This cross-sectional study investigates the association between brain metabolism and motor scores of patients with early Huntington disease.
Key Points
Question
What is the meaning of cerebral hypermetabolism in Huntington disease?
Findings
This cross-sectional study found that in 60 patients with early Huntington disease, less severe bradykinesia and rigidity were associated with hypermetabolism in the cuneus, lingual gyrus, and crus I/II of the cerebellum. Conversely, hypermetabolism in the inferior parietal and temporal lobules, anterior cingulate, dentate nucleus, and motor regions of the cerebellum was associated with more severe hyperkinetic symptoms (eg, dystonia and chorea).
Meaning
Brain regional hypermetabolism in early Huntington disease can either have a compensatory role or presumably reflect synaptic hyperactivity contributing to motor symptoms, although both roles could be found in the cerebellar associative or motor regions.
Abstract
Importance
Brain hypometabolism is associated with the clinical consequences of the degenerative process, but little is known about regional hypermetabolism, sometimes observed in the brain of patients with clinically manifest Huntington disease (HD). Studying the role of regional hypermetabolism is needed to better understand its interaction with the motor symptoms of the disease.
Objective
To investigate the association between brain hypometabolism and hypermetabolism with motor scores of patients with early HD.
Design, Setting, and Participants
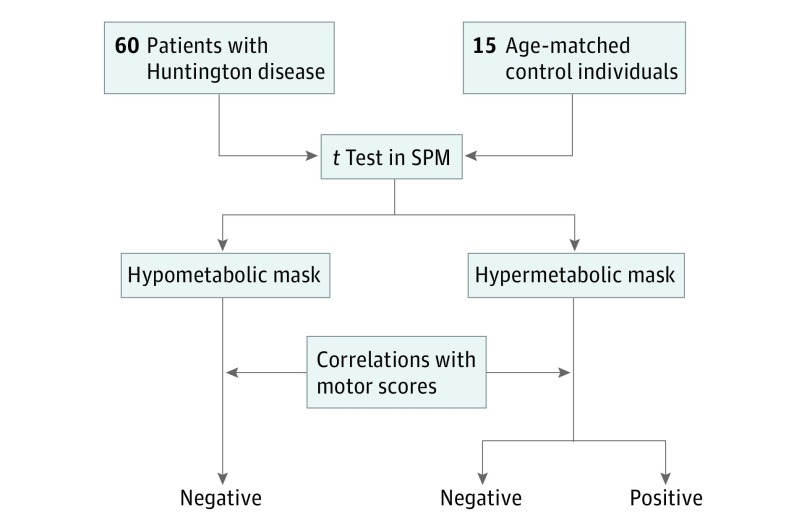
This study started in 2001, and analysis was completed in 2016. Sixty symptomatic patients with HD and 15 healthy age-matched control individuals underwent positron emission tomography to measure cerebral metabolism in this cross-sectional study. They also underwent the Unified Huntington’s Disease Rating Scale motor test, and 2 subscores were extracted: (1) a hyperkinetic score, combining dystonia and chorea, and (2) a hypokinetic score, combining bradykinesia and rigidity.
Main Outcomes and Measures
Statistical parametric mapping software (SPM5) was used to identify all hypo- and hypermetabolic regions in patients with HD relative to control individuals. Correlation analyses (P < .001, uncorrected) between motor subscores and brain metabolic values were performed for regions with significant hypometabolism and hypermetabolism.
Results
Among 60 patients with HD, 22 were women (36.7%), and the mean (SD) age was 44.6 (7.6) years. Of the 15 control individuals, 7 were women (46.7%), and the mean (SD) age was 42.2 (7.3) years. In statistical parametric mapping, striatal hypometabolism was significantly correlated with the severity of all motor scores. Hypermetabolism was negatively correlated only with hypokinetic scores in the cuneus (z score = 3.95, P < .001), the lingual gyrus (z score = 4.31, P < .001), and the crus I/II of the cerebellum (z score = 3.77, P < .001), a region connected to associative cortical areas. More severe motor scores were associated with higher metabolic values in the inferior parietal lobule, anterior cingulate, inferior temporal lobule, the dentate nucleus, and the cerebellar lobules IV/V, VI, and VIII bilaterally corresponding to the motor regions of the cerebellum (z score = 3.96 and 3.42 in right and left sides, respectively; P < .001).
Conclusions and Relevance
Striatal hypometabolism is associated with clinical disease severity. Conversely, hypermetabolism is likely compensatory in regions where it is associated with decreasing motor scores. Hypermetabolism might be detrimental in other structures in which it is associated with more severe motor symptoms. In the cerebellum, both compensatory and detrimental contributions seem to occur. This study helps to better understand the motor clinical relevance of hypermetabolic brain regions in HD.
Introduction
Huntington disease (HD) is an autosomal dominant neurodegenerative disorder associated with severe striatal atrophy and widespread cortical brain changes that occur early during the course of the disease.
