Key Points
Question
Is plasma total tau level a prognostic marker for cognitive decline and dementia, and do these potential associations differ by elevated brain amyloid β levels?
Findings
In this cohort study of 458 patients, high levels of plasma total tau were associated with a risk of mild cognitive impairment among cognitively normal participants. However, total tau levels were not associated with risk of dementia among participants with mild cognitive impairment. Elevated brain amyloid β was not a confounder or effect modifier when examining any outcome.
Meaning
Elevated plasma total tau levels may be associated with cognitive decline, and this association is independent of elevated brain amyloid β.
Abstract
Importance
The utility of plasma total tau level as a prognostic marker for cognitive decline and dementia is not well understood.
Objectives
To determine (1) the association between plasma total tau level, cognitive decline, and risk of mild cognitive impairment (MCI) and dementia; (2) whether this association differs by the presence of elevated brain amyloid β (Aβ); and (3) whether plasma total tau level is associated with cognitive decline over a short interval of 15 months.
Design, Setting, and Participants
The present analyses included 458 participants who were enrolled in a population-based cohort study between October 2008 and June 2013. All included participants had available plasma total tau levels, Aβ positron emission tomography imaging, and a complete neuropsychological examine at the same visit, as well as at least 1 follow-up visit.
Exposures
Concentration of plasma total tau.
Main Outcomes and Measures
Risk of MCI and dementia; global and domain-specific cognitive decline.
Results
Of the 458 participants, 287 (62.7%) were men; mean (SD) age was 80.6 (5.6) years. Among cognitively normal (CN) participants oversampled for elevated brain Aβ, both the middle (hazard ratio [HR], 2.43; 95% CI, 1.25-4.72) and highest (HR, 2.02; 95% CI, 1.01-4.06) tertiles of plasma total tau level, compared with the lowest, were associated with an increased risk of MCI. Among participants with MCI, higher plasma total tau levels were not significantly associated with risk of dementia (all-cause dementia or Alzheimer disease). Among all participants, higher levels of plasma total tau, examined as a continuous variable, were associated with significant (P < .05) declines in global cognition, memory, attention, and visuospatial ability over a median follow-up of 3.0 years (range, 1.1-4.9 years). In additional analyses restricting the follow-up to 15 months, plasma total tau did not predict decline among CN participants. However, among participants with MCI, higher plasma total tau levels were associated with greater decline in both visuospatial ability (regression coefficient [b] = −0.50 [0.15], P < .001) and global cognition (b = −0.27 [0.10], P = .009) at 15 months. Adjusting for elevated brain Aβ did not attenuate any association. There was no interaction between plasma total tau level and brain Aβ for prognosis with any outcome.
Conclusions and Relevance
These results suggest that elevated plasma total tau levels are associated with cognitive decline, but the results differ based on cognitive status and the duration of follow-up. The association between plasma total tau levels and cognition is independent of elevated brain Aβ.
This cohort study evaluates the association between plasma total tau level and cognitive decline as well as dementia in cognitively normal individuals.
Introduction
Multiple studies suggest that total tau levels can be measured in plasma. Cross-sectionally, some studies have reported elevated plasma total tau levels among participants with Alzheimer disease (AD) dementia or mild cognitive impairment (MCI), but other studies have not found a difference. Few studies have examined whether plasma total tau level could be used as a prognostic marker or risk factor for cognitive decline and dementia. One recent study using Alzheimer’s Disease Neuroimaging Initiative (ADNI) data reported that elevated plasma total tau levels were associated with greater cognitive decline and a larger increase in ventricular volume over 4 years among MCI and AD participants. We sought to build on these findings by examining the association between plasma total tau level, cognitive decline, and risk of MCI and dementia in the Mayo Clinic Study of Aging (MCSA). Considering the ongoing secondary prevention amyloid β (Aβ) trials, we also examined whether there was an interaction between elevated brain Aβ and plasma total tau levels for rate of cognitive decline and progression to MCI. Lastly, we determined whether plasma total tau level could be utilized as prognostic indicator of cognitive decline over a short trial duration of 15 months.
