Abstract
Importance
Even though 15% to 50% of patients with head and neck squamous cell carcinoma (HNSCC) experience recurrence, relatively little is known regarding patterns of treatment failure and postrecurrence outcomes after chemoradiotherapy using modern radiation techniques (intensity-modulated radiotherapy [IMRT]). Recurrence patterns are significantly affected by variations in the quality of radiotherapy, which may confound findings from multicenter trials.
Objective
To assess patterns of treatment failure and postrecurrence outcomes for patients with HNSCC treated with contemporary radiotherapy techniques.
Design, Setting, and Participants
This large single-institution cohort study reviewed the outcomes of 1000 consecutive patients with stage III to IVB oropharyngeal carcinoma (n = 703), laryngeal carcinoma (n = 126), or hypopharyngeal carcinoma (n = 46) treated with definitive IMRT with or without concurrent chemotherapy, as well as patients with oral cavity carcinoma (n = 125) treated with postoperative IMRT with or without concurrent systemic therapy, from December 1, 2001, to December 31, 2013, with a median follow-up of 65.1 months among surviving patients. Data analysis was performed from January 31, 2016, to February 17, 2017.
Main Outcomes and Measures
Patterns of treatment failure and overall survival following locoregional failure or distant metastasis.
Results
Among the 1000 patients (186 women and 814 men; mean [SD] age, 59.3 [10.8] years), there were no marginal or isolated out-of-radiation-field failures. Among subsites, the cumulative incidence of local failure was highest among patients with oral cavity carcinoma vs those with oropharyngeal carcinoma (hazard ratio, 5.2; 95% CI, 3.1-8.6; P < .001). Furthermore, patients with oral cavity carcinoma experienced significantly shorter survival following distant metastasis (hazard ratio, 3.66; 95% CI, 1.98-6.80; P < .001). Patients with oropharyngeal carcinoma positive for human papillomavirus or p16 lived longer after locoregional failure compared with patents with oropharyngeal carcinoma negative for human papillomavirus or p16 (median survival, 36.5 vs 13.6 months; P = .007) but not after distant metastasis. Salvage surgery was associated with improved overall survival following locoregional failure (hazard ratio, 0.51; 95% CI, 0.34-0.77; P = .001); oligometastatic disease (1 vs ≥2 lesions: hazard ratio, 0.32; 95% CI, 0.16-0.63; P = .001) was associated with improved overall survival following distant metastasis.
Conclusions and Relevance
Overall survival after recurrence of HNSCC is influenced by the HNSCC subsite and human papillomavirus or p16 status, as well surgical and systemic interventions. An oligometastatic phenotype characterizes patients with solitary metastasis after chemoradiotherapy. These findings have important implications for clinical trial designs for HNSCC in the recurrent and oligometastatic setting.
This cohort study assesses patterns of treatment failure and postrecurrence outcomes among patients with head and neck squamous cell carcinoma after chemoradiotherapy using contemporary radiotherapy techniques.
Key Points
Question
What are the patterns of treatment failure and postrecurrence outcomes among patients with head and neck squamous cell carcinoma (HNSCC) treated with modern definitive therapy?
Findings
This cohort study of 1000 patients with HNSCC found that locoregionally recurrent disease among patients positive for human papillomavirus is associated with an improved prognosis and that, compared with other HNSCCs, patients with oral cavity carcinoma displayed unique patterns of treatment failure and particularly poor outcomes after metastasis. The study also found a subset of patients with oligometastasis who experienced prolonged survival.
Meaning
Survival after recurrence of HNSCC is influenced by the HNSCC subsite and human papillomavirus status. An oligometastatic phenotype is associated with improved survival.
Introduction
Intensity-modulated radiotherapy (IMRT) has become the standard of care for definitive and postoperative treatment of head and neck squamous cell carcinoma (HNSCC), replacing conventional radiation techniques. Intensity-modulated radiotherapy has allowed for more precise delivery of radiation to areas of tumor and lymphatic basins while sparing surrounding structures in the head and neck, which has resulted in a significant decrease in toxic effects.
