the percentage of patients with atherosclerotic cardiovascular disease who would require proprotein convertase subtilisin/kexin type 9 inhibitor therapy when oral therapy to lower lipid levels is intensified first.
Key Points
Question
How many patients with atherosclerotic cardiovascular disease would require proprotein convertase subtilisin/kexin type 9 inhibitor therapy?
Findings
In this simulation model study based on a large, representative cohort of 105 269 patients with atherosclerotic cardiovascular disease, only 53.2% were receiving statins at baseline and only 25.2% achieved low-density lipoprotein cholesterol levels of less than 70 mg/dL. Simulation of maximal lipid-lowering treatment intensification indicated that 99.3% could achieve low-density lipoprotein cholesterol levels of less than 70 mg/dL, including 86% receiving statins and ezetimibe and 14% with add-on proprotein convertase subtilisin/kexin type 9 inhibitors.
Meaning
An opportunity exists to improve achievement of low-density lipoprotein cholesterol goals in the population with atherosclerotic cardiovascular disease by giving oral-only lipid-lowering treatment, with a modest percentage requiring a proprotein convertase subtilisin/kexin type 9 inhibitor.
Abstract
Importance
In patients with atherosclerotic cardiovascular disease (ASCVD), guidelines recommend optimizing statin treatment, and consensus pathways suggest use of ezetimibe and proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors in patients with persistently elevated low-density lipoprotein cholesterol (LDL-C) levels despite use of statins. Recent trials have provided evidence of benefit in reduction of cardiovascular events with these agents.
Objective
To estimate the percentage of patients with ASCVD who would require a PCSK9 inhibitor when oral lipid-lowering therapy (LLT) is intensified first.
Design, Setting, and Participants
This simulation model study used a large administrative database of US medical and pharmacy claims to identify a cohort of 105 269 patients with ASCVD enrolled from January 1, 2012, through December 31, 2013, who met the inclusion criteria (database cohort). Patients were sampled with replacement (bootstrapping) to match the US epidemiologic distribution and entered into a Monte Carlo simulation (simulation cohort) that applied stepwise treatment intensification algorithms in those with LDL-C levels of at least 70 mg/dL. All patients not initially receiving a statin were given atorvastatin, 20 mg, and the following LLT intensification steps were applied: uptitration to atorvastatin, 80 mg; add-on ezetimibe therapy; add-on alirocumab therapy, 75 mg (a PCSK9 inhibitor); and uptitration to alirocumab, 150 mg. Sensitivity analyses included evolocumab as a PCSK9 inhibitor. Efficacy was estimated from published studies and incorporated patient-level variation. Data were analyzed from December 2015 to May 2017.
Exposures
Treatment intensification strategies with LLT.
Main Outcomes and Measures
Use of LLT among the population with ASCVD and distributions of LDL-C levels under various treatment intensification scenarios.
Results
Inclusion criteria were met by 105 269 individuals in the database cohort (57.2% male and 42.8% female; mean [SD] age, 65.1 [12.1] years). In the simulation cohort (1 million patients; 54.8% male and 45.2% female; mean [SD] age, 66.4 [12.2] years), before treatment intensification, 51.5% used statin monotherapy and 1.7% used statins plus ezetimibe. Only 25.2% achieved an LDL-C level of less than 70 mg/dL. After treatment intensification, 99.3% could achieve an LDL-C level of less than 70 mg/dL, including 67.3% with statin monotherapy, 18.7% with statins plus ezetimibe, and 14% with add-on PCSK9 inhibitor.
Conclusions and Relevance
Large gaps exist between recommendations and current practice regarding LLT in the population with ASCVD. In our model that assumes no LLT intolerance and full adherence, intensification of oral LLT could achieve an LDL-C level of less than 70 mg/dL in most patients, with only a modest percentage requiring a PCSK9 inhibitor.
Introduction
Lowering low-density lipoprotein cholesterol (LDL-C) levels with statins reduces the risk of cardiovascular events (CVE) in individuals with established atherosclerotic cardiovascular disease (ASCVD) and in certain primary prevention populations. The 2013 American College of Cardiology and American Heart Association (ACC-AHA) guidelines on lipid-lowering therapy (LLT) recommended statin treatment in 4 patient groups. In 2014, IMPROVE-IT (Improved Reduction of Outcomes: Vytorin Efficacy International Trial) reported CVE risk reduction with the addition of ezetimibe to statin therapy in patients with recent acute coronary syndrome. In 2015, 2 proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors were approved as adjunct to diet and maximally tolerated statin therapy for the treatment of adults with heterozygous familial hypercholesterolemia or clinical ASCVD who require additional lowering of LDL-C levels. In line with an editorial discussing implementation of PCSK9 inhibitor therapy, the ACC published Expert Consensus Decision Pathway (ECDP) recommendations on the use of ezetimibe and PCSK9 inhibitors in clinical practice.
We developed a simulation model that implemented a patient-level treatment intensification algorithm with LLT to understand the effect of intensification on LDL-C goal attainment. The treatment intensification algorithm was based largely on the ACC-AHA guidelines and the ACC ECDP recommendations, which suggest maximizing statin use before nonstatin LLT. The key assumptions on the efficacy of lowering LDL-C levels were based on trial evidence. We applied the simulation to a contemporary ASCVD cohort derived from a large US claims database to investigate the proportion of patients requiring various LLT options and the proportion achieving LDL-C goals with full treatment intensification. We further investigated the effect of varying assumptions in the treatment intensification algorithm on these measures.
