Key Points
Question
Does race affect outcomes at 1 year in patients undergoing percutaneous coronary intervention at US Veterans Affairs hospitals?
Findings
This study used data recorded in the Veterans Affairs Clinical Assessment, Reporting, and Tracking System for Cath Laboratories (CART-CL) program to study 42 391 patients undergoing percutaneous coronary intervention. There was no difference in adjusted mortality between black and white patients undergoing percutaneous coronary intervention at the Veterans Affairs hospitals at 1 year.
Meaning
Race was not independently associated with 1-year mortality among black and white patients undergoing percutaneous coronary intervention at Veterans Affairs hospitals.
Abstract
Importance
Current comparative outcomes among black and white patients treated with percutaneous coronary intervention (PCI) in the Veterans Affairs (VA) health system are not known.
Objective
To compare outcomes between black and white patients undergoing PCI in the VA health system.
Design, Setting, and Participants
This study compared black and white patients who underwent PCI between October 1, 2007, and September 30, 2013, at 63 VA hospitals using data recorded in the VA Clinical Assessment, Reporting, and Tracking System for Cardiac Catheterization Laboratories (CART-CL) program. A generalized linear mixed model with a random intercept for site assessed the relative difference in odds of outcomes between black and white patients. The setting was integrated institutionalized hospital care. Excluded were all patients of other races or those with multiple listed races and those with missing data regarding race or the diagnostic cardiac catheterization. The dates of analysis were January 7, 2016, to April 17, 2017.
Exposure
Percutaneous coronary intervention at a VA hospital.
Main Outcomes and Measures
The primary outcome was 1-year mortality. Secondary outcomes were 30-day all-cause readmission rates, 30-day acute kidney injury, 30-day blood transfusion, and 1-year readmission rates for myocardial infarction. In addition, variations in procedural and postprocedural care were examined, including the use of intravascular ultrasound, optical coherence tomography, fractional flow reserve measurements, bare-metal stents, postprocedural medications, and radial access.
Results
A total of 42 391 patients (13.3% black and 98.4% male; mean [SD] age, 65.2 [9.1] years) satisfied the inclusion and exclusion criteria. In unadjusted analyses, black patients had higher rates of 1-year mortality (7.1% vs 5.9%, P < .001) as well as secondary outcomes of 30-day acute kidney injury (20.8% vs 13.8%, P < .001), 30-day blood transfusion (3.4% vs 2.7%, P < .01), and 1-year readmission rates for myocardial infarction (3.3% vs 2.7%, P = .01) compared with white patients. After adjustment for demographics, comorbidities, and procedural characteristics, odds for 1-year mortality (odds ratio, 1.04; 95% CI, 0.90-1.19) were not different between black and white patients. There were also no differences in secondary outcomes with the exception of a higher rate of adjusted 30-day acute kidney injury (odds ratio, 1.22; 95% CI, 1.10-1.36).
Conclusions and Relevance
While black patients had a higher rate of mortality than white patients in unadjusted analyses, race was not independently associated with 1-year mortality among patients undergoing PCI in VA hospitals.
This study using data recorded in the Veterans Affairs Clinical Assessment, Reporting, and Tracking System for Cath Labs (CART-CL) program compares outcomes between black and white patients undergoing percutaneous coronary intervention in the Veterans Affairs hospitals system.
Introduction
Race has well-established associations with the quality of care and outcomes of patients with coronary artery disease. Black patients are less likely to be recommended to undergo invasive angiography compared with white patients. Moreover, once coronary artery disease has been found, black patients are less likely to undergo revascularization via percutaneous or surgical methods. Among contemporary Medicare patients, black patients have higher intermediate and long-term adjusted mortality rates after percutaneous coronary intervention (PCI). These trends persist after controlling for socioeconomic factors.
The Veterans Affairs (VA) health system represents a unique nationalized, single-payer health care system within the United States. In the case of coronary artery disease management within the VA system, there may exist both a diminished financial incentive among physicians for unnecessary revascularization as well as a diminished financial burden for the patient when revascularization is performed. While black patients have worse intermediate and long-term outcomes after PCI than their white counterparts in non-VA hospitals, analyses of this issue in the VA system have not been performed. In addition, potential racial variation in procedural and postprocedural care among patients undergoing PCI at VA hospitals has not been studied to date.
We hypothesized that black patients treated at VA hospitals have worse adjusted 1-year outcomes after PCI than their white counterparts. Furthermore, we hypothesized that differences in 1-year mortality are at least partially mediated by variations in procedural and postprocedural care, including stent type choice, access site, completeness of revascularization, and post-PCI medication prescription.
Methods
Data for this analysis were derived from the VA Clinical Assessment, Reporting, and Tracking System for Cardiac Catheterization Laboratories (CART-CL) program. The CART-CL program compiles data from the patient’s clinical history embedded in the VA electronic health record (EHR) and captures standard patient and procedural data from all procedures performed at VA catheterization laboratories across the nation. Data elements have been engineered to comply with definitions as reported in the National Cardiovascular Data Registry database. The VA CART-CL data are also merged with the VA EHR to derive longitudinal patient data, including pharmacy prescription and refills, inpatient data, outpatient clinic visit data, and laboratory data. Patient mortality was ascertained from the Veterans Health Administration Vital Status File, which extracts from multiple VA and non-VA data sources, including the VA beneficiary death file, the VA Medicare Vital Status File, and the Social Security Administration Death Master File. The Colorado Multiple Institutional Review Board provided waiver of consent and approval for this study.
Study Population
Patients who underwent PCI between October 1, 2007, and September 30, 2013, with records in the CART-CL database were eligible for inclusion. The dates of analysis were January 7, 2016, to April 17, 2017. Analyses were restricted to patients listing their race as black or white, excluding all patients with multiple listed races or other races, such as Hispanic and Asian. Sites with no black patients in the cohort or that were outside of the 50 states or District of Columbia (ie, US territories) were excluded from analyses. Furthermore, we limited our analyses to patients whose diagnostic catheterization was recorded in the CART-CL database because information from the diagnostic catheterization included key covariates.
