Key Points
Question
What are the screening yield and tumor characteristics detected by combined mammography and magnetic resonance imaging (MRI) or ultrasonography in women diagnosed at 50 years or younger who underwent breast conservation and radiotherapy for breast cancer?
Findings
In this multicenter comparison study of 754 women, MRI screening detected 3.8 additional cancers and ultrasonography detected 2.4 additional cancers, most of which were stage 0 or stage 1, per 1000 women and increased sensitivity over mammography alone.
Meaning
In younger women who had undergone breast conservation therapy, the addition of MRI screening or ultrasonography to mammography can be considered.
Abstract
Importance
Younger women (aged ≤50 years) who underwent breast conservation therapy may benefit from breast magnetic resonance imaging (MRI) screening as an adjunct to mammography.
Objective
To prospectively determine the cancer yield and tumor characteristics of combined mammography with MRI or ultrasonography screening in women who underwent breast conservation therapy for breast cancers and who were 50 years or younger at initial diagnosis.
Design, Setting, and Participants
This multicenter, prospective, nonrandomized study was conducted from December 1, 2010, to January 31, 2016, at 6 academic institutions. Seven hundred fifty-four women who were 50 years or younger at initial diagnosis and who had undergone breast conservation therapy for breast cancer were recruited to participate in the study. Reference standard was defined as a combination of pathology and 12-month follow-up.
Interventions
Participants underwent 3 annual MRI screenings of the conserved and contralateral breasts in addition to mammography and ultrasonography, with independent readings.
Main Outcomes and Measures
Cancer detection rate, sensitivity, specificity, interval cancer rate, and characteristics of detected cancers.
Results
A total of 754 women underwent 2065 mammograms, ultrasonography, and MRI screenings. Seventeen cancers were diagnosed, and most of the detected cancers (13 of 17 [76%]) were stage 0 or stage 1. Overall cancer detection rate (8.2 vs 4.4 per 1000; P = .003) or sensitivity (100% vs 53%; P = .01) of mammography with MRI was higher than that of mammography alone. After the addition of ultrasonography, the cancer detection rate was higher than that by mammography alone (6.8 vs 4.4 per 1000; P = .03). The specificity of mammography with MRI or ultrasonography was lower than that by mammography alone (87% or 88% vs 96%; P < .001). No interval cancer was found.
Conclusions and Relevance
After breast conservation therapy in women 50 years or younger, the addition of MRI to annual mammography screening improves detection of early-stage but biologically aggressive breast cancers at acceptable specificity. Results from this study can inform patient decision making on screening methods after breast conservation therapy.
This cohort study examines magnetic resonance imaging or ultrasonography plus mammography in screening for a second breast cancer in women 50 years or younger who underwent breast conservation therapy.
Introduction
Women who are treated with breast conservation surgery and radiotherapy (BCT) remain at an increased risk for second breast cancers, which can be either a local recurrence or a new primary in the conserved and contralateral breast at 5-year (approximately 10%) and 10-year (approximately 20%) follow-up visits. Because early detection of second breast cancers at the asymptomatic phase in breast cancer survivors can improve their relative survival by 27% to 47%, the American Society of Clinical Oncology and the National Comprehensive Cancer Network recommend annual mammography screening or surveillance for women who received BCT. However, although mammography screening detects early-stage second breast cancers, lower sensitivity and higher interval cancer rates were observed in women with a personal history of breast cancer compared with women without, especially in women 50 years or younger and those with extremely dense breasts.
The American Cancer Society guideline suggests that there is insufficient evidence to recommend or advise against annual magnetic resonance imaging (MRI) screenings for women with a personal history of breast cancer, while MRI screening is recommended as an adjunct to mammography for women with genetic mutations or women with more than a 20% to 25% lifetime risk of breast cancer. Women with a personal history of breast cancer, however, have a heterogeneous risk for developing a second breast cancer. According to a model of cancer risk, women with early-stage, hormone receptor–positive breast cancer who were not BRCA mutation carriers fit a 20% lifetime cancer-risk threshold for developing a second breast cancer if they were 50 years or younger and had undergone BCT but not a total mastectomy. As MRI or ultrasonography screenings to detect second breast cancers in women after BCT have been increasingly used despite limited evidence regarding their comparative effectiveness, it has been reported that 45% to 54% of women who were screened for breast cancer using MRI or ultrasonography had a personal history of breast cancer. Retrospective studies have shown similar cancer detection rates but significantly lower false-positive screening, defined as an unnecessary recall, biopsy, or short-term follow-up of MRI or ultrasonography screening in women with a personal history of breast cancer compared with those without a personal history of breast cancer or those with a genetic risk for or a family history of breast cancer.
To our knowledge, there has been no prospective study to compare, among the same participants, the performance of a combination of imaging techniques for breast cancer screening: mammography and MRI or ultrasonography vs mammography alone for women after BCT. Thus, the purpose of our study was to compare outcomes of a combined mammography and MRI or ultrasonography screening in women who had received BCT for breast cancer before age 50 years.