Methods
A Monte Carlo simulation model was applied to a cohort of patients with ASCVD identified in a claims database. The study did not involve enrollment of human participants and was based on an existing data source that cannot be used to identify an individual because the patient information was deidentified in accordance with established privacy guidelines under the Health Insurance Portability and Accountability Act. Therefore, the study was exempt under the Department of Health and Human Services policy 45 CFR 46.101(b)(4), and a separate institutional review board approval or patient informed consent were not sought.
Development of the Database Cohort for the Simulation Model
We used the MarketScan Research database, a large (>18 million unique members) administrative database of medical and pharmacy claims. All inpatient and outpatient medical and pharmacy encounters were captured while a patient remained enrolled in the plan. The following inclusion criteria were used: LDL-C level measured from January 1, 2012, through December 31, 2013 (last LDL-C level measured during this period defined as index date); 21 years or older; 2 years of continuous enrollment before the index date; and ASCVD defined as (1) recent acute coronary syndrome, (2) other coronary heart disease, (3) ischemic cerebrovascular disease, and (4) peripheral arterial disease.
Atherosclerotic cardiovascular disease conditions and non-ASCVD comorbidities were identified using codes from the International Classification of Diseases, Ninth Revision, Current Procedural Terminology, and Healthcare Common Procedure Coding System, which were reviewed and independently verified by expert physicians (including one of us [C.P.C.]) to ensure greater specificity for representing these conditions (eTable 1 in the Supplement). Application of many of these codes for identifying the conditions of interest has been studied previously. For example, codes used for myocardial infarction and ischemic stroke were reported to have positive predictive values of 97% and 90%, respectively.
Definitions of statin therapy intensity were based on 2013 ACC-AHA guidelines. Treatment status for LLT and other medications was determined as of the index date from evidence of filled prescriptions and medication supply availability on or within 30 days of the index date to ensure concurrency of LLT status and LDL-C level measurement (eFigure 1 in the Supplement).
Monte Carlo Simulation Model
A Monte Carlo simulation model was developed in the Visual Basic programming language with Excel (Microsoft Corporation) as a host application executing the Visual Basic code. A study population of 1 million patients was generated by randomly sampling with replacement (bootstrapping method) from the database cohort and then entered in the simulation model. This bootstrap-sampled cohort of 1 million individuals is termed the simulation cohort. The resulting greater size of the simulation cohort relative to the database cohort is consistent with the principles of Monte Carlo simulation, with bootstrapping allowing for multiple replications per individual. Each patient in the simulation cohort traced a different probabilistic path in the simulation. The sample size of 1 million represented a figure in which diagnostic plots representing key summary results from simulation vs sample size were stable (variation <0.01% with successive runs) and insensitive to further increase in the size of the simulation cohort. Sampling weights (eMethods in the Supplement) were used in the bootstrap-sampling method to account for quantifiable differences in the characteristics of the database cohort relative to the overall US population.
The base-case simulation used a treatment intensification logic to achieve an LDL-C goal of less than 70 mg/dL (to convert to millimoles per liter, multiply by 0.0259), maximizing the use of statins before augmenting with ezetimibe and alirocumab (a PCSK9 inhibitor) if needed. At each step in the treatment intensification pathway for an individual, the achieved LDL-C level was modeled probabilistically from the distribution of LDL-C level reduction with a given LLT. For statins and ezetimibe, treatment effect was sampled from a β probability density function (PDF), with parameters estimated from the means and SDs as reported in the literature (eTable 2 in the Supplement). We validated the estimated PDFs by comparing them with the detailed distributions of percentage of LDL-C level reduction for a subset of statins and doses as reported in the literature. For alirocumab, the PDF was estimated directly from the pooled ODYSSEY program data for trials representing populations at high risk of CVE who were receiving a statin with placebo as the comparator. The implicit mean percentage of LDL-C level reductions underlying the PDFs for alirocumab were 48.6% and 64.4% for the 75- and 150-mg doses, respectively. For a patient receiving a specific LLT at a particular step in the simulation (eg, an 80-mg dose of atorvastatin), a specific efficacy for that patient was randomly sampled from the PDF. Thus, each patient traced a unique path in the simulation depending on their baseline characteristics and probabilistic sampling of LDL-C level reduction with a given LLT.