Fluorodeoxyglucose [18F] (18F-FDG) positron emission tomography (PET) has consistently revealed reduced striatal and cortical metabolism in patients with HD that correlates in magnitude with global clinical changes. Squitieri et al demonstrated a linear correlation between the rate of decrease in 18F-FDG uptake in parietal, occipital, and cingulate cortices and the worsening of the Unified Huntington’s Disease Rating Scale (UHDRS) motor scores in patients with HD. Brain hypometabolism is thus associated with the clinical consequences of the degenerative process. Moreover, the improvement of striatal and cortical hypometabolism may be associated with clinical improvement induced by cell therapy.
Little is known about regional hypermetabolism sometimes observed in the brain of patients with clinically manifest HD. Focal hypermetabolism has been reported in presymptomatic HD mutation carriers. However, the detailed pattern and meaning of regional hypermetabolism in patients with HD with clinical symptoms are poorly understood. Three explanations are conceivable: (1) regional hypermetabolism reflects neuronal mechanisms to compensate for the consequences of the disease (ie, involvement of alternative circuits); (2) regional hypermetabolism results from the altered global distribution of cerebral glucose consumption, unrelated to the specific HD clinical status; or (3) regional hypermetabolism reflects local synaptic hyperactivity related to typical HD symptoms (eg, abnormal overflow activation associated with abnormal movements).
We analyzed the distribution of metabolic changes in a cohort of 60 patients at an early stage of the disease to better understand the pathophysiology of regional hypermetabolism in patients with clinically manifest HD. We evaluated the relation of these metabolic changes to hyper- and hypokinetic motor subscores derived from the UHDRS. We identified regions with a probable compensatory role and regions where hypermetabolism presumably reflects synaptic hyperactivity that contributes to motor symptoms.
Methods
Participants
Sixty symptomatic patients with genetically confirmed HD participated in this cross-sectional study. All patients were included either in cell (54 patients) (Multicentric Intracerebral Grafting in Huntington Disease, ClinicalTrials.gov NCT00190450) or gene (6 patients) therapy programs, but only baseline evaluations were analyzed here. The mean (SD) age of the patients was 44.6 (7.6) years (range, 28-59 years), and the mean disease duration ranged between 2 months and 16 years (mean [SD], 3.9 [2.8] years). The patients were early in the course of the disease with a mean Total Functional Capacity scale score of higher than 10 at inclusion (mean [SD], 10.7 [1.4]; range, 6-13). The mean (SD) CAG repeat length and Dementia Rating Scale score were 45.2 (7.6) and 129.7 (7.2), respectively. Clinical evaluation was performed using the UHDRS. The Total Motor Score can range from 0 to 124, with higher values indicating greater impairment. Two subscores were extracted: the hyperkinetic score as a combination of dystonia and chorea (sum of items 11 A-E and 12 A-G; range, 0-48) and the hypokinetic score as a combination of bradykinesia and rigidity (sum of items 6 A-B, 7 A-B, 9 A-B, and 10; range, 0-28). The mean (SD) total motor UHDRS score was 35.5 (16.0), ranging from 6 to 87. Hyperkinetic scores ranged from 0 to 48 (mean [SD], 15.4 [8.5]) and hypokinetic scores from 0 to 22 (mean [SD], 8.4 [4.1]).
Fifteen age-matched healthy volunteers served as control individuals for the 18F-FDG PET study (mean [SD] age, 42.2 [7.4] years). They had no history of medical or neurologic illness, had normal brain magnetic resonance imaging, and did not take any medication known to affect FDG uptake.
Ethical permission for this study was obtained from the French National Ethics Committee and the Créteil University Hospital Ethics Committee. Written informed consent was obtained from each patient and volunteer after detailed explanation of the procedures. The study was performed in conformity with the Declaration of Helsinki.
PET Image Acquisition
All PET examinations were performed using a high-resolution EXACT HR+ tomograph (Siemens-CTI), allowing acquisition of 63 simultaneous 2.4-mm–thick axial slices in 3-dimensional mode with an isotropic intrinsic resolution of 4.5 mm on a 128 × 128 voxel matrix. All study participants were placed in a supine position with the head in the middle of the field of view using 3-dimensional laser alignment. A thermoplastic mask molded to each participant’s face minimized head movements. All acquisitions were carried out in a quiet, dark environment while the participants were in a resting state with the eyes closed. To correct for attenuation, a 15-minute transmission scan was performed using germanium 68 rods. Metabolic images were acquired 30 to 50 minutes after a mean (SD) injection of 151 (6.9) MBq of 18F-FDG. Positron emission tomography emission scans were reconstructed with filtered back projection with a ramp filter producing images with a resolution of 6.8-mm full-width at half-maximum. Positron emission tomography images were corrected for scatter, γ-ray attenuation, and 18F decay and then summed from 30 to 50 minutes. All PET images were anonymized and transmitted to the Service Hospitalier Frédéric Joliot site for storage and image processing and analysis.
18F-FDG PET Image Analysis
Spatial preprocessing and statistical analysis were performed using SPM5 (Wellcome Trust Centre for Neuroimaging, http://www.fil.ion.ucl.ac.uk/spm/) running in Matlab 2008 (MathWorks Inc; https://www.mathworks.com). To take into account brain atrophy observed in patients with HD, all PET scans from each patient and control individual were realigned and spatially normalized to an optimized template created using SPM software, mixing control individuals without HD and patients with HD and previously validated. After normalization, all PET scans were smoothened using an 8-mm full-width at half-maximum isotropic gaussian spatial filter to improve the signal-to-noise ratio. The effects of global metabolism were removed by normalizing the count of each voxel to the total count of the brain using proportional scaling. Global normalization was set to 10.
