Abstract
Background
Five-grass pollen tablet is an effective and well-tolerated therapy for patients with allergic rhinoconjunctivitis (ARC). This trial sought to determine the satisfaction and health-related quality of life (HRQoL) of patients undergoing this treatment.
Methods
This was a cross-sectional, multicentre, observational, naturalistic study, following a discontinuous pre- and co-seasonal five-grass pollen regimen over two seasons in Spain (2012, 2013). The HRQoL of the patients was measured with the specific Rhinoconjunctivitis Quality of Life Questionnaire (RQLQ) for adults, adolescent (AdolRQLQ), or paediatric (PRQLQ) patients. Treatment satisfaction was assessed by the Satisfaction Scale for Patients Receiving Allergen Immunotherapy (ESPIA) questionnaire. Patients/investigators were surveyed on beliefs and attitudes towards the five-grass pollen tablet. ARC evolution according to allergic rhinitis and its impact on asthma (ARIA) criteria and treatment adherence were evaluated.
Results
Among the 591 ARC patients included, the mean (SD) HRQoL scores were 1.40 (1.1) in adults, 1.33 (1.1) in adolescents, and 1.15 (1.1) in children, indicating low levels of impairment (scale 0–6). ESPIA answers showed high levels of satisfaction, with an average score of 69.2 (scale 0–100). According to ARIA criteria, 88.2% of patients reported improvement of ARC. Moreover, this was accompanied by a reduced use of symptomatic medication. Adherence to treatment was estimated at 96.8%. In general, both patients and specialists exhibited a positive attitude towards five-grass pollen tablet treatment.
Conclusion
ARC patients treated with five-grass pollen tablet showed favourable levels of HRQoL and treatment satisfaction, with concomitant improvements in ARC and symptomatic medication use, which translated into high levels of treatment adherence and a positive attitude towards five-grass pollen tablet.
Keywords: sublingual immunotherapy, pollen, rhinitis allergic seasonal, conjunctivitis allergic, health-related quality of life, patient satisfaction, symptom improvement, cross-sectional study
Introduction
Allergic rhinoconjunctivitis (ARC) is considered a significant health problem, which is estimated to affect approximately 23% of adult population in Spain [1]. It is a major risk factor for the development of comorbidities including asthma, associated with sleep and mood disturbances, and has been shown to impair daily activities and performance at work or school, exerting a negative impact on patient’s health-related quality of life (HRQoL) [2,3]. Allergen-specific immunotherapy (AIT) is the only etiologic treatment for ARC [4]. Although traditional pharmacotherapy is widely used, this approach only targets the symptoms of the condition [5,6]. AIT is recommended for patients with moderate-to-severe ARC who have not responded to symptomatic pharmacotherapy [4]. Traditional AIT, which is administered by subcutaneous injections (SCIT), typically every month for 3–5 years, has shown notable efficacy in several trials [7–12].
Orally administered once-daily sublingual five-grass pollen tablet immunotherapy (five-grass pollen tablet; Oralair®) is an alternative form of AIT, which has proven efficacy, with a more favourable safety and tolerability profile than SCIT [11]. The active constituents in five-grass pollen tablet comprises purified and calibrated freeze-dried extract of pollen from sweet vernal grass (Anthoxanthum odoratum), cocksfoot/orchard grass (Dactylis glomerata), perennial rye grass (Lolium perenne), meadow grass (Poa pratensis), and timothy grass (Phleum pratense) [13]. Inclusion of these five components (rather than one) better mimics the exposure profile in Europe, sensitization conditions, and the polysensitization of allergic patients across the continent [14,15].
Recent studies indicate that five-grass pollen tablet exhibits a positive influence on the patient’s HRQoL, largely reflecting symptom improvement and a reduction in the use of symptomatic medication [16–20]. To further investigate these findings, this observational study was specifically conducted to assess the level of treatment satisfaction and HRQoL with five-grass pollen tablet in patients with grass-pollen-related ARC.
Methods
Patients and study design
This was a cross-sectional, observational, multicentre, naturalistic study conducted in Spain. Patients aged ≥6 years with moderate-to-severe ARC to grass pollen uncontrolled with symptomatic treatment, who had received five-grass pollen tablet in a discontinuous pre- and co-seasonal regimen (before and during the previous grass-pollen season, principally Spring/Summer) were eligible for participation. Patients who had received any other form of AIT or those who were unable to comply with the trial protocol were excluded. Written informed consent was obtained from participating patients, their parents, or legal representatives. Moreover, the study was approved by the Research Ethics Committee at the Hospital Universitario de La Princesa (Madrid).
Eligible patients were identified from clinical records and attended a single clinic visit during which demographics, medical, and treatment history were confirmed. Patients were then required to complete self-administered questionnaires (if necessary with the help of parents or caregivers) to evaluate HRQoL, treatment satisfaction, and attitude towards medication. Symptom severity was evaluated retrospectively. Physician demographics were also recorded, and physician attitude towards medication was evaluated by self-administered questionnaire. Analyses were conducted separately for the previous 2012 and 2013 pollen seasons.
Assessments
HRQoL
HRQoL was evaluated using validated age-specific instruments [21–24]. Adult patients (≥18 years of age) completed the Spanish version of the Rhinoconjunctivitis Quality of Life Questionnaire (RQLQ) [21–24], consisting of 28 items distributed in 7 dimensions (activity limitations, sleep disturbances, general problems, practical problems, nose symptoms, eye symptoms, and emotional function). Adolescents aged 12–17 years were asked to complete the Spanish adolescent RQLQ (AdolRQLQ) [22], consisting of 25 items distributed in 6 dimensions (activity limitation, nasal symptoms, eye symptoms, practical problems, non-hay fever symptoms, and emotional function); whereas, patients under 12 years of age completed the paediatric RQLQ (PRQLQ) [24], which consists of 23 items distributed in 5 dimensions (nose symptoms, eye symptoms, practical problems, activity limitation, and other symptoms). In all three questionnaires, each item was scored from 0 (not impaired at all) to 6 (severely impaired).
Treatment satisfaction
To determine the level of satisfaction with five-grass pollen tablet, adult patients were asked to complete the Satisfaction Scale for Patients Receiving Allergen Immunotherapy (ESPIA) questionnaire [25], comprising 16 items specifically designed for patients treated with AIT. Overall satisfaction in this validated instrument is graded from 0 to 100 (100 denoting the highest level of satisfaction). Psychometric properties of the (unvalidated) paediatric version of the ESPIA questionnaire were also evaluated in an exploratory analysis.