Methods
Study Participants and Study Conduct
For this prospective, nonrandomized, multicenter, observational cohort study (clinicaltrials.gov Identifier: NCT01257152), we recruited asymptomatic women who were 50 years or younger at the initial diagnosis of breast cancer, who had undergone BCT, and who came in for a mammogram at 6 different academic centers (Seoul National University Hospital, Samsung Medical Center, Asan Medical Center, Seoul St Mary's Hospital, Severance Hospital, and Seoul National University Bundang Hospital) between December 1, 2010, and January 31, 2016. Each facility met the American College of Radiology Imaging Network (ACRIN) MRI trial standards and the American College of Radiology Breast MRI Accreditation Program standards. This study was approved by the respective institutional review board of Seoul National University Hospital, Samsung Medical Center, Asan Medical Center, Seoul St Mary's Hospital, Severance Hospital, and Seoul National University Bundang Hospital. Written informed consent was obtained from all participants.
The inclusion criteria were as follows: women 20 years or older and 50 years or younger at the initial diagnosis of ductal carcinoma in situ or invasive breast cancer; women whose final margins were negative, defined as no ink on tumor and who had finished radiotherapy at least 6 months before the study; women with no history of a breast biopsy within 6 months prior to the study; women who had not had contralateral mastectomies; women who had no known metastatic disease; women who were not pregnant or lactating; women who had no present signs or symptoms of breast cancer; women with no contraindications to MRI examinations; and women with no prior breast MRI, breast ultrasonography, or mammography screenings within 6 months before the study. Radiotherapy and systemic treatment protocol was standardized across the academic centers according to the National Comprehensive Cancer Network guidelines. Annual mammography, breast ultrasonography, and breast MRI were performed for both conserved and contralateral breasts during the 3-year study period—from December 1, 2010, to January 31, 2016—with a maximum interval of 2 months between each imaging modality. Clinical breast examinations were performed every 6 months. Two-view mammograms, including mediolateral oblique and craniocaudal view, were performed using full-field digital mammography units. Whole-breast screening ultrasonography was performed by radiologists (N.C., B.-K.H., M.S.B., E.S.K., E.Y.C., S.H.K., B.J.K., E.-K.K., H.J.M., S.M.K., H.H.K., and W.K.M.). Breast MRI was performed using a 1.5-T or 3.0-T scanner with a dedicated breast coil according to previously detailed protocols.
Image Interpretation and Outcome Measures
Radiologists with at least 5 years of breast MRI experience at each site and blinded to the results of the other studies (N.C., B.-K.H., M.S.B., E.S.K., E.Y.C., S.H.K., B.J.K., E.-K.K., H.J.M., S.M.K., H.H.K., and W.K.M.) interpreted mammography, ultrasonography, and MRI data and recorded the American College of Radiology Breast Imaging Reporting and Data System (BI-RADS) final assessment category and likelihood of malignancy score (score range, 0%-100%; higher scores indicate higher possibility of malignancy). Although radiologists were blinded to the other modality images, previous images were allowed to be compared within the same imaging modality; thus, preoperative MRIs were compared for interpretation of MRIs. Then, a site investigator (N.C., B.-K.H., M.S.B., E.S.K., E.Y.C., S.H.K., B.J.K., E.-K.K., H.J.M., S.M.K., H.H.K., or W.K.M.), a breast radiologist who did not participate in the initial interpretation of images, recorded a combined category after reviewing both mammogram and ultrasonogram, mammogram and MRI, and a combination of the 3 modalities, respectively. A clinical recommendation was made on the basis of combined interpretations. For lesions with BI-RADS final assessment category 4 or more, image-guided needle biopsy was performed. Guidance modality was determined at the discretion of the radiologists who interpreted images. The MRI-guided, vacuum-assisted biopsy was performed for lesions identified on MRI alone. Determination of cancer was made on the basis of the biopsy or imaging follow-up results within 365 days after the imaging examinations. Cancer detection rate (CDR), sensitivity, specificity, recall rate, positive predictive value for recall (PPV1), short-term follow-up rate, biopsy rate, PPV for biopsy (PPV3), and diagnostic accuracy assessed by area under the (receiver operating characteristic) curve (AUC) were calculated for each imaging modality and its respective combinations. The CDR was defined as the number of detected cancers per 1000 examinations. A negative examination result was defined as a BI-RADS category of 1 or 2, and a positive examination result was defined as a BI-RADS category of 3, 4A, 4B, 4C, or 5. An interval cancer was defined as a breast cancer diagnosed after the last negative examination result because of clinical symptoms but before the next scheduled examination.
