This epidemiologic register-based study examines the 20-year epidemiologic trends of amyotrophic lateral sclerosis in the Piemonte and Valle d’Aosta regions of Italy.
Key Points
Question
Are the incidence and prevalence of amyotrophic lateral sclerosis changing over time?
Findings
In this epidemiologic register-based study of 2702 patients in Italy, the crude incidence rate of amyotrophic lateral sclerosis increased by 14% during the 1995 to 2014 period, but the increase was reduced to 9% and was mostly limited to women after age and sex adjustment. The age-period-cohort model revealed that the increase can be partly ascribed to a birth cohort effect, affecting women born before 1930.
Meaning
The incidence of amyotrophic lateral sclerosis is increasing in part because of the increase of the mean age of the general population, with a residual component attributable to a birth cohort effect in women.
Abstract
Importance
This study reports the long-term epidemiologic trends of amyotrophic lateral sclerosis (ALS) based on a prospective register.
Objective
To examine the 20-year epidemiologic trends of ALS in the Piemonte and Valle d’Aosta regions of Italy.
Design, Setting, and Participants
The Piemonte and Valle d’Aosta Register for ALS (PARALS) is an epidemiologic prospective register that covers 2 Italian regions (population of 4 476 931 inhabitants according to the 2011 census) from January 1, 1995, through December 31, 2014. Case ascertainment is based on multiple sources (neurologic departments, hospital discharge archives, and mortality records). Incidence rates are age and sex standardized for the Italian population of the 2011 census. Age-period-cohort (APC) analysis was performed using a Poisson regression model.
Main Outcomes and Measures
The primary study outcomes were long-term incidence and prevalence rates of ALS using a prospective design and their determinants.
Results
During the study period, a total of 2702 patients (mean [SD] age at onset, 65.7 [11.1] years; 1246 [46.1%] female and 1456 [53.9%] male) received a diagnosis of ALS between 1995 and 2014, corresponding to a crude annual incidence rate of 3.03 per 100 000 population (95% CI, 2.85-3.23) and an adjusted incidence rate of 2.78 per 100 000 population (95% CI, 2.57-2.96). The age-adjusted incidence rate increased in the 2 decades of the study (1995-2004: 2.66; 95% CI, 2.50-2.83; 2005-2014: 2.89; 95% CI, 2.71-3.07; P = .04), mostly in women. The adjusted rate ratio of men to women decreased from 1.27:1 (1995-2004) to 1.17:1 (2005-2014). The analysis of deviance for the APC regression models indicated that the drift variable is relevant in explaining the variation of ALS incidence rates over time in the overall population (change in deviance, 4.6553; P = .03) and in women (change in deviance, 3.8821; P = .05) but not in men (change in deviance, 0.77215; P = .38). A total of 479 patients with ALS were alive and had not undergone tracheostomy at the prevalence day (December 31, 2014), corresponding to a crude prevalence rate of 10.54 per 100 000 population (95% CI, 9.64-11.52).
Conclusions and Relevance
During the 1995 to 2014 period, the crude and adjusted incidences of ALS increased in Piemonte and Valle d’Aosta, mostly in women. The APC model revealed that the increase of ALS incidence is attributable to a birth cohort effect in women, with a peak in the 1930 cohort. The different increase of ALS incidence in men and women points to an effect of exogenous factors with a differential effect on the 2 sexes, acting on a genetic background.
Introduction
Amyotrophic lateral sclerosis (ALS) is a degenerative disorder of the central nervous system characterized by progressive muscle loss caused by the involvement of lower (spinal and bulbar) motor neurons and pyramidal signs caused by the involvement of upper (cortical) motor neurons. Approximately 50% of patients have different degrees of cognitive impairment, ranging from dysexecutive impairment to frontotemporal dementia. Amyotrophic lateral sclerosis is familial in 10% of cases and apparently sporadic in the remaining 90%. Overall, besides the genetic forms, the cause(s) of ALS still remains elusive. Several exogenous factors have been proposed, namely, cigarette smoking, exposure to electromagnetic fields, heavy metals, organic chemicals, professional sports, occupations related to strenuous work, physical traumas, and personal characteristics, such as lower body mass index and lower educational level, but none of these, with the possible exception of smoking in women, is definitely causative of the disease. Descriptive epidemiologic studies, in particular on long-term trends of incidence and prevalence rates, may help to elucidate the respective role of genes and environment in the pathogenesis of ALS.
Although the epidemiology of ALS has been widely studied, in particular in Western countries, there are few data on the long-term epidemiologic trends of ALS. The Piemonte and Valle d’Aosta Register for ALS (PARALS) was established in 1995 to assess the epidemiology of the disease in 2 regions of Northwestern Italy. The register is still active and, over time, has used the same method of patient ascertainment. The aim of this study was to examine the 20-year epidemiologic trends of ALS in Piemonte and Valle d’Aosta, Italy, assessing its demographic variations.