First, we compared the 18F-FDG scans of the 60 patients with HD with those of the 15 healthy volunteers using an unpaired 2-group t test to detect clusters displaying significant regional differences in 18F-FDG uptake (Figure 1). The P value (uncorrected) was set at < .001, and only clusters containing more than 20 voxels were deemed to be significant. t Tests were performed with age as covariate.
Figure 1. Flowchart Depicting Study Design.
SPM indicates statistical parametric mapping.
In each mask, a correlation analysis was performed using a multiple regression model with disease duration as covariate to investigate the association between motor scores and brain metabolism. For the 60 patients with HD, we used an explicit mask to restrict analyses to hypo- or hypermetabolic regions. The statistically significant threshold was fixed at P = .001, uncorrected. Only negative correlations were considered in the regions defined by the hypometabolic mask as they reveal the classic association between low metabolic values and higher motor scores, reflecting more severe disease. The hypermetabolic mask was used to better understand the clinical relevance of hypermetabolic regions: (1) negative correlations associating greater metabolism with a less severe motor score and (2) positive correlations associating greater metabolism with a more severe motor score.
Results
Among 60 patients with HD, 22 were women (36.7%), and of 15 control individuals, 7 were women (46.7%).
Regions With Abnormal Metabolic Values
We observed significant hypometabolism in patients with HD relative to matched control individuals (P < .001; 20 voxels). Analysis using SPM revealed a marked reduction of activity in caudate and putamen nuclei and in multiple cortical areas, such as the frontal and cingular regions. Conversely, we found a pattern of hypermetabolism (P < .001; 20 voxels) in the parietal, occipital, thalamus, and several areas in the cerebellum.
Correlation of Motor Scores With Regional Hypometabolism
Only classic association between low 18F-FDG values and disease severity (higher motor scores) was considered and referred as negative correlation. The extracted regions were therefore related to motor clinical worsening. Hypokinetic motor scores negatively correlated with the metabolic rate (P < .001, uncorrected) in the right caudate body (z score = 3.44) and bilaterally in the middle part of the putamen and pallidum (z score = 3.33 and 3.58 in right and left sides, respectively) (Table 1 and Figure 2A).
Table 1. Regions With Significant Correlations Between Hypometabolism and Hypokinetic and Hyperkinetic Motor Scores Obtained Using SPM.
| Anatomical Region | Side | MNI Coordinates | FDR-Corrected P Valuea | t | z Score | Voxels, No.b | ||
|---|---|---|---|---|---|---|---|---|
| x | y | z | ||||||
| Hypokinetic Motor Score | ||||||||
| Middle putamen/pallidum | Left | −24 | −2 | 4 | .04 | 3.80 | 3.58 | 38 |
| Caudate body | Right | 20 | 4 | 18 | .04 | 3.64 | 3.44 | 24 |
| Middle putamen/pallidum | Right | 22 | 4 | 0 | .04 | 3.52 | 3.33 | 34 |
| Hyperkinetic Motor Score | ||||||||
| Caudate head/caudate body/anterior putamen/cingulate gyrus (anterior, middle) | Left | −18 | 14 | 10 | <10−3 | 5.85 | 5.16 | 3963 |
| Caudate head/caudate body | Right | 20 | 10 | 16 | <10−3 | 5.83 | 5.14 | NA |
| Posterior insula lobe | Left | −34 | −10 | 6 | <10−3 | 5.61 | 4.99 | NA |
| Angular gyrus/inferior and superior parietal/BA 40/7 | Right | 36 | −58 | 48 | <10−3 | 5.24 | 4.71 | 214 |
| Thalamus | Right/left | −6 | −22 | 8 | <10−3 | 4.87 | 4.43 | 600 |
| Postcentral gyrus/inferior parietal lobule/BA 40 | Right | 54 | −30 | 50 | .002 | 3.98 | 3.73 | 32 |
| Precuneus/inferior and superior parietal lobule/postcentral gyrus/BA 40/7/5 | Right | 6 | −52 | 62 | .003 | 3.70 | 3.49 | 101 |
| Parahippocampa gyrus/GH30 | Right | 18 | −34 | −2 | .003 | 3.70 | 3.49 | 23 |
| Parahippocampa gyrus/GH30 | Left | −16 | −34 | 0 | .005 | 3.46 | 3.28 | 11 |
Abbreviations: BA, Brodmann area; FDR, false discovery rate; MNI, Montreal Neurological Institute; NA, not applicable; SPM, statistical parametric mapping.
Significance was set at P < .001, uncorrected for multiple comparisons across the whole brain volume, and FDR-corrected P values are indicated.
Number of voxels for each cluster, 1 voxel = 8 mm3.