Statistical Analysis
We compared screening outcomes between each image modality and its combinations using generalized estimating equations, where a participant was defined as a cluster, or McNemar test. The independence working correlation structure was used for PPV or negative predictive value, and the exchangable correlation structure was used for the other outcomes in the generalized estimating equation. The AUCs were estimated and compared using the method accounting for the correlations. The 95% CIs of CDR, specificity, recall rate, PPV1, short-term follow-up, and biopsy rate were estimated considering a participant as a cluster. Because the number of detected or diagnosed cancers was small, exact CI for sensitivity was estimated by the Wilson score method. The adjusted P values for multiple testing were calculated using the Sidak method for each screening outcome. Clinicopathologic features and treatment data of the initial breast cancers were also obtained and compared between the women with and without second breast cancers using the Fisher exact test. The features showing P < .20 were included for multivariable logistic regression analysis to identify independent factors associated with second breast cancers. The stepwise variable selection method was applied. Statistical analyses were performed using SAS software, version 9.2 (SAS Institute Inc). A 2-sided P < .05 was considered to indicate a significant difference, and CIs are shown at the 95% confidence level.
Results
Participant Characteristics
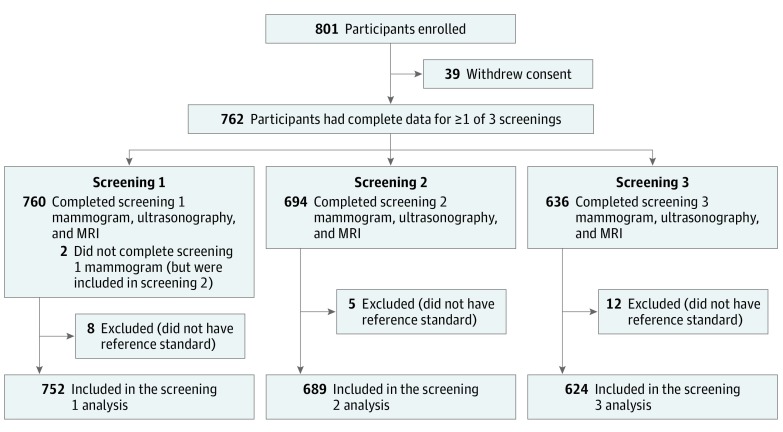
From December 1, 2010, through January 31, 2016, a total of 754 eligible women participated in the study. Of these women, 2 (0.3%) did not undergo mammography but underwent ultrasonography and MRI in the first round, completed the second and third rounds, and were included for the second-year and third-year analyses but excluded from the first-year analysis (Figure). Systemic metastasis was found in 7 women (0.9%), and they were excluded from the study. No MRI examination results were excluded because of poor image quality. Thus, a total of 754 women with reference standards completed 2065 screenings: 752 screenings in the first year, 689 in the second year, and 624 in the third year (Table 1). Six hundred ninety-three participants (91.9%) underwent a preoperative MRI. Sixty-one women (8.1%) underwent genetic testing and 17 (2.3%) were found to be BRCA mutation carriers, and 2 of the 17 had second breast cancers. Characteristics of the study cohort, including clinicopathologic features and treatment data of initial breast cancers, can be found in eAppendix in the Supplement.
Figure. Flowchart of Study Protocol.
MRI indicates magnetic resonance imaging.
Table 1. Characteristics of the Study Participants and Their Initial Cancers.
| Characteristic | No. (%) | ||
|---|---|---|---|
| Screening 1 (n = 752) |
Screening 2 (n = 689) |
Screening 3 (n = 624) |
|
| Participants | |||
| Age at imaging examinations, mean (SD), y | 43.0 (5.7) | 44.1 (5.7) | 45.2 (5.6) |
| Median (IQR), y | 44.0 (8) | 45.0 (8) | 46.0 (8) |
| Age group at imaging examinations, y | |||
| <40 | 184 (24.5) | 134 (19.4) | 84 (13.5) |
| 40-54 | 568 (75.5) | 555 (80.6) | 540 (86.5) |
| Menopausal status | |||
| Premenopausal | 326 (43.4) | 284 (41.2) | 241 (38.6) |
| Perimenopausal | 14 (1.9) | 13 (1.9) | 12 (1.9) |
| Postmenopausal | 412 (54.8) | 392 (56.9) | 371 (59.5) |
| BRCA1 or BRCA2 mutation | |||
| Positive | 17 (2.3) | 12 (1.7) | 8 (1.3) |
| Negative | 44 (5.9) | 35 (5.1) | 30 (4.8) |
| Unknown | 691 (91.9) | 642 (93.2) | 586 (93.9) |
| Family history of breast cancer | |||
| Absent | 577 (76.7) | 534 (77.5) | 490 (78.5) |
| Present | 70 (9.3) | 65 (9.4) | 58 (9.3) |
| Unknown | 105 (14.0) | 90 (13.1) | 76 (12.2) |
| Mammographic breast density | |||
| Fatty | 7 (0.9) | 11 (1.6) | 11 (1.8) |
| Scattered fibroglandular | 109 (14.5) | 120 (17.4) | 128 (20.5) |
| Heterogeneously dense | 442 (58.8) | 402 (58.3) | 355 (56.9) |
| Extremely dense | 194 (25.8) | 156 (22.6) | 130 (20.8) |
| Initial Cancer | |||
| Stage | |||
| DCIS | 57 (7.6) | 50 (7.3) | 43 (6.9) |
| Invasive | |||
| Stage I | 356 (47.3) | 332 (48.2) | 300 (48.1) |
| Stage II | 292 (38.8) | 265 (38.5) | 241 (38.6) |
| Stage III | 47 (6.3) | 42 (6.1) | 40 (6.4) |
| Histologic grade | |||
| Low to intermediate | 420 (55.9) | 396 (57.5) | 360 (57.7) |
| High | 247 (32.8) | 220 (31.9) | 197 (31.6) |
| Unknown | 85 (11.3) | 73 (10.6) | 67 (10.7) |
| Hormone receptor statusa | |||
| Positive | 554 (73.7) | 512 (74.3) | 470 (75.3) |
| Negative | 196 (26.1) | 176 (25.5) | 153 (24.5) |
| Unknown | 2 (0.3) | 1 (0.1) | 1 (0.2) |
| HER2 statusb | |||
| Positive | 91 (12.1) | 84 (12.2) | 75 (12.0) |
| Negative | 649 (86.3) | 597 (86.6) | 541 (86.7) |
| Unknown | 12 (1.6) | 8 (1.2) | 8 (1.3) |
Abbreviations: DCIS, ductal carcinoma in situ; HER2, human epidermal growth factor receptor 2; IQR, interquartile range.