ARC frequency and severity
ARC was classified as persistent or intermittent in frequency and as mild or moderate-to-severe ARC, according to the Allergic Rhinitis and its Impact on Asthma (ARIA) criteria [26]. Typical symptoms associated to ARC (sneezing, rhinorrhoea, nasal itching, nasal congestion, ocular itching, and tearing) were classified as mild, moderate, or severe, according to the Center for Drug Evaluation and Research guidelines [27]. Both ARC severity and associated symptoms before and after five-grass pollen tablet were assessed retrospectively, based on the data retrieved from the clinical records of the patients.
Patients survey on beliefs and attitudes towards five-grass pollen tablet
All patients were asked to complete a descriptive survey, specifically designed for this study, which included 17 questions/statements divided in three sections, aimed at assessing the following aspects of five-grass pollen tablet therapy: beliefs/attitudes, effectiveness/security, and compliance/adherence. The section on compliance and adherence included the Spanish version of the validated Haynes–Sackett questionnaire [28]. Compliance was estimated as the difference between prescriptions written and collected (%); whereas, adherence was calculated as the proportion of tablets returned unused (%).
Investigators attitude towards five-grass pollen tablet
Investigator beliefs and attitudes to five-grass pollen tablet were assessed by a self-administered questionnaire, specifically designed for this study, before patient recruitment. The clinical practice of investigators in relation to five-grass pollen tablet was assessed by determining their level of agreement with each of 10 statements scored from 1 (totally agree) to 5 (totally disagree). The factors that investigators typically consider when prescribing five-grass pollen tablet were evaluated in 19 statements scored again from 1 (always) to 5 (never). The preferences of investigators for oral (five-grass pollen tablet) compared to other routes of administration were determined by the number and percentage of investigators answering ‘better’, ‘equal’, or ‘worse’. In addition, the investigators were asked about the primary advantages and disadvantages of the five-grass pollen tablet and their level of adherence with treatment guidelines.
Statistics
Statistical analyses were performed using the SAS system version 9.2 (SAS Institute Inc., Cary, NC, USA). Data were stratified by patient age (6–11 years, 12–17 years, and 18–80 years). Baseline demographics were summarized descriptively. The correlation between sociodemographic and clinical variables with the level of satisfaction and HRQoL was analysed using the ANOVA test (categorical variables) or the Pearson and Spearman correlation coefficients (continuous variables). A significance level of 0.05 was used in all comparisons between groups. A descriptive analysis of the answers to the questionnaire on beliefs and attitudes towards five-grass pollen tablet was conducted. Likert-type answers were described as percentage of patients in each category. The global level of satisfaction of patients with five-grass pollen tablet was described as a continuous variable in a 0–100 scale.
Results
A total of 591 evaluable patients with moderate-to-severe ARC participated in this observational study, who were treated with five-grass pollen tablet over two seasons. Among the participants, 116 (19.6%) were children aged 6–11 years, 87 (14.7%) were adolescents aged 12–17 years, and 388 (65.7%) were adults. Table 1 presents the most relevant sociodemographic and clinical data. Among the 46 patients (7.8%) who discontinued the treatment prematurely, the most common reason was clinical improvement (33.3%), followed by adverse reactions (15.6%).
Table 1.
Socio-demographic and clinical data of patients included in the study.
| Variable | Children (6–11) | Adolescents (12–17) | Adults (≥18) | Total | p-value | |
|---|---|---|---|---|---|---|
| Age | n | 116† | 87† | 388† | 591† | <0.0001 |
| Average (SD) | 9.0 (1.5) | 14.5 (1.8) | 33.9 (11.2) | 26.2 (14.2) | ||
| Gender | Male | 75 (64.7%) | 54 (62.1%) | 194 (50.0%) | 323 (54.7%) | 0.0067 |
| Habitat | Rural (<10,000 pop.) | 11 (9.5%) | 10 (11.5%) | 51 (13.2%) | 72 (12.2%) | 0.4199 |
| Semi-urban (>10,000–<30,000 pop.) | 11 (9.5%) | 7 (8.0%) | 50 (13.0%) | 68 (11.5%) | ||
| Urban (>30,000–<200,000 pop.) | 68 (58.6%) | 48 (55.2%) | 184 (47.7%) | 300 (50.9%) | ||
| Metropolitan (>200,000 pop.) | 26 (22.4%) | 22 (25.3%) | 101 (26.2%) | 149 (25.3%) | ||
| Level of education | No formal education | 6 (9.4%) | - | 4 (1.1%) | 10 (2.0%) | <0.0001 |
| Primary education | 57 (89.1%) | 20 (30.8%) | 52 (14.0%) | 129 (25.8%) | ||
| Secondary education | 1 (1.6%) | 43 (66.2%) | 139 (37.5%) | 183 (36.6%) | ||
| University education | 2 (3.1%) | 176 (47.4%) | 178 (35.6%) | |||
| Employment status | Unemployed | 23 (6.2%) | 23 (4.7%) | <0.0001 | ||
| Self-employed | 37 (10.0%) | 37 (7.5%) | ||||
| Employed by other | 210 (56.9%) | 210 (42.5%) | ||||
| Unable to work | 1 (0.3%) | 1 (0.2%) | ||||
| Pensioner | 10 (2.7%) | 10 (2.0%) | ||||
| Housework | 18 (4.9%) | 18 (3.6%) | ||||
| Student | 58 (100.0%) | 67 (100.0%) | 70 (19.0%) | 195 (39.5%) | ||
| Duration of ARC | Average (SD) years | 3.9 (2.0) | 5.9 (3.3) | 10.4 (8.7) | 8.45 (7.7) | <0.0001 |
| Diagnosis method | Skin prick-test | 115 (99.1%) | 87 (100.0%) | 385 (99.2%) | 587 (99.3%) | 0.7027 |
| Specific classic IgE | 71 (61.2%) | 45 (51.7%) | 181 (46.6%) | 297 (50.3%) | 0.0217 | |
| Molecular diagnosis | 27 (23.3%) | 25 (28.7%) | 89 (22.9%) | 141 (23.9%) | 0.5113 | |
| Other | 3 (2.6%) | 1 (1.1%) | 12 (3.1%) | 16 (2.7%) | 0.5984 | |
| Comorbidities | Yes | 94 (81.0%) | 66 (75.9%) | 263 (67.8%) | 423 (71.6%) | 0.0134 |
| Asthma | 72 (62.1%) | 54 (62.1%) | 206 (53.1%) | 332 (56.2%) | 0.1129 | |
| Sinusitis | 4 (3.4%) | 2 (2.3%) | 23 (5.9%) | 29 (4.9%) | 0.2640 | |
| Nasal polyposis | 1 (0.9%) | 2 (0.5%) | 3 (0.5%) | 0.6932 | ||
| Medium otitis | 5 (4.3%) | 1 (1.1%) | 6 (1.5%) | 12 (2.0%) | 0.1475 | |
| Eczema | 13 (11.2%) | 3 (3.4%) | 19 (4.9%) | 35 (5.9%) | 0.0235 | |
| Urticaria | 2 (1.7%) | 6 (6.9%) | 12 (3.1%) | 20 (3.4%) | 0.1130 | |
| Atopic dermatitis | 32 (27.6%) | 10 (11.5%) | 25 (6.4%) | 67 (11.3%) | <0.0001 | |
| Headache | 5 (4.3%) | 5 (5.7%) | 36 (9.3%) | 46 (7.8%) | 0.1604 | |
| Food allergy | 15 (12.9%) | 8 (9.2%) | 44 (11.3%) | 67 (11.3%) | 0.7081 | |
| Drugs allergy | 1 (1.1%) | 16 (4.1%) | 17 (2.9%) | 0.0383 | ||
| Other | 5 (4.3%) | 1 (1.1%) | 14 (3.6%) | 20 (3.4%) | 0.4290 |
Total evaluated unless specified otherwise.