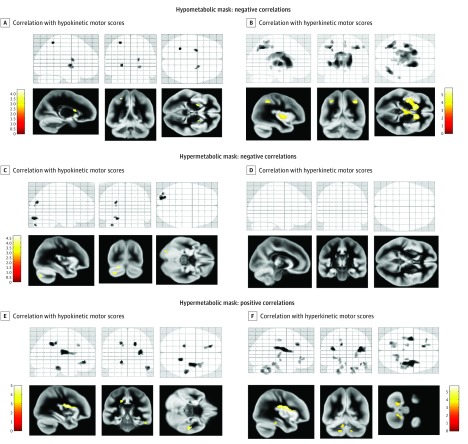
Figure 2. Statistical Parametric Mapping Presenting Correlations of Motor Scores With Regional Metabolism.
t Statistics maps of the correlation analyses computed between the extracted glucose metabolism values and clinical motor scores (P < .001, uncorrected) using the hypometabolic and hypermetabolic masks are overlaid on 3 intersecting slices of a glass brain. Clusters of these maps are overlaid on the mean gray matter volume with axial, sagittal, and coronal views. Correlations with hypokinetic motor scores (A, C, and E) and correlations with hyperkinetic motor scores (B, D, and F).
Hyperkinetic motor scores correlated with the metabolic rate bilaterally in the head and body of the caudate (z score = 5.14 and 5.16 in right and left sides, respectively), in the thalami (z score = 4.43), and in the anterior part of the left putamen (z score = 5.16) (Table 1 and Figure 2B). Other correlations were found in the following cortical regions: bilaterally in the cingulate gyrus (anterior and middle), the parahippocampal gyrus (z score = 3.49 and 3.28 in right and left sides, respectively), the left posterior insula (z score = 4.99), and the right angular area (z score = 4.71; Brodmann area [BA] 40/7) (Figure 2B).
Correlation of Motor Scores With Regional Hypermetabolism
Negative Correlations
Hypokinetic motor scores correlated with hypermetabolic values in the cuneus (z score = 3.70), lingual gyrus (z score = 4.31), and cerebellum (crus I/II, z score = 3.77) (Table 2, Figure 2C, and Figure 3A). We observed no significant negative correlation for hyperkinetic motor scores (Figure 2D).
Table 2. Regions With Significant Correlations Between Hypermetabolism and Motor Scores Obtained Using SPM.
| Anatomical Region | Side | MNI Coordinates | FDR-Corrected P Valuea | t | z Score | Voxels, No.b | ||
|---|---|---|---|---|---|---|---|---|
| x | y | z | ||||||
| Negative Correlationsc | ||||||||
| Hypokinetic Motor Score | ||||||||
| Lingual gyrus/inferior occipital gyrus/BA 18/19 | Left | −22 | −86 | −18 | .04 | 4.70 | 4.31 | 75 |
| Cerebellum crus I/II | Left | −34 | −82 | −36 | .04 | 4.04 | 3.77 | 64 |
| Cuneus/superior and middle occipital gyrus/BA 19 | Left | −24 | −78 | 28 | .04 | 3.95 | 3.70 | 16 |
| Positive Correlations | ||||||||
| Hypokinetic Motor Score | ||||||||
| Inferior parietal lobule | Right | 34 | −6 | 20 | .018 | 4.96 | 4.51 | 260 |
| Cingulate gyrus/BA 31 | Right | 20 | −42 | 40 | .018 | 4.74 | 4.33 | 74 |
| Anterior cingulate/BA 32 | Left | −18 | 42 | −4 | .019 | 4.45 | 4.11 | 70 |
| Cingulate gyrus/BA 31 | Left | −18 | −32 | 42 | .022 | 4.19 | 3.90 | 42 |
| Inferior temporal lobule/ BA 20 | Right | 54 | −28 | −20 | .022 | 4.19 | 3.89 | 111 |
| Superior frontal gyrus/BA 6/premotor | Left | −12 | 28 | 38 | .036 | 3.69 | 3.48 | 19 |
| Hyperkinetic Motor Score | ||||||||
| Inferior parietal lobule | Right | 36 | −4 | 22 | .002 | 5.72 | 5.06 | 500 |
| Inferior parietal lobule | Left | −36 | −8 | 26 | .003 | 5.01 | 4.54 | 163 |
| Medial frontal gyrus/BA 9/anterior cingulate 32 | Left | −12 | 30 | 36 | .005 | 4.41 | 4.08 | 49 |
| Cerebellar lobules IV/V, lobule VI, lobule VIII, lobule IX, dentate/midbrain/pons | Right | 14 | −48 | −30 | .007 | 4.23 | 3.96 | 186 |
| Cerebellum ant lobe (white matter) | Right | 20 | −44 | −38 | .014 | 3.84 | 3.61 | NA |
| Cerebellar lobules IV/V, lobule VI | Right | 28 | −46 | −30 | .014 | 3.84 | 3.61 | NA |
| Cerebellar lobules IV/V, lobule VIII | Left | −22 | −64 | −36 | .009 | 4.16 | 3.87 | 228 |
| Dentate | Left | −10 | −54 | −26 | .017 | 3.71 | 3.50 | NA |
| Cerebellar lobules IV/V, lobule VI, lobule VIII, lobule IX | Left | −18 | −56 | −42 | .019 | 3.62 | 3.42 | NA |
| Inferior temporal lobule/BA 20 | Right | 46 | −34 | −14 | .012 | 3.96 | 3.71 | 108 |
| Middle frontal gyrus | Left | −32 | 38 | 0 | .013 | 3.91 | 3.66 | 21 |
| Parahippocampa gyrus/BA 36 | Right | 38 | −36 | −18 | .013 | 3.90 | 3.66 | 11 |
| Corpus geniculatum lateral | Right | 28 | −22 | 6 | .016 | 3.78 | 3.55 | 42 |
| Anterior cingulate/BA 32 | Left | −16 | 44 | −2 | .017 | 3.71 | 3.50 | 50 |
| Midbrain | Left | −18 | −16 | −6 | .017 | 3.71 | 3.50 | 20 |
Abbreviations: BA, Brodmann area; FDR, false discovery rate; MNI, Montreal Neurological Institute; NA, not applicable; SPM, statistical parametric mapping.