Scenario Analyses
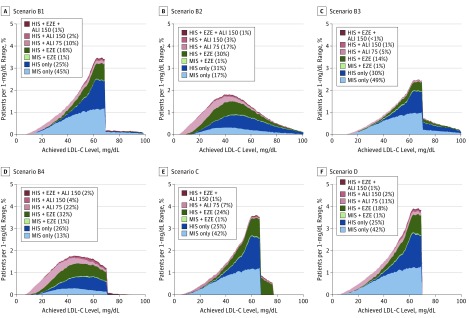
We conducted additional analyses representing scenarios in which some assumptions were varied relative to the base case (scenario A). In scenarios B1 to B4, we modeled the ACC ECDP recommendations. In scenario B1, patients with and without a comorbidity (eTable 3 in the Supplement) had LDL-C goals of less than 70 mg/dL and less than 100 mg/dL, respectively. In scenario B2, the LDL-C goal was a 50% reduction in the LDL-C level relative to the baseline LDL-C level before any LLT treatment. In scenario B3, the goal was defined as achievement of either scenario B1 or B2 goals. In scenario B4, the goal was defined as achievement of both scenario B1 and B2 goals. In scenarios B1 to B4, if the LDL-C goal was not achieved with add-on ezetimibe therapy, then ezetimibe was removed before intensification to alirocumab. In scenario C, we increased the threshold for intensification to alirocumab to an LDL-C goal of less than 80 mg/dL, which could be envisioned as a more pragmatic use of PCSK9 inhibitors. In scenario D, if the LDL-C level of less than 70 mg/dL was not achieved with high-intensity statins and ezetimibe, ezetimibe was removed before intensification to alirocumab. We also conducted scenario analyses assessing the options of going straight to high-dose alirocumab treatment, using evolocumab as the add-on PCSK9 inhibitor based on data reported in the Further Cardiovascular Outcomes Research With PCSK9 Inhibition in Subjects With Elevated Risk (FOURIER) trial and using an LDL-C target of less than 55 mg/dL.
Statistical Analyses
Data were analyzed from December 2015 to May 2017. Demographic and clinical characteristics and use of medication at baseline in the database and simulation cohorts were summarized descriptively as means and proportions as appropriate. Results from the simulation model were summarized as means, proportions, and area graphs as appropriate. The 95% CIs for the base case were estimated using a bootstrap method. Analyses for the development of the database cohort were conducted with SAS software (version 9.2; SAS Institute Inc).
Results
Inclusion criteria were met by 105 269 individuals in the database cohort (57.2% male and 42.8% female; mean [SD] age, 65.1 [12.1] years) (eFigure 2 in the Supplement). The Table provides a summary of baseline characteristics for the database and simulation cohorts (1 million patients; 54.8% male and 45.2% female; mean [SD] age, 66.4 [12.2] years). All subsequent results are based on the simulation cohort. At baseline, approximately 53.2% were treated with a statin (with or without ezetimibe); 1.2%, ezetimibe without statins; 0.8%, only niacin or bile-acid sequestrants; and 44.8%, no LLT. The latter group included patients with no evidence of LLT during the previous 2 years (26.9%) or those with evidence of LLT during the past 2 years and subsequent discontinuation of treatment (17.9%). Achievement of LDL-C goals of less than 70 mg/dL at baseline was 25.2% in the overall cohort and 35% for those receiving a statin. Our simulation cohort was broadly similar to other US populations with ASCVD (eTable 4 in the Supplement).
Table. Baseline Characteristics of the Database and Simulation Cohorts.
| Characteristic | Database Cohort (n = 105 269) |
Simulation Cohort (n = 1 000 000) |
|---|---|---|
| Demographic | ||
| Age, mean (SD), y | 65.1 (21.1) | 66.4 (12.2) |
| Age ≥75 y, % | 23.4 | 26.9 |
| Male, % | 57.2 | 54.8 |
| Medicare, %a | 45.9 | 52.6 |
| US region, % | ||
| South | 20.2 | 18.4 |
| Northeast | 21.4 | 21.6 |
| Midwest | 22.1 | 22.2 |
| West/other | 36.4 | 37.8 |
| Baseline clinical characteristics, % | ||
| Recent ACS | 5.6 | 5.4 |
| Other CHD | 68.4 | 66.3 |
| Ischemic cerebrovascular disease, % | 24.5 | 26.6 |
| PAD | 29.4 | 31.4 |
| Type 1 or 2 diabetes | 35.9 | 36.2 |
| Other comorbidities of interest, % | ||
| ACC ECDP comorbidities | 89.2 | 90.4 |
| Hypertension | 82.1 | 82.8 |
| History of CHF | 18.3 | 18.9 |
| CKD stage III | 11.3 | 12.3 |
| CKD stages IV-Vb | 4.3 | 4.5 |
| Concomitant medication use, % | ||
| β-Blockers | 44.3 | 44.6 |
| ACEI/ARBs, | 46.9 | 47.1 |
| Antiplateletsc | 23.8 | 23.4 |
Abbreviations: ACC ECDP, American College of Cardiology Expert Consensus Decision Pathway; ACEI, angiotensin-converting enzyme inhibitor; ACS, acute coronary syndrome; ARB, angiotensin II receptor blocker; CHD, coronary heart disease; CHF, congestive heart failure; CKD, chronic kidney disease; PAD, peripheral arterial disease.
Represents health plan offered by a private company that contracts with Medicare to provide patients with hospital and medical insurance benefits.
Includes dialysis.
Inlcudes clopidogrel bisulfate, ticagrelor, and/or prasugrel.