Patient race was recorded in the VA Corporate Data Warehouse administrative tables. These tables contain the most recent race entered in the Veterans Information Systems and Technology Architecture (VistA) system. Because different sites may have different VistA systems, multiple entries may be found in the tables, in which case priority was given to race that was self-reported or reported by a proxy. Patients with differing self-reported races were excluded. Patient zip codes were used to generate demographic information, including region of the country, median household income stratified into quartiles, and urban or rural status. Region of the country was defined by US Census divisions and median household income by zip code using 2006 to 2010 US Census data.
Outcomes
The primary outcome was 1-year mortality. It was calculated as a dichotomous variable denoted as death during the hospitalization or within 1 year after hospital discharge from the index PCI.
All secondary outcomes were dichotomous variables and included 30-day all-cause readmission rates, 30-day acute kidney injury (AKI), 30-day blood transfusion, and 1-year readmission rates for myocardial infarction (MI). All-cause readmissions included all inpatient and observation stays that either occurred at a VA hospital or in which the VA system was invoiced for care. Admissions paid for through private insurers or Medicare at non-VA hospitals were not captured. One-year rehospitalization for MI excluded events within 14 days of the index PCI due to inability to determine if the MI diagnosis was related to the index procedure. Acute kidney injury was defined as at least one creatinine level at least 0.3 mg/dL above baseline creatinine within 30 days of the index PCI (to convert creatinine level to micromoles per liter, multiply by 88.4). Baseline creatinine was defined as the most recent available value within 30 days before the index PCI.
Procedural and Postprocedural Care
If a significant adjusted mortality difference was found between races, a prespecified mediation analysis was planned to investigate the potential role of variations in procedural and postprocedural care in driving this difference. Procedural and postprocedural factors considered in the analyses included the following: (1) the use of advanced intracoronary imaging or physiologic testing (fractional flow reserve [FFR], intravascular ultrasound [IVUS], or optical coherence tomography [OCT]); (2) bare-metal stent (BMS) vs drug-eluting stent (DES) use; (3) incomplete revascularization; (4) radial access use; and (5) postprocedural medication prescriptions (high-intensity statins, β-blockers, angiotensin-converting enzyme inhibitor [ACEi] or aldosterone receptor blocker [ARB] therapy, and clopidogrel bisulfate, ticagrelor, or prasugrel). Performance of FFR, IVUS, or OCT included the use of this technology during the diagnostic catheterization, PCI, or both. Bare-metal stent use was defined as at least one BMS placed during the index PCI. Incomplete revascularization was defined as at least one vessel with at least 70% stenosis (50% for the left main coronary artery) noted in the diagnostic catheterization that was not treated with PCI. Cases with multiple access sites were defined by the first access site used during the PCI procedure. Postprocedural medication prescription was defined by prescription of a medication in each class or group at any time within 14 days after hospital discharge. High-intensity statin prescription was defined as 80 mg of simvastatin, 40 mg or greater of atorvastatin calcium, or 20 mg or greater of rosuvastatin calcium, consistent with current guidelines.
Statistical Analysis
Patient characteristics stratified by race were summarized with proportions (total numbers) for categorical variables and means (SDs) for continuous variables. χ2 Tests for categorical variables and t tests for continuous variables assessed if there was a significant difference based on race for each variable. Variables based on procedural characteristics also included information collected in all staged procedures associated with the index PCI.
Variables were reviewed for outliers and missing data. Two covariates, PCI status and PCI indication, had missing values. Because of the difficulty in imputing these variables, patients missing these variables were excluded from analyses (Figure 1). Two additional covariates, obesity (dichotomized by body mass index >30 [calculated as weight in kilograms divided by height in meters squared]) and fluoroscopy time, contained missing values, and fluoroscopy time also had extreme values. We discarded any fluoroscopy times greater than 200 minutes and treated them as missing data. Fluoroscopy time was summed across the diagnostic catheterization and PCI and was logarithmically transformed in the analysis to account for skew in its distribution. Obesity and fluoroscopy time results were then imputed by simulating random values from distributions, with patient-specific parameters estimated using models generated from observed data. Similar results were observed across multiple imputations; therefore, only a single imputation was used for final analyses. A sensitivity analysis performed to explore the potential influence of missing data revealed minimal influence of missing data on the final results.
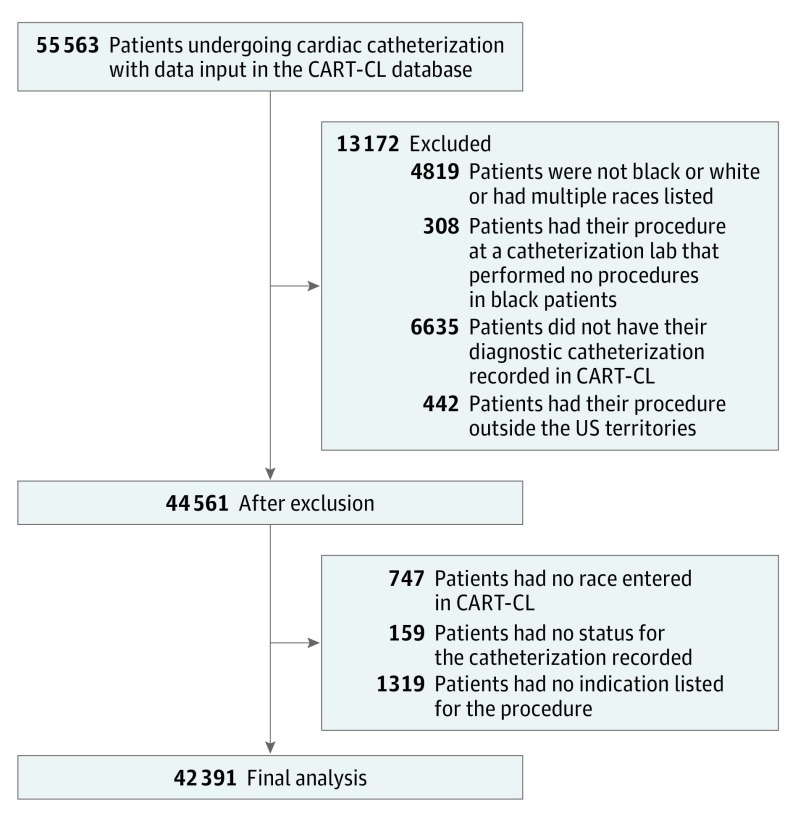
Figure 1. Patients Included and Excluded in the Analysis.