Significance was set at P < .001, uncorrected for multiple comparisons across the whole brain volume, and FDR-corrected P values are indicated.
Number of voxels for each cluster, 1 voxel = 8 mm3.
No significant correlation for hyperkinetic motor score was found.
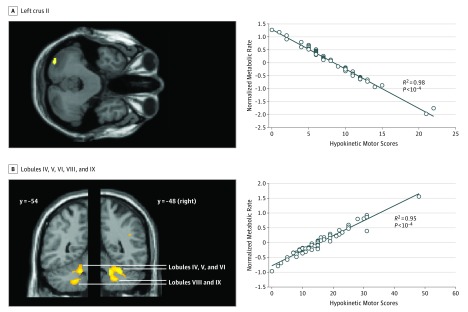
Figure 3. Correlation of Motor Scores With Hypermetabolism in the Cerebellum.
A, Left: clusters overlaid on axial slice (z = −36) from a canonical brain T1 image showing negative correlations. Hypokinetic motor scores correlated with hypermetabolic values in the left crus II of the cerebellum. Right: correlation plot on the left crus II region (R2 = 0.98).
B, Left: clusters overlaid on coronal slices at 2 different y coordinates showing positive correlations between hypermetabolic values and hyperkinetic motor scores bilaterally in the lobules IV-V, VI, VIII, and IX in the cerebellum. Right: correlation plot on the right lobules IV, V, VI, VIII, and IX (R2 = 0.95).
Positive Correlations
Hypokinetic motor scores correlated with hypermetabolic values in the right inferior parietal lobule, cingulate gyrus (bilaterally, BA 31/32), the right inferior temporal cortex, and the left superior frontal cortex (premotor area) (Table 2 and Figure 2E).
Hyperkinetic motor scores correlated with hypermetabolism bilaterally in the inferior parietal lobule (z score = 4.51), cingulate gyrus (z score = 4.33 and 4.11 in right and left sides, respectively), and left middle frontal gyri (z score = 4.08) (Table 2 and Figure 2F). In addition, such correlations were found in several regions of the cerebellum, including the vermis, the dentate nuclei, and the cerebellar lobules IV/V, VI, VIII, and IX bilaterally (z score = 3.96 and 3.42 in right and left sides, respectively) (Table 2, Figure 2F, and Figure 3B).
Discussion
The main goal of this study was to better understand the association between metabolic abnormalities of the brain and the severity of motor symptoms observed in patients with early symptomatic HD. We observed the main expected correlation between striatal hypometabolism and the degree of motor impairment. We also analyzed the association between hypermetabolic changes in HD brains and motor disorders. We found that some hypermetabolic values correlated negatively with motor scores, such as the cerebellum crus I/II for hypokinetic signs. Conversely, hypermetabolic values positively associated with more severe motor symptoms were found in the inferior parietal lobule; the anterior cingulate; the inferior temporal lobule; and bilaterally in the cerebellar lobules IV/V, VI, VIII, and IX (Figure 2).
We found the striatum of patients with HD to be hypometabolic, as well as several cortical areas, including the cingulate and parietal regions, as previously reported in presymptomatic and symptomatic patients with HD. We confirm the association between hypokinetic motor scores (bradykinesia and rigidity) and striatal hypometabolism. In addition, the correlation between hyperkinetic motor scores and hypometabolism in the anterior part of the left putamen and bilaterally in the caudate nucleus suggests that the anterior part of the striatum may also contribute to hyperkinetic movement disorders.
Striatal hypometabolism is the principal trait of HD, but abnormal hyperactivity has also been reported in several brain regions, such as the cerebellum, the thalamus, and occipital cortex. The pattern of hypermetabolism found in our study is similar to that reported by Feigin and colleagues(Figure 2). Until now, this regional hyperactivity has been considered to be a marker of processes that compensate for the clinical deficits resulting from the disease. Hypermetabolism in these regions would thus be expected to increase when motor scores decrease. Conversely, a positive correlation between metabolism and motor impairment would support the notion that hypermetabolism reflects neuronal hyperactivity detrimental to motor function.