Methods
Participants
The present study was conducted with data from the MCSA, a prospective, population-based study that began in 2004 in Olmsted County, Minnesota. The study initially recruited Olmsted County residents between the ages of 70 and 89 years using an age- and sex-stratified random sampling design. Since 2004, the population has been re-enumerated several times and extended to cover the ages of 50 to 90 years or older following the same sampling strategy. Residents randomly chosen for recruitment were invited to participate in the MCSA, and those without a medical contraindication (eg, pacemaker) were invited to participate in imaging studies. The present analyses included 458 participants aged 56 to 95 years with plasma total tau measures, Aβ positron emission tomography (PET) imaging, and cognitive testing at the same study visit and at least 1 follow-up visit with cognitive testing. All participants were enrolled between October 2008 and June 2013.
The study protocols were approved by the Mayo Clinic and Olmsted Medical Center institutional review boards. All participants provided written informed consent and received financial compensation.
Participant Assessment
The MCSA visits occurred every 15 months and included a physician examination, an interview by a study coordinator, and neuropsychological testing administered by a psychometrist. Physician examinations included a review of the participant’s medical history, a neurologic examination, and administration of the Short Test of Mental Status. Study coordinator interviews included participant demographic information and medical history and the participant and informant Clinical Dementia Rating scale.
The neuropsychological battery included 9 tests covering 4 domains: (1) memory (Auditory Verbal Learning Test Delayed Recall Trial; Wechsler Memory Scale–Revised Logical Memory II and Visual Reproduction II), (2) language (Boston Naming Test and Category Fluency), (3) executive function (Trail Making Test B and Wechsler Adult Intelligence Scale-Revised [WAIS-R] Digit Symbol subtest), and (4) visuospatial (WAIS-R Picture Completion and Block Design subtests). Using the mean (SD) from all participants included in this study, test scores were converted to z scores. Global cognition was calculated using the z-score–transformed means of the 4 cognitive domains.
MCI and Dementia Diagnostic Determination
For each participant, performance in a cognitive domain was compared with the age-adjusted scores of cognitively normal (CN) individuals previously obtained using Mayo’s Older American Normative Studies. This approach relies on prior normative work and extensive experience with the measurement of cognitive abilities in an independent sample of participants from the same population. Participants with scores approximately 1.5 SDs below the age-specific mean in the general population were considered for possible cognitive impairment. A final decision was made after considering educational level, occupation, visual or hearing deficits, and reviewing all other participant information. The diagnosis of MCI was made by a consensus agreement between the study coordinator, examining physician, and neuropsychologist using published criteria. Participants with MCI were classified as having amnestic MCI (aMCI) if the memory domain was impaired. The diagnosis of dementia and AD were based on published criteria. Participants who performed in the normal range and did not meet criteria for MCI or dementia were deemed CN.
Plasma Total Tau Enzyme-Linked Immunosorbent Assay of MCSA Samples
Participants’ blood was collected in the clinic after an overnight fast. The blood was centrifuged, aliquoted, and stored at −80°C. Plasma total tau levels were measured on the Simoa HD-1 Analyzer (Quanterix), as described previously.
Assessment of Covariates
Participant demographics (eg, sex, age, and years of education) were ascertained at the in-clinic examination. Apolipoprotein E (APOE) ɛ4 genotyping was performed from a blood sample drawn at the in-clinic examination.
Imaging Methods
Amyloid β PET imaging was performed with Pittsburgh Compound B. Participants also completed computed tomography scanning for attenuation correction. Amyloid β PET images were acquired from 40 to 60 minutes after injection and were analyzed with our in-house, fully automated image processing pipeline, where image voxel values are extracted from automatically labeled regions of interest propagated from a magnetic resonance imaging template. An Aβ PET standardized uptake value ratio (SUVR) was formed from the prefrontal, orbitofrontal, parietal, temporal, anterior cingulate, and posterior cingulate and precuneus regions of interest normalized to uptake in cerebellar gray matter. The data were partial volume corrected for voxel cerebrospinal fluid content using segmented coregistered magnetic resonance imaging. Elevated Aβ PET was defined as SUVR greater than 1.40 as previously described.