Methods
The PARALS is a prospective register of all cases of ALS in the Piemonte and Valle d’Aosta regions of Italy (total population at the 2011 national census, 4 476 931; total area, 28 692 km2). Epidemiologic data regarding the 1995 to 2004 period have been published. Data on the reference population have been obtained from the demographic websites of the Piemonte (http://www.demos.piemonte.it/) and Valle d’Aosta regions (http://www.regione.vda.it/statistica/default_i.asp). During the study period, the resident population remained stable, ranging from 4 408 414 in 1995 to 4 544 055 in 2014, for a total of 89 200 359 person-years. The Piedmont regional government has recognized the Piemonte ALS Register as a register of high health interest. Accordingly, the PARALS has the right to access all the existing databases owned by the regional administration and to obtain clinical information about patients with ALS from public and private hospitals and general practitioners. The register database is anonymized and treated according to the Italian Data Protection Code. Patients sign written informed consent forms. This study was approved by the ethical committee of the Città della Salute e della Scienza of Turin.
Sources of Cases
The primary sources of cases are the 2 tertiary ALS centers (ALS expert centers), located in Torino and Novara, and the neurology departments of the 2 regions. Every 6 months, a search is also performed for ALS diagnoses (International Classification of Diseases, Ninth Revision [ICD-9] code 335.2) at the Piemonte and Valle d’Aosta Hospital Discharge Archives, which include information about all patients discharged by public and private hospitals of the 2 regions; for reimbursement reasons, the hospital discharge archives also collect data on the hospital admissions of patients resident in the 2 regions who have been admitted to public and private hospitals located in other Italian regions. Annually, a search is also performed for mortality data from the Italian Statistical Bureau (ICD-9 code 335.2). Clinical records of cases found through secondary sources are obtained from the admitting hospitals and the patients’ general practitioner. Relevant clinical information for each case is evaluated to verify whether the patient meets the eligibility criteria. All living patients have been contacted by telephone and visited by one of us (C.M., S.C., A.I., or E.B.) involved in the study.
Diagnostic Criteria
The diagnosis of ALS was originally based on the El Escorial Criteria (EEC), but after 2000, patients were also classified according to the revised El Escorial Criteria (EEC-R). Patients are included in the PARALS if they meet the diagnosis of definite, probable, or probable laboratory-supported ALS according to the EEC-R at any stage of the disease. Electromyographic records of cases diagnosed before 2000 have been evaluated to reclassify suspected or possible ALS according to the EEC-R.
Follow-up
Follow-up visits of each patient are performed at regular intervals (2-4 months). Local investigators use an ad hoc questionnaire to collect patients’ demographic data, disease history, and neurologic and laboratory findings, including ALS functional rating scale and treatments. At each visit, the EEC or EEC-R diagnosis is verified and updated. Patients’ date of death is obtained from the municipality offices where they resided.
Age-Period-Cohort Analysis
To further investigate the trends in incidence rates of ALS, an age-period-cohort (APC) analysis was performed. Temporal incidence trends are determined by the merged effects of age, period, and birth cohort. Because of the exact linear dependency among these 3 variables (the birth cohort can be determined by subtracting the age at diagnosis from the period of diagnosis), APC models are limited by an identification problem, which means that it is impossible to extricate the singular effects of the 3 variables. To estimate the independent effects of age, period, and cohort, we performed an APC analysis based on a Poisson regression model. Through this approach, a drift factor can be obtained, representing the sum of the linear time trend for birth cohort and period: the term drift indicates a temporal variation of rates that does not distinguish the influences of 2 of the 3 temporal variables involved in the analysis. The eventual deviations from linearity can therefore be interpreted as cohort or period effects. Because ALS is extremely rare in young ages and few cases occur in the population older than 85 years, the APC analysis was restricted to the population aged 40 to 85 years. Birth cohort and period effects were given as rate ratios (RRs). The last period in our data (2010) was set as the reference period, whereas the 1930 cohort was set as the reference cohort. The APC effects were calculated for the total population and separately for men and women. The data analysis was conducted using the apc.fit function of the Epi package of R (R Foundation for Statistical Computing).
Statistical Analysis
The prevalence rate was estimated on December 31, 2014. Incidence rates were standardized on age and sex distribution of the 2001 Italian population with the direct method of standardization. The 95% CIs were calculated assuming a Poisson distribution. Differences between mean values were assessed with the 2-tailed t test. Comparison of categorical variables was made with the χ2 test. The capture-recapture estimation is calculated with the maximum likelihood estimator using the 2 independent sources of patient identification (neurologic departments and hospital discharge archives). All tests were 2-sided. P ≤ .05 was considered statistically significant.
