This crossover cohort, case-control study uses medical records from the Research Patient Data Repository to determine the features, triggers, and risk factors of poststroke recrudescence among patients with a previous stroke.
Key Points
Question
What are the clinical features, triggers, and risk factors of poststroke recrudescence?
Findings
This crossover cohort and case-control study of 153 patients admitted for poststroke recrudescence found that it occurs approximately 4 years after the index stroke and is characterized by mild worsening of poststroke deficits that usually resolve within 1 day. Infection, hypotension, hyponatremia, insomnia or stress, and benzodiazepine use are important precipitants; recrudescence is more common in women, African American individuals, and patients with vascular risk factors, severe deficits, or infarcts affecting deep white matter tracts within the middle cerebral artery territory.
Meaning
Results from this study should enable prompt diagnosis and help distinguish poststroke recrudescence from mimics.
Abstract
Importance
Reemergence of previous stroke-related deficits (or poststroke recrudescence [PSR]) is an underrecognized and inadequately characterized phenomenon.
Objective
To investigate the clinical features, triggers, and risk factors for PSR.
Design, Setting, and Participants
This retrospective study incorporated a crossover cohort study to identify triggers and a case-control study to identify risk factors. The study used the Massachusetts General Hospital Research Patient Data Repository to identify patients for the period January 1, 2000, to November 30, 2015, who had a primary or secondary diagnosis of cerebrovascular disease, who underwent magnetic resonance imaging of the brain at least once, and whose inpatient or outpatient clinician note or discharge summary stated the term recrudescence. In all, 153 patients met the preliminary diagnostic criteria for PSR: transient worsening of residual poststroke focal neurologic deficits or transient recurrence of prior stroke-related focal deficits, admission magnetic resonance imaging showing a chronic stroke but no acute infarct or hemorrhage, no evidence of transient ischemic attack or seizure, no acute lesion on diffusion-weighted imaging, and no clinical or electroencephalographic evidence of seizure around the time of the event.
Main Outcomes and Measures
Clinical and imaging features of PSR; triggers (identified by comparing PSR admissions with adjacent admissions without PSR); and risk factors (identified by comparing PSR cases with control cases from the Massachusetts General Hospital Stroke Registry).
Results
Of the 153 patients, 145 had prior infarct, 8 had hypertensive brain hemorrhage, and 164 admissions for PSR were identified. The patients’ mean (SD) age was 67 (16) years, and 92 (60%) were women. Recrudescence occurred a mean (SD) of 3.9 (0.6) years after the stroke, lasted 18.4 (20.4) hours, and was resolved on day 1 for 91 of the 131 episodes with documented resolution time (69%). Deficits were typically abrupt and mild and affected motor-sensory or language function. No patient had isolated gaze paresis, hemianopia, or neglect. During PSR, the National Institutes of Health Stroke Scale (NIHSS) score worsened by a mean (SD) 2.5 (1.9) points, and deficits were limited to a single NIHSS item in 62 episodes (38%). The underlying chronic strokes were variably sized, predominantly affected white matter tracts, and involved the middle cerebral artery territory for 112 patients (73%). Infection, hypotension, hyponatremia, insomnia or stress, and benzodiazepine use were higher during PSR admissions. Compared with the control group (patients who did not experience recrudescence), the PSR group (patients who were hospitalized for recrudescence) had more women, African American individuals, and those who self-identified as being from “other” race. The PSR group also had more diabetes, dyslipidemia, smoking, infarcts from small-vessel disease, and “other definite” causes and worse onset NIHSS scores. Six patients (4%) received intravenous tissue plasminogen activator without complications.
Conclusions and Relevance
The PSR features identified in the study should enable prompt diagnosis and distinguish recrudescence from mimics, such as transient ischemic attacks, migraine, Todd paralysis, and Uhthoff phenomenon. Prospective studies are required to validate the proposed diagnostic criteria and to decipher underlying mechanisms.
Introduction
Transient worsening of poststroke neurologic deficits or reemergence of previous stroke-related deficits (or poststroke recrudescence [PSR]) in the setting of toxic metabolic factors is a frequently encountered phenomenon that has not been adequately characterized. Anecdotal case reports and expert opinions have referred to PSR as anamnestic recall or recrudescence and have associated it with systemic infections and the use of sedative medications and anesthetic drugs. A few clinical studies have demonstrated iatrogenic reemergence of previous stroke symptoms with the use of midazolam hydrochloride and fentanyl citrate. Rodent studies have provided preliminary mechanistic insights; however, the underlying biochemical and molecular mechanisms have yet to be elucidated. The baseline stroke phenotype and potential risk factors, event triggers, and the clinical characteristics, time course, and outcome of PSR have not been studied, to our knowledge. It can be difficult to distinguish PSR from mimics, such as acute stroke, transient ischemic attack (TIA), and seizure; however, accurate diagnosis is important given these conditions’ diverse management. In this study, we describe the clinical-imaging phenotype, risk factors, and clinical outcome of PSR and propose diagnostic criteria.
Methods
This retrospective study design incorporated a crossover cohort study to identify triggers and a case-control study to identify risk factors. The Partners Human Research Committee approved the study and waived the informed consent requirement because the study was a retrospective medical record review without patient contact.
Patients with potential PSR were identified using the Massachusetts General Hospital Research Patient Data Repository (RPDR), an electronic database with natural-language processing query system that contains real-time patient information from numerous sources, including clinical encounters, billing data, radiologic images, and laboratory results. For this study, we queried the RPDR for the period January 1, 2000, to November 30, 2015, to identify patients coded with a primary or secondary diagnosis of “cerebrovascular disease” who underwent magnetic resonance (MR) imaging of the brain at least once and had any type of inpatient or outpatient clinician note or discharge summary in which the term recrudescence was stated. The MR imaging and clinical note were not restricted to the same encounter.