Some patients fit into multiple exclusion categories. CART-CL indicates Clinical Assessment, Reporting, and Tracking System for Catheterization Laboratories.
Unadjusted outcome proportions were calculated for black vs white patients, with a corresponding P value from a χ2 test. Results were further stratified by acute coronary syndrome (ACS) status. A generalized linear mixed model (GLMM) with a logit link compared odds of 1-year mortality for black vs white patients. The model controlled for the following: (1) patient demographics (age, sex, income, region, and urban vs rural), (2) medical history as obtained from the CART-CL database and linkage to the VA EHR (heart failure, chronic kidney disease, chronic obstructive pulmonary disease, cerebrovascular disease, depression, diabetes [with or without insulin use], hyperlipidemia, hypertension, obesity, peripheral artery disease, posttraumatic stress disorder, sleep apnea, and tobacco use), (3) procedural characteristics (fluoroscopy time, intra-aortic balloon pump use, PCI indication, positive functional study, and staged procedure), (4) PCI status (elective, urgent, or emergent salvage), (5) specific vessels stented, and (6) an index length of stay of more than 3 days. The model included a random intercept for site to account for clustering of patients within hospitals. In addition, we adjusted for a site-level variable representing the proportion of all patients receiving PCI that were black. These models were generated in R (version 3.2.5; The R Foundation) using the “glmmML” package. Similar models were then produced for all secondary outcomes.
Prespecified secondary mediation analyses addressing potential variations in procedural and postprocedural care were planned. A similar GLMM was generated adjusting for the previously mentioned covariates and random intercept, with each procedural and postprocedural care mediator as the outcome, followed by mediation analyses designed to elucidate the direct, indirect, and total influence of race on the primary outcome.
To account for multiple testing, a Bonferroni correction was used. The traditional α level of .05 was divided by 13 (accounting for primary outcomes, secondary outcomes, and analyses of procedural and postprocedural care) to produce our updated level of statistical significance, 2-sided P = .004.
Post Hoc Analyses
To more fully understand the attenuation of the unadjusted difference in the primary outcome, stepwise analyses were performed. Adjustment was carried out in 4 steps whereby covariates from previous steps carried forward while assessing the change in association between race and mortality. A separate analysis was also performed adjusting for categories of covariates individually, omitting all covariates from other steps while assessing the change in association between race and mortality. In addition to age, sex, and time trend, step 1 included the following comorbidities: heart failure, chronic kidney disease, chronic obstructive pulmonary disease, cerebrovascular disease, depression, diabetes (with or without insulin use), hemodialysis use, hyperlipidemia, hypertension, obesity, peripheral artery disease, posttraumatic stress disorder, sleep apnea, and tobacco use. Step 2 included the following presentation or anatomic factors: fluoroscopy time, intra-aortic balloon pump use, PCI indication, index length of stay exceeding 3 days, positive functional study, staged procedure, PCI status, and specific vessels stented. Step 3 included socioeconomic status (the median household income [with zip code] and urban vs rural). Step 4 included a site-level racial breakdown (proportion of black patients at the catheterization laboratory). Finally, BMS and β-blocker use were assessed in analyses that stratified patients according to presentation status (ACS vs non-ACS).
Results
Patients
A total of 55 563 patients underwent PCI between October 1, 2007, and September 30, 2013, at 69 VA cardiac catheterization laboratories. After applying exclusion criteria and removing patients with missing data, the final study cohort included 42 391 patients from 63 VA hospitals (Figure 1).
Black patients comprised 13.3% of the cohort. Black patients tended to be younger, of lower socioeconomic status, and from urban areas. Black patients had a higher burden of several medical comorbidities, including heart failure, chronic kidney disease, hemodialysis, diabetes, peripheral artery disease, posttraumatic stress disorder, and tobacco use. Black patients were more likely to be initially seen in the cardiac catheterization laboratory with ACS (33.5% vs 26.2%, P < .01) and were more likely to undergo PCI under urgent or emergent conditions (43.2% vs 36.6%, P < .01). Baseline demographic and procedural characteristics are summarized in Table 1.
Table 1. Baseline Demographic and Procedural Characteristicsa.
| Variable | All (N = 42 391) |
Black Patients (n = 5650) |
White Patients (n = 36 741) |
P Value |
|---|---|---|---|---|
| Demographics | ||||
| Age, mean (SD), y | 65.2 (9.1) | 62.7 (9.6) | 65.6 (9.0) | <.01 |
| Male, No. (%) | 41 713 (98.4) | 5486 (97.1) | 36 227 (98.6) | <.01 |
| Income quartile, No. (%) | ||||
| 1 | 10 598 (25.0) | 2506 (44.4) | 8092 (22.0) | <.01 |