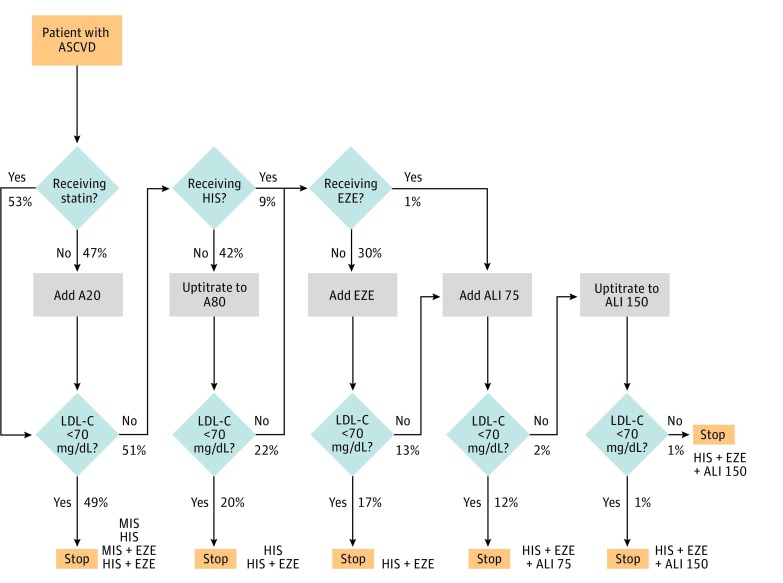
Figure 1 summarizes the base-case scenario (scenario A) and displays the proportion of patients at various steps in treatment intensification logic. When a 20-mg dose of atorvastatin was added for patients not receiving a statin, 49.1% of the overall cohort achieved an LDL-C level of less than 70 mg/dL. Of the remaining 50.9%, 9.1% were already receiving high-intensity statins and 41.8% would undergo uptitration to atorvastatin, 80 mg. The uptitration resulted in an additional 20.2% achieving an LDL-C level of less than 70 mg/dL (overall cohort with LDL-C level <70 mg/dL, 69.3% at this stage). Of the remaining 30.7% not at the LDL-C goal, 0.9% were already taking concomitant ezetimibe; therefore, ezetimibe was added in the remaining 29.8% of the cohort receiving high-intensity statins and not at the LDL-C level goal. After this step in intensification, an additional 16.7% were able to achieve the LDL-C goal (total at LDL-C goal, 86%) and 14% of the original cohort required additional treatment with alirocumab. Addition of a 75-mg dose of alirocumab for patients not at the LDL-C goal resulted in an incremental 12% achieving an LDL-C level of less than 70 mg/dL. The remaining 2% of the cohort received uptitration to alirocumab, 150 mg. At this final step of the intensification, only 0.7% of the original cohort failed to achieve an LDL-C level goal of less than 70 mg/dL. After full treatment intensification, 67.3% were receiving statin monotherapy; 18.7%, statins and ezetimibe; and 14%, add-on alirocumab. The proportion of patients requiring add-on alirocumab with an LDL-C threshold of less than 55 mg/dL increased from 14% to 31.3% (eFigure 3 in the Supplement). eTable 5 in the Supplement provides an estimate of the 95% CIs for the base case.
Figure 1. Logic of Lipid-Lowering Treatment Intensification and Proportion of Patients Flowing Through the Treatment Intensification Logic in the Simulation.
Final treatment combinations are shown in orange. A20 indicates atorvastatin, 20 mg; A80, atorvastatin, 80 mg; ALI 75, alirocumab, 75 mg; ALI 150, alirocumab, 150 mg; ASCVD, atherosclerotic cardiovascular disease; EZE, ezetimibe; HIS, high-intensity statin; LDL-C, low-density lipoprotein cholesterol; and MIS, moderate- to low-intensity statin. To convert LDL-C levels to millimoles per liter, multiply by 0.0259.
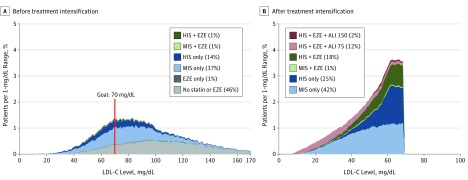
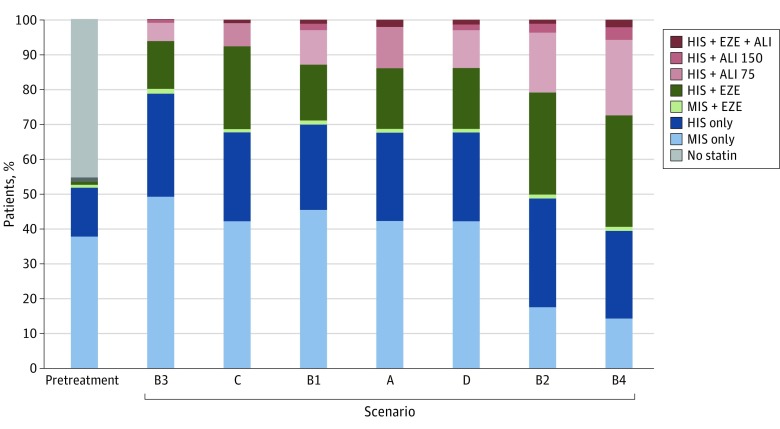
Figure 2 and eTable 6 in the Supplement summarize the distribution of LDL-C levels in the overall cohort at baseline and after the final step in intensification for the base-case scenario (scenario A). There was a wide range in baseline LDL-C levels before treatment intensification with and without statins (Figure 2A). The LDL-C distribution changed markedly after full treatment intensification, with a sharp peak near 70 mg/dL (Figure 2B). Overall, 99.3% achieved LDL-C levels of less than 70 mg/dL after full treatment intensification, and the mean LDL-C level was lowered to 52.2 from 93.7 mg/dL before the simulation. Achievement of very low LDL-C levels (<15 mg/dL) occurred in 6.3% of patients receiving alirocumab and 0.5% in the remaining cohort. Figure 3 summarizes the results for various scenarios representing the ACC ECDP recommendations, whereas Figure 4 and eTable 7 in the Supplement provide a summary of LLT use across all scenarios. eTable 8 in the Supplement provides a summary of achieved LDL-C levels under the options of going straight to high-dose alirocumab and using evolocumab as the add-on PCSK9 inhibitor.