The RPDR query returned 3441 patients, from which a random sample of 1700 medical records (49.4%) was selected for detailed review. To identify the clinical note (and hence the encounter) containing recrudescence, we used a novel indigenous search engine called Queriable Patient Inference Dossier, which interrogates the specified patient’s aggregate electronic health record for the relevant term. For each relevant encounter, we reviewed all clinical notes, laboratory results, MR imaging of the brain (including diffusion-weighted imaging [DWI]), and electroencephalographic results (if performed) to identify patients with PSR.
Criteria for PSR
Following are the preliminary diagnostic criteria for PSR:
Transient worsening of residual poststroke focal neurologic deficits, or transient recurrence of previous stroke-related focal neurologic deficits
Chronic stroke on brain imaging
No acute lesion on DWI
Cerebral ischemia considered unlikely (eg, symptom duration >1 hour without new DWI lesion; no suspicion for low-flow TIA from cerebral artery stenosis or occlusion)
No clinical or electroencephalographic evidence of seizure around the time of the event
Of 1700 patient records, PSR was identified in 164 episodes (153 patients [9%]). The remaining 1547 patients (91%) were excluded as follows: 17 for deficits lasting 1 hour or less (ie, possible TIA), 23 for new transient deficits that differed from the previous stroke-related deficits, 29 for seizure, 317 for new infarcts on DWI, 152 for not undergoing DWI, 447 for recrudescence of nonstroke systemic conditions such as infection or pain, 359 for previous nonstroke neurologic conditions (eg, multiple sclerosis), 146 for nonfocal symptoms such as delirium but no focal neurologic deficits, and 57 for inadequate documentation or use of the term recrudescence for counseling purposes.
Features of PSR
The following data from the PSR admission were extracted: demographics, time interval since index stroke, tempo of onset, duration of recrudescence, presence of fever (body temperature ≥38.5°C), relative hypotension or hypertension in the judgment of the clinician, maximum National Institutes of Health Stroke Scale (NIHSS) score (score range: 0-42, with the higher scores indicating more severe focal deficits from stroke), relevant laboratory results (eg, complete blood cell count, blood glucose level, electrolyte levels, renal and liver function test results, blood cultures, inflammatory markers, urinalysis, chest radiography, and echocardiography), recent surgical operations, medications, brain and cerebrovascular imaging findings, and length of hospital stay. Episodes with possible recrudescence from a chronic asymptomatic (silent) stroke were excluded. Baseline poststroke deficits, functional status, and estimated modified Rankin Scale (mRS) score (score range: 0-6, with the highest score indicating death) were extracted from medical records dated before the PSR admission.
Features of the Index Stroke
The following data were extracted: maximum NIHSS score, stroke type (ischemic or hemorrhagic), ischemic stroke etiology per the Trial of Org 10172 in Acute Stroke (TOAST) classification, arterial territory (anterior, middle, or posterior cerebral; vertebrobasilar; or cerebellar branch arteries), stroke distribution (superficial or deep), stroke size (small, axial diameter 0 to 1.5 cm; medium, >1.5 to 3.0 cm; or large, >3.0 cm), and estimated time to resolution of deficits or plateau of stroke recovery. In patients with more than 1 previous stroke, the event with presenting symptoms consistent with the new recrudescence symptoms was selected.
Identification of PSR Triggers (Crossover Cohort Study)
We compared features of the PSR admissions with discrete hospitalizations when the patient did not experience recrudescence (ie, “control” admission group). If there was more than 1 admission, the admission closest to the date of the recrudescence admission was selected. Data on the following potential triggers were collected for the PSR group and the control admissions group: fever, infection (eg, peripheral white blood cell count >10 000 cells/mm3, urinary tract infection, pneumonia, and gastroenteritis), anemia (hematocrit <40% for men and <36% for women), hypoglycemia or hyperglycemia (blood glucose level, <60 or >200 mg/dL), hyponatremia or hypernatremia (serum sodium level, <135 or >145 mEq/L), hypokalemia or hyperkalemia (serum potassium level, <3.4 or >4.6 mEq/L), dehydration, hypotension, hypertension, congestive heart failure, acute renal failure, and acute liver failure (as documented, per clinical judgment). (To convert white blood cell count to ×109 per liter, multiply by 0.001; hematocrit to a proportion of 1, multiply by 0.01; and glucose level to millimoles per liter, multiply by 0.0555. Conversion of sodium and potassium levels to millimoles per liter is 1:1.) Other potential triggers included alcohol intoxication and medication use.
Identification of Risk Factors for PSR (Case-Control Study)
We compared the vascular risk profile, clinical features, and etiology of the incident ischemic stroke with the features of all patients with DWI-confirmed ischemic stroke in Massachusetts General Hospital Stroke Registry from the period January 1, 2007, to November 5, 2009 (ie, “stroke control” group). Patients with PSR in the Stroke Registry were excluded to avoid an overlap between groups. Given the small number of patients with recrudescence after hemorrhagic stroke, we did not investigate or compare their profile.
Statistical Analysis
Data are displayed here as mean (SD) with interquartile range or percentages. A variable was considered absent if not documented in the record; hence, there were no missing data. Unpaired t test, χ2 test, paired t test, and McNemar test were used as appropriate. Two-sided P < .05 was considered statistically significant. SPSS software, version 21 (IBM), was used for analyses.