| 2 | 10 592 (25.0) | 1059 (18.7) | 9533 (25.9) | |
| 3 | 10 599 (25.0) | 1037 (18.4) | 9562 (26.0) | |
| 4 | 10 602 (25.0) | 1048 (18.5) | 9554 (26.0) | |
| Region, No. (%) | ||||
| New England | 1083 (25.5) | 43 (0.8) | 1040 (2.8) | <.01 |
| Middle Atlantic | 2583 (6.1) | 289 (5.1) | 2294 (6.2) | |
| East North Central | 7123 (16.8) | 987 (17.5) | 6136 (16.7) | |
| West North Central | 4758 (11.2) | 493 (8.7) | 4265 (11.6) | |
| South Atlantic | 9677 (22.8) | 1733 (30.7) | 7944 (21.6) | |
| East South Central | 4185 (9.9) | 624 (11.0) | 3561 (9.7) | |
| West South Central | 6732 (15.9) | 999 (17.7) | 5733 (15.6) | |
| Mountain | 2687 (6.3) | 87 (1.5) | 2600 (7.1) | |
| Pacific | 3563 (8.4) | 395 (7.0) | 3168 (8.6) | |
| Urban, No. (%) | 23 560 (55.6) | 4679 (82.8) | 18 881 (51.4) | <.01 |
| Medical History, No. (%) | ||||
| Congestive heart failure | 9703 (22.9) | 1524 (27.0) | 8179 (22.3) | <.01 |
| Chronic kidney disease | ||||
| No | 34 354 (81.0) | 4018 (71.1) | 30 336 (82.6) | <.01 |
| Without hemodialysis use | 7347 (17.3) | 1368 (24.2) | 5979 (16.3) | |
| With hemodialysis use | 690 (1.6) | 264 (4.7) | 426 (1.2) | |
| Chronic obstructive pulmonary disease | 9215 (21.7) | 842 (14.9) | 8373 (22.8) | <.01 |
| Cerebrovascular disease | 7199 (17.0) | 901 (15.9) | 6298 (17.1) | .03 |
| Depression | 12 461 (29.4) | 1571 (27.8) | 10 890 (29.6) | .01 |
| Diabetes | ||||
| No | 22 641 (53.4) | 2822 (49.9) | 19 819 (53.9) | <.01 |
| With insulin use | 8112 (19.1) | 1264 (22.4) | 6848 (18.6) | |
| Without insulin use | 11 638 (27.5) | 1564 (27.7) | 10 074 (27.4) | |
| Hyperlipidemia | 37 681 (88.9) | 4833 (85.5) | 32 848 (89.4) | <.01 |
| Hypertension | 37 858 (89.3) | 5274 (93.3) | 32 584 (88.7) | <.01 |
| Obesity | 19 946 (47.1) | 2343 (41.5) | 17 603 (47.9) | <.01 |
| Peripheral artery disease | 8680 (20.5) | 1208 (21.4) | 7472 (20.3) | .07 |
| Posttraumatic stress disorder | 6624 (15.6) | 993 (17.6) | 5631 (15.3) | <.01 |
| Sleep apnea | 7678 (18.1) | 780 (13.8) | 6898 (18.8) | <.01 |
| Tobacco use | 26 451 (62.4) | 3691 (65.3) | 22 760 (61.9) | <.01 |
| Procedural Characteristics | ||||
| Fluoroscopy time, mean (SD), min | 22.2 (16.6) | 21.1 (15.8) | 22.3 (16.7) | <.01 |
| Intra-aortic balloon pump use, No. (%) | 537 (1.3) | 77 (1.4) | 460 (1.3) | .53 |
| PCI indication, No. (%) | ||||
| NSTEMI | 8650 (20.4) | 1362 (24.1) | 7288 (19.8) | <.01 |
| STEMI | 2889 (6.8) | 533 (9.4) | 2356 (6.4) | |
| Stable angina, chest pain, or asymptomatic | 17 008 (40.1) | 1951 (34.5) | 15 057 (41.0) | |
| Unstable angina | 11 090 (26.2) | 1443 (25.5) | 9647 (26.3) | |
| Other or valvular disease | 2754 (6.5) | 361 (6.4) | 2393 (6.5) | |
| Index length of stay >3 d, No. (%) | 11 295 (26.6) | 1716 (30.4) | 9579 (26.1) | <.01 |
| Positive functional study, No. (%) | ||||
| No study | 23 148 (54.6) | 3250 (57.5) | 19 898 (54.2) | <.01 |
| Negative | 2015 (4.8) | 292 (5.2) | 1723 (4.7) | |
| Positive | 17 228 (40.6) | 2108 (37.3) | 15 120 (41.2) | |
| Staged procedure, No. (%) | 1844 (4.3) | 208 (3.7) | 1636 (4.5) | .01 |
| PCI status, No. (%) | ||||
| Elective | 26 496 (62.5) | 3209 (56.8) | 23 287 (63.4) | <.01 |
| Urgent | 12 930 (30.5) | 1876 (33.2) | 11 054 (30.1) | |
| Emergent salvage | 2965 (7.0) | 565 (10.0) | 2400 (6.5) | |
| Specific vessels stented, No. (%) | ||||
| Left anterior descending artery | 14 684 (34.6) | 2008 (35.5) | 12 676 (34.5) | .13 |
| Left circumflex artery | 11 034 (26.0) | 1572 (27.8) | 9462 (25.8) | <.01 |
| Left main coronary artery | 1181 (2.8) | 106 (1.9) | 1075 (2.9) | <.01 |
| Right coronary artery | 12 791 (30.2) | 1651 (29.2) | 11 140 (30.3) | .10 |
Abbreviations: NSTEMI, non–ST-segment elevation myocardial infarction; PCI, percutaneous coronary intervention; STEMI, ST-segment elevation myocardial infarction.
Absolute values are presented, with percentages of each cohort in parentheses.
Outcomes
In unadjusted analyses, black patients overall had higher 1-year mortality than white patients (7.1% vs 5.9%, P < .001). Black patients had higher rates of 30-day AKI (20.8% vs 13.8%, P < .001), 30-day blood transfusion (3.4% vs 2.7%, P < .01), and 1-year readmission for MI (3.3% vs 2.7%, P = .01), with similar rates of 30-day all-cause readmission (13.6% vs 12.9%, P = .14). Full unadjusted results stratified by presence or absence of ACS are listed in eTable 1 in the Supplement.
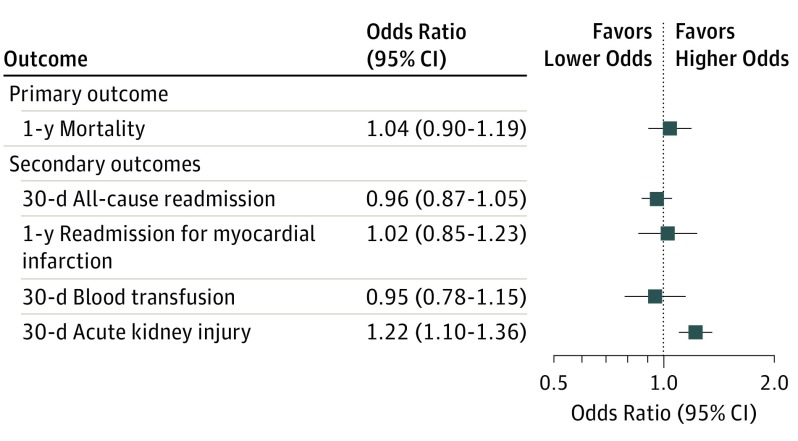
In adjusted primary analyses, there was no significant difference in odds of 1-year mortality (odds ratio [OR], 1.04; 95% CI, 0.90-1.19), with similar nonsignificant results for the secondary outcomes of 30-day all-cause readmission rates, 30-day blood transfusion, and 1-year readmission rates for MI between black and white patients. Analyses of AKI involved a reduced cohort of 33 331 after exclusion of patients receiving chronic hemodialysis and those missing creatinine measurements. The adjusted odds of 30-day AKI were significantly higher in black patients than white patients (OR, 1.22; 95% CI, 1.10-1.36). Because no significant results were found in the primary analysis, the prespecified secondary mediation analyses were not reported. Full results of the adjusted analyses are shown in Figure 2.