Results
During the study period, a total of 2702 patients (mean [SD] age at onset, 65.7 [11.1] years; 1246 [46.1%] female and 1456 [53.9%] male) who met the enrollment criteria were included in the PARALS. The increase of the age at onset paralleled the increase of the mean age of the population of Piemonte and Valle d’Aosta in the same period (eFigures 1 and 2 in the Supplement). The main demographic and clinical data, comparing the two 10-year periods (1995-2004 vs 2005-2014), are reported in Table 1.
Table 1. Demographic and Clinical Characteristics of Patients With ALS in the Two 10-Year Periodsa.
| Characteristic | 1995-2004 (n = 1243) |
2005-2014 (n = 1459) |
P Value |
|---|---|---|---|
| Male | 678 (54.5) | 778 (53.3) | .54 |
| Age at onset, mean (SD), y | 65.0 (11.1) | 66.3 (11.1) | .002 |
| Diagnostic delay, mean (SD), mo | 11.3 (9.6) | 10.9 (10.3) | .01 |
| Bulbar onset | 465 (37.4) | 508 (34.8) | .17 |
| Familial ALS | 53 (4.3) | 110 (7.5) | <.001 |
| Followed by an ALS multidisciplinary center | 601 (48.4) | 1154 (79.1) | <.001 |
Abbreviation: ALS, amyotrophic lateral sclerosis.
Data are presented as number (percentage) of patients unless otherwise indicated.
EEC at Diagnosis
During the study period, at time of diagnosis, 1305 patients (48.3%) had definite ALS, 844 (31.2%) had probable ALS, 142 (5.3%) had probable laboratory-supported ALS, 298 (11%) had possible ALS, and 113 (4.2%) had suspected ALS. There was no difference in the two 10-year periods, although there was a slight increase of patients with possible ALS (eTable 1 in the Supplement).
Site of Onset and Diagnostic Delay
A total of 972 patients (36.0%) had bulbar onset, and 1730 (64.0%) had spinal onset, with no difference between the two 10-year periods (P = .16). When considering the 2 sexes, no period difference was found among women (246 [43.5%] with bulbar onset in 1995-2004 vs 294 [43.2%] in 2005-2014 and 319 [56.5%] with spinal onset in 1995-2004 vs 387 [56.8%] in 2014; P = .94), whereas, among men, there was a decrease of the frequency of bulbar onset in the second decade (219 [32.3%] with bulbar onset in 1995-2004 vs 213 [27.4%] in 2005-2014 and 459 [67.7%] with spinal onset in 1995-2004 vs 565 [72.6%] in 2005-2014; P = .05) (eTable 2 in the Supplement). The mean (SD) diagnostic delay decreased from 11.3 (9.6) months in the 1995 to 2004 period to 10.9 (10.3) months in the 2005 to 2014 period (P = .01).
Incidence Rate
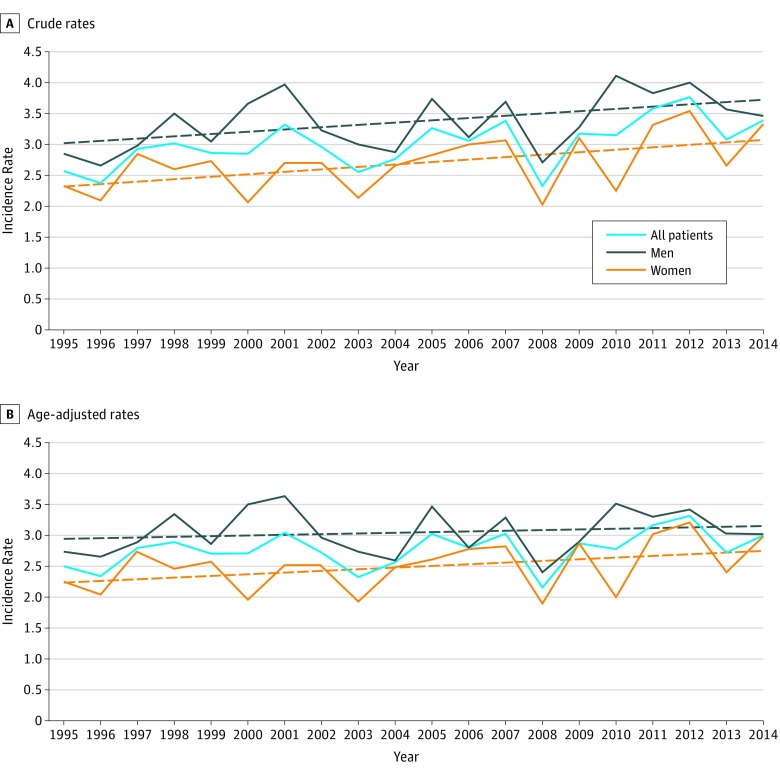
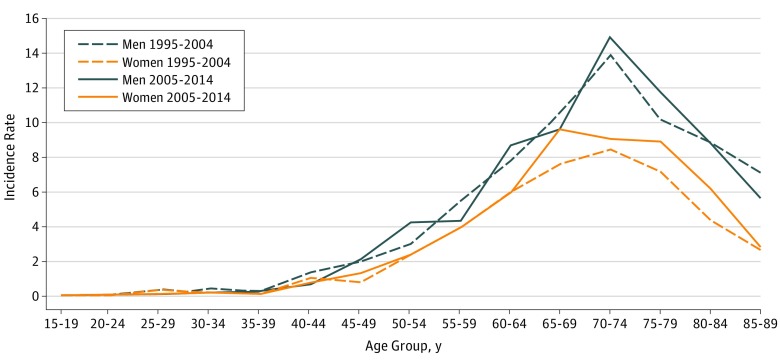