Statistical Analysis
The distribution of plasma total tau level was right skewed so that the variable was log base 10 transformed prior to analyses. We used Cox proportional hazards regression models to determine whether the baseline plasma total tau level, as a continuous variable and in tertiles, was associated with risk of MCI among CN participants and risk of dementia (both all-cause and AD) among participants with MCI. All participants were censored at their last follow-up visit, diagnosis of MCI or dementia (depending on analyses), or death. Age was used as the time scale. Multivariable models were adjusted for sex, educational level, and APOE ɛ4. We also examined other variables previously associated with plasma total tau level (eg, diabetes, hypertension, atrial fibrillation, myocardial infarction, and statin use) as potential confounders. Adjusting for these additional variables did not affect the strength of any of the associations; they were therefore not included. Lastly, we adjusted for elevated brain Aβ to determine whether it was a potential confounder. We also examined whether there was an interaction between brain Aβ and plasma total tau levels for predicting risk of MCI and dementia. Kaplan-Meier survival curves were constructed for each outcome.
We used linear mixed-effects models, with an unstructured correlation matrix and random intercepts and slopes, to examine the association between baseline plasma total tau levels and change in global and domain-specific cognition. The models included baseline plasma total tau level (indicating the association between baseline plasma total tau level and baseline cognitive z score), time (indicating annual change in the cognitive z score), and the interaction between plasma total tau level and time (indicating whether baseline plasma total tau level predicted change in the cognitive z score). Change score analyses were used to examine the association between baseline plasma total tau level and 15-month change in each global and domain-specific z score to simulate the timeline of a clinical trial. Multivariable models were adjusted for age, sex, educational level, and APOE ɛ4 status. We again examined elevated brain Aβ as a potential confounder and/or effect modifier.
For all analyses, we extensively examined the data, including plotting the associations between plasma total tau level and the slopes, assessing for influential outliers, assessing model assumptions, and examining marginal and conditional residuals. There was no evidence of influential outliers and no concerns after assessing the marginal and conditional residuals. Linear mixed-effect and Cox proportional hazards regression model analyses were completed using SAS, version 9.4 (SAS Institute Inc). Kaplan-Meier survival curves were created using the survival package in R, version 3.3.1 (R Foundation). An unpaired, 2-tailed, P < .05 level was considered statistically significant.
Results
The characteristics of all 458 participants at baseline and after stratification by cognitive status (335 CN and 123 MCI) are reported in Table 1. The mean (SD) age of the participants was 80.6 (5.6) years, and 287 (62.7%) were men. Plasma total tau levels increased with age (Spearman ρ = 0.19, P < .001). Plasma total tau levels also did not differ significantly by cognitive diagnosis (MCI vs CN), sex, APOE ɛ4 carrier status, or elevated brain Aβ (all P > .05). The median follow-up was 3.0 years (range, 1.1-4.9 years). Of the 458 participants, all had a follow-up visit at 15 months, 352 (76.9%) had a visit at 30 months, and 206 (45.0%) had a visit at 45 months.
Table 1. Baseline Characteristics of Participants.
