Key Points
Question
What is the association between plasma phospho-tau and total-tau levels and traumatic brain injury presence, severity, type of pathoanatomic lesions, and patient outcome?
Findings
In a cohort study, plasma samples from the TRACK-TBI pilot study were collected at a single time point from 196 patients with acute traumatic brain injury and 21 patients with severe traumatic brain injury admitted to receive inpatient rehabilitation. Plasma phospho-tau levels and phospho tau–total tau ratio during the acute phase and chronic traumatic brain injury were superior to total-tau levels for discriminating the severity and status of neurotrauma patients from healthy controls.
Meaning
Plasma phospho-tau levels and phospho tau–total tau ratio might be useful diagnostic and prognostic biomarkers for both acute and chronic traumatic brain injury.
Abstract
Importance
Annually in the United States, at least 3.5 million people seek medical attention for traumatic brain injury (TBI). The development of therapies for TBI is limited by the absence of diagnostic and prognostic biomarkers. Microtubule-associated protein tau is an axonal phosphoprotein. To date, the presence of the hypophosphorylated tau protein (P-tau) in plasma from patients with acute TBI and chronic TBI has not been investigated.
Objective
To examine the associations between plasma P-tau and total-tau (T-tau) levels and injury presence, severity, type of pathoanatomic lesion (neuroimaging), and patient outcomes in acute and chronic TBI.
Design, Setting, and Participants
In the TRACK-TBI Pilot study, plasma was collected at a single time point from 196 patients with acute TBI admitted to 3 level I trauma centers (<24 hours after injury) and 21 patients with TBI admitted to inpatient rehabilitation units (mean [SD], 176.4 [44.5] days after injury). Control samples were purchased from a commercial vendor. The TRACK-TBI Pilot study was conducted from April 1, 2010, to June 30, 2012. Data analysis for the current investigation was performed from August 1, 2015, to March 13, 2017.
Main Outcomes and Measures
Plasma samples were assayed for P-tau (using an antibody that specifically recognizes phosphothreonine-231) and T-tau using ultra-high sensitivity laser-based immunoassay multi-arrayed fiberoptics conjugated with rolling circle amplification.
Results
In the 217 patients with TBI, 161 (74.2%) were men; mean (SD) age was 42.5 (18.1) years. The P-tau and T-tau levels and P-tau–T-tau ratio in patients with acute TBI were higher than those in healthy controls. Receiver operating characteristic analysis for the 3 tau indices demonstrated accuracy with area under the curve (AUC) of 1.000, 0.916, and 1.000, respectively, for discriminating mild TBI (Glasgow Coma Scale [GCS] score, 13-15, n = 162) from healthy controls. The P-tau level and P-tau–T-tau ratio were higher in individuals with more severe TBI (GCS, ≤12 vs 13-15). The P-tau level and P-tau–T-tau ratio outperformed the T-tau level in distinguishing cranial computed tomography–positive from -negative cases (AUC = 0.921, 0.923, and 0.646, respectively). Acute P-tau levels and P-tau–T-tau ratio weakly distinguished patients with TBI who had good outcomes (Glasgow Outcome Scale–Extended GOS-E, 7-8) (AUC = 0.663 and 0.658, respectively) and identified those with poor outcomes (GOS-E, ≤4 vs >4) (AUC = 0.771 and 0.777, respectively). Plasma samples from patients with chronic TBI also showed elevated P-tau levels and a P-tau–T-tau ratio significantly higher than that of healthy controls, with both P-tau indices strongly discriminating patients with chronic TBI from healthy controls (AUC = 1.000 and 0.963, respectively).
Conclusions and Relevance
Plasma P-tau levels and P-tau–T-tau ratio outperformed T-tau level as diagnostic and prognostic biomarkers for acute TBI. Compared with T-tau levels alone, P-tau levels and P-tau–T-tau ratios show more robust and sustained elevations among patients with chronic TBI.
This cohort study evaluates the use of phospho-tau levels, total-tau levels, and the phospho tau–total tau ratio as diagnostic and prognostic biomarkers in patients with traumatic brain injury.
Introduction
Traumatic brain injury (TBI), considered as an event and/or a disease, initiates a complex pathophysiologic central nervous system cascade with acute effects and secondary insults that may lead to chronic functional, neurocognitive, and neuropsychiatric deficits. Traumatic brain injury is classified according to its degree of severity (mild, moderate, and severe). In the United States, there are more than 3.5 million emergency department visits for TBI and more than 280 000 patients are hospitalized annually with TBI, with most of these classified as mild TBI (mTBI). There are also many more individuals with mTBI who never seek medical attention. More than 300 000 armed service members sustained TBI during combat and training from 2000 to 2014. Approximately half of patients with TBI in the United States incur at least some short-term disability. Traumatic brain injury is associated with an increased risk of neurodegenerative disorders, such as Alzheimer disease, years or decades after injury. In addition, repetitive mTBI is a risk factor for a neurodegenerative condition called chronic traumatic encephalopathy (CTE). Chronic traumatic encephalopathy, which can be confirmed only by postmortem neuropathologic examination, is defined by the abnormal accumulation of hyperphosphorylated tau protein (P-tau) in neurons, astroglia, and cell processes distributed around small blood vessels at the depth of cortical sulci in an irregular pattern.
Tau protein is involved in regulating axonal microtubule assembly and disassembly. Posttranslational modifications of tau seem to precede brain tauopathy. These modifications include (1) hyperphosphorylation by multiple kinases, (2) proteolysis by calpain and caspase, and (3) oxidative modifications. The abnormal P-tau protein forms paired helical filaments and progressively aggregates to form the main component of neurofibrillary tangles—a hallmark of tauopathy, including Alzheimer disease. The major pathologic P-tau sites include, but are not limited to, threonine (Thr)181, serine (Ser)202, Thr205, Thr231, Ser396, and Ser404. Abnormal accumulation of P-tau deposits in postmortem human brains are found years after repetitive mTBIs have been sustained. Single, simulated blast exposure and closed head injury in mice also result in increased deposition of P-tau. Amyloid β1-42 and/or tau levels are elevated in cerebrospinal fluid samples after severe TBI. The use of a high-sensitivity assay platform (Simoa; Quanterix Corp) has permitted detection of acute and chronic tau elevations following mTBI in ice hockey players with sports-related concussion and military personnel with chronic postconcussive disorder symptoms. In contrast to the present report, those studies did not measure P-tau levels.
We recently used another high-sensitivity assay platform (multi-arrayed fiberoptics conjugated with rolling circle amplification [a-EIMAF]) and detected serum total (T)-tau and P-tau levels in patients with severe TBI, as well as in 1- to 30-day mouse serum after single and/or repetitive mTBI. However, to our knowledge, the profile of P-tau in blood samples in humans with mild and moderate TBI has yet to be defined. Incorporating the 3 tau indices to distinguish abnormal cranial computed tomography (CT) (pathoanatomic lesions) from normal CT findings would aid in the diagnosis and prognosis for acute TBI. Furthermore, to our knowledge, monitoring the 3 tau indices simultaneously in blood samples from patients with chronic TBI has not been done previously. Therefore, we used the a-EIMAF platform to examine whether P-tau and T-tau might be sensitive and specific biomarkers, using plasma from patient cohorts in the completed TRACK-TBI Pilot study.