A detailed understanding of the natural history and patterns of treatment failure associated with HNSCC is critical for the design of clinical trials in the setting of recurrence. However, studies of recurrence after radiotherapy for HNSCC from multicenter trials can be significantly affected by variations in the quality of IMRT and treatment center volume. As such, even though 15% to 50% of patients with HNSCC will experience recurrence, true patterns of treatment failure, as well as their associations with IMRT treatment fields with the use of adequate and modern treatment techniques, are not well established. The study of the patterns of disease spread after recurrence following IMRT, therefore, needs to first ensure adequate locoregional therapy to differentiate recurrences due to suboptimally delivered radiotherapy from recurrences resulting from truly treatment-resistant disease.
We describe the natural history following recurrence of HNSCC in a large population treated with contemporary chemoradiotherapy techniques, with few treatment failures associated with the quality of radiotherapy. By studying this population, in which treatment failures due to inadequate radiation targeting are minimal, we reveal nuances in treatment failure patterns and postrecurrence outcomes that reflect disease course and biology and are relatively unbiased by artifacts of suboptimal radiotherapy.
Methods
The Memorial Sloan Kettering Cancer Center tumor registry and radiation oncology departmental databases were used to identify patients with stage III to IVB HNSCC with a diagnosis of oropharyngeal carcinoma (OPC), laryngeal carcinoma (LC), or hypopharyngeal carcinoma (HPC) treated with definitive IMRT or patients with oral cavity carcinoma (OCC) treated with postoperative IMRT between December 1, 2001, and December 31, 2013, without recurrent or distant metastatic disease at the time of treatment. Only patients who completed the prescribed course of IMRT were included. Approval for this study was provided by the Memorial Sloan Kettering Cancer Center Institutional Review Board, who granted a waiver of informed consent for retrospective analysis of individual patient data.
Patient and Staging Information
A complete medical history, a physical examination with fiberoptic nasopharyngoscopy, and computed tomography (CT) and/or magnetic resonance imaging of the head and neck were performed as part of the pretreatment evaluation. Additional imaging included a plain radiograph, CT of the chest, or positron emission tomography (PET). All patients were restaged according to the 7th edition of the American Joint Committee on Cancer staging manual.
For patients with OPC, human papillomavirus (HPV) or p16 positivity was defined as either p16 positivity determined by immunohistochemistry and/or HPV positivity by in situ hybridization (ISH). Immunohistochemical results for p16 were considered positive when strong and diffuse nuclear and cytoplasmic staining was observed in 70% or more of tumor cells. Human papillomavirus ISH was performed with a probe for HPV types 16, 18, 31, 33, and 51; positivity for HPV ISH was defined as any positive staining. Cases were defined as negative for HPV or p16 with negative p16 immunohistochemical results or negative results on HPV ISH. Cases were defined as HPV unknown if neither p16 immunohistochemistry nor HPV ISH information was available.
Radiotherapy
For each patient, the recommended course of treatment was formulated with the input of a multidisciplinary team including a radiation oncologist, medical oncologist, head and neck surgeon, pathologist, and radiologist. Details regarding head and neck IMRT planning have previously been described in detail; the IMRT field design evolved over the course of the study period based on observed patterns of treatment failure (eAppendix in the Supplement).
Systemic Therapy
High-risk patients were treated with concurrent systemic therapy based on the treatment recommendation of the multidisciplinary team, taking into account clinicopathologic factors and patient comorbidity and preference. In most cases, cisplatin was administered at a dosage of 100 mg/m2 every 3 weeks or 30 to 40 mg/m2 weekly. Alternatively, patients were treated with cetuximab given as an initial loading dose of 400 mg/m2 followed by weekly cycles of 250 mg/m2. In a minority of cases, other systemic regimens were used, including cisplatin and bevacizumab (as part of a clinical trial), carboplatin and paclitaxel, or carboplatin and fluorouracil.
Follow-up
Patients were evaluated weekly during IMRT, every 2 to 3 months for the first 2 years following treatment, and, subsequently, every 4 to 6 months. Follow-up visits consisted of a physical examination and flexible fiberoptic nasopharyngoscopy. Three months after treatment, PET and CT or magnetic resonance imaging of the neck were performed. Afterward, imaging studies were performed as clinically indicated. Chest radiographs or other imaging were performed annually to assess for distant metastases (DMs) as indicated. Patients with evidence of metastatic disease underwent systemic restaging, typically with whole-body PET and CT scan.