Figure 2. Primary and Secondary Adjusted Outcomes After Percutaneous Coronary Intervention for Black and White Patients in Veterans Affairs Hospitals.
Values greater than 1 indicate higher odds for black vs white patients.
Post hoc analyses revealed that adjustment for comorbidities and presentation or anatomic factors attenuated most of the difference in the unadjusted primary outcome, with socioeconomic status and the proportion of black patients at a site having minimal influence on the attenuation after accounting for the previous factors. These results are summarized in Table 2.
Table 2. Results of Post Hoc Analysesa.
| Variable | Odds Ratio (95% CI) | |
|---|---|---|
| Individual Adjustment | Sequential Adjustment | |
| Unadjusted | 1.18 (1.05-1.32) | 1.18 (1.05-1.32) |
| Step 1: comorbidities | 1.12 (0.99-1.27) | 1.12 (0.99-1.27) |
| Step 2: presentation or anatomic factors | 1.07 (0.95-1.20) | 1.05 (0.93-1.20) |
| Step 3: socioeconomic status | 1.12 (0.99-1.26) | 1.06 (0.92-1.21) |
| Step 4: site-level racial breakdown | 1.16 (1.03-1.31) | 1.04 (0.90-1.19) |
Shown is the change in association between race and mortality after adjustment for categories of factors. The individual adjustment results show the change in association after adjustment for the listed category of covariates, omitting all other categories. The sequential adjustment adjusts for the listed category of covariates as well as covariates from all previous steps.
Procedural and Postprocedural Care
In unadjusted analyses, black patients were more likely to be treated with a BMS (21.9% vs 18.7%, P < .001), have their PCI performed via radial access (16.9% vs 11.8%, P < .001), undergo complete revascularization (51.6% vs 49.6%, P < .01), receive postprocedural ACEi or ARB therapy (63.0% vs 58.8%, P < .001), and be prescribed high-intensity statin therapy (33.9% vs 30.6%, P < .001). Black patients were less likely to have advanced intracoronary imaging or physiologic testing (FFR, IVUS, or OCT) performed (20.0% vs 22.2%, P < .001). Rates of postprocedural β-blocker use (73.4% vs 72.8%, P = .38) and adenosine diphosphate receptor antagonist (clopidogrel, ticagrelor, or prasugrel) use (90.5% vs 91.1%, P = .13) were similar between black and white patients.
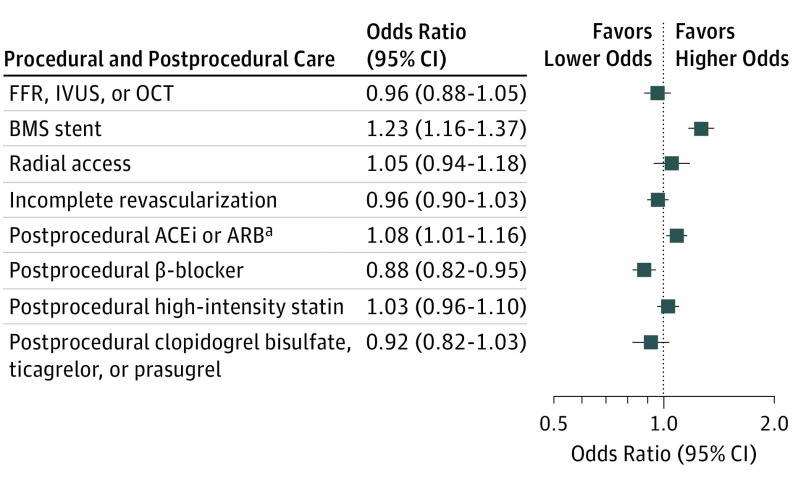
After adjustment, black patients had higher odds of BMS treatment (OR, 1.26; 95% CI, 1.16-1.37), similar odds of receiving ACEi or ARB therapy (after Bonferroni correction) (OR, 1.08; 95% CI, 1.01-1.16), and lower odds of being prescribed β-blocker therapy (OR, 0.88; 95% CI, 0.82-0.95) than their white counterparts. There was no significant racial variation in other assessed measures of procedural and postprocedural care after adjustment. Full adjusted results are shown in Figure 3.
Figure 3. Adjusted Rates of Procedural and Postprocedural Care Outcomes for Black and White Patients.
Values greater than 1 indicate higher odds for black vs white patients. ACEi indicates angiotensin-converting enzyme inhibitor; ARB, aldosterone receptor blocker; BMS, bare-metal stent; FFR, fractional flow reserve; IVUS, intravascular ultrasound; and OCT, optical coherence tomography. aResults are nonsignificant after adjustment for multiple tests.
Stratified analyses demonstrated that black patients were more likely to receive a BMS regardless of their presentation status, although this result did not meet statistical significance after Bonferroni correction among non-ACS patients (OR, 1.21; 95% CI, 1.06-1.37). Black patients with ACS remained less likely to receive β-blockers (OR, 0.85; 95% CI, 0.76-0.94). Full results are listed in eTable 2 in the Supplement.
Discussion
The present analysis reveals no significant association between black race and adjusted 1-year mortality for patients receiving PCI at VA hospitals in the United States. In addition, there were no significant associations with adjusted 30-day all-cause readmission rates, 30-day blood transfusion, or 1-year readmission rates for MI. Black patients had significantly higher associated adjusted rates of 30-day AKI after PCI than white patients, although this secondary analysis was performed on a limited cohort due to missing data. Also, black and white patients had modestly different treatment patterns during and after the PCI procedure, with black patients being more likely to receive a BMS during the procedure and being less likely to be prescribed β-blockers after the procedure.