Methods
TBI Patients and Biosample Collection
Four sites (3 level I trauma centers and 1 rehabilitation center) participated in this study. On arrival, participants were classified along the spectrum of neurotrauma severity (Glasgow Coma Scale [GCS)] score of 3-8, severe; 9-12, moderate; and 13-15, mild) and recruited into the multicenter, prospective TRACK-TBI Pilot study. The TRACK-TBI Pilot study was conducted from April 1, 2010, to June 30, 2012. Data analysis for the current investigation was performed from August 1, 2015, to March 13, 2017.
Study protocols were approved by the institutional review boards of participating centers (acute sites: San Francisco General Hospital, San Francisco, California; University of Pittsburgh Medical Center, Pittsburgh, Pennsylvania; and University Medical Center Brackenridge, Austin, Texas; rehabilitation site: Mount Sinai Rehabilitation Center, New York). Participants or their legally authorized representative provided written informed consent.
Patients with acute TBI must have presented within 24 hours of injury to the emergency department and have had a noncontrast head CT scan performed based on American College of Emergency Physicians/Centers for Disease Control and Prevention guidelines; patients with chronic TBI must have had sufficient neurologic impairment to be admitted to inpatient TBI rehabilitation units. Single blood samples from patients with acute TBI across the full spectrum of injury severity (GCS, 3-8 [n = 25]; GCS, 9-12 [n = 8]; and GCS, 13-15 [n = 163]) were collected within 24 hours of injury. Among these patients with acute TBI, 108 (55.1%) had normal head CT (CT−) scans, while 88 (44.9%) showed CT abnormality (CT+) (Table). Single blood samples from 21 patients with chronic TBI were collected during the inpatient rehabilitation stay (Table). Sample collection and processing for the TBI and healthy control cohorts were performed identically at all sites (including the commercial vendor for controls) according to the recommendations from the National Institutes of Health–National Institute of Neurological Disorders and Stroke Common Data Elements Biospecimens and Biomarkers Working Group (eMethods in the Supplement provides details). Plasma was extracted after centrifugation of whole blood collected in K2-EDTA blood tubes for 5 minutes at 3000g. In addition, 20 commercially obtained samples (Bioreclamation Inc) from healthy control plasma (K2-EDTA tubes) (n = 20; mean [SD] age, 40.5 [14.2] years; 70% male), which were sex- and age-matched with the acute (n = 196; age, 42.1 [18.1] years; 73% male) and chronic (n = 21; age, 44.4 [20.5] years; 76% male) TBI samples, were assayed. There were no significant differences in age or sex across the patient and control cohorts (Table). In the 217 patients with TBI, 161 (74.2%) were men; mean age was 42.5 (18.1) years.
Table. Demographics and Injury Characteristics of TRACK-TBI Pilot Subjects.
| Characteristic | Control (n = 20) |
TBI | |
|---|---|---|---|
| Acute (n = 196) |
Chronic (n = 21) |
||
| Age, mean (SD) [range], y | 40.5 (14.2) [22-61] | 42.4 (17.8) [16-86] | 44.4 (20.5) [19-81] |
| Sex, No. (%) | |||
| Male | 14 (70.0) | 145 (74.0) | 16 (76.2) |
| Female | 6 (30.0) | 51 (26.0) | 5 (23.8) |
| Race, No. (%) | |||
| White | 15 (75.0) | 164 (83.7) | 19 (90.5) |
| African American or African | 3 (15.0) | 18 (9.2) | 1 (4.8) |
| Asian | 1 (5.0) | 4 (2.0) | 1 (4.8) |
| Hawaiian or Pacific Islander | 0 | 1 (0.5) | 0 |
| American Indian or Alaskan Native | 0 | 1 (0.5) | 0 |
| ≥1 Race | 1 (5.0) | 8 (4.1) | 0 |
| Ethnicity, No. (%) | |||
| Hispanic | 3 (15.0) | 27 (13.8) | 5 (23.8) |
| Non-Hispanic | 17 (85.0) | 167 (85.2) | 12 (57.1) |
| Unknown | 0 | 2 (1.0) | 4 (19.0) |
| Highest academic degree, No. (%) | |||
| Below high school | 26 (13.3) | 1 (4.8) | |
| High school or GED | 108 (55.1) | 12 (57.1) | |
| College | 32 (16.3) | 5 (23.8) | |
| Graduate school | 18 (9.2) | 2 (10.0) | |
| Unknown | 20 (100) | 12 (6.1) | 1 (4.8) |
| Employment, No. (%) | |||
| Full-time | 78 (39.8) | 11 (52.4) | |
| Part-time | 33 (16.8) | 3 (14.3) | |
| Retired, student, or disabled | 32 (16.3) | 6 (28.6) | |
| Unemployed | 42 (21.4) | 1 (4.8) | |
| Unknown | 20 (100) | 11 (5.6) | 0 |
| Marriage status, No. (%) | |||
| Single, never married | 94 (48.0) | 11 (52.4) | |
| Married | 75 (38.3) | 7 (33.3) | |
| Separated or divorced | 14 (7.1) | 0 | |
| Widowed | 6 (3.1) | 3 (14.3) | |
| Unknown | 20 (100) | 7 (3.6) | 0 |
| GCS score, No. (%) | NA | NAa | |
| 3-8 | 12 (6.1) | ||
| 9-12 | 6 (3.1) | ||
| 13-15 | 160 (81.6) | ||
| Unknown | 18 (9.2) | ||
| Admission cranial CT, No. (%)b | NA | ||
| Normal | 108 (55.1) | 0 | |
| Abnormal | 88 (44.9) | 11 (52.4) | |
| Unknown | 0 | 10 (47.6) | |
| Negative | 108 (55.1) | NA | |
| Extra-axial only | 22 (11.2) | 5 (23.8) | |
| Intra-axial only | 24 (12.2) | 3 (14.3) | |
| Extra-axial and intra-axial | 42 (21.4) | 3 (14.3) | |
| Unknown | 0 | 10 (47.6) | |
| Marshall CT scale, No. (%)b | NA | ||
| 1 | 96 (49.0) | 0 | |
| 2 | 78 (39.8) | 2 (20.0) | |
| 3 | 8 (4.1) | 3 (30.0) | |
| 4 | 1 (0.5) | 0 | |
| 5 | 12 (6.1) | 5 (50.0) | |
| 6 | 1 (0.5) | 0 | |
| Outcome [6 mo], No. (%)c | NA | ||
| GOS-E | |||
| 1 | 7 (5.2) | 0 | |
| 2 | 1 (0.7) | 0 | |
| 3 | 10 (7.5) | 1 (5.9) | |
| 4 | 2 (1.5) | 5 (29.4) | |
| 5 | 13 (9.7) | 5 (29.4) | |
| 6 | 21 (15.7) | 1 (5.9) | |
| 7 | 38 (28.4) | 1 (5.9) | |
| 8 | 43 (32.1) | 4 (23.5) | |
| Sample collection time postinjury, mean (SD) [range] | NA | 10.6 (6.4) [0.5-23.4], h | 176.4 (44.5) [16.0-249.6], d |
Abbreviations: CT, computed tomographic; GCS, Glasgow Coma Scale; GED, general educational development (GED Testing Service); GOS-E, Glasgow Outcome Scale–Extended; TBI, traumatic brain injury.