Event Definitions
Locoregional or distant recurrences were documented by biopsy unless there was clear radiographic evidence of metastatic disease. Local treatment failure was defined as recurrence at the site of the initial primary tumor, regional treatment failure as the development of recurrence in cervical lymph nodes, and distant treatment failure as recurrence in an organ outside of the head and neck. The presence of oligometastatic disease was determined based on the results of a clinical evaluation and systemic radiographic imaging (typically with whole-body PET and CT scan) demonstrating fewer than 5 metastatic lesions.
Statistical Analysis
Statistical analysis was performed from January 31, 2016, to February 17, 2017. Local, regional, and distant treatment failure following the end of IMRT were estimated separately using cumulative incidence analysis and compared using the Gray test. The time to each event following upfront treatment was measured from the end of IMRT. The time to each event following recurrence was measured from the date of diagnosis of recurrence. Deaths without the event of interest or treatment failure patterns different from the event of interest (ie, local, regional, or distant) were considered competing risk events. Overall survival (OS) was estimated using the Kaplan-Meier method with log-rank testing for comparison between groups. Univariate and multivariate analyses were conducted with Cox proportional hazards regression models. Statistical analyses were performed with SPSS version 24 (IBM Corp) and R version 3.2 (http://www.R-project.org); all analyses were 2-sided and used a significance level of P < .05.
Results
Baseline patient and tumor characteristics are shown in eTable 1 in the Supplement. The median follow-up among surviving patients was 65.1 months. A total of 908 of 1000 patients (90.8%) received concurrent systemic therapy; among these patients, no significant difference was observed with regard to the proportion that received cisplatin every 3 weeks or cetuximab vs other agents or regimens (determined by the χ2 test). The median follow-up after initial treatment was 56.6 months, and median follow-up after recurrence was 12.1 months.
Treatment Failure Patterns Across HNSCC Subsites
A total of 243 patients ultimately developed recurrence. A total of 147 patients developed locoregional failure (LRF): 112 were isolated, 28 were synchronous with DM, and 7 occurred more than 3 months following DM. A total of 143 patients experienced DM: 96 were isolated, 28 were synchronous, and 19 occurred more than 3 months following LRF.
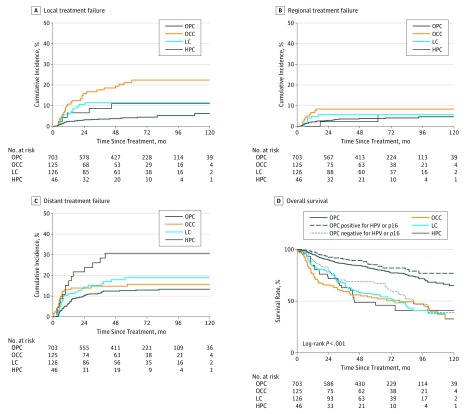
At 5 years, the cumulative incidence of local treatment failure was 4.2% among patients with OPC, 21.3% among patients with OCC, 11.4% among patients with LC, and 11.1% among patients with HPC (Figure 1A). The cumulative incidence of regional nodal failure was 2.9% among patients with OPC, 8.4% among patients with OCC, 5.6% among patients with LC, and 2.2% among patients with HPC (Figure 1B). The cumulative incidence of DM was 12.7% among patients with OPC, 15.7% among patients with OCC, 18.9% among patients with LC, and 30.1% among patients with HPC (Figure 1C). Local, regional, and distant treatment failures were less common among patients with OPC positive for HPV or p16 vs those with OPC negative for HPV or p16, with 2-year cumulative incidences of 0.9% vs 12.3% (P < .001) for local treatment failure, 2.7% vs 6.2% (P = .06) for regional treatment failure, and 10.1% vs 16.8% (P = .05) for distant treatment failure (eFigure 1 in the Supplement). Five-year rates of OS by subsite were 80.8% for OPC (HPV or p16 positive, 85.2%; HPV or p16 negative, 66.9%), 53.2% for OCC, 56.3% for LC, and 48.9% for HPC (Figure 1D), with survival significantly improved among patients with OPC positive for HPV or p16 compared with those with OPC negative for HPV or p16 and all other subsites (P < .001).