| Characteristic | Data Available, No. | Total (N = 458) |
Data Available, No. | CN (n = 335) |
Data Available, No. | MCI (n = 123) | P Value |
|---|---|---|---|---|---|---|---|
| Age, mean (SD) | 458 | 80.6 (5.6) | 335 | 80.8 (4.8) | 123 | 79.9 (7.4) | .69 |
| Men, No. (%) | 458 | 287 (62.7) | 335 | 209 (62.4) | 123 | 78 (63.4) | .84 |
| Education, mean (SD), y | 458 | 14.4 (3.0) | 335 | 14.6 (3.0) | 123 | 14.0 (3.0) | .03 |
| ≥1 APOE ɛ4 allele, No. (%) | 458 | 130 (28.4) | 335 | 88 (26.3) | 123 | 42 (34.1) | .10 |
| Plasma total tau level, mean (SD), ng/L | 458 | 4.3 (1.6) | 335 | 4.2 (1.5) | 123 | 4.5 (1.8) | .28 |
| z Score, mean (SD) | |||||||
| Global cognition | 439 | 0.1 (0.7) | 324 | 0.3 (0.6) | 115 | −0.5 (0.6) | <.001 |
| Memory | 454 | 0.1 (0.8) | 334 | 0.4 (0.7) | 120 | −0.7 (0.7) | <.001 |
| Attention | 438 | 0.1 (0.9) | 324 | 0.3 (0.7) | 114 | −0.5 (1.0) | <.001 |
| Language | 445 | 0.1 (0.8) | 329 | 0.3 (0.6) | 116 | −0.5 (0.8) | <.001 |
| Visuospatial ability | 436 | 0.1 (0.9) | 341 | 0.2 (0.8) | 114 | −0.5 (1.0) | <.001 |
| Abnormal PiB-PET (SUVR>1.40), No. (%) | 478 | 263 (57.4) | 335 | 192 (57.3) | 123 | 71 (57.7) | .94 |
Abbreviations: APOE, apolipoprotein E; CN, cognitively normal; MCI, mild cognitive impairment; PET, positron emission tomography; PiB, Pittsburgh Compound B; SUVR, standardized uptake value ratio.
Plasma Total Tau Level and Risk of MCI or Dementia
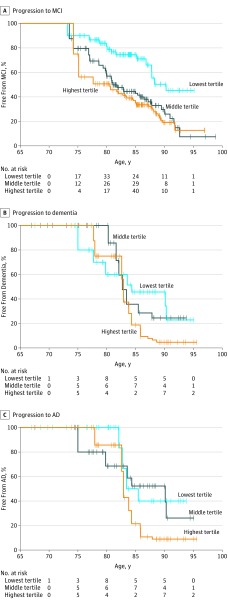
Of the 335 CN individuals, 67 (20.0%) progressed to MCI over the follow-up period. Each log-unit increase in plasma total tau level, as a continuous variable, was associated with a 2.5-fold increased risk of MCI (hazard ratio [HR], 2.45; 95% CI, 1.20-5.02). This association was attenuated after adjusting for sex, educational level, and APOE ɛ4 (HR, 2.05; 95% CI, 0.99-4.28). Further adjustment for elevated brain Aβ did not alter this association (HR, 2.05; 95% CI, 0.98-4.31). Examining tertiles of plasma total tau levels, both the middle (HR, 2.43; 95% CI, 1.25-4.72) and highest (HR, 2.02; 95% CI, 1.01-4.06) tertiles compared with the lowest tertile were associated with an increased risk of MCI in multivariable models. Kaplan-Meier plots of this association are shown in Figure 1A. In additional analyses, we did not observe an interaction between plasma total tau level as a continuous measure or in tertiles and elevated Aβ level for risk of MCI.
Figure 1. Tertiles of Plasma Total Tau Levels .
A, Progression from cognitively normal to mild cognitive impairment (MCI). B, Progression from MCI to dementia. C, Progression from MCI to Alzheimer disease (AD).
Forty-eight of the 67 (71.6%) incident MCI cases were aMCI. In sensitivity analyses, we repeated the analyses using aMCI as the outcome. In multivariable models, each log-unit increase in plasma total tau level was associated with an increased risk of aMCI, but the results were not significant (HR, 1.63; 95% CI, 0.69-3.88). Compared with the lowest tertile of plasma total tau level, both the middle (HR, 2.40; 95% CI, 1.12-5.13) and highest (HR, 1.93; 95% CI, 0.85-4.37) tertiles were associated with an increased risk of aMCI. Adjusting for elevated brain Aβ did not further attenuate this association, and there was not an interaction between plasma total tau level and Aβ for predicting risk of aMCI.