This study represents the largest modern analysis to date of the influence of race on cardiovascular outcomes among US veterans. A strength of the study is the ability to adjust for a wide range of confounders through the merging of clinical registry data with administrative claims. These data include certain medical comorbidities (major depression, posttraumatic stress disorder, and obstructive sleep apnea), socioeconomic status, regional variation, and urbanization of populations, each of which has individually been implicated in worse outcomes in prior studies of cardiac patients but may not be as easily captured in pure administrative dataset analyses.
Several prior analyses of the influence of race on PCI outcomes in non-VA populations have been performed. The effort most comparable to the present study linked data from the National Cardiovascular Data Registry CathPCI Registry with administrative data from the Centers for Medicare & Medicaid Services to analyze post-PCI outcomes among Medicare recipients. That study demonstrated a significantly higher adjusted mortality rate in black patients compared with white patients over a 30-month follow-up period. There are several possible explanations for the difference in significance seen between the present VA analysis and that of Medicare patients treated in non-VA hospitals. First, the Medicare analysis reported a hazard ratio of 1.08 (95% CI, 1.04-1.12) for long-term mortality. The estimate for the adjusted OR for 1-year mortality in the present analysis is 1.04 (95% CI, 0.90-1.19). With the low mortality rates in these studies, ORs approximate hazard ratios, so the point estimates from the prior Medicare study and our work are similar. One explanation for the difference in statistical significance between the studies could be the larger sample size and narrower 95% CI in the Medicare study. The 95% CI in the present study indicates a range of likely mortality influences and excludes extreme differences in mortality between racial groups. It should be noted that the VA system has key system differences that could also have a role in creating differential PCI outcomes compared with the private sector. The VA system is an integrated health system that provides primary and specialty care, inpatient services, and outpatient pharmaceuticals. Integrated health systems have been associated with reduced racial outcome disparities in some medical conditions.
In post hoc analyses, we found that differences in the unadjusted primary outcome were primarily attenuated by adjustment for comorbidities, presentation, and anatomic factors, as summarized in Table 2. Black patients had a greater burden of medical comorbidities and manifested higher-acuity presentations than their white counterparts. Adjustment for socioeconomic status and site-level racial mix had minimal influence on our point estimate after adjustment for the above, indicating that most of the unadjusted mortality difference seen between races was not based on patients’ relative wealth or where they were treated. However, reduction in racial disparities relating to prevention and treatment of chronic comorbidities may lead to improved absolute outcomes among black veterans undergoing PCI.
Despite similar mortality and recurrent MI rates, black patients had higher rates of associated postprocedural AKI after PCI in our analysis. Given the large number of patients with missing creatinine measurements during follow-up, we believe that this result should be interpreted with caution. Data from prior large epidemiologic investigations have suggested that black patients may be more vulnerable to AKI than white patients. However, there is little research investigating possible pathophysiologic mechanisms for this finding. Despite the issues with missing data noted above, the present study represents the largest comparison to date of postprocedural kidney injury between racially distinct populations.
In addition, 2 important differences were manifest in procedural and postprocedural treatment patterns between black and white patients. First, black patients were less likely to receive a DES over the study period regardless of whether they were initially seen with stable coronary artery disease or ACS. This finding is consistent with multiple prior analyses of non-VA patients. Drug-eluting stent use is influenced by individual case characteristics and socioeconomic status, and the disparity in DES use in our analysis persisted after adjustment for these factors. Prior DES vs BMS analyses have shown that randomized trials do not demonstrate mortality differences between the stent types, while observational studies often do, likely because of residual treatment selection bias even after attempts at adjustment. The lack of mortality difference seen in the present study despite differential DES use may reflect our ability to more fully adjust for confounders compared with prior large observational studies of PCI. Second, black patients were less likely to be treated with β-blockers after PCI regardless of their presentation status. Prior analyses of veterans have demonstrated reduced efficacy of β-blockers as an antihypertensive agent in black patients. Results of these seminal studies could be influencing post-PCI prescription patterns. Most important, while β-blockade is an important performance measure for patients having MI, it does not clearly improve mortality in patients with stable coronary artery disease.
Limitations
Several limitations of this study should be noted. Most important, as an observational study, unmeasured confounding cannot be excluded. However, we used multiple datasets to account for a wide range of demographic, clinical, procedural, and socioeconomic variables. A 1-year period for our primary end point was chosen to focus on mortality rates that might be attributable to the decisions made during and immediately after the PCI procedure. Differences in mortality between races may emerge over greater lengths of time, which may or may not be influenced by the PCI procedure. In addition, all secondary outcomes may be limited by some degree of potential underreporting due to care provision at non-VA facilities that is paid for through private insurers or Medicare. We do not have information to judge whether this underreporting is differential by race. Also, we cannot account for a higher rate of case fatality for black patients with coronary artery disease before their presentation in the interventional suite. Finally, for many of the reasons listed previously, the VA population and delivery of care are unique, and our results may not apply to non-VA populations.
Conclusions
Black patients had a higher rate of mortality than white patients after PCI in unadjusted analyses. However, race was not independently associated with 1-year mortality among patients receiving PCI in VA hospitals.