Figure 1. Cumulative Incidence of Treatment Failures Across Subsites.
A, Local treatment failure. B, Regional treatment failure. C, Distant failure. D, Overall survival across subsites. Compared with oropharyngeal carcinoma (OPC), oral cavity carcinoma (OCC) demonstrated the highest cumulative incidence of local treatment failure (hazard ratio [HR], 5.2; 95% CI, 3.1-8.6; P < .001), followed by laryngeal carcinoma (LC) (HR, 2.6; 95% CI, 1.4-4.9; P = .004) and hypopharyngeal carcinoma (HPC) (HR, 2.5; 95% CI, 1.0-6.3; P = .06). Compared with OPC, OCC also demonstrated the highest cumulative incidence of regional treatment failure (HR, 2.2; 95% CI, 1.0-45; P = .04), followed by HPC (HR, 1.6; 95% CI, 0.5-5.1; P = .40) and LC (HR, 1.4, 95% CI, 0.6-3.3; P = .40). Hypopharyngeal carcinoma demonstrated the highest incidence of distant treatment failure compared with OPC (HR, 2.6; 95% CI, 1.5-4.6), followed by LC (HR, 1.5; 95% CI, 0.9-2.4; P = .08) and OCC (HR, 1.3; 95% CI, 0.8-2.2; P = .31). Overall survival was significantly longer for patients with OPC positive for human papillomavirus (HPV) or p16 compared with all other subsites combined (HR, 0.45; 95% CI, 0.36-0.57; P < .001).
Comparisons of the patterns of treatment failure between OCC and non-OCC subsites demonstrated a higher proportion of isolated LRF as opposed to DM or synchronous LRF and DM among 29 of 45 patients with OCC (64.4%) (eFigure 2 in the Supplement) despite the fact that all patients with non-OCC HNSCC received unimodality or bimodality treatment and 51 of 125 patients with OCC (40.8%) received trimodality therapy. Pairwise comparisons of cumulative incidence across subsites demonstrated increased rates of local and regional treatment failure associated with OCC (Figure 1A and B). Fifty-one patients with OCC had indications for concurrent systemic therapy (32 extracapsular extension, 12 positive margin, and 7 both), of which 48 received systemic therapy (94.1%; 2 refused, and 1 was not a medical candidate). Three of the remaining 74 patients (4.1%), without positive margin or extracapsular extension, received systemic therapy based on multiple other adverse features. No difference in LRF was observed between patients with OCC based on receipt of systemic therapy, and higher risk of local and regional treatment failure among patients with OCC was still observed when excluding patients who did not receive systemic therapy. Rates of local failure and regional failure were no different between patients who received cisplatin every 3 weeks compared with those who received weekly cisplatin or carboplatin and fluoruracil (2-year local and regional control, 94.7% vs 96.8%; P = .21; and 94.6% vs 95.3%, P = .45, respectively). Patients with HPC demonstrated the highest rates of DM.
Outcomes Following Locoregional Recurrence
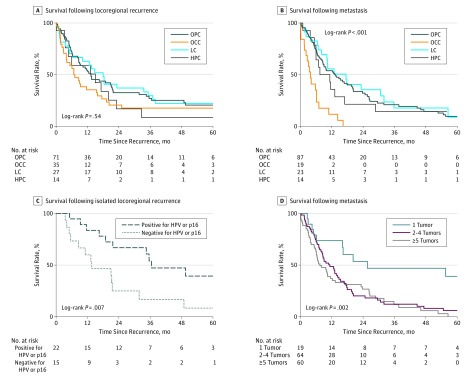
Patient characteristics at the time of LRF are shown in eTable 2 in the Supplement. Overall survival following LRF did not differ between patients with OPC, OCC, LC, or HPC (Figure 2A). Results of univariate and multivariate analysis performed among patients with LRF are shown in the Table. Univariate analysis revealed a Karnofsky performance status (KPS) greater than 70, an age younger than 70 years, receipt of salvage surgery (eFigure 3 in the Supplement), and salvage irradiation to be associated with survival following LRF. On multivariate analysis, a KPS greater than 70 and receipt of salvage surgery were associated with survival. In patients with OPC, survival following LRF was significantly longer among those positive for HPV or p16 compared with those negative for HPV or p16 (median OS, 36.5 vs 13.6 months; P = .007) (Figure 2C). Among patients with OCC and LRF, no significant difference in OS following LRF was observed based on margin status (positive vs negative margin) or the presence of extracapsular extension (presence vs absence). Among patients who underwent a modified radical neck dissection, a median of 32 lymph nodes were removed (range, 12-78 lymph nodes), and only 1 patient had fewer than 18 nodes removed.