SD=standard deviation.
HRQoL
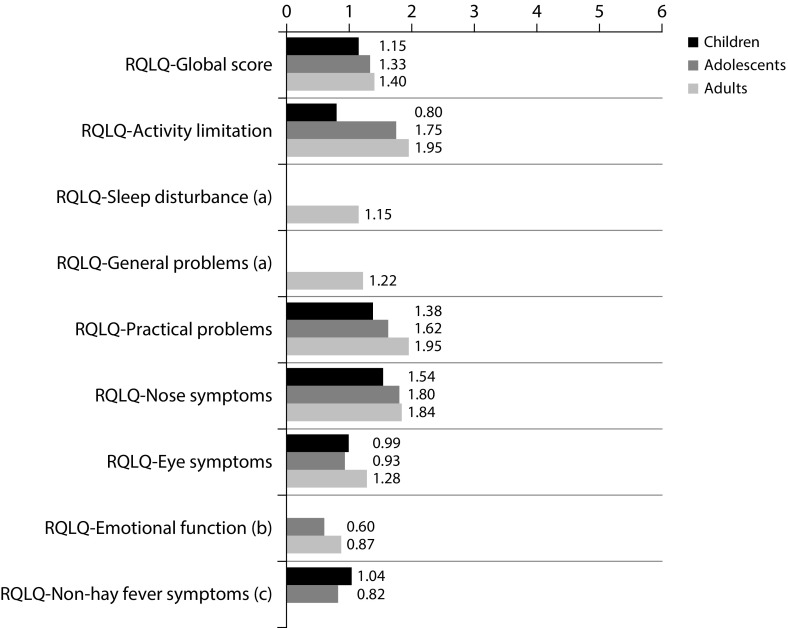
The mean (SD) scores of RQLQ, AdolRQLQ, and PRQLQ were 1.40 (1.1) in adults, 1.33 (1.1) in adolescents, and 1.15 (1.1) in children, respectively (Figure 1), indicating low levels of impairment. Of note, 26.5% of the patients exhibited scores between 0 and 0.5 (denoting no symptoms). Regarding the dimensions included in the questionnaires, the differences on daily activities impairment emerge across different ages; older patients exhibited higher levels of impairment and practical problems (p<0.005). Higher scores from HRQoL questionnaires were associated with the following: work or school impairment, presence of troublesome symptoms during treatment, taking other allergy medication, difficulties to get to places with presence of grass pollen, and increased medication taken for allergy since starting five-grass pollen tablet (p<0.05). As expected, the scores were inversely related to the level of satisfaction of patients with five-grass pollen tablet (p<0.0001).
Figure 1. Mean score from patients’ RQLQ by dimension after treatment with five-grass pollen tablet.
(a) Dimensions not included in the AdolRQLQ or the PRQLQ.
(b) Dimensions not included in the PRQLQ.
(c) Dimensions not included in the adults RQLQ Mean score from patients RQLQ questionnaire by dimensions after five-grass pollen tablet treatment.
AdolRQLQ=Rhinoconjunctivitis Quality of Life Questionnaire for adolescent patients; PRQLQ= Rhinoconjunctivitis Quality of Life Questionnaire for pediatric patients; RQLQ=Rhinoconjunctivitis Quality of Life Questionnaire for adult patients.
Treatment satisfaction
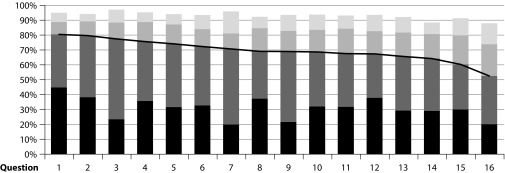
Adult patients showed a high level of satisfaction following five-grass pollen tablet treatment, as shown by an ESPIA questionnaire mean (SD) score of 69.2 (23.7) (Figure 2). High levels of satisfaction were also recorded for those dimensions related to cost–benefit balance and general satisfaction with five-grass pollen tablet; mean (SD) scores were 68.6 (28.4) and 75.4 (25.9), respectively. Data for adolescent and paediatric patients were evaluated in an exploratory analysis, because the questionnaire used had not been validated. Nevertheless, the results showed reasonable correlation with those in the adult population (data not shown).
Figure 2. Frequency of agreement of patients with the ESPIA questionnaire in adult patientsa.
(a) This English language version has not been subject to the standard process of translation–back translation in accordance with the recommendations of the specialized bibliography. It is merely a free translation included here for informational purposes only.
ESPIA=Satisfaction Scale for Patients Receiving Allergen Immunotherapy.