The mean annual crude incidence rate in the whole period was 3.03 per 100 000 population (95% CI, 2.85-3.23) (men: 3.31 per 100 000; 95% CI, 3.21-3.52; women: 2.65; 95% CI, 2.48-2.82; RR for men to women, 1.25:1). The mean annual crude incidence rate increased from 2.83 per 100 000 population (95% CI, 2.66-3.01) in the 1995 to 2004 decade to 3.23 per 100 000 (95% CI, 3.03-3.44) in the 2005 to 2014 decade, with a 14% increase (Figure 1A). The mean annual incidence rate, adjusted for age and sex, in the 2001 Italian population was 2.78 per 100 000 population (95% CI, 2.57-2.96) (men: 3.06 per 100 000; women: 2.51 per 100 000; RR for men to women, 1.22:1). The age-adjusted incidence rate increased in the 2 decades of the study (1995-2004: 2.66; 95% CI, 2.50-2.83; 2005-2014: 2.89; 95% CI, 2.71-3.07; P = .04), mostly in women (Figure 1B). Consequently, the adjusted RR for men to women decreased from 1.27:1 (1995-2004) to 1.17:1 (2005-2014) (eFigure 3 in the Supplement). The age-specific mean annual incidence rates peaked in the 70- to 74-year age group in both sexes, with a shift to the 65- to 69-year age group in women in the 2005 to 2014 decade (Figure 2).
Figure 1. Incidence Rates During the 20-Year Study.
A, Crude incidence rates; B, age-adjusted incidence rates. Dashed straight lines indicate the linear regression.
Figure 2. Incidence Rates by Age Groups and Period (1995-2004 vs 2005-2014).
Prevalence Rate
A total of 479 patients with ALS were alive and had not undergone tracheostomy on the prevalence day (December 31, 2014), corresponding to a crude prevalence rate of 10.54 per 100 000 population (95% CI, 9.64-11.52), with a small difference between men (246 cases; 11.19; 95% CI, 9.88-12.71) and women (233 cases; 9.94; 95% CI, 8.77-11.27) (P = .33). When the 78 patients (26 women and 52 men) who had undergone tracheostomy and were alive on the prevalence day were included, the prevalence rate increased to 12.26 (95% CI, 11.27-13.34) and was higher in men (298 cases; 13.55; 95% CI, 12.09-15.18) than in women (259 cases; 11.04; 95% CI, 9.74-12.51) (P = .04).
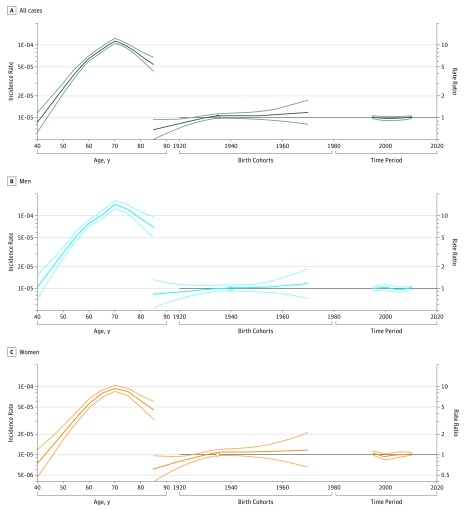
APC Modeling
The effects of age (plotted as incidence rates), birth cohort, and period (plotted as RRs) evaluated through APC analysis are shown in Figure 3. The analysis of deviance for the APC regression models indicated that the drift variable (ie, the linear variation of the incidence in time attributable to a birth cohort effect) is relevant in explaining the variation of ALS incidence rates over time in the overall population (age-period model for all cases: deviance, 4.6553; P = .03) and in females (deviance, 3.8821; P = .05) but not in males (deviance, 0.77215; P = .38). We found a birth cohort effect of increasing incidence up to the 1930 cohort; however, this finding was limited to women (eTable 3 in the Supplement). Neither the age-period model nor the age-cohort model was statistically significant (age period model for all cases: deviance, −3.6881; P = .30; age-cohort model for all cases: deviance, 3.0783; P = .38).