We found such hypermetabolism associated with both increased hypokinetic and hyperkinetic motor symptoms in a consistent subset of regions: the inferior parietal lobule; the anterior cingulate; the inferior temporal lobule; the cerebellar lobules IV/V, VI, VIII, and IX bilaterally; the cerebellum crus I/II; and the dentate nucleus. Hypermetabolism in this subset of regions may be considered to be a marker of the collective motor symptoms of early HD, which fits with several observations in other movement disorders. For example, overactivity of the parietal cortex (BA 40) has been observed in patients with Parkinson disease performing complex manual tasks and has been considered to represent a shift from the deficient striato-mesial frontal motor system to the lateral motor system to compensate for bradykinesia. The same region is also hyperactive in DYT1 and DYT6 gene carriers when they exhibit dystonia but not in asymptomatic carriers. These convergent results observed for different movement disorders suggest that the parietal hypermetabolism observed in patients with HD is unlikely to be a compensatory mechanism but rather detrimental for motor deficit.
Our results suggest that the situation is more complex for the cerebellum. Again, hypermetabolism or hyperactivity in the cerebellum has often been considered to be a compensatory process in patients with movement disorders. Here, we found negative correlations in cerebellar structures connected with associative cortical areas but also positive correlations in motor areas of the cerebellum. Indeed, the severity of both hyperkinetic and hypokinetic motor symptoms was associated with a bilateral increase of metabolism in the dentate nucleus and cerebellar lobules IV, V, VI, VIII, and IX (Figure 3B). These regions belong to the motor structures of the cerebellum and have been found to be overactive in many movement disorders, either hypokinetic (eg, Parkinson disease) or hyperkinetic (eg, tremor, dystonia, and tics). However, previous studies did not investigate the correlation between motor performance or disease severity and cerebellar overactivity. Conversely, our results do not support a consistent compensatory role of hyperactivity in the cerebellum to counteract motor deficits. Both hypokinetic and hyperkinetic clinical manifestations in patients with early HD are associated with hypermetabolism in the same regions. Thus, hyperactivity of these motor cerebellar regions is more likely to be a marker of the disease itself, reflecting a global disorganization of the neuronal cerebral network involved in motor control. This motor cerebellar hyperactivity is less likely explained by a compensatory process. Indeed, the intensity of this hypermetabolic pattern increases when patients with pre-HD become clinically symptomatic patients.
We also found that increased metabolism in crus I/II of the cerebellum was associated with less severe hypokinetic symptoms (Figure 3A). Therefore, the hyperactivity in this region is interpreted as an indication of a compensatory process. This region is connected to prefrontal (crus I) and visual (crus II) associative areas and may be integrated in a global system involving visuomotor structures to compensate for hypokinetic movement disturbances.
Higher metabolism is associated with less severe hypokinetic motor scores in the cuneus, the lingual gyrus, and the area of visual integration (BA 18/19). The presence of glucose hypermetabolism in occipital regions is in agreement with the previously mentioned HD-related pattern. Hence, Johnson et al reported a reduction of cortical thickness in the occipital cortex and lingual gyrus in patients with HD that correlated with visuospatial performance. In addition, Carella et al showed that, when movement accuracy is required, patients with HD are more dependent on visual control than individuals without HD or any other abnormalities. Our results also suggest that increased metabolic activity in these regions may participate in compensatory mechanisms for motor disturbances in early HD.
Finally, we did not find any region exhibiting a compensatory effect for hyperkinetic movement disorders. This can be generalized to all hyperkinetic movement disorders: abnormal hyperactivity in brain regions increases with the severity of movement disorders, and the reduction of the movement severity is rather associated with a reduction of this hyperactivity. Therefore, compensation or efficient therapeutic action in these cases is associated with reduced, rather than increased, brain activity. This has been shown in tremor, dystonia, and tics. However, because tics can be voluntarily suppressed, reduction of activation in several regions, but also cortical frontal activations, have been associated with the reduction of tics. It remains to be determined whether the compensatory mechanisms in hyperkinetic movement disorders are actually absent or not detectable. For example, the benefits seen from deep brain stimulation in tremor are associated with deep brain stimulation–evoked activations in the same regions that are overactivated by the tremor itself, such as cerebellum and sensorimotor cortex.
Limitations
There were limitations of this study. The correlation analysis performed in this study and shown in Figure 3 cannot rule out a risk of overfitting of the data. We performed an error analysis through a cross-validation by splitting the data obtained in the 60 patients into 50% training and 50% validation subsets with 500 permutations. This did not evidence an overfitting in the present population, although this would need further confirmation in an independent population.
Conclusions
Hypermetabolic changes in early HD are likely associated with both compensatory processes and detrimental effects on motor symptoms. Our hypothesis needs confirmation using longitudinal analysis of metabolic changes during the progression of motor symptoms in patients with HD. Analysis needs to be conducted at the subregional level to infer pathophysiologic understanding from associations between metabolism and clinical symptoms in HD. In particular, coexistence of both compensatory and detrimental processes within the cerebellum requires precise anatomofunctional analysis of such abnormalities.