GCS data were unavailable for all patients with chronic TBI.
Admission cranial CT data were not available for 11 of 21 patients with
chronic TBI.
Data were available for 137 patients with acute TBI and 17 of those with chronic TBI.
Plasma P-tau and T-tau Analysis by a-EIMAF
T-tau and P-tau a-EIMAF assay performance, lower limit of detection, lower limit of quantitation, and linearity range of standard curves for calculating T-tau and P-tau concentration are shown in eFigures 1 and 2 in the Supplement, respectively (eMethods in the Supplement).
Statistical Analysis
The T-tau and P-tau data for the acute and chronic TBI cohorts and controls were not normally distributed; thus, results are presented as median and interquartile range. Median differences for more than 2 groups were compared with the Kruskal-Wallis test. For 2-group comparisons, the nonparametric Mann-Whitney test was performed. The ability of T-tau level, P-tau level, and P-tau–T-tau ratio to distinguish patients with acute TBI vs controls, mild (GCS, 13-15) vs moderate/severe (GCS, 3-12) TBI, normal vs abnormal cranial CT, 6-month outcomes on Glasgow Outcome Scale–Extended (GOS-E), and chronic TBI vs controls were assessed using the receiver operating characteristic (ROC) curve and associated area under the curve (AUC). Statistical analyses were performed using Prism, version 6 (GraphPad Software). Statistical significance was defined by an unpaired, 2-tailed P value ≤.05.
Results
Plasma P-tau levels, T-tau levels, and P-tau–T-tau ratios were determined for all patients with acute and chronic TBI. We have previously described detection of T-tau and P-tau in cerebrospinal fluid (EIMAF format) and serum (a-EIMAF format) from patients with severe TBI during the first 5 days after injury. Herein, we expand these findings by using plasma samples from 2 cohorts of the TRACK-TBI Pilot study and quantitate concentrations of T-tau and P-tau (eFigure 1 and eFigure 2 in the Supplement).
In controls, we found no significant correlations between any of the 3 tau indices and age. For CT− patients, age had statistically significant Pearson correlation coefficients with P-tau level (r = 0.200, P = .04) and P-tau–T-tau ratio (r = 0.233, P = .02), but not with T-tau level (r = −0.075, P = .45). For CT+ patients, age had a statistically significant Pearson correlation coefficient with P-tau–T-tau ratio (r = 0.219, P = .04), but not for P-tau (r = 0.157, P = .14) or T-tau (r = −0.082, P = .44) levels.
T-tau and P-tau Levels in TBI of Different Severities vs Controls
Plasma values of T-tau were 62.59 fg/mL for healthy controls, 79.21 fg/mL for patients with acute TBI with initial GCS 13 to 15, 84.10 fg/mL for GCS 9 to 12, and 79.21 for GCS 3 to 8 (P < .001 across groups) (Figure 1A, eTable 1 in the Supplement). Median P-tau level was 20.85 fg/mL × 100 for healthy controls, 200.16 fg/mL × 100 for patients with acute TBI with GCS 13 to 15, 307.19 fg/mL × 100 for GCS 9 to 12, and 294.68 fg/mL × 100 for GCS 3 to 8 (P < .001) (Figure 1A, eTable 1 in the Supplement). P-tau–T-tau ratio ( × 10 000) for healthy controls was 30.94, compared with 263.19 for patients with acute TBI with GCS 13 to 15, 348.38 for GCS 9 to12, and 363.02 for GCS 3 to 8 (P < .001) (Figure 1A, eTable 1 in the Supplement). Healthy controls compared with each severity group were significantly different for T-tau level, P-tau level, and P-tau–T-tau ratio (eTable 2 in the Supplement). P-tau values for GCS 13 to 15 vs GCS 9 to 12 and GCS 13 to 15 vs GCS 3 to 8 were significantly different, as were P-tau–T-tau ratios for GCS 13 to 15 vs GCS 3 to 8 (eTable 2 in the Supplement).
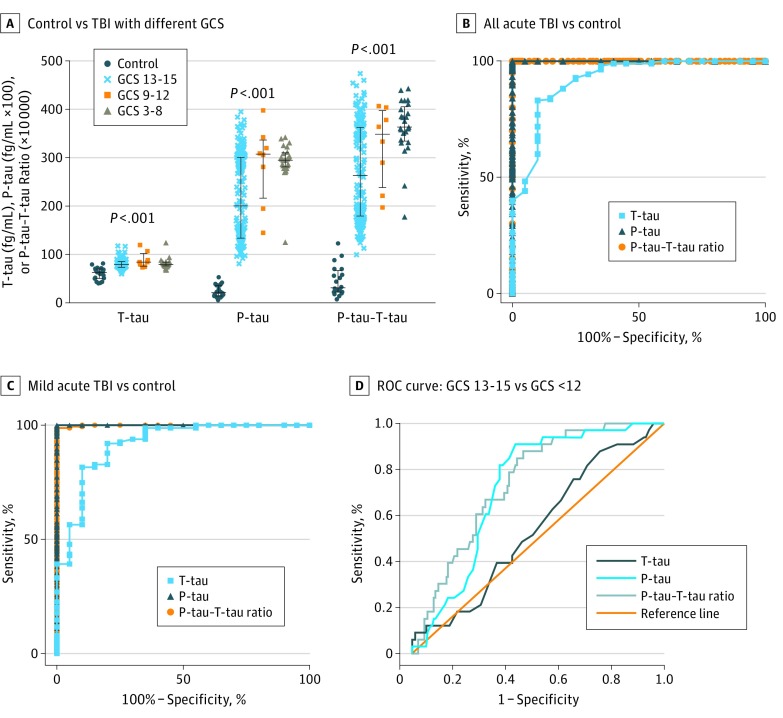
Figure 1. Comparison of Plasma Total-Tau (T-tau) Level, Phospho-Tau (P-tau) Level, and P-tau–T-tau Ratio in Patients With Acute Traumatic Brain Injury (TBI) With Different Severity and Healthy Controls.