Results
Features of PSR
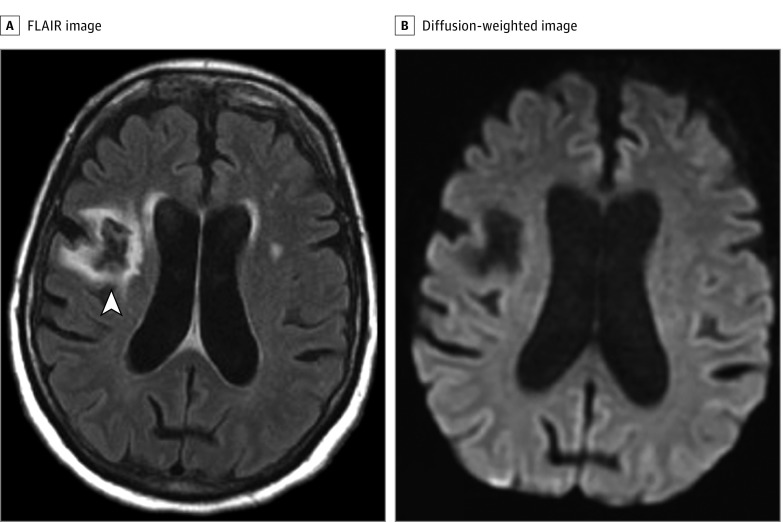
Diagnostic criteria were confirmed in 164 episodes (153 patients: 145 previous infarcts and 8 hypertensive brain hemorrhages). Eleven patients (7.2%), all with previous ischemic stroke, had more than 1 episode of PSR; triggers and symptoms were not identical for these recurrences. Of the 153 patients, the mean (SD) age was 67 (16) years, and 92 (60%) were women. All races were affected. A typical episode is depicted in Figure 1.
Figure 1. Recrudescence After Ischemic Stroke.
A woman in her 50s with chronic hypertension, diabetes, and a cryptogenic ischemic stroke 7 years prior to admission developed acute dysarthria, mild left facial weakness, and mild sensory loss in the left arm. The admission National Institutes of Health Stroke Scale (NIHSS) score was 3. Magnetic resonance imaging of the brain showed a chronic embolic-appearing ischemic stroke in the right middle cerebral artery territory (arrowhead in A, axial fluid-attenuated inversion recovery [FLAIR] image) but no acute lesion on diffusion-weighted images (B). Findings on magnetic resonance angiography of the head and neck were normal. Blood test results were normal except for an elevated blood glucose level (262 mg/dL) (to convert to millimoles per liter, multiply by 0.0555). Urinalysis results were positive, prompting treatment for urinary tract infection. Electroencephalogram showed no evidence of ongoing seizures. Her symptoms resolved completely in 10 hours. A review of her medical records showed that she had an NIHSS score of 12 at the time of the incident stroke with left face, arm, and leg weakness and left hemisensory loss, from which she recovered completely (NIHSS score of 0) over a period of 3 months. The clinical diagnosis was poststroke recrudescence from urinary tract infection and hyperglycemia.
We were unable to extract reliable information about the tempo of onset symptoms, however. In most cases, the onset appeared to be abrupt, similar to a stroke, and not gradual. The mean (SD) duration of the deficits, documented in 131 episodes, was 18.4 (20.4) hours with 91 episodes (69.5%) resolving on day 1, 23 (17.5%) on day 2, 8 (6.1%) on day 3, and 9 (6.9%) on day 4 or beyond. Resolution appeared to be gradual and usually correlated with removal or treatment of the presumed trigger. The mean (SD) interval from index stroke to recrudescence was 3.9 (0.6) years. A total of 111 episodes (67.6%) resulted in hospitalization, the rest discharged from the emergency department. The mean (SD) length of stay was 4.0 (4.9) days. Six patients (4.0%) received intravenous tissue plasminogen activator without hemorrhagic complications.
The mean (SD) NIHSS score immediately before the recrudescence spell was 2.8 (4.2). The maximum mean (SD) NIHSS score during recrudescence was 5.2 (4.6), and the mean (SD) change in NIHSS score was +2.5 (1.9). The range of NIHSS score worsening was 1 to 12 points, with a change of 4 or more in 28 episodes (17.0%). There was no significant difference in the NIHSS change score or event duration between recrudescence after ischemic and hemorrhagic stroke (Figure 2). Subscale analysis of the NIHSS change score showed a wide variability in the distribution of neurologic deficits: Level of Consciousness, 10%; Gaze, 1%; Visual, 4%; Facial Palsy, 33%; Motor arm or leg, 49%; Limb Ataxia, 9%; Sensory, 26%; Language, 21%; Dysarthria, 26%; and Extinction-Inattention, 1%. Neurologic deficits were limited to a single subscale NIHSS item in 62 episodes (38%). These single-item deficits predominantly localized to the motor-sensory and language pathways. No patient had isolated gaze paresis, isolated hemianopia, or isolated neglect. Mental status appeared normal in 124 patients (75.6%); the rest had delirium or confusion, but none were comatose.