Of the 123 individuals with MCI at baseline, 28 (22.8%) progressed to dementia (20 attributed to AD). There were no associations between plasma total tau level as a continuous variable or in tertiles and risk of either all-cause dementia (multivariable analysis with plasma total tau level as a continuous variable: HR, 1.70; 95% CI, 0.57-5.07) or AD (multivariable analysis with plasma total tau level as a continuous variable: HR, 1.78; 95% CI, 0.61-5.19). Kaplan-Meier plots of these associations are shown in Figure 1B and C. Elevated brain Aβ was not a confounder or effect modifier.
Given the strong associations with age and both plasma total tau level and our outcomes, we used age as the time scale in the Kaplan-Meier plots and for the Cox proportional hazard hazards regression models. In sensitivity analyses, we reran the models using time in study as the time scale. The results of these analyses, including strengths of the HRs, were similar.
Plasma Total Tau Level and Global and Domain-Specific Cognitive Decline
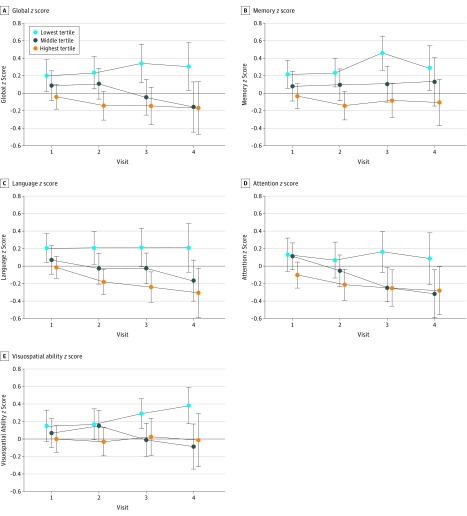
The associations between plasma total tau level and cognitive decline are reported in Table 2 and Figure 2. Higher baseline plasma total tau levels were associated with greater declines (indicated by the baseline plasma log tau × time coefficient) in global and domain-specific z scores in multivariable analyses. In additional analyses, we observed a significant interaction between plasma total tau level and diagnostic group (P = .02) such that higher plasma total tau level was associated with visuospatial ability declines among participants with MCI (regression coefficient [b] [SE] = −0.18 [0.06], P = .004) but not among CN individuals (b [SE] = −0.02 [0.03], P = .46). Elevated brain Aβ was not a confounder or effect modifier.
Table 2. Association Between Baseline Plasma Tau Level and Global and Domain-Specific Cognitive Decline Among All Participantsa.
| Outcome (z Score) | Baseline Plasma Log Tau | Time | Baseline Plasma Log Tau × Time | |||
|---|---|---|---|---|---|---|
| b (SE) | P Value | b (SE) | P Value | b (SE) | P Value | |
| Total (N = 458) | ||||||
| Memory | −0.15 (0.12) | .20 | 0.04 (0.04) | .26 | −0.05 (0.03) | .04 |
| Language | −0.14 (0.11) | .22 | −0.04 (0.04) | .32 | −0.03 (0.03) | .25 |
| Attention | −0.12 (0.12) | .34 | −0.03 (0.04) | .48 | −0.06 (0.03) | .03 |
| Visuospatial ability | −0.003 (0.12) | .98 | 0.05 (0.04) | .18 | −0.06 (0.03) | .02 |
| Global | −0.16 (0.12) | .17 | 0.01 (0.04) | .85 | −0.07 (0.02) | .006 |
Abbreviation: APOE, apolipoprotein E.
Models adjusted for age, sex, years of education, and presence of an APOE ɛ4 allele.
Figure 2. Plots of Global and Domain-Specific Cognitive Decline as a Function of Baseline Plasma Tau in Tertiles.
Global (A), memory (B), language (C), attention (D), and visuospatial (E) z scores.