Hormone receptor–positive status was estrogen receptor positive or progesterone receptor positive. It was defined as the presence of 10% or more positively stained nuclei at ×10 magnification.
HER2-positive status was defined as ERBB2/HER2 gene amplification (fluorescent in situ hybridization) or was scored as 3+ on immunohistochemical staging for ERBB2/HER2, which was scored as 0, 1+, 2+, or 3+.
Cancer Detection, Interval Cancer Rate, and Tumor Characteristics
A total of 17 breast cancers were identified in 17 women (2.3%): 12 identified in the first year, 3 in the second year, and 2 in the third year. The mean time from the initial surgery to the detection of a second breast cancer was 17.8 (range, 6-41) months (range, 6-41 months). All cancers were detected by 1 of 3 imaging modalities. No cancer was detected on clinical breast examinations. No cancer was found during the intervals between screenings and at the 12-month follow-up after the third-year interpretation.
Of the 17 women with detected cancers, 10 (58.8%) were diagnosed with cancer in the ipsilateral breast and 7 (41.2%) were diagnosed with cancer in the contralateral breast, and 13 (76.5%) were in stage 0 or stage 1. Only 1 cancer (5.9%) had nodal micrometastasis, and 10 (58.8%) were invasive ductal carcinomas with a median (interquartile range) size of 10.5 (14.5) mm. Two cancers (11.8%) were detected by mammography alone, 0 by ultrasonography alone, 3 (17.6%) by MRI alone, 1 (5.9%) by mammogram and MRI, 5 (29.4%) by ultrasonography and MRI, and 6 (35.3%) by all 3 imaging modalities (eTable 1 in the Supplement). Two cancers detected by mammography alone presented with suspicious calcifications on mammography, but they did not show suspicious enhancement on MRI. The clinicopathologic features and treatment data of initial breast cancers in women with recurrent cancers and the characteristics of the detected cancers are summarized in eTable 2 in the Supplement. Most of the detected cancers (12 [70.6%]) were biologically aggressive hormone receptor–negative and/or ERBB2/HER2 (OMIM 164870)–positive tumors.
Incremental Cancer Detection Yield
The overall CDR was 8.2 per 1000 examinations (17 of 2065) (Table 2). The CDR of mammography with MRI was higher than that of mammography alone (8.2 vs 4.4 per 1000; P = .003). The CDR of mammography with ultrasonography was higher than that of mammography alone (6.8 vs 4.4 per 1000; P = .03).
Table 2. Three-Year Mammography, Ultrasonography, and MRI Screening Performances for 754 Participants.