References
- 1.Douaud G, Behrens TE, Poupon C, et al. . In vivo evidence for the selective subcortical degeneration in Huntington’s disease. Neuroimage. 2009;46(4):958-966. [DOI] [PubMed] [Google Scholar]
- 2.Vonsattel JP, Myers RH, Stevens TJ, Ferrante RJ, Bird ED, Richardson EP Jr. Neuropathological classification of Huntington’s disease. J Neuropathol Exp Neurol. 1985;44(6):559-577. [DOI] [PubMed] [Google Scholar]
- 3.Reading SA, Dziorny AC, Peroutka LA, et al. . Functional brain changes in presymptomatic Huntington’s disease. Ann Neurol. 2004;55(6):879-883. [DOI] [PubMed] [Google Scholar]
- 4.Antonini A, Leenders KL, Spiegel R, et al. . Striatal glucose metabolism and dopamine D2 receptor binding in asymptomatic gene carriers and patients with Huntington’s disease. Brain. 1996;119(pt 6):2085-2095. [DOI] [PubMed] [Google Scholar]
- 5.Ciarmiello A, Cannella M, Lastoria S, et al. . Brain white-matter volume loss and glucose hypometabolism precede the clinical symptoms of Huntington’s disease. J Nucl Med. 2006;47(2):215-222. [PubMed] [Google Scholar]
- 6.Herben-Dekker M, van Oostrom JC, Roos RA, et al. . Striatal metabolism and psychomotor speed as predictors of motor onset in Huntington’s disease. J Neurol. 2014;261(7):1387-1397. [DOI] [PubMed] [Google Scholar]
- 7.Squitieri F, Orobello S, Cannella M, et al. . Riluzole protects Huntington disease patients from brain glucose hypometabolism and grey matter volume loss and increases production of neurotrophins. Eur J Nucl Med Mol Imaging. 2009;36(7):1113-1120. [DOI] [PubMed] [Google Scholar]
- 8.Gaura V, Bachoud-Lévi AC, Ribeiro MJ, et al. . Striatal neural grafting improves cortical metabolism in Huntington’s disease patients. Brain. 2004;127(pt 1):65-72. [DOI] [PubMed] [Google Scholar]
- 9.Eidelberg D, Surmeier DJ. Brain networks in Huntington disease. J Clin Invest. 2011;121(2):484-492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feigin A, Leenders KL, Moeller JR, et al. . Metabolic network abnormalities in early Huntington’s disease: an [(18)F]FDG PET study. J Nucl Med. 2001;42(11):1591-1595. [PubMed] [Google Scholar]
- 11.Feigin A, Tang C, Ma Y, et al. . Thalamic metabolism and symptom onset in preclinical Huntington’s disease. Brain. 2007;130(pt 11):2858-2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grafton ST, Mazziotta JC, Pahl JJ, et al. . A comparison of neurological, metabolic, structural, and genetic evaluations in persons at risk for Huntington’s disease. Ann Neurol. 1990;28(5):614-621. [DOI] [PubMed] [Google Scholar]
- 13.Tang CC, Feigin A, Ma Y, et al. . Metabolic network as a progression biomarker of premanifest Huntington’s disease. J Clin Invest. 2013;123(9):4076-4088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cleret de Langavant L, Sudraud S, Verny C, et al. . Longitudinal study of informed consent in innovative therapy research: experience and provisional recommendations from a multicenter trial of intracerebral grafting. PLoS One. 2015;10(5):e0128209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bloch J, Bachoud-Lévi AC, Déglon N, et al. . Neuroprotective gene therapy for Huntington’s disease, using polymer-encapsulated cells engineered to secrete human ciliary neurotrophic factor: results of a phase I study. Hum Gene Ther. 2004;15(10):968-975. [DOI] [PubMed] [Google Scholar]
- 16.World Medical Association World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053. [DOI] [PubMed] [Google Scholar]
- 17.Douaud G, Gaura V, Ribeiro MJ, et al. . Distribution of grey matter atrophy in Huntington’s disease patients: a combined ROI-based and voxel-based morphometric study. Neuroimage. 2006;32(4):1562-1575. [DOI] [PubMed] [Google Scholar]
- 18.Allain P, Gaura V, Fasotti L, et al. . The neural substrates of script knowledge deficits as revealed by a PET study in Huntington’s disease. Neuropsychologia. 2011;49(9):2673-2684. [DOI] [PubMed] [Google Scholar]
- 19.Kuwert T, Lange HW, Langen KJ, Herzog H, Aulich A, Feinendegen LE. Cortical and subcortical glucose consumption measured by PET in patients with Huntington’s disease. Brain. 1990;113(pt 5):1405-1423. [DOI] [PubMed] [Google Scholar]
- 20.Young AB, Penney JB, Starosta-Rubinstein S, et al. . PET scan investigations of Huntington’s disease: cerebral metabolic correlates of neurological features and functional decline. Ann Neurol. 1986;20(3):296-303. [DOI] [PubMed] [Google Scholar]
- 21.Kim JS, Reading SA, Brashers-Krug T, Calhoun VD, Ross CA, Pearlson GD. Functional MRI study of a serial reaction time task in Huntington’s disease. Psychiatry Res. 2004;131(1):23-30. [DOI] [PubMed] [Google Scholar]
- 22.Ikeda M, Tsukagoshi H. Monochorea caused by a striatal lesion. Eur Neurol. 1991;31(4):257-258. [DOI] [PubMed] [Google Scholar]