Association Between Plasma Tau Level and Change in Cognition Over 15 Months
Last, we determined whether plasma total tau level could be used as a prognostic marker of cognitive decline over a short 15-month interval using change scores (Table 3). There were significant interactions between plasma total tau level and diagnostic group, so the analyses were stratified by cognitive status. There were no associations between plasma total tau level and cognitive decline among the CN participants. Among participants with MCI, each log-unit increase in plasma total tau levels was associated with a greater 15-month decline for both visuospatial ability (b [SE] = −0.50 [0.15], P < .001) and global (b = −0.27 [0.10], P = .009) cognition. As in the previous analyses, elevated brain Aβ was not a confounder or effect modifier.
Table 3. Association Between Baseline Plasma Tau Level and 15-Month Change in Global and Domain-Specific Cognitiona,b.
| Outcome (z Score) | Total (N = 452) |
CN (n = 330) |
MCI (n = 122) |
|||
|---|---|---|---|---|---|---|
| b (SE) | P Value | b (SE) | P Value | b (SE) | P Value | |
| Memory | −0.05 (0.07) | .47 | 0.01 (0.08) | .89 | −0.18 (0.13) | .17 |
| Language | −0.05 (0.06) | .46 | −0.09 (0.08) | .28 | −0.01 (0.11) | .96 |
| Attention | −0.08 (0.08) | .33 | −0.02 (0.08) | .85 | −0.11 (0.16) | .48 |
| Visuospatial ability | −0.06 (0.07) | .44 | 0.13 (0.08) | .13 | −0.50 (0.15) | <.001 |
| Global | −0.06 (0.05) | .20 | 0.02 (0.06) | .67 | −0.27 (0.10) | .009 |
Abbreviations: APOE, apolipoprotein E; CN, cognitively normal; MCI, mild cognitive impairment.
Models adjusted for age, sex, years of education, presence of an APOE ɛ4 allele, and baseline cognition.
The numbers are slightly less than those of the previous analyses because 6 individuals did not have a 15-month follow-up evaluation.
Discussion
This study examined the association between plasma total tau level, cognitive decline, and risk of MCI and dementia. Higher levels of plasma total tau were associated with an increased risk of MCI and with global- and domain-specific cognitive decline. Simulating a short 15-month trial, higher plasma total tau levels were associated only with declines in global cognition and visuospatial abilities among participants with MCI. In all analyses, the association between plasma total tau levels and all outcomes was independent of elevated brain Aβ.
The search for a blood-based biomarker with utility for AD has been confounded by many studies showing initial interesting findings but very little replication. The results of previous studies across 4 cohorts and 2 independent laboratories examining the diagnostic utility of plasma total tau level for MCI or AD using the Simoa HD-1 hTau assay have been consistent. In all studies, participants with MCI or AD had higher plasma total tau levels compared with CN participants. However, there was considerable overlap between groups and no significant differences between CN individuals and those with MCI. Thus, the plasma total tau level has limited utility as a diagnostic marker.
Two studies examined the use of the plasma total tau level as a prognostic marker for cognitive decline and dementia. In the first study of patients with MCI, there was no significant difference in plasma total tau levels between those who progressed to AD dementia and those who remained as MCI and did not convert to dementia over a mean follow-up of 8 years. In contrast, a recent study using ADNI data reported that elevated plasma total tau levels were associated with greater declines in measures of global cognition over 4 years. There was a significant interaction between plasma total tau levels and diagnostic group such that the plasma total tau level was only associated with accelerated cognitive decline among participants with aMCI and AD dementia but not CN participants.
In the present study, higher plasma total tau levels were associated with an increased risk of MCI. There was also an increased risk of dementia, but the results were not significant. It is likely that our power was limited since we only had 20 cases of MCI that progressed to AD. Indeed, when we examined cognitive decline, higher plasma total tau levels were associated with global and domain-specific cognitive decline among both CN participants and those with MCI.