Figure 2. Survival Following Locoregional Recurrence or Metastasis.
A, Survival following locoregional recurrence according to head and neck primary tumor subsite. B, Survival following metastasis according to head and neck primary tumor subsite. C, Survival following isolated locoregional failure according to human papillomavirus (HPV) or p16 status. D, Survival following metastasis according to number of metastatic tumors. HPC indicates hypopharyngeal carcinoma; LC, laryngeal carcinoma; OCC, oral cavity carcinoma; and OPC, oropharyngeal carcinoma
Table. Univariate and Multivariate Analyses of Predictors of Death Following Locoregional Failure or Distant Metastasis.
| Variable | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Death following locoregional failure | ||||
| KPS >70 | 0.34 (0.18-0.59) | <.001 | 0.46 (0.26-0.81) | .007 |
| Age <70 y | 0.62 (0.40-0.96) | .03 | 0.74 (0.47-1.14) | .17 |
| Subsite (oral cavity vs other) | 1.40 (0.91-2.15) | .13 | NA | NA |
| Salvage surgery | 0.44 (0.30-0.64) | <.001 | 0.51 (0.34-0.77) | .001 |
| Salvage re-irradiation | 0.54 (0.30-0.96) | .04 | 0.72 (0.40-1.31) | .29 |
| Time to locoregional failure | 0.99 (0.97-1.00) | .10 | NA | NA |
| Death following metastasis | ||||
| KPS >70 | 0.28 (0.18-0.42) | <.001 | 0.39 (0.25-0.61) | <.001 |
| Age <70 y | 0.65 (0.39-1.07) | .09 | NA | NA |
| Solitary metastatic tumor | 0.34 (0.17-0.65) | .001 | 0.32 (0.16-0.63) | .001 |
| Cetuximab treatment | 0.54 (0.36-0.80) | .002 | 0.48 (0.31-0.73) | .001 |
| Subsite (oral cavity vs other) | 3.85 (2.26-6.54) | <.001 | 3.66 (1.98-6.80) | <.001 |
| Time to metastasis | 0.97 (0.95-0.99) | .002 | 0.98 (0.96-1.00) | .05 |
| Organ of metastasis (lung vs other) | 0.72 (0.45-1.17) | .19 | NA | NA |
Abbreviations: HR, hazard ratio; KPS, Karnofsky performance status; NA, not applicable.
Patterns of Regional Nodal Recurrence
A total of 78 patients experienced regional nodal recurrence as the first site of treatment failure (eFigure 4 in the Supplement). No marginal treatment failures were observed, and 4 out-of-field treatment failures occurred in conjunction with in-field treatment failures (3 in superficial anterior cervical nodes in patients with OCC) (eFigure 5 in the Supplement). In definitive IMRT cases (OPC, LC, and HPC [n = 875]), 55 of 63 regional treatment failures (87.3%) involved recurrence at sites of treated gross disease (eTable 3 in the Supplement). In such cases, isolated regional treatment failure in the elective treatment volumes, outside of sites of gross disease, occurred in 8 of 875 patients (0.9%). Recurrence in elective volumes was more frequent in OCC (12 of 125 [9.6%]), most commonly outside of the dissected neck (eTable 4 and eFigure 6 in the Supplement).