ARC frequency and severity
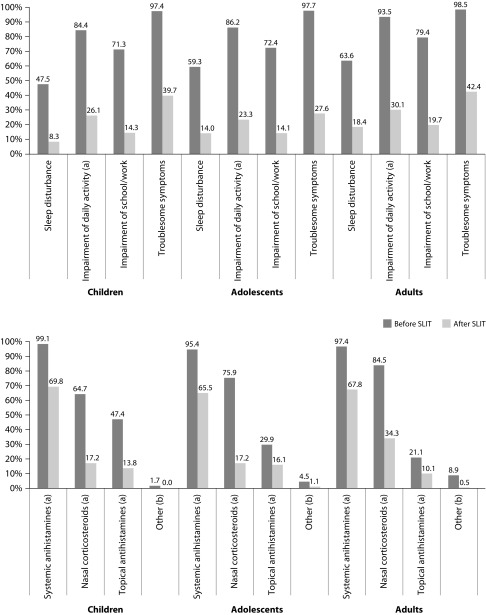
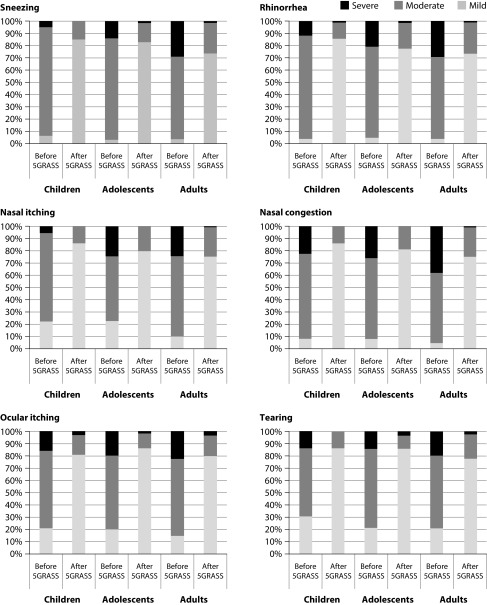
ARC followed a more favourable clinical course after the five-grass pollen tablet treatment. Symptoms transitioned from being persistent to intermittent for 60.9% of patients; whereas, the severity of ARC was reduced from severe or moderate to mild in 49.1% of patients. Improvements were recorded for all the ARIA measures across all age groups, reaching statistical significance for the reduction in impairment of daily living activities (Figure 3). These outcomes were reflected in a statistically significant reduction in the use of medications for symptomatic relief; specifically for systemic antihistamines, topical antihistamines, and nasal corticosteroids (Figure 3). Symptoms exhibited after the five-grass pollen tablet were similar across all ages, sneezing (71.3%) being the most common, followed by rhinorrhoea (66.5%) and nasal itching (64.1%). A general reduction in the intensity of symptoms was reported after five-grass pollen tablet (Figure 4). These results were similar irrespective of the sensitization status. Only 15 patients (2.5%) showed no improvement in the ARIA criteria, ARC symptoms, or use of symptomatic medication.
Figure 3. Presence of alterations related to ARC following ARIA criteria (top) and use of other pharmacological treatment (bottom) before and after treatment with five-grass pollen tablet.
(a) Statistically significant reduction (p<0.05). (b) Systemic corticosteroids, nasal descongestants and oral descongestants.
ARC=Allergic rhinoconjunctivitis; ARIA=Allergic Rhinitis and its Impact on Asthma; SLIT=Sub-lingual immunotherapy (five-grass pollen tablet).
Figure 4. Symptom intensity before and after treatment with five-grass pollen tablet.
5GRASS=Five-grass pollen tablet.
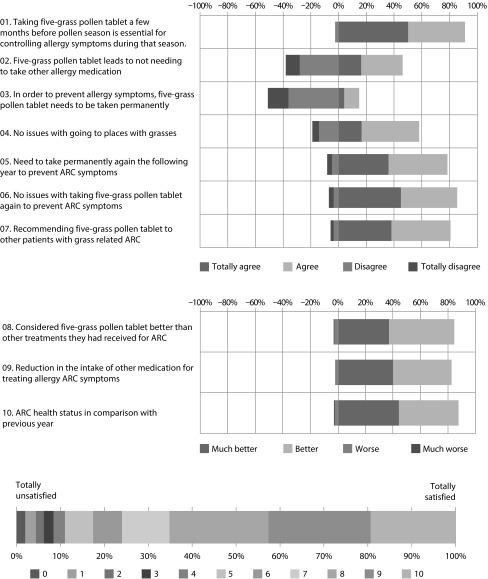
Patients survey on beliefs and attitudes towards five-grass pollen tablet
The results of the patient survey on beliefs and attitudes towards the five-grass pollen tablet revealed generally positive opinions relating to the prior use of this therapy (Figure 5). For instance, 91.2% of patients considered that taking the five-grass pollen tablet a few months before and during the pollen season was essential for controlling the allergy symptoms during that season; 46.2% agreed that five-grass pollen tablet had not led to the requirement of other allergy medication; and only 14.9% of patients believed that, in order to prevent allergy symptoms, five-grass pollen tablet should be taken permanently. In terms of effectiveness, 58.2% claimed to have no issues with going to places with grasses and 78.6% claimed they would need to take the five-grass pollen tablet again the following year to prevent ARC symptoms. The overall mean (SD) level of satisfaction with the five-grass pollen tablet, on a scale of 0 (lowest) to 10 (highest), was 7.5 (2.4), indicating a high level of satisfaction with the agent (Figure 5).
Figure 5. Results from the patient survey on beliefs and attitudes towards five-grass pollen tableta.
(a) This English language version has not been subject to the standard process of translation–back translation in accordance with the recommendations of the specialized bibliography. It is merely a free translation included here for informational purposes only.
ARC=Allergic rhinoconjunctivitis.
Estimates of patient compliance and adherence were high; 93.3% of patients were calculated to be compliant to the prescribed regimen, and 96.8% of patients had good treatment adherence. In relation to compliance, 25.1% of patients stated that they had difficulties to take the tablets; whereas, only 27.5% of patients claimed that they had not forgotten to take any tablet, and 34% forgot to take five or more tablets.