In contrast to the ADNI results, the only group interaction that we found was for visuospatial ability such that the plasma total tau level was only associated with decline among participants with MCI. It is possible that this association was spurious. However, when we restricted the follow-up time to 15 months to simulate a short clinical trial, elevated plasma total tau levels were specifically associated with declines in global cognition and visuospatial ability among participants with MCI but not among CN individuals. These results may suggest that plasma total tau levels could help to identify participants in whom MCI will progress fastest. The 2 previous studies did not examine plasma total tau levels and short-term cognitive changes, so our proposed explanation is only a hypothesis, and additional studies are needed to confirm this observation.
Although the present MCSA results are similar to the ADNI results, there are a few methodologic differences that could account for some of the discrepancies described above. First, ADNI enrolls participants with aMCI, while the MCSA includes all participants with MCI. Restricting some of our analyses to individuals with aMCI, however, did not alter the results. Second, the only MCSA enrollment exclusionary criterion is a terminal illness. ADNI has some restrictions regarding vascular and other diseases. If the plasma total tau level is a nonspecific marker of cognitive decline (discussed below) and the CN MCSA participants have more comorbid diseases and pathologic conditions, this could explain why the association among CN participants was observed in the MCSA but not in ADNI. However, participants were initially oversampled on elevated brain Aβ to cross-sectionally examine plasma total tau level differences by both cognitive diagnosis and elevated brain Aβ, as previously reported.
Adjusting for elevated brain Aβ did not attenuate the association between plasma total tau levels and cognition. There were also no interactions between plasma total tau level and elevated brain Aβ for any outcome. These findings suggest that the association between plasma total tau levels and cognitive decline is independent of elevated brain Aβ and that plasma total tau level could be a useful prognostic marker for nonspecific cognitive decline, regardless of etiology. This finding is not surprising given that cerebrospinal fluid total tau levels appear to reflect the intensity of neuronal degeneration in AD at a specific point in time, and thus is less specific for AD, while phospho-tau appears to be more specific to AD-type tau pathology. Future longitudinal studies will need to assess the association between plasma total tau level and other brain changes and pathologies. Furthermore, the development and validation of a plasma phospho-tau method might prove to be more specific to AD.
Strengths and Limitations
Strengths of the study include the community-based population and the large number of CN individuals. There are also limitations. First, the plasma sample set was used for 2 separate studies to conserve samples and, thus, underwent at least 1 freeze-thaw cycle before analysis. Second, the study did not include participants with AD dementia. As a result, we were not able to determine whether the plasma total tau level was associated with cognitive decline among individuals with AD or other dementia types. Lastly, we did not adjust for other brain pathologies that could attenuate the association. Future research is needed to discern the association between other brain abnormalities, including brain tau deposition measured via tau-PET, on plasma total tau levels.
Conclusions
Plasma total tau level may be a useful prognostic risk factor or biomarker for cognitive decline, but is not an AD-specific biomarker. Given some of the discrepancies between the 3 studies examining plasma total tau levels and longitudinal cognitive changes, we cannot currently suggest that patients be stratified or selected on plasma total tau levels. Additional studies using other samples and specifically testing short vs long follow-up are needed before this question can be answered. Longitudinal studies examining serial measures of plasma total tau levels, across both the AD cognitive spectrum and in other neurodegenerative diseases, are also needed to determine how to best utilize plasma total tau level as a risk factor or biomarker for cognitive decline.