A, T-tau level, P-tau level, and P-tau–T-tau ratio in plasma of patients with TBI of different severity (Glasgow Coma Scale [GCS] score: mild, 13-15 [n = 160]; moderate, 9-12 [n = 6]; and severe, 3-8 [n = 12]) vs healthy control plasma (n = 20). Median and first and third quartiles are shown (black bars). Statistical significance was based on Kruskal-Wallis test comparison. Mann-Whitney test multiple group comparison results are given in eTable 2 in the Supplement. B, Receiver operating characteristic (ROC) curve of plasma T-tau level, P-tau level, and P-tau–T-tau ratio for all acute TBI (n = 195) vs control (n = 20) samples. C, ROC of T-tau level, P-tau level, and P-tau–T-tau ratio of all patients with acute mild TBI (n = 162) vs controls (n = 20). D, ROC curve of acute plasma T-tau level, P-tau level, and P-tau–T-tau ratio in distinguishing patients with TBI with GCS scores of 13 to 15 vs those with GCS scores of 12 or lower. Detailed characterization analysis of ROC curves is available in eTables 3 and 4 in the Supplement.
The ROC analyses showed the accuracy of all 3 plasma tau indices in distinguishing all acute TBI cases from healthy controls (Figure 1B). The AUC was 0.919 (P < .001) for T-tau level, 1.000 (P < .001) for P-tau level, and 1.000 (P < .001) for P-tau–T-tau ratio (eTable 3 in the Supplement, all TBI vs controls). All 3 plasma tau indices also showed accuracy in distinguishing mTBI (GCS 13-15) from normal control plasma (Figure 1C), with AUC = 0.916 (P < .001) for T-tau level, 1.000 (P < .001) for P-tau level, and 1.000 (P < .001) for P-tau–T-tau (eTable 3 in the Supplement, mTBI vs controls).
Because our results suggested that P-tau indices could distinguish mTBI (GCS 13-15) from severe and moderate TBI (eTable 2 in the Supplement), we further applied ROC plots (Figure 1D). Plasma P-tau levels and P-tau–T-tau ratio showed fair accuracy for distinguishing mTBI (initial GCS 13-15) in patients with severe/moderate TBI (GCS≤12) (AUC = 0.711 and 0.748, respectively; both P < .001), whereas T-tau showed poor, but significant results (AUC 0.527, P = .04) (Figure 1D; eTable 4 in the Supplement).
Pathoanatomic Lesion Correlation
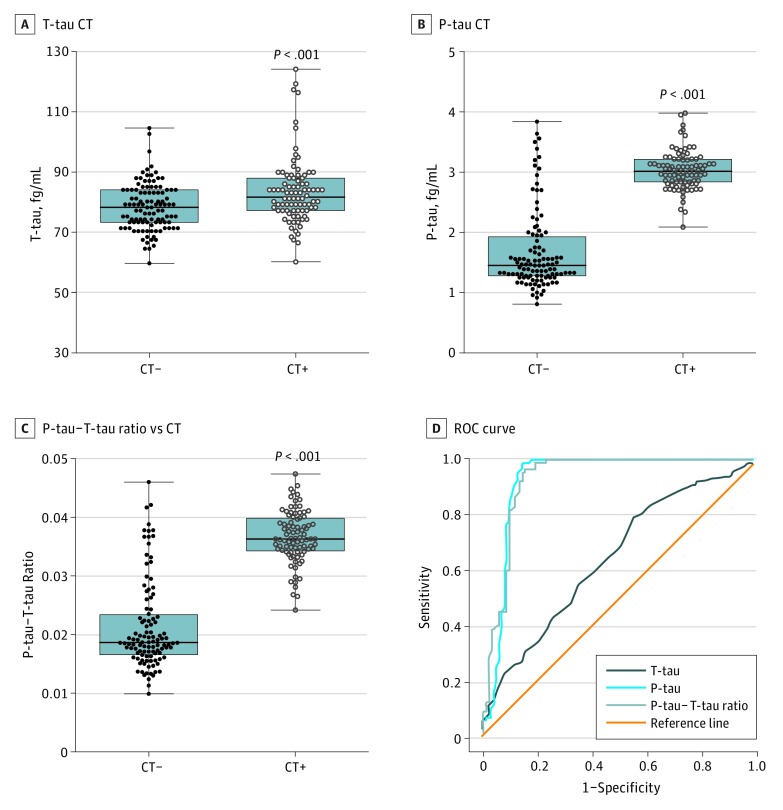
Neuroimaging using CT assessed the extent of the TBI, thereby allowing pathoanatomic classification. We assessed the ability of the 3 plasma tau indices to distinguish CT+ scan patients with TBI from those with normal findings on scans (CT–). As displayed in Figure 2A-C, median T-tau levels were 78.24 fg/mL for CT– and 83.13 fg/mL for CT+ scans (P < .001) (Figure 2A). P-tau levels were 1.45 fg/mL for CT– and 3.02 fg/mL for CT+ scans (P < .001) (Figure 2B), and the P-tau–T-tau ratio had a median of 0.0187 for CT– and 0.0363 for CT+ scans (P < .001) (Figure 2C). We also performed ROC analysis of 3 tau indices in distinguishing CT+ from CT– scans (Figure 2D). Both P-tau level and P-tau–T-tau ratio had excellent accuracy (AUC = 0.921 and 0.923, respectively), while T-tau had poor accuracy (AUC = 0.646) (eTable 5 in the Supplement).
Figure 2. Comparison of Plasma Total-Tau (T-tau) Level, Phospho-Tau (P-tau_ Level, and P-tau–T-tau Ratio in Acute Traumatic Brain Injury With Abnormal Cranial Computed Tomography (CT) (CT+) vs Normal Cranial CT (CT−).
T-tau level (A), P-tau level (B), and P-tau–T-tau ratio (C) for CT− (n = 105) and CT+ (n = 91) patients. Median and first and third quartiles are shown (black bars). The receiver operating characteristic (ROC) curve is for T-tau level, P-tau level, and P-tau–T-tau ratio in distinguishing CT+ vs CT– (D). eTable 5 in the Supplement provides detailed characterization. Error bars indicate SE. P values based on Mann-Whitney test comparison.
We compared the acute TBI tau indices of the mTBI (GCS 13-15) groups that had scans that were either CT+ (n = 61) or CT− (n = 102) with those of healthy controls (n = 20) (eFigure 3 in the Supplement). We identified significant group differences for T-tau level, P-tau level, and P-tau–T-tau ratio. Healthy controls could be distinguished from both CT+ and CT− cases based on all 3 tau indices (eFigure 3 in the Supplement). Furthermore, P-tau levels and P-tau–T-tau ratio could distinguish CT+ vs CT− scans among all patients with mTBI, although T-tau levels could not (eFigure 3 in the Supplement).