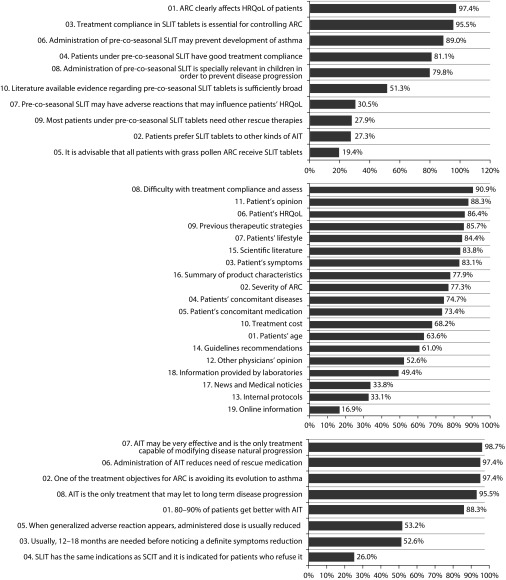
Investigators attitude towards five-grass pollen tablet
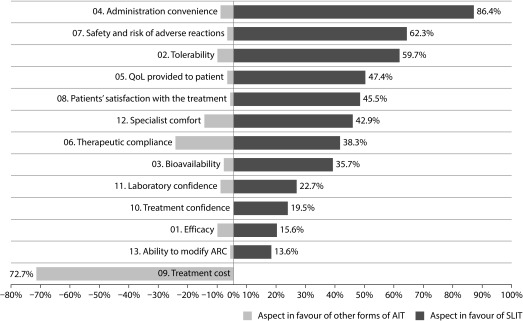
A total of 154 investigators completed the online survey on beliefs and attitudes towards the five-grass pollen tablet. The level of agreement of investigators with statements regarding general clinical management of ARC, factors to be considered when prescribing five-grass pollen tablet, and treatment guidelines followed are detailed in Figure 6. Of note, 89.0% of investigators believed that five-grass pollen tablet could prevent the occurrence of asthma, and 81.1% considered that the patients showed good treatment compliance. In general, the investigators considered the five-grass pollen tablet to be better than other AIT across nearly all measures. More than half of the investigators rated the five-grass pollen tablet as being more favourable owing to ease of administration (86.4%), safety and risk of adverse reactions (62.3%), and tolerability (59.7%) (Figure 7). In contrast, 72.7% of the investigators considered the five-grass pollen tablet to be disadvantaged by its higher cost (Figure 7). Nevertheless, 86.4% of the physicians claimed that they would take five-grass pollen tablet if they suffered ARC related to grass pollen.
Figure 6. Level of agreement with investigators with statements included in the survey. General clinical management of ARC (top), factors considered always and almost always when prescribing five-grass pollen tablet (middle) and agreement with general ARC guidelines (bottom)a.
(a) This English language version has not been subject to the standard process of translation–back translation in accordance with the recommendations of the specialized bibliography. It is merely a free translation included here for informational purposes only.
AIT=Allergen immunotherapy; ARC=Allergic rhinoconjunctivitis; HRQoL=Health related quality of life; SCIT=Sub-cutaneous immunotherapy; SLIT=Sub-lingual immunotherapy (five-grass pollen tablet).
Figure 7. Aspects of five-grass pollen tablet that investigators consider better or worse in comparison with AITa.
(a) This English language version has not been subject to the standard process of translation–back translation in accordance with the recommendations of the specialized bibliography. It is merely a free translation included here for informational purposes only.
AIT=Allergen-specific immunotherapy; SLIT=Sub-lingual immunotherapy (five-grass pollen tablet).
Safety
Overall, 29.1% of the patients experienced at least one adverse event when taking five-grass pollen tablet, the most common adverse events being oral pruritus (32.5%), followed by throat irritation (18.1%), ear pruritus (12.4%), tongue swelling (11.2%), and mouth swelling (9.2%). Treatment withdrawal owing to adverse events was recorded for 4% of patients, and none was due to severe adverse reactions.
Discussion
Controlled clinical studies have demonstrated the efficacy of five-grass pollen tablet in terms of symptom control and a reduced need for additional medication to provide symptom relief [16–20]. However, ARC also exhibits a significant negative impact on other patient outcomes, including HRQoL and productivity [2,29], which are also positively modified with five-grass pollen tablet treatment [16–20]. We confirm this and add new findings in relation to five-grass pollen tablet through our novel naturalistic study, conducted over two seasons in a wide age range of patients with grass-pollen-related ARC.
In this study, self-reported scores for HRQoL showed generally low levels of impairment across all age groups, with approximately a quarter of patients reporting scores denoting no symptoms. These low levels of HRQoL scores are consistent with the findings of a randomized, double-blind, placebo-controlled clinical trial conducted in Spain, where patients experienced an improvement in HRQoL and low levels of impairment following sublingual immunotherapy with grass plus olive pollen extract, after completing the treatment [30]. Similarly, our HRQoL outcomes corroborated those from investigations with five-grass pollen tablet performed in other countries, where patients with pollen-related ARC evaluated HRQoL using RQLQ [31–33]. Furthermore, our results extend the apparent beneficial effects of five-grass pollen tablet on HRQoL from the adult patient population to adolescents and children. In general, answers to the ESPIA questionnaire indicated a high level of satisfaction in all dimensions among adult patients previously treated with the five-grass pollen tablet, with similar results reported for adolescents and children in an exploratory analysis. These findings further corroborate high levels of satisfaction from prior observational studies of patients with grass-pollen ARC treated with the five-grass pollen tablet, across a variety of age groups including children [34–37]. Underpinning these findings, our study showed a more favourable clinical course of the condition following five-grass pollen tablet treatment, with 60.9% of patients experiencing less frequent symptoms, when evaluations were made using ARIA criteria. A significant improvement in daily living activities was particularly noteworthy, as was the significant reduction in the use of most-used symptomatic medications.
Overall, the above pattern of positive outcomes was recorded in this study, also translated into high levels of treatment adherence (96.8%) and compliance (93.3%). Patient and physician opinions on the utility of the five-grass pollen tablet were also positive. Patients, generally, reported high levels of satisfaction with the agent, and considered it to exhibit a positive impact on their condition. These findings were in line with the opinions of physicians, who believed that five-grass pollen tablet exhibited a clear positive effect on the HRQoL of the patients. In addition, most physicians claimed that they would use five-grass pollen tablet treatment themselves for ARC.
Although this study was not designed to evaluate safety, adverse drug reactions were generally infrequent, rarely resulted in withdrawal, and no adverse event was of a serious nature.
Strengths of the study include the use of age- and disease-specific validated instruments to evaluate HRQOL and adult patient satisfaction, the use of standardized criteria to assess disease dimensions, and the employment of specific instruments designed for this study to evaluate patient and physician opinions on therapy. Provided the seasonal nature of ACR, we also conducted this investigation to cover two different pollen seasons (2012, 2013), with data collection afterwards. These periods covered the main pollen season in Europe, which typically occurs between March and July, with minor variations occurring with changes in latitude [14]. The limitations of this study were its retrospective design, involving one-visit evaluation, and the subjective nature of the self-reported survey data collected. The selection of investigators and lack of both a centralized laboratory and intensive monitoring, among others, may also have hampered the internal validity. However, the sites and investigators for this study were selected to ensure a fair representation of the Spanish territory.
In conclusion, this observational, naturalistic study in ARC patients, treated with the five-grass pollen tablet, showed favourable levels of HRQoL and treatment satisfaction, with concomitant improvements in ARC and symptomatic medication use, which translated into high levels of treatment adherence and a positive attitude towards the five-grass pollen tablet.
Acknowledgements
The authors thank the scientific and editorial assistance provided by IMS Health and NewMed Publishing funded by Stallergenes Iberica SA. The authors also thank all participating patients and investigators.