| Clinicopathologic Feature | Mammo | US | MRI | Mammo + US | P Value for Mammo vs Mammo + USa | Mammo + MRI | P Value for Mammo vs Mammo + MRIa | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Women, No./Total No. | Estimate (95% CI) | Women, No./Total No. | Estimate (95% CI) | Women, No./Total No. | Estimate (95% CI) | Women, No./Total No. | Estimate (95% CI) | Women, No./Total No. | Estimate (95% CI) | |||
| Yield, per 1000 examinations | 9/2065 | 4.4 (1.5-7.2) | 11/2065 | 5.3 (2.2-8.5) | 15/2065 | 7.3 (3.6-10.9) | 14/2065 | 6.8 (3.2-10.3) | .03 | 17/2065 | 8.2 (4.3-12.2) | .003 |
| AUC | 0.79 (0.65-0.93) | 0.87 (0.77-0.97) | 0.96 (0.90-1.00) | 0.92 (0.84-1.00) | .18 | 0.99 (0.94-1.00) | .01 | |||||
| Sensitivity, %b | 9/17 | 52.9 (31.0-73.8) | 11/17 | 64.7 (41.3-82.7) | 15/17 | 88.2 (65.7-96.7) | 14/17 | 82.4 (59.0-93.8) | .07c | 17/17 | 100 (81.6-100) | .01c |
| Specificity, % | 1966/2048 | 96.0 (94.9-97.1) | 1850/2048 | 90.3 (88.9-91.8) | 1842/2048 | 89.9 (88.4-91.4) | 1794/2048 | 87.6 (85.9-89.3) | <.001 | 1781/2048 | 87.0 (85.2-88.7) | <.001 |
| Recall rate, % | 91/2065 | 4.4 (3.3-5.5) | 209/2065 | 10.1 (8.6-11.6) | 221/2065 | 10.7 (9.2-12.2) | 268/2065 | 13.0 (11.2-14.7) | <.001 | 284/2065 | 13.8 (12.0-15.5) | <.001 |
| PPV1, % | 9/91 | 9.9 (3.6-16.2) | 11/209 | 5.3 (2.2-8.3) | 15/221 | 6.8 (3.5-10.1) | 14/268 | 5.2 (2.5-7.9) | .007 | 17/284 | 6.0 (3.2-8.8) | .09 |
| Short-term follow-up rate, % | 75/2065 | 3.6 (2.6-4.7) | 181/2065 | 8.8 (7.4-10.1) | 154/2065 | 7.5 (6.2-8.7) | 230/2065 | 11.1 (9.5-12.8) | <.001 | 210/2065 | 10.2 (8.6-11.8) | <.001 |
| Biopsy rate, % | 11/2065 | 0.5 (0.2-0.8) | 23/2065 | 1.1 (0.7-1.6) | 51/2065 | 2.5 (1.8-3.1) | 29/2065 | 1.4 (0.9-1.9) | <.001 | 56/2065 | 2.7 (2.0-3.4) | <.001 |
| PPV3, %b | 8/11 | 72.7 (43.4-90.3) | 8/23 | 34.8 (18.8-55.1) | 12/51 | 23.5 (14.0-36.8) | 11/29 | 37.9 (22.7-56.0) | .03 | 16/56 | 28.6 (18.4-41.5) | .006 |
Abbreviations: AUC, area under the (receiver operating characteristic) curve; Mammo, mammography; MRI, magnetic resonance imaging; PPV1, positive predictive value for recalls including BI-RADS 3, 4A, 4B, 4C, or 5; PPV3, positive predictive value for biopsies performed; US, ultrasonography.
The P value was adjusted using the Sidak method for each screening outcome.
95% CI estimated from the Wilson score method.
P value calculated using the McNemar test.
When MRI was added to mammography, 3.8 more cancers per 1000 women (95% CI, 2.8-4.9; P = .003) were detected. When ultrasonography was added to mammography, 2.4 more cancers per 1000 women (95% CI, 1.7-3.1; P = .03) were detected.
Regarding the yield for invasive carcinoma, when MRI was added to mammography, 2.4 more cancers per 1000 women were detected (95% CI, 1.5-3.3; P = .03). When ultrasonography was added to mammography, 1.5 more cancers per 1000 women were detected (95% CI, 0.9-2.0; P = .12) (eTable 3 in the Supplement).
Sensitivity, Specificity, and AUC
The sensitivity of mammography with MRI screening (100% [17 of 17]; 95% CI, 81.6%-100%) was higher than that of mammography alone (52.9% [9 of 17]; 95% CI, 31.0%-73.8%; P = .01) (Table 2). However, the sensitivity of a screening with mammography and ultrasonography (82.4% [14 of 17]; 95% CI, 59.0%-93.8%) was not different from that of mammography alone (52.9% [9 of 17]; 95% CI, 31.0%-73.8%; P = .07).
The specificity of mammography with MRI (87.0% [1781 of 2048]; 95% CI, 85.2%-88.7%) or mammography with ultrasonography (87.6% [1794 of 2048]; 95% CI, 85.9%-89.3%) was lower than that of mammography alone (96.0% [1966 of 2048]; 95% CI, 94.9%-97.1%; P < .001 vs P < .001) (Table 2).
The AUC increased from 0.79 (95% CI, 0.65-0.93) to 0.99 (95% CI, 0.94-1.00) (P = .01) when MRI was added to mammography; however, the AUC was not significantly differently increased from 0.79 (95% CI, 0.65-0.93) to 0.92 (95% CI, 0.84-1.00) (P = .18) when ultrasonography was added to mammography (Table 2).