Finally, we investigated the correlation between acute TBI plasma tau indices and the Marshall CT scale. Owing to small individual counts, the more severe Marshall CT classifications (3-6) were grouped into a single category similar to previous approaches. Marshall group comparisons showed statistical significance for all 3 tau indices (all P < .001) (eFigure 4 in the Supplement). We also found significant subgroup differences in Marshall CT scores: (1) T-tau level, 1 vs 2 (P < .001) and 1 vs 3 or higher (P < .05); (2) P-tau level, 1 vs 2 (P < .001) and 1 vs 3 or higher (P < .001); and P-tau–T-tau ratio, 1 vs 2 (P < .001) and 1 vs 3 or higher (P < .001) (eFigure 4 in the Supplement).
Outcome Correlation
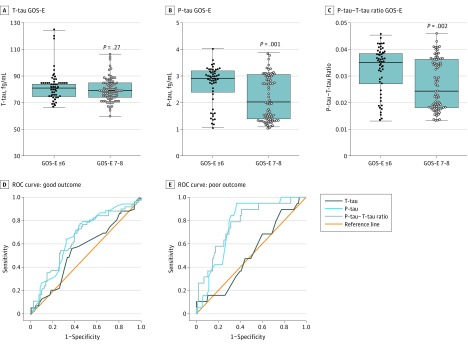
Since P-tau might be related to tauopathy formation, we examined the ability of the plasma tau indices to predict outcome in patients with TBI using the GOS-E. Six-month outcome data were available in 137 patients (69.9%), 134 of whom had available plasma samples (68.3%) (Table). A GOS-E score of 7 to 8 is considered a good outcome, and a GOS-E score of 6 or lower is considered a poor outcome. Figure 3B and C demonstrate that both acute TBI plasma P-tau level and P-tau–T-tau ratio have an inverse association with GOS-E. P-tau level (P = .001) and P-tau–T-tau ratio (P = .002) are lower in patients with TBI with a GOS-E of 7 to 8 vs those with a GOS-E of 6 or lower. In contrast, T-tau did not differ significantly between the 2 outcome groups (P = .27) (Figure 3A). In the good outcome (GOS-E, 7-8) column for P-tau level and P-tau–T-tau ratio, there appear to be 2 clusters (above or below 2 fg/mL P-tau level; and above or below 0.025 P-tau–T-tau ratio, respectively). These upper clusters closely match the respective ranges of P-tau level and P-tau–T-tau ratio for the poor outcome group (GOS-E, ≤6). This finding may suggest a natural clustering of acute post-TBI P-tau levels that are independent of our patient outcome measures (eg, GOS-E). In ROC curves for GOS-E of 7 to 8 (good outcome) vs GOS-E of 6 or lower (Figure 3D), the AUC for P-tau level (0.663) and P-tau–T-tau ratio (0.658) show that they are poor predictors of good outcome (eTable 6 in the Supplement, panel A). T-tau level failed to discriminate between those with good outcome (AUC = 0.552). In ROC curves for GOS-E of 4 or lower (poor outcome) vs GOS-E higher than 4 (Figure 3E), AUC for P-tau level (0.771) and for P-tau–T-tau ratio (0.777) shows that both of these P-tau indices are fair predictors of poor outcome (eTable 6 in the Supplement, panel B). The AUC for T-tau level (0.516) was again not significant.
Figure 3. Plasma Total-Tau (T-tau) Level, Phospho-Tau (P-tau) Level, and P-tau–T-tau Ratio in Acute Traumatic Brain Injury in Predicting Patient Outcomes at 6 Months.
T-tau level (A), P-tau level (B), and P-tau–T-tau ratio, Glascow Outcome Scale-Extended (GOS-E) of 7 to 8 (good outcome) vs GOS-E of 6 or lower (poor outcome). Median and first and third quartiles are shown (black bars). Statistical significance was based on Mann-Whitney test comparison. Receiver operating characteristic (ROC) curves for T-tau level, P-tau level, and P-tau–T-tau ratio in distinguishing ROC curves for good outcome GOS-E of 7 to 8 vs GOS-E of 6 or lower (D), and ROC curves for poor outcome GOS-E of 4 or lower vs GOS-E of 5 to 8 (E). eTable 6 in the Supplement provides detailed analysis.
Characterization of Plasma Tau Indices in Patients With Chronic TBI
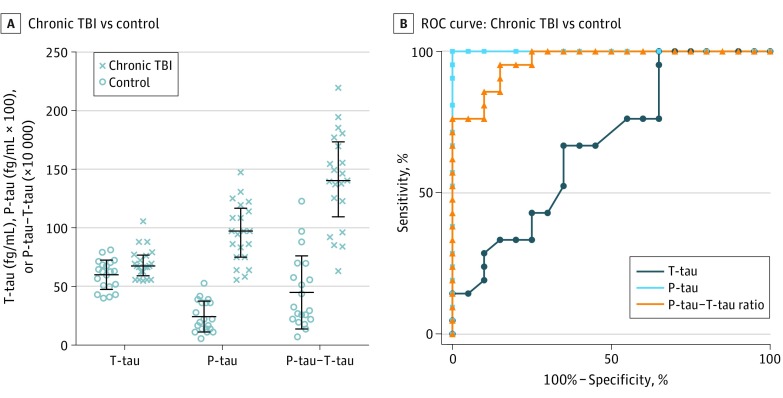
For the chronic TBI cohort, the mean (SD) postinjury time at enrollment was 176.4 (44.5) days (range, 16.0-249.6 days). The plasma T-tau level, P-tau level, and P-tau–T-tau ratio were compared with those of healthy controls (n = 20) (Figure 4A). Median plasma T-tau level for chronic TBI was 69.48 vs 62.59 fg/mL for controls (P = .02); P-tau level was 97.30 fg/mL × 100 for chronic TBI vs 20.85 fg/mL × 100 for controls (P < .001); and the P-tau–T-tau ratio ( × 10 000) was 140.33 for chronic TBI vs 30.95 for controls (P < .001).
Figure 4. Comparison of Plasma Phospho-Tau (P-tau) Level, Total-Tau (T-tau) Level, and P-tau–T-tau Ratio in Patients With Chronic Traumatic Brain Injury (TBI) vs Healthy Controls.
A, T-tau level, P-tau, and P-tau–T-tau ratio. Median and first and third quartiles are shown (black bars). Statistical significance was based on Mann-Whitney test comparison; for the 2 subgroups (control vs chronic TBI), T-tau level (P = .02), P-tau level (P < .001), and P-tau–T-tau ratio (P < .001) are significantly different. B, Receiver operating characteristic (ROC) curve of plasma T-tau level, P-tau level, and P-tau–T-tau ratio for patients with chronic TBI vs healthy controls. eTable 7 in the Supplement provides detailed ROC analysis.
Finally, we performed ROC curves of plasma T-tau level, P-tau level, and P-tau–T-tau ratio from patients with chronic TBI vs healthy controls (Figure 4B). P-tau level (AUC = 1.000) and P-tau–T-tau ratio (AUC = 0.963) showed accuracy and outperformed T-tau level (AUC = 0.674) (eTable 7 in the Supplement).