Figure 2. Use of Lipid-Lowering Treatment and Low-Density Lipoprotein Cholesterol (LDL-C) Level Distribution Before and After Treatment Intensification (Base-Case Scenario A).
The graphs depict base-case scenario A (for definition of scenarios, see Scenario Analyses subsection of the Methods). ALI 75 indicates alirocumab, 75 mg; ALI 150, alirocumab, 150 mg; EZE, ezetimibe; HIS, high-intensity statin; and MIS, moderate- to low-intensity statin. To convert LDL-C levels to millimoles per liter, multiply by 0.0259.
Figure 3. Use of Lipid-Lowering Treatment and Low-Density Lipoprotein Cholesterol (LDL-C) Level Distribution After Treatment Intensification for Other Scenarios.
Scenario B1 consists of patients with LDL-C goals of less than 70 mg/dL with comorbidities and less than 100 mg/dL without comorbidities; scenario B2, 50% reduction of LDL-C levels from baseline; scenario B3, scenario B1 or B2; scenario B4, scenarios B1 and B2; scenario C, threshold for adding alirocumab increased to LDL-C goal of less than 80 mg/dL; and scenario D, ezetimibe removed before adding alirocumab if LDL-C goal of less than 70 mg/dL was not achieved. ALI 75 indicates alirocumab, 75 mg; ALI 150, alirocumab, 150 mg; EZE, ezetimibe; HIS, high-intensity statin; and MIS, moderate- to low-intensity statin. To convert LDL-C levels to millimoles per liter, multiply by 0.0259. For additional information on scenarios, see the Scenario Analyses subsection of the Methods.
Figure 4. Use of Lipid-Lowering Treatment (LLT) With Full Treatment Intensification Across All Scenarios.
Scenarios are ordered by increasing use of proprotein convertase subtilisin/kexin type 9 inhibitor therapy (for definition of scenarios, see Scenario Analyses subsection of the Methods). Scenario A includes reduction of low-density lipoprotein cholesterol (LDL-C) levels to less than 70 mg/dL for all patients and LLT with alirocumab (ALI) as third-line therapy after statins and add-on ezetimibe (EZE). Scenarios B1-B4, C, and D are described in Figure 3. Scenarios A and C used ALI as third-line therapy after statins and add-on EZE. Other scenarios removed EZE before adding ALI if goal LDL-C level was not achieved. ALI75 indicates alirocumab, 75 mg; ALI150, alirocumab, 150 mg; HIS, high-intensity statin; and MIS, moderate-to-low intensity statin. To convert LDL-C levels to millimoles per liter, multiply by 0.0259.
Discussion
In a contemporary US population with ASCVD, only 53.2% received a statin at baseline and only 15.3% received a high-intensity statin; this treatment resulted in only 25.2% achieving LDL-C levels of less than 70 mg/dL. Therefore, we explored various LLT intensification strategies, all of which used statin therapy initially before adding nonstatin agents if LDL-C levels remained above a predefined threshold (or if a 50% LDL-C level reduction was not achieved). When following this approach, we found that 69.3% of patients could achieve LDL-C levels of less than 70 mg/dL with statin initiation and/or uptitration only, and add-on ezetimibe could increase this percentage to 86%. Adding a PCSK9 inhibitor to therapy for the remaining 14% still above the LDL-C threshold could result in more than 99% of the population with ASCVD having LDL-C levels of less than 70 mg/dL. In other scenarios, we found that if a PCSK9 inhibitor was added only for those individuals with LDL-C levels of 80 mg/dL or more, only 7.7% would need a PCSK9 inhibitor. However, in accordance with ACC ECDP recommendations, which recommend the goal of at least 50% reduction in LDL-C levels with an optional threshold of less than 70 mg/dL (scenario B2 representing the 50% reduction case), a higher proportion of patients (20.9% compared with 14%) would need a PCSK9 inhibitor. Collectively, these findings indicated an opportunity in improving the achievement of an LDL-C goal with oral-only LLT, with 14% to 20.9% of the population with ASCVD requiring a PCSK9 inhibitor.
Implications on PCSK9 Inhibitor Use
When using a simple algorithm that added a PCSK9 inhibitor when the LDL-C level was 70 mg/dL or higher, despite maximal oral therapy, we observed that only 14% of the simulation cohort required these agents. In a modeled scenario, we found that this proportion of patients decreased considerably as the threshold for initiation of PCSK9 inhibitor therapy was increased from 70 mg/dL or higher to 80 mg/dL or higher, with PCSK9 inhibitor use changing from 14% to 7.7%. This scenario was meant to simulate a patient with an LDL-C level of, for example, 72 mg/dL who is receiving a high-intensity statin and ezetimibe and might be an unlikely candidate to receive a PCSK9 inhibitor in clinical practice. Our simulation model can be used to explore and test other thresholds and scenarios to help clinicians and payers with the identification of treatment strategies that promote thoughtful use of these newer agents.