Footnotes
Contributions: Stallergenes was responsible for the study design, collection, analysis and interpretation of data, and writing the manuscript. All authors contributed by data acquisition, critical appraisal of the manuscript, and approval of the final version of the manuscript. All authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Disclosure and potential conflict of interest: All authors with the exception of Isabel Botella received personal fees from Stallergenes for conducting the study. Isabel Botella is a full-time employee of Stallergenes Iberica SA. The international Committee of Medical Journal Editors (ICMJE) Potential Conflicts of Interest form for the authors is available for download at: http://www.drugsincontext.com/wp-content/uploads/2017/10/dic.212309-COI.pdf
Funding declaration: This study was funded by Stallergenes Iberica SA.
Correct attribution: Copyright © 2017 Antolín-Amerigo D, Tabar IA, Fernández-Nieto M, Callejo-Melgosa AM, Muñoz-Bellido FJ, Martínez-Alonso JC, Méndez-Alcalde JD, Reche M, Rodríguez-Trabado A, Rosado-Ingelmo A, Alonso-Gómez A, Blanco-González R, Alvarez-Fernandez JA, Botella I, Valls A, Cimarra M, Blanco C. https://doi.org/10.7573/dic.212309. Published by Drugs in Context under Creative Commons License Deed CC BY NC ND 4.0.
Provenance: submitted; externally peer reviewed.
Drugs in Context is published by BioExcel Publishing Ltd. Registered office: Plaza Building, Lee High Road, London, England, SE13 5PT.
BioExcel Publishing Limited is registered in England Number 10038393. VAT GB 252772009.
For all manuscript and submissions enquiries, contact the Editorial office: dic.editorial@bioexcelpublishing.com
For all permissions, rights and reprints, contact David Hughes: david.hughes@bioexcelpublishing.com
Peer review comments to author: 30 June 2017
References
- 1.Pereira C, Valero A, Loureiro C, Dávila I, Martinez-Cócera C, Murio C, Rico P, Palomino R. Iberian study of aeroallergens sensitisation in allergic rhinitis. Eur Ann Allergy Clin Immunol. 2006;38:186–94. [PubMed] [Google Scholar]
- 2.Spector SL. Overview of comorbid associations of allergic rhinitis. J Allergy Clin Immunol. 1997;99:S773–80. doi: 10.1016/s0091-6749(97)70126-x. http://dx.doi.org/10.1016/S0091-6749(97)70126-X. [DOI] [PubMed] [Google Scholar]
- 3.Tripathi A, Patterson R. Impact of allergic rhinitis treatment on quality of life. Pharmacoeconomics. 2001;19:891–9. doi: 10.2165/00019053-200119090-00001. http://dx.doi.org/10.2165/00019053-200119090-00001. [DOI] [PubMed] [Google Scholar]
- 4.Jacobsen L, Niggemann B, Dreborg S, Ferdousi HA, Halken S, Høst A, Koivkko A, Norberg LA, Valovirta E, Wahn U, Möller C The PAT Investigator Group. Specific immunotherapy has long-term preventive effect of seasonal and perennial asthma: 10-year follow-up on the PAT study. Allergy. 2007;62:943–8. doi: 10.1111/j.1398-9995.2007.01451.x. http://dx.doi.org/10.1111/j.1398-9995.2007.01451.x. [DOI] [PubMed] [Google Scholar]
- 5.Fokkens WJ, Jogi R, Reinartz S, Sidorenko I, Sitkauskiene B, van Oene C, Faris MA, Ellsworth A, Caldwell MF. Once daily fluticasone furoate nasal spray is effective in seasonal allergic rhinitis caused by grass pollen. Allergy. 2007;62:1078–84. doi: 10.1111/j.1398-9995.2007.01522.x. http://dx.doi.org/10.1111/j.1398-9995.2007.01522.x. [DOI] [PubMed] [Google Scholar]
- 6.Philip G, Nayak AS, Berger WE, Leynadier F, Vrijens F, Dass SB, Reiss TF. The effect of montelukast on rhinitis symptoms in patients with asthma and seasonal allergic rhinitis. Curr Med Res Opin. 2004;20:1549–58. doi: 10.1185/030079904x3348. http://dx.doi.org/10.1185/030079904X3348. [DOI] [PubMed] [Google Scholar]
- 7.Walker SM, Varney VA, Gaga M, Jacobson MR, Durham SR. Grass pollen immunotherapy: efficacy and safety during a 4-year follow-up study. Allergy. 1995;50:405–13. doi: 10.1111/j.1398-9995.1995.tb01170.x. http://dx.doi.org/10.1111/j.1398-9995.1995.tb01170.x. [DOI] [PubMed] [Google Scholar]
- 8.Bousquet J, Lockey R, Malling HJ. Allergen immunotherapy: therapeutic vaccines for allergic diseases. A WHO Position Paper. J Allergy Clin Immunol. 1998;102:558–62. doi: 10.1016/s0091-6749(98)70271-4. [DOI] [PubMed] [Google Scholar]
- 9.Cox L, Calderon MA. Subcutaneous specific immunotherapy for seasonal allergic rhinitis: a review of treatment practices in the US and Europe. Curr Med Res Opin. 2010;26:2723–33. doi: 10.1185/03007995.2010.528647. http://dx.doi.org/10.1185/03007995.2010.528647. [DOI] [PubMed] [Google Scholar]
- 10.Tabar AI, Arroabarren E, Echechipía S, García BE, Martin S, Alvarez-Puebla MJ. Three years of specific immunotherapy may be sufficient in house dust mite respiratory allergy. J Allergy Clin Immunol. 2011;127:57–63. doi: 10.1016/j.jaci.2010.10.025. http://dx.doi.org/10.1016/j.jaci.2010.10.025. [DOI] [PubMed] [Google Scholar]
- 11.Burks AW, Calderon MA, Casale T, Cox L, Demoly P, Jutel M, Nelson H, Akdis CA. Update on allergy immunotherapy: American Academy of Allergy, Asthma & Immunology/European Academy of Allergy and Clinical Immunology/PRACTALL consensus report. J Allergy Clin Immunol. 2013;131:1288–96. doi: 10.1016/j.jaci.2013.01.049. http://dx.doi.org/10.1016/j.jaci.2013.01.049. [DOI] [PubMed] [Google Scholar]