Outcomes Following DM
The time from DM to death was significantly shorter among patients with OCC than among patients with OPC, LC, or HPC (Figure 2B; median OS: OCC, 3.9 months; non-OCC, 12.9 months; P < .001). On univariate analysis, the factors associated with survival following metastasis included a KPS greater than 70, a solitary metastatic tumor, receipt of a cetuximab-containing palliative regimen, a non-OCC primary tumor, and the time to metastasis. On multivariate analysis, the factors associated with survival following DM included the time to metastasis, a KPS greater than 70, a non-OCC primary tumor (Figure 2B), receipt of a cetuximab-containing palliative regimen (eFigure 7 in the Supplement), and a solitary metastatic tumor (Figure 2D and Table). The time from DM to death was not significantly different between patients with OPC positive for HPV or p16 and those with OPC negative for HPV or p16 (hazard ratio, 1.1; 95% CI, 0.6-2.3; P = .72; eFigure 8 in the Supplement). No difference in survival was observed according to organ of metastasis (lung vs other; hazard ratio, 0.72; 95% CI, 0.45-1.17; P = .19; eTable 5 in the Supplement). Across the entire cohort, OS following isolated LRF was significantly longer than following DM (hazard ratio, 0.7; 95% CI, 0.5-0.9; P = .01).
Oligometastasis
Nineteen patients presented with a single site of DM, 13 of which were in the lung, 2 in noncervical lymph nodes, 1 in bone, 1 in soft tissue, 1 in skin, and 1 in the heart. Seventeen of the lesions were detected on routine follow-up imaging, and 2 patients presented with symptoms prompting further evaluation. All solitary metastatic lesions were verified by biopsy. Median OS was significantly improved among patients with a single metastasis (25.7 months) vs those with 2 to 4 (11.3) or 5 or more metastases (7.5 months) (P = .002) (Figure 2D). Patients who received definitive local therapy with surgery or radiotherapy (n = 14) for a single metastasis survived longer than those who did not (n = 5; 2-year OS, 55.7% vs 20%; P < .001), although the patients who did not receive local therapy were of poorer KPS. Among patients with solitary metastasis, 7 of 19 (36.8%) were from OPC positive for HPV or p16 (eTable 6 in the Supplement), but no significant difference in survival following DM between HPV- or p16-positive and HPV- or p16-negative OPC solitary metastatic disease was seen (5-year survival, 21% vs 33%; P = .65). Furthermore, no difference was seen in survival following DM when comparing OPC positive for HPV or p16 and the remainder of HNSCC subsites (5-year survival, 21% vs 46%; P = .66). No difference was seen with regard to median time to metastasis between patients with 1 (8.7 months), 2 to 4 (9.0 months), or 5 or more lesions (9.7 months) (P = .91).
Discussion
In this cohort of 1000 patients with locally advanced HNSCC treated with definitive locoregional therapy, we have performed a detailed analysis of the patterns of treatment failure and outcomes following recurrence. Accurate analysis of recurrence patterns and outcomes after recurrence can be significantly affected by variations or errors in radiation target delineation that can affect rates of LRF and OS by approximately 20% and hamper interpretation of multi-institutional prospective trials in both the upfront and recurrent or metastatic settings. As such, this large, single-institution study in which patients were treated in a relatively uniform manner minimizes the confounder of variation in the quality of locoregional treatment with that of biologically aggressive disease and allows for analysis of the true patterns of recurrence and natural history after adequate treatment. This finding is supported by the fact that no marginal or isolated out-of-field recurrences were observed in our treatment cohort. In addition to IMRT, the quality of other treatment modalities, including supportive care, likely contribute to the improved outcomes achieved at high-volume centers.
Our study reveals that patients with OCC experience unique patterns of treatment failure and outcomes compared with patients with other HNSCC primary tumor sites. In particular, the primary pattern of treatment failure is locoregional, within the radiation field, despite many patients receiving trimodality therapy. In addition, following DM, patients with OCC experience poor OS (median, 3.9 months). In smaller studies, poor outcomes have been seen in the setting of pulmonary metastasis from OCC. This finding may be a reflection of more aggressive biology and resistance to chemotherapy and radiation, as well as poor performance status resulting from upfront trimodality treatment of OCC and associated morbidity. Genomic data have begun to reveal subsets of oral cancers with distinct molecular profiles and unique molecular drivers, including a subset of patients with CASP8 (OMIM 601763) with or without a FAT1 (OMIM 600976) mutation. Further work is needed to better characterize the underlying genomic and biological basis for particularly aggressive and treatment-refractory disease in the setting of metastatic OCC to identify effective treatments for this population.