Our algorithm for LLT intensification used 2 doses of alirocumab as the PCSK9 inhibitor therapy, with the lower dose administered first as suggested in the US Food and Drug Administration–approved prescribing information. With this treatment strategy, 85.6% of patients receiving the initial low dose of alirocumab were able to achieve LDL-C levels of less than 70 mg/dL. This proportion matches the experience in the ODYSSEY program in trials with patients with ASCVD or a high CVE risk who received a dose titration to reach LDL-C levels of less than 70 mg/dL, in which approximately 83% of patients required only the 75-mg dose. Although we considered alirocumab as the PCSK9 inhibitor therapy in the base-case simulation, the key results regarding proportion of patients requiring PCSK9 inhibitors also apply to evolocumab because PCSK9 inhibitors were considered in the simulation as the last step in treatment intensification after statins and ezetimibe.
A change in approach to using PCSK9 inhibitors may result from the clinical benefit being demonstrated in the FOURIER trial (with ODYSSEY Outcomes still ongoing). Entry criteria for both of these trials included patients being on maximally tolerated statins and having an LDL-C level of 70 mg/dL or higher. If an LDL-C threshold of higher than 70 mg/dL is regarded as evidence-based in light of these trials, we could anticipate that approximately 14% of all patients with ASCVD would be eligible for this class of agent, if oral LLT were optimally maximized before PCSK9 inhibitor use. If only high-intensity statin therapy is required (and not also ezetimibe) as background therapy, our model would anticipate that 30.7% of all patients with ASCVD would require a PCSK9 inhibitor. A reference point from the recent literature regarding these estimates is an analysis from the US Veterans Affairs database that concluded that approximately 25% of patients with ASCVD would be eligible for evolocumab with the application of broad FOURIER inclusion and exclusion criteria and approximately 10% would be eligible if full LLT intensification with statins and ezetimibe occurs before treatment with evolocumab.
Achieved LDL-C Levels
An additional output from the simulation model is the detailed distribution of achieved LDL-C levels with various treatment intensification strategies. As seen in Figure 2A and eTable 6 in the Supplement, we found a wide variation in LDL-C levels at baseline. With treatment intensification, in those achieving LDL-C levels of less than 70 mg/dL who received statins with or without ezetimibe only, 97.2% achieved LDL-C levels from 25 to 70 mg/dL. In those receiving add-on alirocumab (not achieving LDL-C levels <70 mg/dL with statins with or without ezetimibe only), approximately 72.0% achieved LDL-C levels from 25 to 70 mg/dL; 22%, from 10 to 25 mg/dL; and 0.8% (0.1% of the overall cohort), less than 10 mg/dL. To date, achievement of these lower levels of LDL-C has not been associated with a safety signal; however, the long-term effects of very low levels of LDL-C with PCSK9 inhibitors are not well established. The relatively low proportion of patients achieving very low LDL-C levels (eg, <10 mg/dL) was attributable to the stepwise approach to LLT intensification, including initial use of low-dose PCSK9 inhibitors. As shown in eTable 8 in the Supplement, although the proportion of patients achieving LDL-C levels of less than 70 mg/dL was largely similar across scenarios with and without uptitration of the alirocumab dose, the proportion of patients achieving very low LDL-C levels (eg, <10 mg/dL) was much higher with the use of evolocumab or only high-dose alirocumab (10.2%-13.6%) compared with alirocumab dose uptitration (0.8%).
Simulation Model
An important feature of our study is the explicit handling of the variation in the percentage of LDL-C level reduction at a patient level as a result of LLT. In the JUPITER (Justification for the Use of Statins in Primary Prevention: An Intervention Trial Evaluating Rosuvastatin) study, the variation in percentage LDL-C level reduction with a 20-mg dose of rosuvastatin was wide, ranging from a modest increase in LDL-C levels to a greater than 80% reduction, with a median reduction of 50%. Similar findings were reported for a wider selection of statins and trials. The explicit handling of variation in the LDL-C level–lowering response in the simulation is expected to provide a better estimate of the relative proportion of patients who would take specific paths in a given treatment decision logic, becoming candidates for specific therapies, and their expected outcomes in terms of achievement of LDL-C goals. In addition, because the simulation was based on sampling individuals, the results also reflect an outcome that explicitly accounts for intrinsic heterogeneity in patient baseline characteristics, such as the distribution of baseline LDL-C levels and types of LLT. Because the sampling approach used a bootstrap method with application of sampling weights, it also helped ensure that the study population of 1 million individuals entering the simulation model is representative of the overall US population with ASCVD.
Limitations
We used a claims database in the United States for developing the study population, which can introduce specific biases, such as the population being fully insured, and groups such as those covered under Medicaid programs not being represented. Although we accounted for quantifiable biases based on age, sex, and clinical conditions with use of an adjusted sampling method, not all biases can be measured or corrected. We also have not provided a measure of statistical uncertainty around our estimates, because the Monte Carlo method with a large sample size (in this case, 1 million) converges around expectations with effective 95% CIs of nearly 0. We realize that most health care systems and clinicians would be concerned with populations with ASCVD that are smaller. eTable 5 in the Supplement provides 95% CIs for the base-case analysis for several sizes of populations with ASCVD. Medication use at baseline was estimated using evidence of filled prescriptions at a point in time, thus accounting for factors such as primary nonadherence (ie, written prescription never filled) and discontinuation of treatment over time. Our estimates of statin use at baseline (53.2%) are thus somewhat lower than those from other studies, which have reported statin use in patients with ASCVD based on prescription only from 58% to 70%. Finally, our simulation model assumed perfect adherence with LLT, no tolerability issues (representing relatively mild effects that may not warrant stopping treatment) or adverse effects, and that the decisions to escalate LLT in response to reductions of LDL-C levels are instantaneous and strictly according to assumed logic with no deviations. Although many of these assumptions would not be met in usual clinical practice, our study provides a reference point for implications based on certain well-defined assumptions.