Discussion
Biomarkers are emerging diagnostic tools for TBI. Since P-tau is pathologically linked to tauopathy found in Alzheimer disease, CTE, and other neurodegenerative diseases associated with TBI, we examined peripheral T-tau and P-tau levels in the full spectrum of patients with TBI by using TRACK-TBI Pilot cohorts. We demonstrate that plasma P-tau (P-Thr231) level, T-tau level, and P-tau–T-tau ratio are robustly higher in patients with all severities of acute TBI compared with healthy controls (Figure 1A). However, both P-tau indices differed according to TBI severity (Figure 1D). Although all patients with TBI and cranial CT+ scans had higher levels of all 3 tau indices than their CT− counterparts, P-tau levels and P-tau–T-tau ratio were much higher and predicted CT scan abnormality more accurately than did T-tau levels (Figure 2). In patients with acute TBI, P-tau levels and P-tau–T-tau ratio outperformed T-tau level in identifying and possibly predicting good outcomes (Figure 3). Taken together, to our knowledge, this is the first report showing that acute plasma P-tau levels and the P-tau–T-tau ratio are superior diagnostic biomarkers for TBI than T-tau levels. Because of the likely differences in T-tau antigen standards and antibodies used in the different assay formats, making direct comparisons between the absolute plasma T-tau values from the acute mild and chronic TBI cohorts reported in our study with the previous reports on concussed ice-hockey players and military personnel is not possible.
Recent studies have examined the use of blood biomarkers, which included ubiquitin C-terminal hydrolase L1 (UCH-L1) and glial fibrillary acidic protein (GFAP), for the diagnosis and prognosis of TBI. Each of these biomarkers was effective alone and, in 1 study, more specific and sensitive when combined in diagnosing TBI. However, a potential limitation of these studies is the short half-lives of UCH-L1 (<12 hours) and GFAP (<2 days) combined with the sensitivity limits of currently available assays. P-tau and T-tau levels in cerebrospinal fluid have diagnostic value for identifying individuals with Alzheimer disease. However, detection of these proteins in blood have been challenging owing to their low levels, which may now be overcome by the availability of new high-sensitivity assay platforms (single molecule array and a-EIMAF).
We also found that plasma P-tau levels and P-tau–T-tau ratio in patients with chronic TBI are significantly higher than in healthy controls (Figure 4). Chronic traumatic encephalopathy was first described in 1928 by Martland to describe the clinical tremors and impaired cognition that affected some boxers. Being “punch drunk” was later called “dementia pugilistica” and then refined as CTE. Patients with CTE show progressively worsening dementia, poor executive function, confusion, anxiety, depression, and irritability that appear years after the initial trauma events. Omalu and colleagues reported neuropathologic lesions consistent with CTE in the brain of an American football player and a professional wrestler. Although CTE is associated with a history of repetitive mTBI or concussion, it is possible that a single but significant TBI event could also lead to the initiation of a tau phosphorylation cascade similar to those observed in repetitive mTBI. Consistent with this, our study showed elevations of plasma P-tau and T-tau levels in both the acute and the chronic phases of TBI. If, in fact, the tau phosphorylation cascade extends from the acute post-TBI phase into the chronic phase, it is possible that, in vulnerable individuals or those who experience successive TBI events, there may be sufficient cumulative tauopathy to initiate and/or accelerate the development of CTE.
For detection of tauopathy in particularly vulnerable TBI cohorts (athletes and blast-exposed military), postmortem neuropathologic examination along with blood T-tau and P-tau level quantitation is ideal; however, conducting both is often infeasible. The development of specific tau tracers for positron emission tomographic imaging have enabled the analysis of the presence and intensity of tau pathology in patients suspected of having tauopathies. Thus, future studies correlating tau positron emission tomographic imaging data with the levels of P-tau and P-tau–T-tau ratios may offer a novel path for differential diagnosis, prognosis, and disease progression in patients with TBI.
Limitations
The fact that control samples were not collected at the study sites is a major limitation of the study. These commercially obtained control plasma samples had limited demographic and health status data of donors. Although all control and TBI patient samples collected for this study followed best practice guidelines, it is possible that the reliance on commercial control samples may have affected the comparisons between controls and patients with TBI due to possible differences in sample collection and processing. To address this limitation, we will further validate our T-tau and P-tau results using blood samples collected within 24 hours, at 1 to 2 weeks, and at 6 months after the TBI from approximately 1000 patients with TBI in the current TRACK-TBI study supplemented with 2 groups of controls: friends/family controls (n = 300) and non-TBI orthopedic injury controls (n = 300). Thus far, we were able to successfully contact and recruit a friend or family member for 92% of the case patients. This represented 46 friend controls and 54 family controls.
An additional limitation is that our study focused on a single P-tau epitope (P-Thr231). It will be important to examine other major P-tau epitopes. Follow-up studies should seek to examine the time course of the plasma or serum levels of P-tau vs T-tau in the same patients in a larger longitudinal cohort study.
Conclusions
Acute T-tau and P-tau level elevations occur not only in severe/moderate TBI, but also in patients with mTBI. We also identified the P-tau–T-tau ratio as an excellent biomarker index for TBI. We found that both acute P-tau levels and P-tau–T-tau ratio outperformed T-tau levels in discriminating by severity, CT abnormality, and outcome category. Finally, we report, for what we believe to be the first time, plasma P-tau level and P-tau–T-tau ratio elevations among patients with chronic TBI. Taken together, P-tau levels and P-tau–T-tau ratio might be useful biomarkers for both acute and chronic TBI.
eMethods. Detailed Methodology
eFigure 1. Standard Curve for T-Tau Assay (a-EIMAF)
eFigure 2. Standard Curve for P-Tau (T231) Assay (a-EIMAF)
eFigure 3. Comparison of Plasma P-Tau, T-Tau and P-Tau/T-Tau Ratio in Acute TBI (CT+, CT-) vs Normal Controls
eFigure 4. Comparison of Plasma P-Tau, T-Tau and P-Tau/T-Tau Ratio in Acute TBI With Different Marshall CT Finding Scores
eTable 1. Acute Plasma P-Tau, T-Tau Levels and P-Tau/T-Tau Ratio in TBI Patients From Different GCS Severity Categories and Normal Control
eTable 2. Comparing Acute Plasma P-Tau, T-Tau, P-Tau/T-Tau Ratio in TBI Patients From Different GCS Severity Category and Normal Control by Mann-Whitney U-Test Multiple Comparisons
eTable 3. Acute Plasma P-Tau, T-Tau and P-Tau/T-Tau Ratio ROC Curve Characterization for All TBI vs Control and Mtbi vs Control
eTable 4. Acute Plasma P-Tau, T-Tau and P-Tau/T-Tau Ratio ROC Curve Characterization for GCS 13-15 vs GCS≤12
eTable 5. Acute Plasma P-Tau, T-Tau and P-Tau/T-Tau Ratio ROC Curve Characterization for CT+ vs CT-
eTable 6. Acute Plasma P-Tau, T-Tau and P-Tau/T-Tau Ratio ROC Curve Characterization for Predicting Patient Outcome at 6 mo. (GOS-E)
eTable 7. Plasma P-Tau, T-Tau and P-Tau/T-Tau Ratio ROC Curve Characterization for Chronic TBI Subjects vs Normal Control
References
- 1.DeKosky ST, Kochanek PM, Clark RS, Ciallella JR, Dixon CE. Secondary injury after head trauma: subacute and long-term mechanisms. Semin Clin Neuropsychiatry. 1998;3(3):176-185. [PubMed] [Google Scholar]
- 2.DoD Numbers for Traumatic Brain Injury. http://dvbic.dcoe.mil/dod-worldwide-numbers-tbi. Updated February 17, 2017. Accessed April 6, 2017.