Figure 3. Age-Period-Cohort Model for All Incident Cases of Amyotrophic Lateral Sclerosis in Piemonte and Valle d’Aosta, Italy, 1995-2014.
Dark lines indicate the rates; light lines, 95% CIs.
Capture-Recapture Estimation
The results of the 2-source capture-recapture method are reported in eTable 4 in the Supplement. The main source (neurologic departments) identified 2417 cases (89.5% of all cases), 420 of which were unique to that source; the secondary source (Piemonte and Valle d’Aosta Central Regional Archives) identified 2281 cases (84.5% of all cases), 284 of which were unique to that source. The capture-recapture model estimated that 61 patients were unobserved (26 in the 1995-2004 period and 35 in the 2005-2014 period), increasing the annual estimated crude incidence to 3.31 per 100 000 population. The percentage of unobserved patients was similar in the 2 decades (2% vs 2.4%).
Discussion
During the 1995 to 2014 period, the crude incidence rate of ALS progressively increased in the Piemonte and Valle d’Aosta regions by 14%. The age- and sex-adjusted incidence rates revealed a lower increase, almost exclusively in women. These findings indicate that the modification of the burden of ALS in the Italian population is mostly related to the aging of the general population, with a noteworthy deviation in women. We found that this deviation may be ascribed to a birth cohort effect in women, with a peak in the 1930 cohort.
The ALS incidence rates have been extensively studied in European and Far Eastern populations, whereas relatively few data are available from developing countries. Available figures indicate a large range of incidence rates; however, this rate is reduced when considering only those epidemiologic studies that have used a homogeneous identification of cases and a prospective design. A recent meta-analysis of the literature reported a median estimated incidence rate in Europe of 2.39, slightly higher than the corresponding estimate of 1.80 for North America.
The age-adjusted incidence rate of ALS in Piemonte and Valle d’Aosta is among the highest reported in the literature, probably reflecting the accurate case ascertainment based on the collaboration of the neurologic departments of the 2 regions and the use of multiple sources of case identification. It is unlikely that the increase of ALS incidence observed in Piemonte is attributable to methodologic factors, in particular an improvement of identification of patients, because the sources of patients and the diagnostic criteria did not change during the study period and the capture-recapture estimation revealed a substantially similar level of ascertainment of cases during the entire study period.
Retrospective data on ALS incidence spanning at least 10 years are available from some geographic areas with conflicting results, indicating stability or an increase. These differences are likely to be related to the largely different methods of case ascertainment, the assessment of different periods, and the use of diverse diagnostic criteria. Besides the PARALS, the Irish ALS register, which is the only prospective epidemiologic register that has been constantly active for more than 10 years, reported an increase of ALS incidence in the 1996 to 2014 period, limited to patients older than 75 years and without evidence of a differential increase in the 2 sexes.
Few APC studies have been performed in ALS (Table 2). Most of them are based on mortality data, which are available for long periods but have several pitfalls, including the change of ICD-9 classification of ALS and the limited accuracy of death certificates. All mortality studies found an increase in the mortality rate over time, and all but one study reported a possible birth cohort effect in those born before 1920. To our knowledge, besides ours, there are only 2 APC studies based on incidence data. A nationwide study performed in Denmark reported a significant increase in ALS incidence rates in Denmark in succeeding birth cohorts from 1880 to 1920, with a subsequent plateau. As opposed to our study, a study from Ireland reported an initial period effect but did not find a birth cohort effect, but data were not assessed separately for men and women. In our prospective, population-based register, we found a birth cohort effect limited to women up to the 1930 cohort, partly explaining the increase of incidence of ALS in women observed from 1995 to 2014 in Piemonte and Valle d’Aosta.
Table 2. Summary of Age-Period-Cohort Studies Performed in Amyotrophic Lateral Sclerosis.