- 12.Canonica GW, Bousquet J, Casale T, Lockey RF, Baena-Cagnani CE, Pawankar R, Potter PC, Bousquet PJ, Cox LS, Durham SR, Nelson HS, Passalacqua G, Ryan DP, Brozek JL, Compalati E, Dahl R, Delgado L, van Wijk RG, Gower RG, Ledford DK, Filho NR, Valovirta EJ, Yusuf OM, Zuberbier T, Akhanda W, Almarales RC, Ansotegui I, Bonifazi F, Ceuppens J, Chivato T, Dimova D, Dumitrascu D, Fontana L, Kateralis CH, Kaulsay R, Kuna P, Larenas-Linnemann D, Manoussakis M, Nekam K, Nunes C, O’Hehir R, Olaguibel JM, Onder NB, Park JW, Priftanji A, Puy R, Sarmiento L, Scadding, Schmid-Grendelmeier P, Seberova E, Sepiashvili R, Solé D, Togias A, Tomino C, Toskala E, Van Beever H, Vieth S. Sub-lingual immunotherapy: World Allergy Organization position paper 2009. Allergy. 2009;64:1–59. doi: 10.1097/WOX.0b013e3181c6c379. http://dx.doi.org/10.1111/j.1398-9995.2009.02309.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hong J, Bielory L. Oralair®: sublingual immunotherapy for the treatment of grass pollen allergic rhinoconjunctivitis. Expert Rev Clin Immunol. 2011;7:437–44. doi: 10.1586/eci.11.36. http://dx.doi.org/10.1586/eci.11.36. [DOI] [PubMed] [Google Scholar]
- 14.Moingeon P, Hrabina M, Bergmann KC, Jaeger S, Frati F, Bordas V, Peltre G. Specific immunotherapy for common grass pollen allergies: pertinence of a five grass pollen vaccine. Int Arch Allergy Immunol. 2008;146:338–42. doi: 10.1159/000121468. http://dx.doi.org/10.1159/000121468. [DOI] [PubMed] [Google Scholar]
- 15.Chabre H, Gouyon B, Huet A, Baron-Bodo V, Nony E, Hrabina M, Fenaille F, Lautrette A, Bonvalet M, Maillère B, Bordas-Le, Floch V, Van Overtvelt L, Jain K, Ezan E, Batard T, Moingeon P. Molecular variability of group 1 and 5 grass pollen allergens between Pooideae species: implications for immunotherapy. Clin Exp Allergy. 2010;40:505–19. doi: 10.1111/j.1365-2222.2009.03380.x. http://dx.doi.org/10.1111/j.1365-2222.2009.03380.x. [DOI] [PubMed] [Google Scholar]
- 16.Rak S, Yang WH, Pedersen MR, Durham SR. Once-daily sublingual allergen-specific immunotherapy improves quality of life in patients with grass pollen-induced allergic rhinoconjunctivitis: a double-blind, randomised study. Qual Life Res. 2007;16:191–201. doi: 10.1007/s11136-006-9110-3. http://dx.doi.org/10.1007/s11136-006-9110-3. [DOI] [PubMed] [Google Scholar]
- 17.Wise SK, Woody J, Koepp S, Schlosser RJ. Quality of life outcomes with sublingual immunotherapy. Am J Otolaryngol. 2009;30:305–11. doi: 10.1016/j.amjoto.2008.06.003. http://dx.doi.org/10.1016/j.amjoto.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 18.Ciprandi G, Cadario G, Valle C, Ridolo E, Verini M, di Gioacchino M, Minelli M, Gangemi S, Sillano V, Colangelo C, Pravettoni V, Pellegrino R, Borrelli P, Fiorina A, Carosso A, Gasparini A, Riario-Sforza GG, Incorvaia C, Puccinelli P, Scurati S, Frati F. Sublingual immunotherapy in polysensitized patients: effect on quality of life. J Invest Allergol Clin Immunol. 2010;20:274–9. [PubMed] [Google Scholar]
- 19.Laury AM, Schlosser RJ, Wise SK. Sublingual immunotherapy and quality of life. Curr Opin Otolaryngol Head Neck Surg. 2013;21:252–5. doi: 10.1097/MOO.0b013e32835fcb22. http://dx.doi.org/10.1097/MOO.0b013e32835fcb22. [DOI] [PubMed] [Google Scholar]
- 20.Katotomichelakis M, Riga M, Tripsianis G, Balatsouras D, Kourousis C, Danielides G, Giotakis E, Danielides V. Predictors of quality of life improvement in allergic rhinitis patients after sublingual immunotherapy. Ann Otol Rhinol Laryngol. 2015;124:130–6. doi: 10.1177/0003489414565001. http://dx.doi.org/10.1177/0003489414565001. [DOI] [PubMed] [Google Scholar]
- 21.Juniper EF, Guyatt GH. Development and testing of a new measure of health status for clinical trials in rhinoconjunctivitis. Clin Exp Allergy. 1991;21:77–83. doi: 10.1111/j.1365-2222.1991.tb00807.x. http://dx.doi.org/10.1111/j.1365-2222.1991.tb00807.x. [DOI] [PubMed] [Google Scholar]
- 22.Juniper EF, Guyatt GH, Dolovich J. Assessment of quality of life in adolescents with allergic rhinoconjunctivitis: development and testing of a questionnaire for clinical trials. J Allergy Clin Immunol. 1994;93:413–23. doi: 10.1016/0091-6749(94)90349-2. http://dx.doi.org/10.1016/0091-6749(94)90349-2. [DOI] [PubMed] [Google Scholar]
- 23.Juniper EF, Howland WC, Roberts NB, Thompson AK, King DR. Measuring quality of life in children with rhinoconjunctivitis. J Allergy Clin Immunol. 1998;101:163–70. doi: 10.1016/s0091-6749(98)70380-x. http://dx.doi.org/10.1016/S0091-6749(98)70380-X. [DOI] [PubMed] [Google Scholar]
- 24.Soler R, de la Hoz B, Badia X, Mercadal J, Loranzo R, Benavides A, Roset M Grupo Carino. Validation of the Spanish version of the Rhinoconjunctivitis Quality of Life Questionnaire (RQLQ) Rev Clin Esp. 2004;204:131–8. doi: 10.1157/13058825. [DOI] [PubMed] [Google Scholar]
- 25.Justicia JL, Cardona V, Guardia P, Ojeda P, Olaguíbel JM, Vega JM, Vidal C, Baró E, Garcia MA. Validation of the first treatment-specific questionnaire for the assessment of patient satisfaction with allergen-specific immunotherapy in allergic patients: the ESPIA questionnaire. J Allergy Clin Immunol. 2013;131:1539–46. doi: 10.1016/j.jaci.2012.11.049. http://dx.doi.org/10.1016/j.jaci.2012.11.049. [DOI] [PubMed] [Google Scholar]