- 3.Centers for Disease Control and Prevention (CDC) CDC grand rounds: reducing severe traumatic brain injury in the United States. MMWR Morb Mortal Wkly Rep. 2013;62(27):549-552. [PMC free article] [PubMed] [Google Scholar]
- 4.Sivanandam TM, Thakur MK. Traumatic brain injury: a risk factor for Alzheimer’s disease. Neurosci Biobehav Rev. 2012;36(5):1376-1381. [DOI] [PubMed] [Google Scholar]
- 5.Omalu BI, Fitzsimmons RP, Hammers J, Bailes J. Chronic traumatic encephalopathy in a professional American wrestler. J Forensic Nurs. 2010;6(3):130-136. [DOI] [PubMed] [Google Scholar]
- 6.McKee AC, Stern RA, Nowinski CJ, et al. The spectrum of disease in chronic traumatic encephalopathy. Brain. 2013;136(Pt 1):43-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith DH, Johnson VE, Stewart W. Chronic neuropathologies of single and repetitive TBI: substrates of dementia? Nat Rev Neurol. 2013;9(4):211-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McKee AC, Cairns NJ, Dickson DW, et al. ; TBI/CTE group . The first NINDS/NIBIB consensus meeting to define neuropathological criteria for the diagnosis of chronic traumatic encephalopathy. Acta Neuropathol. 2016;131(1):75-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spillantini MG, Goedert M; FRS PMGS . Tau pathology and neurodegeneration. Lancet Neurol. 2013;12(6):609-622. [DOI] [PubMed] [Google Scholar]
- 10.Park SY, Tournell C, Sinjoanu RC, Ferreira A. Caspase-3- and calpain-mediated tau cleavage are differentially prevented by estrogen and testosterone in beta-amyloid–treated hippocampal neurons. Neuroscience. 2007;144(1):119-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu MC, Kobeissy F, Zheng W, Zhang Z, Hayes RL, Wang KKW. Dual vulnerability of tau to calpains and caspase-3 proteolysis under neurotoxic and neurodegenerative conditions. ASN Neuro. 2011;3(1):e00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rohn TT, Rissman RA, Davis MC, Kim YE, Cotman CW, Head E. Caspase-9 activation and caspase cleavage of tau in the Alzheimer’s disease brain. Neurobiol Dis. 2002;11(2):341-354. [DOI] [PubMed] [Google Scholar]
- 13.Nonnis S, Cappelletti G, Taverna F, et al. Tau is endogenously nitrated in mouse brain: identification of a tyrosine residue modified in vivo by NO. Neurochem Res. 2008;33(3):518-525. [DOI] [PubMed] [Google Scholar]
- 14.Reyes JF, Reynolds MR, Horowitz PM, et al. A possible link between astrocyte activation and tau nitration in Alzheimer’s disease. Neurobiol Dis. 2008;31(2):198-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Horiguchi T, Uryu K, Giasson BI, et al. Nitration of tau protein is linked to neurodegeneration in tauopathies. Am J Pathol. 2003;163(3):1021-1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smith DH, Chen XH, Nonaka M, et al. Accumulation of amyloid beta and tau and the formation of neurofilament inclusions following diffuse brain injury in the pig. J Neuropathol Exp Neurol. 1999;58(9):982-992. [DOI] [PubMed] [Google Scholar]
- 17.Duan Y, Dong S, Gu F, Hu Y, Zhao Z. Advances in the pathogenesis of Alzheimer’s disease: focusing on tau-mediated neurodegeneration. Transl Neurodegener. 2012;1(1):24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McKee AC, Cantu RC, Nowinski CJ, et al. Chronic traumatic encephalopathy in athletes: progressive tauopathy after repetitive head injury. J Neuropathol Exp Neurol. 2009;68(7):709-735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Omalu BI, Hamilton RL, Kamboh MI, DeKosky ST, Bailes J. Chronic traumatic encephalopathy (CTE) in a National Football League Player: case report and emerging medicolegal practice questions. J Forensic Nurs. 2010;6(1):40-46. [DOI] [PubMed] [Google Scholar]
- 20.Goldstein LE, Fisher AM, Tagge CA, et al. Chronic traumatic encephalopathy in blast-exposed military veterans and a blast neurotrauma mouse model. Sci Transl Med. 2012;4(134):134ra60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang Z, Wang P, Morgan D, et al. Temporal MRI characterization, neurobiochemical and neurobehavioral changes in a mouse repetitive concussive head injury model. Sci Rep. 2015;5:11178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Franz G, Beer R, Kampfl A, et al. Amyloid beta 1-42 and tau in cerebrospinal fluid after severe traumatic brain injury. Neurology. 2003;60(9):1457-1461. [DOI] [PubMed] [Google Scholar]
- 23.Tsitsopoulos PP, Marklund N. Amyloid-β peptides and tau protein as biomarkers in cerebrospinal and interstitial fluid following traumatic brain injury: a review of experimental and clinical studies. Front Neurol. 2013;4:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blennow K, Nellgård B. Amyloid beta 1-42 and tau in cerebrospinal fluid after severe traumatic brain injury. Neurology. 2004;62(1):159-160. [DOI] [PubMed] [Google Scholar]
- 25.Shahim P, Tegner Y, Wilson DH, et al. Blood biomarkers for brain injury in concussed professional ice hockey players. JAMA Neurol. 2014;71(6):684-692. [DOI] [PubMed] [Google Scholar]
- 26.Olivera A, Lejbman N, Jeromin A, et al. Peripheral total tau in military personnel who sustain traumatic brain injuries during deployment. JAMA Neurol. 2015;72(10):1109-1116. [DOI] [PubMed] [Google Scholar]
- 27.Rubenstein R, Chang B, Davies P, Wagner AK, Robertson CS, Wang KKW. A novel, ultrasensitive assay for tau: potential for assessing traumatic brain injury in tissues and biofluids. J Neurotrauma. 2015;32(5):342-352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yue JK, Vassar MJ, Lingsma HF, et al. ; TRACK-TBI Investigators . Transforming research and clinical knowledge in traumatic brain injury pilot: multicenter implementation of the common data elements for traumatic brain injury. J Neurotrauma. 2013;30(22):1831-1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jagoda AS, Bazarian JJ, Bruns JJ Jr, et al. Clinical policy: neuroimaging and decision making in adult mild traumatic brain injury in the acute setting. J Emerg Nurs. 2009;35(2):e5-e40. [DOI] [PubMed] [Google Scholar]