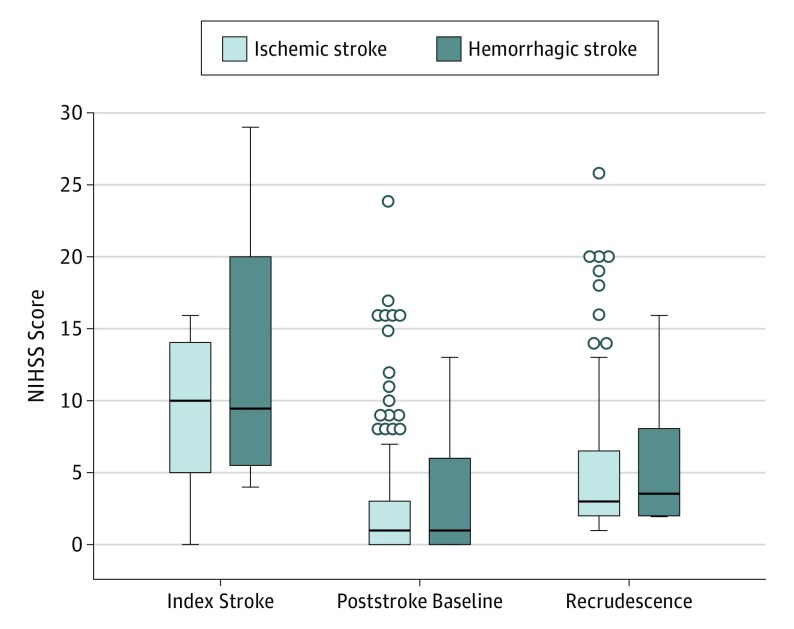
Figure 2. Serial National Institutes of Health Stroke Scale (NIHSS) Scores.
Box-and-whisker plots show the distribution of NIHSS scores at the time of the index stroke, at poststroke baseline, and during recrudescence in patients with ischemic stroke and hemorrhagic stroke. There were no significant differences between NIHSS scores in the ischemic and hemorrhagic groups at the time of stroke onset (mean [SD], 10.0 [5.6] vs 12.9 [9.1]; P = .19), poststroke baseline (mean [SD], 2.7 [4.1] vs 3.4 [5.1]; P = .63), or recrudescence (mean [SD], 5.1 [4.7] vs 5.6 [5.3]; P = .75). There were no significant differences between groups in the degree of NIHSS score worsening (mean [SD], 2.4 [2.0] vs 2.3 [0.9]; P = .81) or the duration of recrudescence episodes (mean [SD], 18.5 [20.7] vs 16.6 [15.5] hours; P = .81). Boxes indicate the lower (25th) and upper (75th) quartiles; horizontal lines in the boxes, median values; whiskers, minimum and maximum values; and open circles beyond the whiskers, outliers.
By definition, the electroencephalogram showed no active seizures in all 45 episodes (27.4%) where performed, and DWI showed no acute infarcts in 164 episodes (100%). Cerebral vascular imaging was performed in 158 episodes (96.3%). New arterial pathology was ruled out with (in order of preference) head-neck computed tomography angiography in 64 episodes (40.5%), head-neck MR angiography in 84 (53.2%), head-only MR angiography in 5 (3.2%), and carotid duplex or transcranial Doppler ultrasonography in 5 (3.2%). However, vascular imaging showed more than 50% cerebral arterial stenosis or occlusion in 33 episodes (20.1%), which was judged by the clinicians to be stable since the previous stroke; in all such cases, the final clinical diagnosis was recrudescence and not TIA.
Triggers
The control group comprised 65 admissions before (16 [25%]) and after (49 [75%]) the PSR encounter. There were no significant differences in demographic characteristics and index stroke features between the patients with recrudescence who did or did not (n = 99) have a control admission.
The mean (SD) interval between the PSR episode and the control episode was 671 (86) days. There was no significant difference between the PSR group and the control group in the mean (SD) length of stay (4.1 [4.7] days vs 4.1 [4.0] days; P = .98). The indications for the 65 control admissions included 13 trauma or falls; 7 back or neck pain; 3 orthopedic surgical procedures; 3 lower-limb vascular, 16 cardiorespiratory, 5 gastrointestinal, and 3 genitourinary tract conditions; 3 psychiatric events; 2 systemic infections; and 10 nonstroke neurologic symptoms, such as headache, vertigo, and seizure.
As shown in Table 1, PSR episodes had a significantly higher frequency of infections, hypotension, and hyponatremia. Insomnia or stress, benzodiazepine use, and fever were more frequent but did not reach statistical significance. There were no significant differences in the use of antiseizure medications or stroke preventive medications, such as anticoagulants, or in admission international normalized ratio values among patients treated with warfarin sodium.
Table 1. Comparison of Potential Triggers of Poststroke Recrudescence Among 65 Patients.