eTable 1. Unadjusted Outcomes Stratified by Presence or Absence of ACS
eTable 2. Adjusted Analysis of Bare-Metal Stent Use and β-Blocker Prescription Stratified by Presence or Absence of ACS
References
- 1.Chen J, Rathore SS, Radford MJ, Wang Y, Krumholz HM. Racial differences in the use of cardiac catheterization after acute myocardial infarction. N Engl J Med. 2001;344(19):1443-1449. [DOI] [PubMed] [Google Scholar]
- 2.McBean AM, Warren JL, Babish JD. Continuing differences in the rates of percutaneous transluminal coronary angioplasty and coronary artery bypass graft surgery between elderly black and white Medicare beneficiaries. Am Heart J. 1994;127(2):287-295. [DOI] [PubMed] [Google Scholar]
- 3.Whittle J, Kressin NR, Peterson ED, et al. . Racial differences in prevalence of coronary obstructions among men with positive nuclear imaging studies. J Am Coll Cardiol. 2006;47(10):2034-2041. [DOI] [PubMed] [Google Scholar]
- 4.Giles WH, Anda RF, Casper ML, Escobedo LG, Taylor HA. Race and sex differences in rates of invasive cardiac procedures in US hospitals: data from the National Hospital Discharge Survey. Arch Intern Med. 1995;155(3):318-324. [PubMed] [Google Scholar]
- 5.Gornick ME, Eggers PW, Reilly TW, et al. . Effects of race and income on mortality and use of services among Medicare beneficiaries. N Engl J Med. 1996;335(11):791-799. [DOI] [PubMed] [Google Scholar]
- 6.Chen MS, Bhatt DL, Chew DP, Moliterno DJ, Ellis SG, Topol EJ. Outcomes in African Americans and whites after percutaneous coronary intervention. Am J Med. 2005;118(9):1019-1025. [DOI] [PubMed] [Google Scholar]
- 7.Peterson ED, Shaw LK, DeLong ER, Pryor DB, Califf RM, Mark DB. Racial variation in the use of coronary-revascularization procedures: are the differences real? do they matter? N Engl J Med. 1997;336(7):480-486. [DOI] [PubMed] [Google Scholar]
- 8.Schulman KA, Berlin JA, Harless W, et al. . The effect of race and sex on physicians’ recommendations for cardiac catheterization. N Engl J Med. 1999;340(8):618-626. [DOI] [PubMed] [Google Scholar]
- 9.Kumar RS, Douglas PS, Peterson ED, et al. . Effect of race and ethnicity on outcomes with drug-eluting and bare metal stents: results in 423 965 patients in the linked National Cardiovascular Data Registry and Centers for Medicare & Medicaid Services payer databases. Circulation. 2013;127(13):1395-1403. [DOI] [PubMed] [Google Scholar]
- 10.Carlisle DM, Leake BD, Shapiro MF. Racial and ethnic disparities in the use of cardiovascular procedures: associations with type of health insurance. Am J Public Health. 1997;87(2):263-267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peterson ED, Wright SM, Daley J, Thibault GE. Racial variation in cardiac procedure use and survival following acute myocardial infarction in the Department of Veterans Affairs. JAMA. 1994;271(15):1175-1180. [PubMed] [Google Scholar]
- 12.Sedlis SP, Fisher VJ, Tice D, Esposito R, Madmon L, Steinberg EH. Racial differences in performance of invasive cardiac procedures in a Department of Veterans Affairs Medical Center. J Clin Epidemiol. 1997;50(8):899-901. [DOI] [PubMed] [Google Scholar]
- 13.Whittle J, Conigliaro J, Good CB, Lofgren RP. Racial differences in the use of invasive cardiovascular procedures in the Department of Veterans Affairs medical system. N Engl J Med. 1993;329(9):621-627. [DOI] [PubMed] [Google Scholar]
- 14.Mathews R, Chen AY, Thomas L, et al. . Differences in short-term vs long-term outcomes of older black vs white patients with myocardial infarction: findings from the Can Rapid Risk Stratification of Unstable Angina Patients Suppress Adverse Outcomes With Early Implementation of American College of Cardiology/American Heart Association Guidelines (CRUSADE). Circulation. 2014;130(8):659-667. [DOI] [PubMed] [Google Scholar]
- 15.Byrd JB, Vigen R, Plomondon ME, et al. . Data quality of an electronic health record tool to support VA cardiac catheterization laboratory quality improvement: the VA Clinical Assessment, Reporting, and Tracking System for Cath Labs (CART) program. Am Heart J. 2013;165(3):434-440. [DOI] [PubMed] [Google Scholar]
- 16.Brindis RG, Fitzgerald S, Anderson HV, Shaw RE, Weintraub WS, Williams JF. The American College of Cardiology–National Cardiovascular Data Registry (ACC-NCDR): building a national clinical data repository. J Am Coll Cardiol. 2001;37(8):2240-2245. [DOI] [PubMed] [Google Scholar]
- 17.Anderson HV, Shaw RE, Brindis RG, et al. ; The American College of Cardiology–National Cardiovascular Data Registry (ACC-NCDR) . A contemporary overview of percutaneous coronary interventions. J Am Coll Cardiol. 2002;39(7):1096-1103. [DOI] [PubMed] [Google Scholar]
- 18.Maddox TM, Stanislawski MA, Grunwald GK, et al. . Nonobstructive coronary artery disease and risk of myocardial infarction. JAMA. 2014;312(17):1754-1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.U.S. Census Bureau. Census regions and divisions of the United States. https://www2.census.gov/geo/pdfs/maps-data/maps/reference/us_regdiv.pdf. Accessed June 1, 2016.
- 20.University of Michigan Population Studies Center, Institute for Social Research. Zip code characteristics: mean and median household income. http://www.psc.isr.umich.edu/dis/census/Features/tract2zip/. Accessed June 6, 2016.
- 21.Chertow GM, Burdick E, Honour M, Bonventre JV, Bates DW. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol. 2005;16(11):3365-3370. [DOI] [PubMed] [Google Scholar]
- 22.Kobayashi N, Hirano K, Yamawaki M, et al. . Ability of fractional flow reserve to predict restenosis after superficial femoral artery stenting. J Endovasc Ther. 2016;23(6):896-902. [DOI] [PubMed] [Google Scholar]
- 23.Miki K, Fujii K, Kawasaki D, et al. . Intravascular ultrasound–derived stent dimensions as predictors of angiographic restenosis following nitinol stent implantation in the superficial femoral artery. J Endovasc Ther. 2016;23(3):424-432. [DOI] [PubMed] [Google Scholar]
- 24.Stone NJ, Robinson JG, Lichtenstein AH, et al. ; American College of Cardiology/American Heart Association Task Force on Practice Guidelines . 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(25, pt B):2889-2934. [DOI] [PubMed] [Google Scholar]
- 25.Li F, Zaslavsky AM, Landrum MB. Propensity score weighting with multilevel data. Stat Med. 2013;32(19):3373-3387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Generalized Linear Models With Clustering [computer program]. Package “glmmML.” https://cran.r-project.org/web/packages/glmmML/glmmML.pdf. Published May 3, 2017. Accessed June 1, 2016.
- 27.The R Foundation. The R Project for Statistical Computing. https://www.R-project.org/. Accessed June 6, 2016.