- 30.Wang KKW, Yang Z, Yue JK, et al. Plasma anti-glial fibrillary acidic protein autoantibody levels during the acute and chronic phases of traumatic brain injury: a Transforming Research and Clinical Knowledge in Traumatic Brain Injury pilot study. J Neurotrauma. 2016;33(13):1270-1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Manley GT, Diaz-Arrastia R, Brophy M, et al. Common data elements for traumatic brain injury: recommendations from the biospecimens and biomarkers working group. Arch Phys Med Rehabil. 2010;91(11):1667-1672. [DOI] [PubMed] [Google Scholar]
- 32.Forslund MV, Roe C, Sigurdardottir S, Andelic N. Predicting health-related quality of life 2 years after moderate-to-severe traumatic brain injury. Acta Neurol Scand. 2013;128(4):220-227. [DOI] [PubMed] [Google Scholar]
- 33.Yuan Q, Wu X, Yu J, et al. Effects and clinical characteristics of intracranial pressure monitoring–targeted management for subsets of traumatic brain injury: an observational multicenter study. Crit Care Med. 2015;43(7):1405-1414. [DOI] [PubMed] [Google Scholar]
- 34.Johnson U, Lewén A, Ronne-Engström E, Howells T, Enblad P. Should the neurointensive care management of traumatic brain injury patients be individualized according to autoregulation status and injury subtype? Neurocrit Care. 2014;21(2):259-265. [DOI] [PubMed] [Google Scholar]
- 35.Diaz-Arrastia R, Wang KKW, Papa L, et al. ; TRACK-TBI Investigators . Acute biomarkers of traumatic brain injury: relationship between plasma levels of ubiquitin C-terminal hydrolase-L1 and glial fibrillary acidic protein. J Neurotrauma. 2014;31(1):19-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Papa L, Brophy GM, Welch RD, et al. Time course and diagnostic accuracy of glial and neuronal blood biomarkers GFAP and UCH-L1 in a large cohort of trauma patients with and without mild traumatic brain injury. JAMA Neurol. 2016;73(5):551-560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Welch RD, Ayaz SI, Lewis LM, et al. ability of serum glial fibrillary acidic protein, ubiquitin C-terminal hydrolase-L1, and S100B to differentiate normal and abnormal head computed tomography findings in patients with suspected mild or moderate traumatic brain injury. J Neurotrauma. 2016;33(2):203-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brophy GM, Mondello S, Papa L, et al. Biokinetic analysis of ubiquitin C-terminal hydrolase-L1 (UCH-L1) in severe traumatic brain injury patient biofluids. J Neurotrauma. 2011;28(6):861-870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Diniz BSO, Pinto Júnior JA, Forlenza OV. Do CSF total tau, phosphorylated tau, and β-amyloid 42 help to predict progression of mild cognitive impairment to Alzheimer’s disease? a systematic review and meta-analysis of the literature. World J Biol Psychiatry. 2008;9(3):172-182. [DOI] [PubMed] [Google Scholar]
- 40.Tabaraud F, Leman JPJ, Milor AMA, et al. Alzheimer CSF biomarkers in routine clinical setting. Acta Neurol Scand. 2012;125(6):416-423. [DOI] [PubMed] [Google Scholar]
- 41.Toledo JB, Zetterberg H, van Harten AC, et al. ; Alzheimer’s Disease Neuroimaging Initiative . Alzheimer’s disease cerebrospinal fluid biomarker in cognitively normal subjects. Brain. 2015;138(pt 9):2701-2715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Martland HS. Punch drunk. JAMA. 1928;91(15):1103-1107. [Google Scholar]
- 43.Critchley M. Medical aspects of boxing, particularly from a neurological standpoint. BMJ. 1957;1(5015):357-362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Corsellis JA, Bruton CJ, Freeman-Browne D. The aftermath of boxing. Psychol Med. 1973;3(3):270-303. [DOI] [PubMed] [Google Scholar]
- 45.Stern RA, Daneshvar DH, Baugh CM, et al. Clinical presentation of chronic traumatic encephalopathy. Neurology. 2013;81(13):1122-1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Montenigro PH, Baugh CM, Daneshvar DH, et al. Clinical subtypes of chronic traumatic encephalopathy: literature review and proposed research diagnostic criteria for traumatic encephalopathy syndrome. Alzheimers Res Ther. 2014;6(5):68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dani M, Brooks DJ, Edison P. Tau imaging in neurodegenerative diseases. Eur J Nucl Med Mol Imaging. 2016;43(6):1139-1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.clinicaltrials.gov. Transforming Research and Clinical Knowledge in Traumatic Brain Injury. NCT02119182. https://clinicaltrials.gov/ct2/show/NCT02119182. Accessed June 9, 2017.
- 49.Zhong C, Cockburn M, Cozen W, et al. Evaluating the use of friend or family controls in epidemiologic case-control studies. Cancer Epidemiol. 2017;46(2):9-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. Detailed Methodology
eFigure 1. Standard Curve for T-Tau Assay (a-EIMAF)
eFigure 2. Standard Curve for P-Tau (T231) Assay (a-EIMAF)
eFigure 3. Comparison of Plasma P-Tau, T-Tau and P-Tau/T-Tau Ratio in Acute TBI (CT+, CT-) vs Normal Controls
eFigure 4. Comparison of Plasma P-Tau, T-Tau and P-Tau/T-Tau Ratio in Acute TBI With Different Marshall CT Finding Scores
eTable 1. Acute Plasma P-Tau, T-Tau Levels and P-Tau/T-Tau Ratio in TBI Patients From Different GCS Severity Categories and Normal Control
eTable 2. Comparing Acute Plasma P-Tau, T-Tau, P-Tau/T-Tau Ratio in TBI Patients From Different GCS Severity Category and Normal Control by Mann-Whitney U-Test Multiple Comparisons
eTable 3. Acute Plasma P-Tau, T-Tau and P-Tau/T-Tau Ratio ROC Curve Characterization for All TBI vs Control and Mtbi vs Control
eTable 4. Acute Plasma P-Tau, T-Tau and P-Tau/T-Tau Ratio ROC Curve Characterization for GCS 13-15 vs GCS≤12
eTable 5. Acute Plasma P-Tau, T-Tau and P-Tau/T-Tau Ratio ROC Curve Characterization for CT+ vs CT-
eTable 6. Acute Plasma P-Tau, T-Tau and P-Tau/T-Tau Ratio ROC Curve Characterization for Predicting Patient Outcome at 6 mo. (GOS-E)
eTable 7. Plasma P-Tau, T-Tau and P-Tau/T-Tau Ratio ROC Curve Characterization for Chronic TBI Subjects vs Normal Control