| Potential Trigger | Recrudescence Episode | Control Episode | P Valuea |
|---|---|---|---|
| Fever, No. (%) | 7 (10.8) | 3 (4.6) | .06 |
| Infection, No. (%) | 34 (52.3) | 5 (7.7) | <.001 |
| WBC count, mean (SD) | 8800 (2928) | 7911 (3107) | .04 |
| WBC count >10 000 cells/mm3, No. (%) | 17 (26.2) | 10 (15.4) | .12 |
| Urinary tract infection, No. (%) | 20 (30.8) | 1 (1.5) | <.001 |
| Pneumonia, No. (%) | 14 (21.5) | 1 (1.5) | <.001 |
| Gastroenteritis, No. (%) | 5 (7.7) | 0 | .06 |
| Metabolic factors, No. (%) | 24 (36.9) | 6 (9.2) | <.001 |
| Glucose level, mean (SD), mg/dL | 139.4 (58.7) | 140.4 (58.1) | .49 |
| Hyperglycemia, No. (%) | 10 (15.4) | 4 (6.2) | .15 |
| Hypoglycemia, No. (%) | 3 (4.6) | 0 | .25 |
| Serum sodium level, mean (SD), mEq/L | 138.5 (3.3) | 139.0 (2.3) | .29 |
| Hypernatremia, No. (%) | 1 (1.5) | 0 | >.99 |
| Hyponatremia, No. (%) | 12 (18.5) | 2 (3.1) | .01 |
| Potassium level, mean (SD), mEq/L | 4.0 (0.5) | 4.1 (0.3) | .90 |
| Hyperkalemia, No. (%) | 3 (4.6) | 0 | .25 |
| Hypokalemia, No. (%) | 4 (6.2) | 0 | .12 |
| Serum urea nitrogen level, mean (SD), mg/dL | 20.4 (14.3) | 19.4 (11.4) | .27 |
| Creatinine level, mean (SD), mg/dL | 1.07 (1.04) | 1.16 (1.22) | .22 |
| Acute renal failure, No. (%) | 4 (6.2) | 0 | .12 |
| Hypertension, No. (%) | 5 (7.7) | 0 | .06 |
| Hypotension, No. (%) | 9 (13.8) | 2 (3.1) | .04 |
| Dehydration, No. (%) | 3 (4.6) | 1 (1.5) | .62 |
| Hematocrit, mean (SD) | 37.8 (4.5) | 37.2 (4.9) | .38 |
| Insomnia or stress, No. (%) | 5 (7.7) | 0 | .06 |
| Congestive heart failure, No. (%) | 9 (13.8) | 6 (9.2) | .37 |
| Alcohol intoxication, No. (%) | 3 (4.6) | 0 | .25 |
| Recent general anesthesia, No. (%) | 2 (3.1) | 0 | .50 |
| Admission medications, No. (%) | |||
| Antiplatelet agents | 48 (73.8) | 48 (73.8) | >.99 |
| Anticoagulants | 23 (35.4) | 26 (40.0) | >.99 |
| Statins | 48 (73.8) | 41 (63.1) | .09 |
| Antihypertensive agents | 47 (72.3) | 52 (80.0) | .12 |
| Oral antidiabetic agents | 16 (24.6) | 18 (27.7) | .70 |
| Insulin | 17 (26.2) | 19 (29.2) | .50 |
| Benzodiazepines | 18 (27.7) | 10 (15.4) | .02 |
| Opiates | 12 (18.5) | 11 (16.9) | >.99 |
| Serotonergic antidepressants | 23 (35.4) | 21 (32.3) | .82 |
| Other antidepressants | 10 (15.4) | 7 (10.8) | .45 |
| Thyroid replacement therapy | 9 (13.8) | 10 (15.4) | >.99 |
| Antiepileptic agents | 21 (32.3) | 22 (33.8) | >.99 |
Abbreviation: WBC, white blood cell.
SI conversion factors: To convert creatinine to micromoles per liter, multiply by 88.4; glucose to millimoles per liter, multiply by 0.0555; hematocrit to a proportion of 1, multiply by 0.01; serum urea nitrogen to millimoles per liter, multiply by 0.357; and WBC count to white blood cell count to ×109 per liter, multiply by 0.001. Conversion of potassium and sodium levels to millimoles per liter is 1:1.
Paired t test or McNemar test used as appropriate.
Features of the Index Stroke
Table 2 shows risk factors and mechanisms of the incident ischemic stroke. Infarct topography was variable. Hemispheric cortical regions were affected in 19 patients (13.1%), subcortical regions in 41 (28.3%), both cortical and subcortical regions in 74 (51.0%), and brainstem or cerebellum in 11 (7.6%). The middle cerebral artery territory was involved in 106 patients (73.1%), anterior cerebral in 4 (2.8%), posterior cerebral in 6 (4.1%), vertebrobasilar in 17 (11.7%), and multiple territories in 12 (8.3%). Infarct volumes were small in 46 patients (31.7%), medium in 24 (16.6%), and large in 75 (51.7%).
Table 2. Incident Stroke Profile and Vascular Risk Factors of the Poststroke Recrudescence Group and the MGH Stroke Registry Group.
| Feature | Recrudescence (n = 145) |
MGH Stroke Registry (n = 1861) |
P Value |
|---|---|---|---|
| Age, mean (SD), y | 67 (16) | 69 (15) | .06 |
| Female, No. (%) | 90 (62.1) | 859 (46.2) | <.001 |
| Race, No. (%) | |||
| White | 101 (69.7) | 1691 (90.9) | <.001 |
| African American | 25 (17.2) | 76 (4.1) | <.001 |
| Other | 19 (13.1) | 94 (5.1) | <.001 |
| Hispanic ethnicity | 9 (6.2) | 118 (6.3) | .99 |
| Dementia | 7 (4.8) | 89 (4.8) | .88 |
| Traditional vascular risk factors, No. (%) | |||
| Hypertension | 112 (77.2) | 1318 (70.8) | .14 |
| Diabetes | 61 (42.1) | 459 (24.7) | <.001 |
| Dyslipidemia | 85 (58.6) | 836 (44.9) | .001 |
| Atrial fibrillation | 36 (24.8) | 415 (22.3) | .48 |
| Coronary artery disease | 41 (28.3) | 437 (23.5) | .19 |
| Smoking | 33 (22.8) | 315 (16.9) | .03 |
| Admission NIHSS score, mean (SD) | 10.2 (5.9) | 7.3 (7.5) | <.001 |
| Stroke cause (TOAST), No. (%) | |||
| Large-artery disease | 30 (20.7) | 412 (22.1) | .67 |
| Cardioembolism | 35 (24.1) | 766 (41.2) | <.001 |
| Small-vessel disease | 23 (15.9) | 145 (7.8) | .001 |
| Other definite | 20 (13.8) | 167 (9.0) | .03 |
| Cryptogenic | 36 (24.8) | 371 (19.9) | .14 |
Abbreviations: MGH, Massachusetts General Hospital; NIHSS, National Institutes of Health Stroke Scale; TOAST, Trial of Org 10172 in Acute Stroke.