Conclusions
In a large and representative US population with ASCVD, 53.2% received statins at baseline and only 25.2% achieved LDL-C levels of less than 70 mg/dL. Our study suggests that, under maximal treatment intensification with LLT, assuming full compliance and no tolerability issues, roughly 86% of patients would achieve LDL-C levels of less than 70 mg/dL with statins and ezetimibe only. Adding a PCSK9 inhibitor to the regimen for the remaining 14% of patients with LDL-C levels of at least 70 mg/dL would result in more than 99% of the population with ASCVD achieving LDL-C levels of less than 70 mg/dL. These data help identify the therapies that could be used to maximize lowering of LDL-C levels in patients with ASCVD.
eTable 1. Diagnosis and Procedure Codes for Identification of ASCVD in the Database
eTable 2. Mean and SD of Percentage Reduction in LDL-C Levels From Statins and Ezetimibe
eTable 3. List of Comorbidities for Scenarios Modeling the American College of Cardiology Expert Consensus Decision Pathway Recommendations
eTable 4. Comparison of Baseline Characteristics for the Simulation Cohort With ASCVD Populations From the NHANES Database and PINNACLE Registry
eTable 5. Estimated 95% CIs for the Proportion of ASCVD Patients Receiving Add-on PCSK9 Inhibitors in the Simulation for the Base Case
eTable 6. Distribution of LDL-C Levels Before and After Treatment Intensification in Base-Case Scenario A
eTable 7. Use of LLTs With Full Treatment Intensification Across All Scenarios
eTable 8. Achieved LDL-C Levels for the Base Case Compared With Scenarios Representing Treatment With Only Alirocumab, 150 mg, or Evolocumab
eFigure 1. Determination of Treatment Status as of the Index Date
eFigure 2. Flowchart of the Cohort Selection for the Study
eFigure 3. LLT Use and LDL-C Level Distribution After Treatment Intensification With an LDL-C Threshold of <55 mg/dL Compared With <70 mg/dL
eMethods. Estimation of Sampling Weights for Bootstrap Sampling
References
- 1.Baigent C, Blackwell L, Emberson J, et al. ; Cholesterol Treatment Trialists’ (CTT) Collaboration . Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376(9753):1670-1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mihaylova B, Emberson J, Blackwell L, et al. ; Cholesterol Treatment Trialists’ (CTT) Collaborators . The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: meta-analysis of individual data from 27 randomised trials. Lancet. 2012;380(9841):581-590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stone NJ, Robinson JG, Lichtenstein AH, et al. ; American College of Cardiology/American Heart Association Task Force on Practice Guidelines . 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(25, pt B):2889-2934. [DOI] [PubMed] [Google Scholar]
- 4.Cannon CP, Blazing MA, Giugliano RP, et al. ; IMPROVE-IT Investigators . Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372(25):2387-2397. [DOI] [PubMed] [Google Scholar]
- 5.Murphy SA, Cannon CP, Blazing MA, et al. . Reduction in total cardiovascular events with ezetimibe/simvastatin post-acute coronary syndrome: the IMPROVE-IT trial. J Am Coll Cardiol. 2016;67(4):353-361. [DOI] [PubMed] [Google Scholar]
- 6.Lloyd-Jones DM, Morris PB, Ballantyne CM, et al. ; Writing Committee . 2016 ACC Expert Consensus Decision Pathway on the role of non-statin therapies for LDL-cholesterol lowering in the management of atherosclerotic cardiovascular disease risk: a report of the American College of Cardiology Task Force on Clinical Expert Consensus Documents. J Am Coll Cardiol. 2016;68(1):92-125. [DOI] [PubMed] [Google Scholar]
- 7.Shrank WH, Barlow JF, Brennan TA. New therapies in the treatment of high cholesterol: an argument to return to goal-based lipid guidelines. JAMA. 2015;314(14):1443-1444. [DOI] [PubMed] [Google Scholar]
- 8.Petersen LA, Wright S, Normand SL, Daley J. Positive predictive value of the diagnosis of acute myocardial infarction in an administrative database. J Gen Intern Med. 1999;14(9):555-558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Andrade SE, Harrold LR, Tjia J, et al. . A systematic review of validated methods for identifying cerebrovascular accident or transient ischemic attack using administrative data. Pharmacoepidemiol Drug Saf. 2012;21(suppl 1):100-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Press WH, Teukolsky SA, Vetterling WT, Flannery BP Numerical Recipes: The Art of Scientific Computing. 3rd ed. Cambridge, England: Cambridge University Press; 2007. [Google Scholar]