| Source | Area | Period | No. of Cases | Major Findings | Notes |
|---|---|---|---|---|---|
| Mortality studies | |||||
| Gordon et al, 2011 | France | 1968-2007 | 38 863 | Birth cohort effect that involved all birth cohorts, more markedly between 1880 and 1920 | Patients 40-89 y, sexes not examined separately |
| Ajdacic-Gross et al, 2012 | Switzerland | 1942-2008 | 5027 | No birth cohort effect, possible age effect | Sexes not examined separately |
| Seals et al, 2013 | Denmark | 1982-2009 | 4265 | Birth cohort effect of increasing mortality over birth cohorts before 1920 | Patients ≥45 y, data by sex not provided |
| Nakken et al, 2016 | Norway | 1951-2014 | 5345 | Birth cohort effect of increasing mortality between 1860 and 1934 | Men and women equally affected |
| Incidence studies | |||||
| Seals et al, 2013 | Denmark | 1982-2009 | 3228 | Cohort effect of increasing incidence over birth cohorts before 1920, stronger birth cohort effect among women | Patients ≥45 y |
| Tobin et al, 2016 | Ireland | 1996-2014 | 1734 | No birth cohort effect, initial period effect | Patients 40-89 y, sexes not examined separately |
| Present study | Piemonte and Valle d’Aosta regions, Italy | 1995-2014 | 2701 | Birth cohort effect among women born before 1930, no birth cohort effect among men, no period effect | Patients 40-85 y |
The prevalence rate of ALS in Piemonte and Valle d’Aosta on December 31, 2014, was 10.5 per 100 000 population, one of the highest reported in the literature and an increase of 34% compared with the prevalence rate on December 31, 2004 (7.9 per 100 000 population). When including patients who had undergone a tracheotomy and who were alive at the prevalence day, the overall prevalence rate of ALS was 12.3 per 100 000 population; this rate can be considered a measure of the disease burden of ALS for the health and social system. Several reasons for the observed increase in the ALS prevalence rate between the 2 estimates of 2004 and 2014 (present study) can be considered. First, the observed trend is partially explained by the increase in the crude incidence rate. Second, the 2014 prevalence estimate includes 62 long-term survivors with ALS diagnosed between 1995 and 2004 (ie, 12.9% of all prevalent patients); conversely, in the 2004 estimate, it was not possible to include the patients who received a diagnosis of ALS before the prospective register was started (1995) but who were still alive at the prevalence day. This observation indicates that even for a disease with as short a survival as ALS, long-term epidemiologic studies are necessary to fully identify the whole prevalent population. Third, an increase of patients’ tracheostomy-free survival between the first and the second decades of the study could be also a factor.
During the study period, we found an increase in the mean age at onset in both sexes, which was partially attributable to the increase in the mean age of the reference population. This observation confirms the hypothesis that the mean age at onset of ALS is directly proportional to the life expectancy in the underlying population and that ALS incidence and prevalence rates are directly proportional to the median age of the reference population. Our finding also reinforces the recent estimate of an increase of the number of ALS cases across the globe in the next 25 years, particularly among developing countries, mainly as a result of the aging of the population.
The site of onset of symptoms did not reveal any modification in women, whereas a significant reduction of the number of bulbar-onset cases was found among men. Regional differences in the distribution of site of onset of ALS have been reported in a study that compared 6 European prospective registers for the period 1998 to 1999, with the highest frequency of bulbar- or generalized-onset cases in Ireland (46.7%) and the lowest in Apulia, Southern Italy (26.1%); however, it is possible that these differences are related to a different modality of classification of onset. There is no obvious explanation for our observation, and no long-term data on ALS site of onset are available in epidemiologic studies.
From the pathogenetic point of view, the relative stability of incidence rates points to a strong genetic background of ALS. However, the birth cohort effect identified in women highlights the role of exogenous nongenetic factors. Notably, in Italy, the cohort of women born in the 1920s is the first to have profoundly modified their lifestyle, for example, having started to smoke cigarettes and having been diffusely engaged in physical activities, including manual work, 2 possible risk factors for ALS. Further studies on the differential action of environmental factors in the 2 sexes will help to elucidate this aspect. The strong increase in the ALS prevalence rate in Piemonte and Valle d’Aosta in the last 2 decades underlines the increasing burden the social and health systems have to face in the next future for the caring of this terrible disease.
Limitations
A possible limitation of this study is that the observed modification of ALS incidence could be attributable to a differential identification of patients during the 1995-2014 period. However, the remarkably stable capture-recapture estimation during the study period indicates that ascertainment of cases did not significantly change.
Conclusions
From 1995 to 2014, the crude and adjusted incidences of ALS increased in Piemonte and Valle d’Aosta, mostly in women. The APC model revealed that the increase of ALS incidence is attributable to a birth cohort effect in women, with a peak in the 1930 cohort. The different increase of ALS incidence in men and women points to an effect of exogenous factors with a differential effect on the 2 sexes, acting on a genetic background.
eFigure 1. Trend of Mean Age at Onset Compared to the Mean Age of the Piemonte and Valle d’Aosta Population, 1995-2014, Males
eFigure 2. Trend of Mean Age at Onset Compared to the Mean Age of the Piemonte and Valle d’Aosta Population, 1995-2014, Females
eFigure 3. Progressive Decline of Men to Women Rate Ratio During the 20 Years of the Study.