All 8 hemorrhagic strokes were hypertensive in origin based on preexisting hypertension and location (5 thalamic and 3 putamen). Two strokes were small (confined to the thalamus), and the others were large and involved white matter tracts.
The time to resolution of deficits or plateau of stroke recovery was adequately documented in 124 patients (85.5%). Recovery occurred within 1 week in 26 patients (21.0%), within 1 month in 55 (44.4%), and beyond 1 month in 43 (35%). The pre-PSR mean (SD) mRS score, estimated in all 145 patients, was 1.3 (1.2), with an mRS score of 0 in 39 patients (26.9%), 1 in 62 (42.8%), 2 in 15 (10.3%), 3 in 18 (12.4%), 4 in 9 (6.2%), and 5 in 2 (1.4%).
Risk Factors
Table 2 compares the 145 patients who had ischemic stroke and recrudescence with 1861 registry patients who had DWI-confirmed ischemic stroke but no recrudescence. A total of 25 patients (17%) had more than 1 previous stroke; for this comparison, we selected the previous ischemic stroke with symptoms similar to the recrudescence episode.
The PSR group had substantially more women (90 [62.1%]), African American individuals (25 [17.2%]), and those who self-identified as being of “other” races (19 [13.1%]). Patients with recrudescence had higher frequencies of diabetes, dyslipidemia, smoking, and more severe neurologic deficits at the time of stroke. There were significantly more small-vessel infarcts and more strokes from other definite causes (eg, dissection) in the recrudescence group.
Discussion
Detailed analysis of this cohort of patients experiencing record-validated recrudescence of stroke symptoms provides new insights into this often-applied but poorly understood classification of transient neurologic symptoms. Our data show that PSR can occur after ischemic and hemorrhagic stroke. Infection, hypotension, hyponatremia, insomnia or stress, and benzodiazepine use are important triggers. New neurologic deficits are usually mild to moderate in severity and do not exceed the previous stroke deficits. Symptoms can start abruptly but can be remedied completely within hours or days after the resolution of the trigger (eg, treatment of an underlying infection). There does not appear to be any age predilection. Women, African American individuals, and those of other race/ethnicity are more frequently affected, possibly because of variances in neuronal susceptibility, disparities in health care resulting in more triggers such as infections, or other unmeasured confounders.
Our data show that nearly all types of focal stroke symptoms can recrudesce. The predominant face, arm, and leg involvement combined with the infrequent altered consciousness, gaze paresis, hemianopia, and extinction or inattention suggests that the long white matter tracts are more susceptible. This notion is supported by the frequent (79%) involvement of subcortical regions, the high prevalence of large strokes, and the significantly greater frequency of small-vessel disease that typically affects the motor sensory tracts. Despite the high frequency of large strokes and severe deficits, 116 patients (80.0%) achieved an mRS score lower than 3 but developed recrudescence, suggesting major recruitment of interhemispheric plasticity pathways during stroke recovery and, in turn, a limited reserve capacity for neurotransmission or a lower threshold for recrudescence.
Consistent with previous anecdotal reports and personal observations, toxic metabolic triggers were common. The identified triggers can be classified into 3 groups, each with mechanistic implications: (1) fever and infection, (2) hypotension and hyponatremia, and (3) insomnia or stress and benzodiazepine use. Previous studies have shown that larger or more severe strokes confer greater susceptibility to infections; in rodents, a second immune challenge induces PSR. It is conceivable that the large strokes in our recrudescence population increased the risk of acute infection, and subsequent infections resulted in recrudescence through cytokine-mediated or other pathways. Similarly, sodium-water imbalances are well known to affect neuronal excitability and transmission along white matter tracts. The association of PSR with insomnia and benzodiazepine use in our study and others implicates neurotransmitter systems pertaining to γ-aminobutyric acid. Similar mechanisms have been implicated in other neurologic conditions associated with transient exacerbations, such as multiple sclerosis (Uhthoff phenomenon), migraine, and brain tumor. The implicated pathways may also explain the reversible stroke-like deficits observed in patients with severe glucose imbalances. Given the diverse and systemic nature of these triggers and the fact that PSR involves a subset of prior stroke symptoms without new neurologic symptoms, the triggers likely act via subtle effects on the undamaged ipsilateral and contralateral brain regions that subserve poststroke plasticity. Brain plasticity pathways are similarly implicated in patients with worsening poststroke deficits from subsequent ipsilateral strokes.