- 11.Ridker PM, Mora S, Rose L; JUPITER Trial Study Group . Percent reduction in LDL cholesterol following high-intensity statin therapy: potential implications for guidelines and for the prescription of emerging lipid-lowering agents. Eur Heart J. 2016;37(17):1373-1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karlson BW, Wiklund O, Palmer MK, Nicholls SJ, Lundman P, Barter PJ. Variability of low-density lipoprotein cholesterol response with different doses of atorvastatin, rosuvastatin, and simvastatin: results from VOYAGER. Eur Heart J Cardiovasc Pharmacother. 2016;2(4):212-217. [DOI] [PubMed] [Google Scholar]
- 13.Sabatine MS, Giugliano RP, Keech AC, et al. ; FOURIER Steering Committee and Investigators . Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376(18):1713-1722. [DOI] [PubMed] [Google Scholar]
- 14.Wong ND, Young D, Zhao Y, et al. . Prevalence of the American College of Cardiology/American Heart Association statin eligibility groups, statin use, and low-density lipoprotein cholesterol control in US adults using the National Health and Nutrition Examination Survey 2011-2012. J Clin Lipidol. 2016;10(5):1109-1118. [DOI] [PubMed] [Google Scholar]
- 15.Maddox TM, Borden WB, Tang F, et al. . Implications of the 2013 ACC/AHA cholesterol guidelines for adults in contemporary cardiovascular practice: insights from the NCDR PINNACLE registry. J Am Coll Cardiol. 2014;64(21):2183-2192. [DOI] [PubMed] [Google Scholar]
- 16.Lloyd-Jones DM. Role of nonstatin therapies for low-density lipoprotein cholesterol lowering in management of atherosclerotic cardiovascular disease risk. JAMA Cardiol. 2017;2(2):218-219. [DOI] [PubMed] [Google Scholar]
- 17.Kereiakes DJ, Robinson JG, Cannon CP, et al. . Efficacy and safety of the proprotein convertase subtilisin/kexin type 9 inhibitor alirocumab among high cardiovascular risk patients on maximally tolerated statin therapy: The ODYSSEY COMBO I study. Am Heart J. 2015;169(6):906-915.e13. [DOI] [PubMed] [Google Scholar]
- 18.Schwartz GG, Bessac L, Berdan LG, et al. . Effect of alirocumab, a monoclonal antibody to PCSK9, on long-term cardiovascular outcomes following acute coronary syndromes: rationale and design of the ODYSSEY outcomes trial. Am Heart J. 2014;168(5):682-689. [DOI] [PubMed] [Google Scholar]
- 19.Virani SS, Akeroyd JM, Nambi V, et al. . Estimation of eligibility for PCSK9 inhibitors and associated costs based on the FOURIER trial: insights from the Department of Veterans Affairs. Circulation. 2017;135(25):2572-2574. [DOI] [PubMed] [Google Scholar]
- 20.Robinson JG, Rosenson RS, Farnier M, et al. . Safety of very low low-density lipoprotein cholesterol levels with alirocumab: pooled data from randomized trials. J Am Coll Cardiol. 2017;69(5):471-482. [DOI] [PubMed] [Google Scholar]
- 21.Giugliano RP, Wiviott SD, Blazing MA, et al. . Long-term Safety and Efficacy of Achieving Very Low Levels of Low-Density Lipoprotein Cholesterol: A Prespecified Analysis of the IMPROVE-IT Trial. JAMA Cardiol. 2017;2(5):547-555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boekholdt SM, Hovingh GK, Mora S, et al. . Very low levels of atherogenic lipoproteins and the risk for cardiovascular events: a meta-analysis of statin trials. J Am Coll Cardiol. 2014;64(5):485-494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johansen ME, Green LA, Sen A, Kircher S, Richardson CR. Cardiovascular risk and statin use in the United States. Ann Fam Med. 2014;12(3):215-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Salami JA, Warraich H, Valero-Elizondo J, et al. . National trends in statin use and expenditures in the US adult population from 2002 to 2013: insights from the medical expenditure panel survey. JAMA Cardiol. 2017;2(1):56-65. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Diagnosis and Procedure Codes for Identification of ASCVD in the Database
eTable 2. Mean and SD of Percentage Reduction in LDL-C Levels From Statins and Ezetimibe
eTable 3. List of Comorbidities for Scenarios Modeling the American College of Cardiology Expert Consensus Decision Pathway Recommendations
eTable 4. Comparison of Baseline Characteristics for the Simulation Cohort With ASCVD Populations From the NHANES Database and PINNACLE Registry
eTable 5. Estimated 95% CIs for the Proportion of ASCVD Patients Receiving Add-on PCSK9 Inhibitors in the Simulation for the Base Case
eTable 6. Distribution of LDL-C Levels Before and After Treatment Intensification in Base-Case Scenario A
eTable 7. Use of LLTs With Full Treatment Intensification Across All Scenarios
eTable 8. Achieved LDL-C Levels for the Base Case Compared With Scenarios Representing Treatment With Only Alirocumab, 150 mg, or Evolocumab
eFigure 1. Determination of Treatment Status as of the Index Date
eFigure 2. Flowchart of the Cohort Selection for the Study
eFigure 3. LLT Use and LDL-C Level Distribution After Treatment Intensification With an LDL-C Threshold of <55 mg/dL Compared With <70 mg/dL
eMethods. Estimation of Sampling Weights for Bootstrap Sampling