eTable 1. Frequency of El Escorial Criteria Revised Levels of Diagnosis, 1995-2004 vs 2005-2014
eTable 2. Site of Onset of ALS Cases According to Sex, 1995-2004 vs 2005-2014
eTable 3. Age-Period-Cohort Rates and Relative Risks (RRs)
eTable 4. Case Ascertainment by Year Using Two Source Capture-Recapture Methods, Piemonte and Valle d’Aosta Register for ALS, 1994-2004
References
- 1.Phukan J, Elamin M, Bede P, et al. The syndrome of cognitive impairment in amyotrophic lateral sclerosis: a population-based study. J Neurol Neurosurg Psychiatry. 2012;83(1):102-108. [DOI] [PubMed] [Google Scholar]
- 2.Montuschi A, Iazzolino B, Calvo A, et al. Cognitive correlates in amyotrophic lateral sclerosis: a population-based study in Italy. J Neurol Neurosurg Psychiatry. 2015;86(2):168-173. [DOI] [PubMed] [Google Scholar]
- 3.Renton AE, Chiò A, Traynor BJ. State of play in amyotrophic lateral sclerosis genetics. Nat Neurosci. 2014;17(1):17-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang MD, Little J, Gomes J, Cashman NR, Krewski D. Identification of risk factors associated with onset and progression of amyotrophic lateral sclerosis using systematic review and meta-analysis [published online June 1, 2016]. Neurotoxicology. 2016;S0161-813X(16)30116-4. doi: 10.1016/j.neuro.2016.06.015 [DOI] [PubMed] [Google Scholar]
- 5.Chiò A, Logroscino G, Traynor BJ, et al. Global epidemiology of amyotrophic lateral sclerosis: a systematic review of the published literature. Neuroepidemiology. 2013;41(2):118-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marin B, Boumédiene F, Logroscino G, et al. Variation in worldwide incidence of amyotrophic lateral sclerosis: a meta-analysis [published online May 13, 2016]. Int J Epidemiol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chiò A, Mora G, Calvo A, Mazzini L, Bottacchi E, Mutani R; PARALS . Epidemiology of ALS in Italy: a 10-year prospective population-based study. Neurology. 2009;72(8):725-731. [DOI] [PubMed] [Google Scholar]
- 8.Brooks BR; Subcommittee on Motor Neuron Diseases/Amyotrophic Lateral Sclerosis of the World Federation of Neurology Research Group on Neuromuscular Diseases and the El Escorial “Clinical limits of amyotrophic lateral sclerosis” workshop contributors . El Escorial World Federation of Neurology criteria for the diagnosis of amyotrophic lateral sclerosis. J Neurol Sci. 1994;124(suppl):96-107. [DOI] [PubMed] [Google Scholar]
- 9.Brooks BR, Miller RG, Swash M, Munsat TL; World Federation of Neurology Research Group on Motor Neuron Diseases . El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord. 2000;1(5):293-299. [DOI] [PubMed] [Google Scholar]
- 10.Carstensen B. Age-period-cohort models for the Lexis diagram. Stat Med. 2007;26(15):3018-3045. [DOI] [PubMed] [Google Scholar]
- 11.Schoenberg BS. Calculating confidence intervals for rates and ratios. Neuroepidemiology. 1983;2(5):257-265. doi: 10.1159/000110529 [DOI] [Google Scholar]
- 12.Sorenson EJ, Stalker AP, Kurland LT, Windebank AJ. Amyotrophic lateral sclerosis in Olmsted County, Minnesota, 1925 to 1998. Neurology. 2002;59(2):280-282. [DOI] [PubMed] [Google Scholar]
- 13.Bonaparte JP, Grant IA, Benstead TJ, Murray TJ, Smith M. ALS incidence in Nova Scotia over a 20-year-period: a prospective study. Can J Neurol Sci. 2007;34(1):69-73. [DOI] [PubMed] [Google Scholar]
- 14.Forbes RB, Colville S, Parratt J, Swingler RJ. The incidence of motor nueron disease in Scotland. J Neurol. 2007;254(7):866-869. [DOI] [PubMed] [Google Scholar]
- 15.Marin B, Gil J, Preux PM, Funalot B, Couratier P. Incidence of amyotrophic lateral sclerosis in the Limousin region of France, 1997-2007. Amyotroph Lateral Scler. 2009;10(4):216-220. [DOI] [PubMed] [Google Scholar]
- 16.Bonvicini F, Vinceti M, Marcello N, Rodolfi R, Rinaldi M. The epidemiology of amyotrophic lateral sclerosis in Reggio Emilia, Italy. Amyotroph Lateral Scler. 2008;9(6):350-353. [DOI] [PubMed] [Google Scholar]
- 17.Govoni V, Cesnik E, Casetta I, Tugnoli V, Tola MR, Granieri E. Temporal trend of amyotrophic lateral sclerosis incidence in southern Europe: a population study in the health district of Ferrara, Italy. J Neurol. 2012;259(8):1623-1631. [DOI] [PubMed] [Google Scholar]