- 23.Ligot N, Krystkowiak P, Simonin C, et al. . External globus pallidus stimulation modulates brain connectivity in Huntington’s disease. J Cereb Blood Flow Metab. 2011;31(1):41-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reese R, Knudsen K, Falk D, Mehdorn HM, Deuschl G, Volkmann J. Motor outcome of dystonic camptocormia treated with pallidal neurostimulation. Parkinsonism Relat Disord. 2014;20(2):176-179. [DOI] [PubMed] [Google Scholar]
- 25.Samuel M, Ceballos-Baumann AO, Blin J, et al. . Evidence for lateral premotor and parietal overactivity in Parkinson’s disease during sequential and bimanual movements: a PET study. Brain. 1997;120(pt 6):963-976. [DOI] [PubMed] [Google Scholar]
- 26.Carbon M, Su S, Dhawan V, Raymond D, Bressman S, Eidelberg D. Regional metabolism in primary torsion dystonia: effects of penetrance and genotype. Neurology. 2004;62(8):1384-1390. [DOI] [PubMed] [Google Scholar]
- 27.Rascol O, Sabatini U, Fabre N, et al. . The ipsilateral cerebellar hemisphere is overactive during hand movements in akinetic parkinsonian patients. Brain. 1997;120(pt 1):103-110. [DOI] [PubMed] [Google Scholar]
- 28.Yu H, Sternad D, Corcos DM, Vaillancourt DE. Role of hyperactive cerebellum and motor cortex in Parkinson’s disease. Neuroimage. 2007;35(1):222-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Payoux P, Brefel-Courbon C, Ory-Magne F, et al. . Motor activation in multiple system atrophy and Parkinson disease: a PET study. Neurology. 2010;75(13):1174-1180. [DOI] [PubMed] [Google Scholar]
- 30.Wu T, Hallett M. A functional MRI study of automatic movements in patients with Parkinson’s disease. Brain. 2005;128(pt 10):2250-2259. [DOI] [PubMed] [Google Scholar]
- 31.Bohlhalter S, Goldfine A, Matteson S, et al. . Neural correlates of tic generation in Tourette syndrome: an event-related functional MRI study. Brain. 2006;129(pt 8):2029-2037. [DOI] [PubMed] [Google Scholar]
- 32.Dresel C, Li Y, Wilzeck V, Castrop F, Zimmer C, Haslinger B. Multiple changes of functional connectivity between sensorimotor areas in focal hand dystonia. J Neurol Neurosurg Psychiatry. 2014;85(11):1245-1252. [DOI] [PubMed] [Google Scholar]
- 33.Wills AJ, Jenkins IH, Thompson PD, Findley LJ, Brooks DJ. A positron emission tomography study of cerebral activation associated with essential and writing tremor. Arch Neurol. 1995;52(3):299-305. [DOI] [PubMed] [Google Scholar]
- 34.Ma Y, Eidelberg D. Functional imaging of cerebral blood flow and glucose metabolism in Parkinson’s disease and Huntington’s disease. Mol Imaging Biol. 2007;9(4):223-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Johnson EB, Rees EM, Labuschagne I, et al. ; TRACK-HD investigators . The impact of occipital lobe cortical thickness on cognitive task performance: an investigation in Huntington’s Disease. Neuropsychologia. 2015;79(pt A):138-146. [DOI] [PubMed] [Google Scholar]
- 36.Carella F, Bressanelli M, Piacentini S, et al. . A study of arm movements in Huntington’s disease under visually controlled and blindfolded conditions. Neurol Sci. 2003;23(6):287-293. [DOI] [PubMed] [Google Scholar]
- 37.Boecker H, Wills AJ, Ceballos-Baumann A, et al. . The effect of ethanol on alcohol-responsive essential tremor: a positron emission tomography study. Ann Neurol. 1996;39(5):650-658. [DOI] [PubMed] [Google Scholar]
- 38.Broersma M, van der Stouwe AM, Buijink AW, et al. . Bilateral cerebellar activation in unilaterally challenged essential tremor. Neuroimage Clin. 2015;11:1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Buijink AW, Broersma M, van der Stouwe AM, et al. . Rhythmic finger tapping reveals cerebellar dysfunction in essential tremor. Parkinsonism Relat Disord. 2015;21(4):383-388. [DOI] [PubMed] [Google Scholar]
- 40.Detante O, Vercueil L, Thobois S, et al. . Globus pallidus internus stimulation in primary generalized dystonia: a H215O PET study. Brain. 2004;127(pt 8):1899-1908. [DOI] [PubMed] [Google Scholar]
- 41.Løkkegaard A, Herz DM, Haagensen BN, Lorentzen AK, Eickhoff SB, Siebner HR. Altered sensorimotor activation patterns in idiopathic dystonia-an activation likelihood estimation meta-analysis of functional brain imaging studies. Hum Brain Mapp. 2016;37(2):547-557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Neuner I, Werner CJ, Arrubla J, et al. . Imaging the where and when of tic generation and resting state networks in adult Tourette patients. Front Hum Neurosci. 2014;8:362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Peterson BS, Leckman JF. The temporal dynamics of tics in Gilles de la Tourette syndrome. Biol Psychiatry. 1998;44(12):1337-1348. [DOI] [PubMed] [Google Scholar]
- 44.Gibson WS, Jo HJ, Testini P, et al. . Functional correlates of the therapeutic and adverse effects evoked by thalamic stimulation for essential tremor. Brain. 2016;139(pt 8):2198-2210. [DOI] [PMC free article] [PubMed] [Google Scholar]