References
- 1.Zetterberg H, Wilson D, Andreasson U, et al. . Plasma tau levels in Alzheimer’s disease. Alzheimers Res Ther. 2013;5(2):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chiu MJ, Yang SY, Horng HE, et al. . Combined plasma biomarkers for diagnosing mild cognition impairment and Alzheimer’s disease. ACS Chem Neurosci. 2013;4(12):1530-1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang T, Xiao S, Liu Y, et al. . The efficacy of plasma biomarkers in early diagnosis of Alzheimer’s disease. Int J Geriatr Psychiatry. 2014;29(7):713-719. [DOI] [PubMed] [Google Scholar]
- 4.Chiu MJ, Chen YF, Chen TF, et al. . Plasma tau as a window to the brain-negative associations with brain volume and memory function in mild cognitive impairment and early Alzheimer’s disease. Hum Brain Mapp. 2014;35(7):3132-3142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dage JL, Wennberg AM, Airey DC, et al. . Levels of tau protein in plasma are associated with neurodegeneration and cognitive function in a population-based elderly cohort. Alzheimers Dement. 2016;12(12):1226-1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mattsson N, Zetterberg H, Janelidze S, et al. ; ADNI Investigators . Plasma tau in Alzheimer disease. Neurology. 2016;87(17):1827-1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roberts RO, Geda YE, Knopman DS, et al. . The Mayo Clinic Study of Aging: design and sampling, participation, baseline measures and sample characteristics. Neuroepidemiology. 2008;30(1):58-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kokmen E, Smith GE, Petersen RC, Tangalos E, Ivnik RC. The Short Test of Mental Status: correlations with standardized psychometric testing. Arch Neurol. 1991;48(7):725-728. [DOI] [PubMed] [Google Scholar]
- 9.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43(11):2412-2414. [DOI] [PubMed] [Google Scholar]
- 10.Rey A. L’examen Clinique en Psychologie. Paris: Presses Universitaires de France; 1964. [Google Scholar]
- 11.Wechsler D. Manual for the Wechsler Memory Scale–Revised. San Antonio, TX: The Psychological Corporation; 1987. [Google Scholar]
- 12.Kaplan E, Goodglass H, Weintraub S. The Boston Naming Test. Philadelphia, PA: Lea & Febiger; 1983. [Google Scholar]
- 13.Strauss E, Sherman EMS, Spreen O. A Compendium of Neuropsychological Tests: Administration, Norms, and Commentary. 3rd ed New York, NY: Oxford University Press; 2006. [Google Scholar]
- 14.Reitan R. Validity of the Trail Making Test as an indicator of organic brain damage. Percept Mot Skills. 1958;8:271-276. [Google Scholar]
- 15.Wechsler D. Wechsler Adult Intelligence Scale-Revised. San Antonio, TX: Psychological Corporation; 1981. [Google Scholar]
- 16.Ivnik RJ, Malec JF, Smith GE, et al. . Mayo’s Older Americans Normative Studies: WAIS-R norms for ages 56 to 97. Clin Neuropsychol. 1992;6(suppl):1-30. [Google Scholar]
- 17.Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256(3):183-194. [DOI] [PubMed] [Google Scholar]
- 18.American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders. 4th ed Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 19.McKhann GM, Knopman DS, Chertkow H, et al. . The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging–Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):263-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klunk WE, Engler H, Nordberg A, et al. . Imaging brain amyloid in Alzheimer’s disease with Pittsburgh Compound-B. Ann Neurol. 2004;55(3):306-319. [DOI] [PubMed] [Google Scholar]
- 21.Senjem ML, Gunter JL, Shiung MM, Petersen RC, Jack CR Jr. Comparison of different methodological implementations of voxel-based morphometry in neurodegenerative disease. Neuroimage. 2005;26(2):600-608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jack CR Jr, Knopman DS, Weigand SD, et al. . An operational approach to National Institute on Aging–Alzheimer’s Association criteria for preclinical Alzheimer disease. Ann Neurol. 2012;71(6):765-775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murray ME, Lowe VJ, Graff-Radford NR, et al. . Clinicopathologic and 11C-Pittsburgh compound B implications of Thal amyloid phase across the Alzheimer’s disease spectrum. Brain. 2015;138(pt 5):1370-1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Olsson B, Lautner R, Andreasson U, et al. . CSF and blood biomarkers for the diagnosis of Alzheimer’s disease: a systematic review and meta-analysis. Lancet Neurol. 2016;15(7):673-684. [DOI] [PubMed] [Google Scholar]
- 25.Blennow K, Hampel H. CSF markers for incipient Alzheimer’s disease. Lancet Neurol. 2003;2(10):605-613. [DOI] [PubMed] [Google Scholar]