- 28.Olafiranye O, Akinboboye O, Mitchell JE, Ogedegbe G, Jean-Louis G. Obstructive sleep apnea and cardiovascular disease in blacks: a call to action from the Association of Black Cardiologists. Am Heart J. 2013;165(4):468-476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lichtman JH, Bigger JT Jr, Blumenthal JA, et al. ; Endorsed by the American Psychiatric Association . AHA science advisory: depression and coronary heart disease: recommendations for screening, referral, and treatment: a science advisory from the American Heart Association Prevention Committee to the Council on Cardiovascular Nursing, Council on Clinical Cardiology, Council on Epidemiology and Prevention, and Interdisciplinary Council on Quality of Care Outcomes Research. Prog Cardiovasc Nurs. 2009;24(1):19-26. [DOI] [PubMed] [Google Scholar]
- 30.Ahmadi N, Hajsadeghi F, Mirshkarlo HB, Budoff M, Yehuda R, Ebrahimi R. Post-traumatic stress disorder, coronary atherosclerosis, and mortality. Am J Cardiol. 2011;108(1):29-33. [DOI] [PubMed] [Google Scholar]
- 31.Mooe T, Franklin KA, Holmström K, Rabben T, Wiklund U. Sleep-disordered breathing and coronary artery disease: long-term prognosis. Am J Respir Crit Care Med. 2001;164(10, pt 1):1910-1913. [DOI] [PubMed] [Google Scholar]
- 32.Kasai T, Floras JS, Bradley TD. Sleep apnea and cardiovascular disease: a bidirectional relationship. Circulation. 2012;126(12):1495-1510. [DOI] [PubMed] [Google Scholar]
- 33.Tang KL, Rashid R, Godley J, Ghali WA. Association between subjective social status and cardiovascular disease and cardiovascular risk factors: a systematic review and meta-analysis. BMJ Open. 2016;6(3):e010137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kaplan GA, Keil JE. Socioeconomic factors and cardiovascular disease: a review of the literature. Circulation. 1993;88(4, pt 1):1973-1998. [DOI] [PubMed] [Google Scholar]
- 35.Stukel TA, Lucas FL, Wennberg DE. Long-term outcomes of regional variations in intensity of invasive vs medical management of Medicare patients with acute myocardial infarction. JAMA. 2005;293(11):1329-1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lucas FL, Sirovich BE, Gallagher PM, Siewers AE, Wennberg DE. Variation in cardiologists’ propensity to test and treat: is it associated with regional variation in utilization? Circ Cardiovasc Qual Outcomes. 2010;3(3):253-260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cohen MG, Fonarow GC, Peterson ED, et al. . Racial and ethnic differences in the treatment of acute myocardial infarction: findings from the Get With the Guidelines–Coronary Artery Disease program. Circulation. 2010;121(21):2294-2301. [DOI] [PubMed] [Google Scholar]
- 38.Rhoads KF, Patel MI, Ma Y, Schmidt LA. How do integrated health care systems address racial and ethnic disparities in colon cancer? J Clin Oncol. 2015;33(8):854-860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ayanian JZ, Landon BE, Newhouse JP, Zaslavsky AM. Racial and ethnic disparities among enrollees in Medicare Advantage plans. N Engl J Med. 2014;371(24):2288-2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xue JL, Daniels F, Star RA, et al. . Incidence and mortality of acute renal failure in Medicare beneficiaries, 1992 to 2001. J Am Soc Nephrol. 2006;17(4):1135-1142. [DOI] [PubMed] [Google Scholar]
- 41.Rao SV, Shaw RE, Brindis RG, et al. . Patterns and outcomes of drug-eluting coronary stent use in clinical practice. Am Heart J. 2006;152(2):321-326. [DOI] [PubMed] [Google Scholar]
- 42.Hannan EL, Racz M, Walford G, et al. . Differences in utilization of drug-eluting stents by race and payer. Am J Cardiol. 2007;100(8):1192-1198. [DOI] [PubMed] [Google Scholar]
- 43.Kirtane AJ, Gupta A, Iyengar S, et al. . Safety and efficacy of drug-eluting and bare metal stents: comprehensive meta-analysis of randomized trials and observational studies. Circulation. 2009;119(25):3198-3206. [DOI] [PubMed] [Google Scholar]
- 44.Veterans Administration Cooperative Study Group on Antihypertensive Agents Comparison of propranolol and hydrochlorothiazide for the initial treatment of hypertension, II: results of long-term therapy. JAMA. 1982;248(16):2004-2011. [PubMed] [Google Scholar]
- 45.Veterans Administration Cooperative Study Group on Antihypertensive Agents Comparison of propranolol and hydrochlorothiazide for the initial treatment of hypertension, I: results of short-term titration with emphasis on racial differences in response. JAMA. 1982;248(16):1996-2003. [PubMed] [Google Scholar]
- 46.Preston RA, Materson BJ, Reda DJ, et al. ; Department of Veterans Affairs Cooperative Study Group on Antihypertensive Agents . Age-race subgroup compared with renin profile as predictors of blood pressure response to antihypertensive therapy. JAMA. 1998;280(13):1168-1172. [DOI] [PubMed] [Google Scholar]
- 47.Andersson C, Shilane D, Go AS, et al. . β-Blocker therapy and cardiac events among patients with newly diagnosed coronary heart disease. J Am Coll Cardiol. 2014;64(3):247-252. [DOI] [PubMed] [Google Scholar]
- 48.Bangalore S, Bhatt DL, Steg PG, et al. . β-Blockers and cardiovascular events in patients with and without myocardial infarction: post hoc analysis from the CHARISMA trial. Circ Cardiovasc Qual Outcomes. 2014;7(6):872-881. [DOI] [PubMed] [Google Scholar]
- 49.Bangalore S, Steg G, Deedwania P, et al. ; REACH Registry Investigators . β-Blocker use and clinical outcomes in stable outpatients with and without coronary artery disease. JAMA. 2012;308(13):1340-1349. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Unadjusted Outcomes Stratified by Presence or Absence of ACS
eTable 2. Adjusted Analysis of Bare-Metal Stent Use and β-Blocker Prescription Stratified by Presence or Absence of ACS