Recall, Biopsy, and Short-term Follow-up Rates
After the addition of MRI to mammography, there was an increase in the recall rate from 4.4% (91 of 2065; 95% CI, 3.3%-5.5%) to 13.8% (284 of 2065; 95% CI, 12.0%-15.5%; P < .001), in the biopsy rate from 0.5% (11 of 2065; 95% CI, 0.2%-0.8%) to 2.7% (56 of 2065; 95% CI, 2.0%-3.4%; P < .001), and in the short-term follow-up rate from 3.6% (75 of 2065; 95% CI, 2.6%-4.7%) to 10.2% (210 of 2065; 95% CI, 8.6%-11.8%; P < .001) (Table 2). After the addition of ultrasonography to mammography, the recall rate, biopsy rate, and short-term follow-up rate (each of which has P < .001) also increased (Table 2).
PPV for Recall and Biopsy
The PPV1 for the recall was not different after the addition of MRI (9.9% [9 of 91]; 95% CI, 3.6%-16.2% vs 6.0% [17 of 284]; 95% CI, 3.2%-8.8%; P = .09), although the PPV1 was significantly decreased when ultrasonography was added to mammography alone (9.9% [9 of 91]; 95% CI, 3.6%-16.2% vs 5.2% [14 of 268]; 95% CI, 2.5%-7.9%; P = .007). The PPV3 for biopsy decreased after the addition of MRI (72.7% [8 of 11]; 95% CI, 43.4%-90.3% vs 28.6% [16 of 56]; 95% CI, 18.4%-41.5%; P = .006) or ultrasonography (72.7% [8 of 11]; 95% CI, 43.4%-90.3% vs 37.9% [11 of 29]; 95% CI, 22.7%-56.0%; P = .03) to mammography alone (Table 2).
Characteristics of Women With Second Breast Cancers
Characteristics of women with second breast cancers and women without second breast cancers are summarized in eTable 4 in the Supplement.
Discussion
In this study, the results showed that for women who had received BCT for breast cancer at 50 years or younger, the addition of MRI to a mammography increased screening sensitivity (100% vs 52.9%; P = .01) and detected 3.8 (95% CI, 2.8-4.9; P = .003) more cancers per 1000 women compared with mammography alone. Most detected cancers (13 of 17 [76.5%]) were stage 0 or stage 1, and all but 1 invasive cancer was node negative. Only 1 cancer was stage IIB, which was comparable to the results of other screening MRI studies performed for high-risk women. No cancer was detected on clinical breast examinations. In addition, no interval cancer was found during the study period, contrary to the reported 7.5 interval cancers per 1000 women that were detected in a mammography screening study in women younger than 50 years with a personal history of breast cancer. A reduction in biologically aggressive, interval breast cancer rates and advanced-stage breast cancer rates can be a useful intermediate surrogate measure for screening benefits.
Regarding the overall CDR, our rate of 8.2 per 1000 examinations (17 of 2065) is lower than the CDR range of 16 to 30 per 1000 for women who have familial breast cancer or are BRCA mutation carriers. The relatively lower CDR in this study might be because 91.9% (693 of 754) of our participants had undergone preoperative MRI that had been reported to be associated with a reduced second breast cancer incidence in contralateral breasts. In addition, women at the highest risk of recurrence (whose final margins were positive or who did not receive radiotherapy) were excluded from the study. In previous retrospective studies carried out in single center for women with a personal history of breast cancers, a highly selected population with negative mammography or nonblinded interpretation of mammography results and MRI might have overestimated CDR for MRI screenings.
One of the major drawbacks of MRI screening is its high false-positive rate and, as a result, the associated costs and morbidity. Compared with mammography alone, the addition of MRI increased the recall rate (from 4.4% to 13.8%; P < .001), biopsy rate (from 0.5% to 2.7%; P < .001), and short-term follow-up rate (from 3.6% to 10.2%; P < .001), which led to decreased specificity (from 96% to 87%; P < .001). However, 47.1% (8 of 17) of cancers might have been missed with mammography alone. Considering the harms caused by false-negative findings, the false-positive findings caused by MRI screening examinations might be within acceptable ranges if informed women choose them.
The addition of ultrasonography to mammography screening tended to increase sensitivity of the screening from 52.9% (9 of 17) to 82.4% (14 of 17); however, this difference was not significant at P = . 07. Therefore, when women who received BCT at 50 years or younger, especially those with dense breasts, are unable to undergo MRI screenings, ultrasonography might be considered.
Limitations
There are several limitations to this study. First, as there was no control group undergoing mammography alone, we were not able to compare the interval cancer rate or advanced stage cancer rate among mammography, ultrasonography, and MRI screening. Second, we could not evaluate the cost-effectiveness and effect of MRI or ultrasonography screening on survival benefit. The use of MRI screening with a 2-year or 3-year interval with abbreviated MRI sequences would be more cost-effective. Metastasis-free survival is better in an annual MRI screening group for women with BRCA mutations or familial risk compared with matched controls. Third, there were 17 women who were BRCA mutation carriers, and 2 of these women had second breast cancers, which may be an overestimated CDR in women after BCT. The National Comprehensive Cancer Network recommends genetic counseling for women 45 years or younger with a personal history of breast cancer. However, in this study, only 8.1% (61 of 754) of women underwent genetic testing before making a treatment decision. Last, only 17 recurrent breast cancers were diagnosed during the study period. The small number of detected cancers caused wide 95% CIs; thus, precise estimate for sensitivity was difficult.