- 26.Bousquet J, Khaltaev N, Cruz AA, Denburg J, Fokkens WJ, Togias A, Zuberbier T, Baena-Cagnani CE, Canonica GW, van Weel C, Agache I, Ait-Khaled N, Bachert C, Blaiss MS, Bonini S, Boulet LP, Bousquet PJ, Camargos P, Carlsen KH, Chen Y, Custovic A, Dahl R, Demoly P, Douagui H, Durham SR, van Wilk RG, Kalayci O, Kaliner MA, Kim YY, Kowalski ML, Kuna P, Le LT, Lemiere C, Li J, Lockey RF, Mavale-Manuel S, Meltzer EO, Mohammad Y, Mullo J, Naclerio R, O’Hehir RE, Ohta K, Ouedraogo S, Palkonen S, Papadopoulos N, Passalacqua G, Pawankar R, Popov TA, Rabe KF, Rosado-Pinto J, Scadding GK, Simons FE, Toskala E, Valovirta E, van Cauwenberge P, Wang DY, Wickman M, Yawn BP, Yorgancioglu A, Yusuf OM, Zar H, Annesi-Maesano I, Bateman ED, Ben Kheder A, Boakye DA, Bouchard J, Burney P, Busse WW, Chan-Yeung M, Chavannes NH, Chuchalin A, Dolen WK, Emuzyte R, Grouse L, Humbert M, Jackson C, Johnson SL, Keith PK, Kemp JP, Klossek JM, Larenas-Linnemann D, Lipworth B, Malo JL, Marshall GD, Naspitz C, Nekam K, Niggemann B, Nizanowska-Mogilnicka E, Okamoto Y, Orru MP, Potter P, Price D, Stoloff SW, Vandenplas O, Viegi G, Williams D World Health Organization; GA(2)LEN; AllerGen. Allergic Rhinitis and its Impact on Asthma (ARIA) 2008 update (in collaboration with the World Health Organization, GA(2)LEN and AllerGen) Allergy. 2008;63(Suppl 86):8–160. doi: 10.1111/j.1398-9995.2007.01620.x. http://dx.doi.org/10.1111/j.1398-9995.2007.01620.x. [DOI] [PubMed] [Google Scholar]
- 27.U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) Guidance for Industry Allergic Rhinitis: Clinical Development Programs for Drug Products. Apr, 2000. [cited 2017 Mar]. Available from: http://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm064981.htm.
- 28.Haynes RB, Sackett DL, Taylor W, Show JC. Annotated and indexed bibliography on compliance of the therapeutic and preventive regimen. In: Haynes RB, Taylor W, Sackett DL, editors. Compliance in Health Care. Baltimore, MD: Johns Hopkins University Press; 1979. pp. 76–81. [Google Scholar]
- 29.de la Hoz Caballer B, Rodríguez M, Fraj J, Cerecedo I, Antolín-Amérigo D, Colás C. Allergic rhinitis and its impact on work productivity in primary care practice and a comparison with other common diseases: the cross-sectional study to evaluate work productivity in allergic Rhinitis compared with other common dIseases (CAPRI) study. Am J Rhinol Allergy. 2012;26:390–4. doi: 10.2500/ajra.2012.26.3799. http://dx.doi.org/10.2500/ajra.2012.26.3799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moreno-Ancillo A, Moreno C, Ojeda P, Domínguez C, Barasona MJ, García-Cubillana A, Martin S. Efficacy and quality of life with once-daily sublingual immunotherapy with grasses plus olive pollen extract without updosing. J Investig Allergol Clin Immunol. 2007;17:399–405. [PubMed] [Google Scholar]
- 31.Nelson HS, Nolte H, Creticos P, Maloney J, Wu J, Bernstein DI. Efficacy and safety of timothy grass allergy immunotherapy tablet treatment in North American adults. J Allergy Clin Immunol. 2011;127:72–80. doi: 10.1016/j.jaci.2010.11.035. http://dx.doi.org/10.1016/j.jaci.2010.11.035. [DOI] [PubMed] [Google Scholar]
- 32.Didier A, Malling HJ, Worm M, Horak F, Sussman G, Melac M, Soulié S, Zeldin RK. Post-treatment efficacy of discontinuous treatment with 300IR 5-grass pollen sublingual tablet in adults with grass pollen-induced allergic rhinoconjunctivitis. Clin Exp Allergy. 2013;43:568–77. doi: 10.1111/cea.12100. http://dx.doi.org/10.1111/cea.12100. [DOI] [PubMed] [Google Scholar]
- 33.Serrano E, Wahn HU, Didier A, Bachert C. 300IR 5-Grass pollen sublingual tablet offers relief from nasal symptoms in patients with allergic rhinitis. Am J Rhinol Allergy. 2014;28:471–76. doi: 10.2500/ajra.2014.28.4112. http://dx.doi.org/10.2500/ajra.2014.28.4112. [DOI] [PubMed] [Google Scholar]
- 34.Trebuchon F, Lhéritier-Barrand M, David M, Demoly P. Characteristics and management of sublingual allergen immunotherapy in children with allergic rhinitis and asthma induced by house dust mite allergens. Clin Transl Allergy. 2014;29:4–15. doi: 10.1186/2045-7022-4-15. http://dx.doi.org/10.1186/2045-7022-4-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Trebuchon F, David M, Demoly P. Medical management and sublingual immunotherapy practices in patients with house dust mite-induced respiratory allergy: a retrospective, observational study. Int J Immunopathol Pharmacol. 2012;25:193–206. doi: 10.1177/039463201202500122. http://dx.doi.org/10.1177/039463201202500122. [DOI] [PubMed] [Google Scholar]
- 36.Pastorello EA, Losappio L, Milani S, Manzotti G, Fanelli V, Pravettoni V, Agostinis F, Flores D’Arcais A, Dell’Albani I, Puccinelli P, Incorvaia C, Frati F. 5-grass pollen tablets achieve disease control in patients with seasonal allergic rhinitis unresponsive to drugs: a real-life study. J Asthma Allergy. 2013;6:127–33. doi: 10.2147/JAA.S53801. https://doi.org/10.2147/JAA.S53801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eberle P, Brueck H, Gall R, Hadler M, Sieber J, Karagiannis E. An observational, real-life safety study of a 5-grass pollen sublingual tablet in children and adolescents. Pediatr Allergy Immunol. 2014;25:760–6. doi: 10.1111/pai.12298. http://dx.doi.org/10.1111/pai.12298. [DOI] [PubMed] [Google Scholar]