A major strength of this study is the use of stringent diagnostic criteria that included DWI. These criteria require prospective validation and could be broadened to include asymptomatic prior stroke and the absence of new lesions on computed tomography of the head, which is far less sensitive but more widely available than DWI. These criteria were based on experience and consideration of features that would distinguish PSR from other conditions that can occur in chronic stroke (Table 3). Although DWI results are often normal in TIA, DWI abnormality is associated with symptom duration, justifying the diagnostic criterion 4, which uses an arbitrary 1-hour symptom duration with negative DWI results to distinguish PSR from TIA or stroke. Ultimately, clinical acumen will be needed for diagnosis. The list of conditions in Table 3 is not comprehensive and omits stroke-like episodes associated with hypoglycemia, mitochondrial disorders, and rare conditions, such as retinal migraine and the syndrome of transient headache and neurologic deficits with cerebrospinal fluid lymphocytosis (or HaNDL). In the PSR cohort, several triggers were identified on the basis of higher frequency in recrudescence when compared with adjacent non-PSR admissions. Similar retrospective crossover cohort studies have been successfully used to uncover stroke triggers and risk factors. We limited the recrudescence comparison to the first adjacent admission to minimize confounding from factors such as advancing age and changes in medications.
Table 3. Differential Diagnosis of Transient Neurologic Deficits After Previous Stroke.
| Feature | Poststroke Recrudescence | Seizure-Related Deficits (eg, Todd Paralysis) | TIA, Aborted Stroke, or Cerebral Ischemia Without Infarction | Migraine-Related Deficits | Functional Neurologic Symptom Disorder | Amyloid Spells |
|---|---|---|---|---|---|---|
| Onset | Usually abrupt; deficits may initially progress | Usually abrupt; associated with seizure disorder; maximum at onset | Abrupt | Gradual over 5-20 min | Variable | Abrupt, with symptoms spread to contiguous body areas, usually arm |
| Symptoms and signs | New deficits that are mild to moderate; a subset of previous stroke symptoms; and mainly affect motor, sensory, or language function | New deficits that may be severe, extending beyond the original stroke symptoms; may be associated with confusion, tongue bite, incontinence, and other ictal or postictal phenomena | New deficits that are usually different from previous stroke symptoms | Migraine aura: positive visual/sensory symptoms or language deficits (more symptoms if basilar-type migraine) but no motor weakness; hemiplegic migraine: motor weakness with ≥1 migraine aura symptom | Variable symptoms; presence of give-way weakness, Hoover sign, etc | “Marching” paresethesias; weakness, or other positive or negative symptoms; may resemble migraine aura |
| Clinical course | Gradual resolution with removal of the triggering factor; less often waxing-and-waning deficits; complete return to baseline usually within 1-2 d | Gradual resolution of deficits over minutes to hours, return to baseline within 2 d | Prompt resolution, usually within 1 h; may occur after stroke thrombolysis (aborted or averted stroke) | Migraine aura: resolution <1 h, usually followed by headache; hemiplegic migraine: resolution <24 h, accompanied with headache; persistent aura without infarct: >1 wk | Variable | Recurrent, lasting minutes |
| Brain and vascular imaging | Chronic infarct or hemorrhage; no evidence of new stroke; no new cerebral artery occlusion or hemodynamically significant stenosis | Chronic infarct or hemorrhage; no evidence of new stroke although DWI may be abnormal if seizure is prolonged | Chronic infarct or hemorrhage; no evidence of new stroke; rarely, evidence for arterial reperfusion (eg, dilated collateral vessels), or new cerebral artery occlusion (aborted stroke) or significant stenosis | Chronic infarct or hemorrhage; no evidence of new stroke; no new cerebral artery occlusion or hemodynamically significant stenosis | Chronic infarct or hemorrhage; no evidence of new stroke; no new cerebral artery occlusion or hemodynamically significant stenosis | Chronic infarct or hemorrhage; no evidence of new stroke; presence of cortical microbleeds, convexal subarachnoid hemorrhage or superficial siderosis |
| Scalp EEG | No evidence of electrographic seizure activity | Ictal or postictal epileptic or epileptiform patterns | No evidence of electrographic seizure activity | No evidence of electrographic seizure activity | No evidence of electrographic seizure activity | No evidence of electrographic seizure activity |
Abbreviations: DWI, diffusion-weighted imaging; EEG, electroencephalogram; TIA, transient ischemic attack.
These results have several management implications. Knowledge of the time course of resolution and the expected length of stay should improve resource use. It is important to prevent and promptly treat infections, hyponatremia, and other medical complications of stroke and to avoid benzodiazepine use. A few patients received tissue plasminogen activator, emphasizing the importance of distinguishing acute stroke from recrudescence (Table 3). However, none of the patients developed tissue plasminogen activator–related complications, the rate of which is known to be extremely low in stroke mimics, supporting the view that tissue plasminogen activator should not be withheld in patients with acute stroke symptoms.
Limitations
The main limitation of this study is its retrospective nature, which relies on medical documentation for determining stroke severity and evolution. Because our RPDR search was restricted to the term recrudescence, we may not have captured all cases. However, capturing more cases of the same phenomenon using terms such as anamnesis or reactivation is unlikely to change study results. The proposed diagnostic criteria were designed to exclude mimics, but Todd paralysis and TIA or DWI-negative stroke still cannot be definitively excluded. Unblinded health record review may have led to an overestimation of subjective triggers, such as infection, although standard definitions were used as far as possible. The list of potential triggers was predefined; hence, there may be as-yet-unidentified factors leading to PSR. We did not perform multivariable analysis because of the relatively small number of cases and controls. The control group had at least 2 poststroke hospitalizations (1 with recrudescence) and may differ from patients with single PSR-related admissions, leading to a possible selection bias. We assessed adjacent admissions but not every readmission; thus, we cannot address whether triggers induce recrudescence at every exposure or require a certain threshold to induce symptom recurrence. These issues could be addressed in future studies.