- 18.Fang F, Valdimarsdóttir U, Bellocco R, et al. Amyotrophic lateral sclerosis in Sweden, 1991-2005. Arch Neurol. 2009;66(4):515-519. [DOI] [PubMed] [Google Scholar]
- 19.Giagheddu M, Puggioni G, Tacconi P, et al. Amyotrophic lateral sclerosis in Sardinia (Italy): epidemiologic features from 1957 to 2000. Acta Neurol Scand. 2013;127(4):251-259. [DOI] [PubMed] [Google Scholar]
- 20.Lareau-Trudel E, Fortin E, Gauthier M, Lavoie S, Morissette E, Mathieu J. Epidemiological surveillance of amyotrophic lateral sclerosis in Saguenay region. Can J Neurol Sci. 2013;40(5):705-709. [DOI] [PubMed] [Google Scholar]
- 21.Georgoulopoulou E, Vinceti M, Bonvicini F, et al. Changing incidence and subtypes of ALS in Modena, Italy: A 10-years prospective study. Amyotroph Lateral Scler. 2011;12(6):451-457. [DOI] [PubMed] [Google Scholar]
- 22.Cima V, Logroscino G, D’Ascenzo C, et al. Epidemiology of ALS in Padova district, Italy, from 1992 to 2005. Eur J Neurol. 2009;16(8):920-924. [DOI] [PubMed] [Google Scholar]
- 23.O’Toole O, Traynor BJ, Brennan P, et al. Epidemiology and clinical features of amyotrophic lateral sclerosis in Ireland between 1995 and 2004. J Neurol Neurosurg Psychiatry. 2008;79(1):30-32. [DOI] [PubMed] [Google Scholar]
- 24.Tobin K, Gilthorpe MS, Rooney J, et al. Age-period-cohort analysis of trends in amyotrophic lateral sclerosis incidence. J Neurol. 2016;263(10):1919-1926. [DOI] [PubMed] [Google Scholar]
- 25.Gordon PH, Artaud F, Aouba A, Laurent F, Meininger V, Elbaz A. Changing mortality for motor neuron disease in France (1968-2007): an age-period-cohort analysis. Eur J Epidemiol. 2011;26(9):729-737. [DOI] [PubMed] [Google Scholar]
- 26.Ajdacic-Gross V, Schmid M, Tschopp A, Gutzwiller F. Birth cohort effects in neurological diseases: amyotrophic lateral sclerosis, Parkinson’s disease and multiple sclerosis. Neuroepidemiology. 2012;38(1):56-63. [DOI] [PubMed] [Google Scholar]
- 27.Seals RM, Hansen J, Gredal O, Weisskopf MG. Age-period-cohort analysis of trends in amyotrophic lateral sclerosis in Denmark, 1970-2009. Am J Epidemiol. 2013;178(8):1265-1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakken O, Lindstrøm JC, Tysnes OB, Holmøy T. Mortality trends of amyotrophic lateral sclerosis in Norway 1951-2014: an age-period-cohort study. J Neurol. 2016;263(12):2378-2385. [DOI] [PubMed] [Google Scholar]
- 29.Lefter S, Hardiman O, Ryan AM. A population-based epidemiologic study of adult neuromuscular disease in the Republic of Ireland. Neurology. 2017;88(3):304-313. [DOI] [PubMed] [Google Scholar]
- 30.Mehta P, Kaye W, Bryan L, et al. Prevalence of Amyotrophic Lateral Sclerosis - United States, 2012-2013. MMWR Surveill Summ. 2016;65(8):1-12. [DOI] [PubMed] [Google Scholar]
- 31.Byrne S, Jordan I, Elamin M, Hardiman O. Age at onset of amyotrophic lateral sclerosis is proportional to life expectancy. Amyotroph Lateral Scler Frontotemporal Degener. 2013;14(7-8):604-607. [DOI] [PubMed] [Google Scholar]
- 32.Arthur KC, Doyle C, Chiò A, Traynor BJ. Use of Genetic Testing in Amyotrophic Lateral Sclerosis by Neurologists. JAMA Neurol. 2017;74(1):125-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Logroscino G, Traynor BJ, Hardiman O, et al. ; EURALS . Incidence of amyotrophic lateral sclerosis in Europe. J Neurol Neurosurg Psychiatry. 2010;81(4):385-390. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Trend of Mean Age at Onset Compared to the Mean Age of the Piemonte and Valle d’Aosta Population, 1995-2014, Males
eFigure 2. Trend of Mean Age at Onset Compared to the Mean Age of the Piemonte and Valle d’Aosta Population, 1995-2014, Females
eFigure 3. Progressive Decline of Men to Women Rate Ratio During the 20 Years of the Study.
eTable 1. Frequency of El Escorial Criteria Revised Levels of Diagnosis, 1995-2004 vs 2005-2014
eTable 2. Site of Onset of ALS Cases According to Sex, 1995-2004 vs 2005-2014
eTable 3. Age-Period-Cohort Rates and Relative Risks (RRs)
eTable 4. Case Ascertainment by Year Using Two Source Capture-Recapture Methods, Piemonte and Valle d’Aosta Register for ALS, 1994-2004