Conclusions
This study suggests that the addition of MRI to mammography screening improved the detection of early-stage breast cancers at acceptable specificity in women who had BCT at 50 years or younger. Our study results can be used not only to inform patient and clinician decision making regarding the best methods of screening after BCT but also to develop more personalized screening guidelines and recommendations for women at increased risk for breast cancer.
eAppendix. Characteristics of the Study Cohort
eTable 1. Summary of Cancer Detection and Tumor Characteristics of 754 Participants
eTable 2. Characteristics of Detected Cancers in Women Treated With Breast Conservation Therapy
eTable 3. Screening Performances for Invasive Cancers in 754 Participants Screened Over 3 Years by Mammography, Ultrasound, and MRI
eTable 4. Characteristics of Women With Second Breast Cancers Versus Those Without Cancers
References
- 1.Darby S, McGale P, Correa C, et al. ; Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) . Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet. 2011;378(9804):1707-1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wapnir IL, Anderson SJ, Mamounas EP, et al. . Prognosis after ipsilateral breast tumor recurrence and locoregional recurrences in five National Surgical Adjuvant Breast and Bowel Project node-positive adjuvant breast cancer trials. J Clin Oncol. 2006;24(13):2028-2037. [DOI] [PubMed] [Google Scholar]
- 3.Cuzick J, Sestak I, Baum M, et al. ; ATAC/LATTE investigators . Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 10-year analysis of the ATAC trial. Lancet Oncol. 2010;11(12):1135-1141. [DOI] [PubMed] [Google Scholar]
- 4.Houssami N, Ciatto S, Martinelli F, Bonardi R, Duffy SW. Early detection of second breast cancers improves prognosis in breast cancer survivors. Ann Oncol. 2009;20(9):1505-1510. [DOI] [PubMed] [Google Scholar]
- 5.Khatcheressian JL, Hurley P, Bantug E, et al. ; American Society of Clinical Oncology . Breast cancer follow-up and management after primary treatment: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2013;31(7):961-965. [DOI] [PubMed] [Google Scholar]
- 6.Runowicz CD, Leach CR, Henry NL, et al. . American Cancer Society/American Society of Clinical Oncology Breast Cancer Survivorship Care Guideline. J Clin Oncol. 2016;34(6):611-635. [DOI] [PubMed] [Google Scholar]
- 7.National Comprehensive Cancer Network Breast cancer, version 2. http://www.nccn.org/professionals/physician_gls/pdf/breast.pdf. Accessed June 15, 2016.
- 8.Houssami N, Abraham LA, Miglioretti DL, et al. . Accuracy and outcomes of screening mammography in women with a personal history of early-stage breast cancer. JAMA. 2011;305(8):790-799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saslow D, Boetes C, Burke W, et al. ; American Cancer Society Breast Cancer Advisory Group . American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin. 2007;57(2):75-89. [DOI] [PubMed] [Google Scholar]
- 10.Houssami N, Abraham LA, Kerlikowske K, et al. . Risk factors for second screen-detected or interval breast cancers in women with a personal history of breast cancer participating in mammography screening. Cancer Epidemiol Biomarkers Prev. 2013;22(5):946-961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Punglia RS, Hassett MJ. Using lifetime risk estimates to recommend magnetic resonance imaging screening for breast cancer survivors. J Clin Oncol. 2010;28(27):4108-4110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schacht DV, Yamaguchi K, Lai J, Kulkarni K, Sennett CA, Abe H. Importance of a personal history of breast cancer as a risk factor for the development of subsequent breast cancer: results from screening breast MRI. AJR Am J Roentgenol. 2014;202(2):289-292. [DOI] [PubMed] [Google Scholar]
- 13.Kim SJ, Chung SY, Chang JM, Cho N, Han W, Moon WK. Ultrasound screening of contralateral breast after surgery for breast cancer. Eur J Radiol. 2015;84(1):54-60. [DOI] [PubMed] [Google Scholar]
- 14.Wernli KJ, DeMartini WB, Ichikawa L, et al. ; Breast Cancer Surveillance Consortium . Patterns of breast magnetic resonance imaging use in community practice. JAMA Intern Med. 2014;174(1):125-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berg WA, Zhang Z, Lehrer D, et al. ; ACRIN 6666 Investigators . Detection of breast cancer with addition of annual screening ultrasound or a single screening MRI to mammography in women with elevated breast cancer risk. JAMA. 2012;307(13):1394-1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lehman CD, Lee JM, DeMartini WB, et al. . Screening MRI in women with a personal history of breast cancer. J Natl Cancer Inst. 2016;108(3):djv349. [DOI] [PubMed] [Google Scholar]