Conclusions
The incidence or prevalence of PSR is not known but, on the basis of our observation, appears to be relatively frequent and more common with ischemic stroke than hemorrhagic stroke. To our knowledge, recognition of PSR remains sporadic. We envision that our diagnostic criteria and the results of this first attempt to characterize PSR will stimulate larger validation studies and ultimately enable prompt diagnosis and distinction from mimics in medical centers across the world.
References
- 1.Elkind MS, Mohr JP. Differential diagnosis of stroke In: Rowland LP, Pedley TA, eds. Merritt’s Neurology. 12th ed Philadelphia, PA: Lippincott Williams & Wilkins; 2010:292. [Google Scholar]
- 2.Bernstock JD, Budinich CS, Cohen LG, Awosika OO. Recrudescence of focal stroke symptoms during pain management with hydromorphone. Front Neurol. 2016;7:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zierath D, Hadwin J, Savos A, Carter KT, Kunze A, Becker KJ. Anamnestic recall of stroke-related deficits: an animal model. Stroke. 2010;41(11):2653-2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lazar RM, Berman MF, Festa JR, Geller AE, Matejovsky TG, Marshall RS. GABAergic but not anti-cholinergic agents re-induce clinical deficits after stroke. J Neurol Sci. 2010;292(1-2):72-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lazar RM, Fitzsimmons BF, Marshall RS, et al. . Reemergence of stroke deficits with midazolam challenge. Stroke. 2002;33(1):283-285. [DOI] [PubMed] [Google Scholar]
- 6.Lazar RM, Fitzsimmons BF, Marshall RS, Mohr JP, Berman MF. Midazolam challenge reinduces neurological deficits after transient ischemic attack. Stroke. 2003;34(3):794-796. [DOI] [PubMed] [Google Scholar]
- 7.Thal GD, Szabo MD, Lopez-Bresnahan M, Crosby G. Exacerbation or unmasking of focal neurologic deficits by sedatives. Anesthesiology. 1996;85(1):21-25. [DOI] [PubMed] [Google Scholar]
- 8.Adams HP Jr, Bendixen BH, Kappelle LJ, et al. ; TOAST Investigators . Classification of subtype of acute ischemic stroke: definitions for use in a multicenter clinical trial. Stroke. 1993;24(1):35-41. [DOI] [PubMed] [Google Scholar]
- 9.Di Pino G, Pellegrino G, Assenza G, et al. . Modulation of brain plasticity in stroke: a novel model for neurorehabilitation. Nat Rev Neurol. 2014;10(10):597-608. [DOI] [PubMed] [Google Scholar]
- 10.Chamorro A, Urra X, Planas AM. Infection after acute ischemic stroke: a manifestation of brain-induced immunodepression. Stroke. 2007;38(3):1097-1103. [DOI] [PubMed] [Google Scholar]
- 11.Dirnagl U, Klehmet J, Braun JS, et al. . Stroke-induced immunodepression: experimental evidence and clinical relevance. Stroke. 2007;38(2)(suppl):770-773. [DOI] [PubMed] [Google Scholar]
- 12.Hug A, Dalpke A, Wieczorek N, et al. . Infarct volume is a major determiner of post-stroke immune cell function and susceptibility to infection. Stroke. 2009;40(10):3226-3232. [DOI] [PubMed] [Google Scholar]
- 13.Giuliani C, Peri A. Effects of hyponatremia on the brain. J Clin Med. 2014;3(4):1163-1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frohman TC, Davis SL, Beh S, Greenberg BM, Remington G, Frohman EM. Uhthoff’s phenomena in MS—clinical features and pathophysiology. Nat Rev Neurol. 2013;9(9):535-540. [DOI] [PubMed] [Google Scholar]
- 15.Yong AW, Morris Z, Shuler K, Smith C, Wardlaw J. Acute symptomatic hypoglycaemia mimicking ischaemic stroke on imaging: a systemic review. BMC Neurol. 2012;12:139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nudo RJ, Wise BM, SiFuentes F, Milliken GW. Neural substrates for the effects of rehabilitative training on motor recovery after ischemic infarct. Science. 1996;272(5269):1791-1794. [DOI] [PubMed] [Google Scholar]
- 17.Ago T, Kitazono T, Ooboshi H, et al. . Deterioration of pre-existing hemiparesis brought about by subsequent ipsilateral lacunar infarction. J Neurol Neurosurg Psychiatry. 2003;74(8):1152-1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fisher CM. Concerning the mechanism of recovery in stroke hemiplegia. Can J Neurol Sci. 1992;19(1):57-63. [PubMed] [Google Scholar]
- 19.Albers GW, Caplan LR, Easton JD, et al. ; TIA Working Group . Transient ischemic attack—proposal for a new definition. N Engl J Med. 2002;347(21):1713-1716. [DOI] [PubMed] [Google Scholar]
- 20.Elkind MS, Carty CL, O’Meara ES, et al. . Hospitalization for infection and risk of acute ischemic stroke: the Cardiovascular Health Study. Stroke. 2011;42(7):1851-1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kamel H, Navi BB, Sriram N, Hovsepian DA, Devereux RB, Elkind MS. Risk of a thrombotic event after the 6-week postpartum period. N Engl J Med. 2014;370(14):1307-1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kumar S, Selim MH, Caplan LR. Medical complications after stroke. Lancet Neurol. 2010;9(1):105-118. [DOI] [PubMed] [Google Scholar]
- 23.Chernyshev OY, Martin-Schild S, Albright KC, et al. . Safety of tPA in stroke mimics and neuroimaging-negative cerebral ischemia. Neurology. 2010;74(17):1340-1345. [DOI] [PMC free article] [PubMed] [Google Scholar]