- 17.DeMartini WB, Ichikawa L, Yankaskas BC, et al. . Breast MRI in community practice: equipment and imaging techniques at facilities in the Breast Cancer Surveillance Consortium. J Am Coll Radiol. 2010;7(11):878-884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gweon HM, Cho N, Han W, et al. . Breast MR imaging screening in women with a history of breast conservation therapy. Radiology. 2014;272(2):366-373. [DOI] [PubMed] [Google Scholar]
- 19.D’Orsi CJ, Bassett LW, Berg WA, et al. . BI-RADS: mammography In: D’Orsi CJ, Mendelson EB, Ikeda DM, et al. , eds. Breast Imaging Reporting and Data System: ACR BI-RADS—Breast Imaging Atlas. 4th ed Reston, VA: American College of Radiology; 2003. [Google Scholar]
- 20.Sickles EA, D’Orsi CJ. ACR BI-RADS follow-up and outcome monitoring In: ACR BI-RADS Atlas, Breast Imaging Reporting and Data System. Reston, VA: American College of Radiology; 2013. [Google Scholar]
- 21.Obuchowski NA. Nonparametric analysis of clustered ROC curve data. Biometrics. 1997;53(2):567-578. [PubMed] [Google Scholar]
- 22.Rao JN, Scott AJ. A simple method for the analysis of clustered binary data. Biometrics. 1992;48(2):577-585. [PubMed] [Google Scholar]
- 23.Kriege M, Brekelmans CT, Boetes C, et al. ; Magnetic Resonance Imaging Screening Study Group . Efficacy of MRI and mammography for breast-cancer screening in women with a familial or genetic predisposition. N Engl J Med. 2004;351(5):427-437. [DOI] [PubMed] [Google Scholar]
- 24.Sardanelli F, Podo F, D’Agnolo G, et al. ; High Breast Cancer Risk Italian Trial . Multicenter comparative multimodality surveillance of women at genetic-familial high risk for breast cancer (HIBCRIT study): interim results. Radiology. 2007;242(3):698-715. [DOI] [PubMed] [Google Scholar]
- 25.Weinstein SP, Localio AR, Conant EF, Rosen M, Thomas KM, Schnall MD. Multimodality screening of high-risk women: a prospective cohort study. J Clin Oncol. 2009;27(36):6124-6128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuhl C, Weigel S, Schrading S, et al. . Prospective multicenter cohort study to refine management recommendations for women at elevated familial risk of breast cancer: the EVA trial. J Clin Oncol. 2010;28(9):1450-1457. [DOI] [PubMed] [Google Scholar]
- 27.Warner E, Hill K, Causer P, et al. . Prospective study of breast cancer incidence in women with a BRCA1 or BRCA2 mutation under surveillance with and without magnetic resonance imaging. J Clin Oncol. 2011;29(13):1664-1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Irwig L, Houssami N, Armstrong B, Glasziou P. Evaluating new screening tests for breast cancer. BMJ. 2006;332(7543):678-679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Berg WA. Supplemental breast cancer screening in women with dense breasts should be offered with simultaneous collection of outcomes data. Ann Intern Med. 2016;164(4):299-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yi A, Cho N, Yang KS, Han W, Noh DY, Moon WK. Breast cancer recurrence in patients with newly diagnosed breast cancer without and with preoperative MR imaging: a matched cohort study. Radiology. 2015;276(3):695-705. [DOI] [PubMed] [Google Scholar]
- 31.Keller DL. In defense of screening for breast cancer with magnetic resonance imaging. JAMA Intern Med. 2014;174(8):1417. [DOI] [PubMed] [Google Scholar]
- 32.Berg WA, Blume JD, Adams AM, et al. . Reasons women at elevated risk of breast cancer refuse breast MR imaging screening: ACRIN 6666. Radiology. 2010;254(1):79-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kuhl CK, Schrading S, Strobel K, Schild HH, Hilgers RD, Bieling HB. Abbreviated breast magnetic resonance imaging (MRI): first postcontrast subtracted images and maximum-intensity projection—a novel approach to breast cancer screening with MRI. J Clin Oncol. 2014;32(22):2304-2310. [DOI] [PubMed] [Google Scholar]
- 34.Saadatmand S, Obdeijn IM, Rutgers EJ, et al. . Survival benefit in women with BRCA1 mutation or familial risk in the MRI screening study (MRISC). Int J Cancer. 2015;137(7):1729-1738. [DOI] [PubMed] [Google Scholar]
- 35.National Comprehensive Cancer Network Genetic/familial high-risk assessment: breast and ovarian, version 2. https://www.nccn.org/professionals/physician_gls/pdf/genetics_screening.pdf. Accessed July 15, 2016.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix. Characteristics of the Study Cohort
eTable 1. Summary of Cancer Detection and Tumor Characteristics of 754 Participants
eTable 2. Characteristics of Detected Cancers in Women Treated With Breast Conservation Therapy
eTable 3. Screening Performances for Invasive Cancers in 754 Participants Screened Over 3 Years by Mammography, Ultrasound, and MRI
eTable 4. Characteristics of Women With Second Breast Cancers Versus Those Without Cancers