Patients with a solitary metastatic lesion at the time of DM experienced significantly improved outcomes, with a significant proportion living 5 or more years. Most of these patients underwent local therapy to the site of oligometastasis with either surgery or radiotherapy with definitive intent. The limited number of patients with a solitary metastatic lesion did not allow for thorough analysis of the effectiveness of such therapies, although it is consistent with reports of improved survival following resection of limited pulmonary metastases in HNSCC and prospective randomized data suggesting a benefit for consolidative local therapy in oligometastatic non–small cell lung cancer. This finding, in addition to the observation that salvage surgery is strongly associated with improved survival following LRF, suggests a real effect of definitive therapies for limited recurrent or metastatic disease. Most of the solitary metastatic lesions in our cohort presented in the lungs, and some may be attributable to second primary squamous cell carcinomas of the lung. Most of the solitary metastatic lesions (13 of 19) were detected in asymptomatic patients with the use of full-body PET or CT scans in routine follow-up, suggesting that this imaging modality may be preferable for follow-up.
We found that patients with OPC positive for HPV or p16 survived significantly longer after LRF compared with those with OPC negative for HPV or p16. Fakhry et al retrospectively assessed the natural history following recurrence of OPC positive for p16 vs OPC negative for p16 among patients enrolled in the Radiation Therapy Oncology Group trials 0129 or 0522 and found that survival following both DM and LRF was significantly better in the group with OPC positive for p16. We did not find a difference in OS after DM or solitary metastasis between patients with OPC positive for HPV or p16 and patients with OPC negative for HPV or p16, but both studies are limited in sample size. Additional larger studies will therefore be needed to clarify whether the prognosis following DM in disease positive for HPV or p16 is distinct from that of disease negative for HPV or p16. Across all subsites, our multivariable analysis additionally revealed a survival difference following salvage surgery after locoregional recurrence, which was also seen by Fakhry et al in the setting of recurrent OPC.
Reduction of electively irradiated nodal basins (treatment of nodal regions without evidence of gross disease but that are at risk for micrometastatic involvement) represents a means to render IMRT less toxic. Our study demonstrated that, in definitive cases, 87.3% of regional recurrences occurred at the sites of gross disease, with isolated recurrences in elective nodal volumes occurring in less than 1% of cases, similar to prior work. The low rate of treatment failures in electively treated nodal basins raises the possibility that the dose of radiation to these areas may be reduced or possibly eliminated in select cases, as suggested by a recent prospective trial that demonstrated no compromise in outcomes with elimination of elective nodal coverage in select patients who responded well to induction chemotherapy.
We identified 3 cases of recurrence in the anterior superficial lymphatic chain after IMRT for OCC, a location that is not traditionally ascribed significant risk, although treatment failures have been previously noted. Consideration should be given to elective nodal coverage of this region in the postoperative setting with the use of a bolus to ensure an adequate dose superficially.
Limitations
Our study is limited by its retrospective nature and associated biases. The subsites were not matched for varying stages of disease, which may affect differences in the outcomes observed but also more accurately reflects differences between subsites in disease stage at presentation. Furthermore, systemic therapy was not delivered uniformly across the entire cohort, although the proportions of patients receiving cisplatin every 3 weeks, for which the most evidence exists, as opposed to an alternative agent or regimen were similar among patients who received systemic therapy across HNSCC subsites. Owing to the evolving epidemiologic patterns of HNSCC and the increased incidence of OPC positive for HPV, other subsites are underrepresented in our cohort.
Conclusions
We have shown that patients with OCC demonstrate unique treatment failure patterns and postrecurrence outcomes. In addition, survival after recurrence of HNSCC is influenced by KPS, subsite, HPV or p16 status, salvage surgery, burden of disease, and the palliative use of cetuximab. These findings have important implications for clinical trials assessing treatment de-escalation and approaches for patients with recurrent or metastatic HNSCC, particularly those with oligometastatic disease.
eAppendix. Methods
eTable 1. Baseline Characteristics
eTable 2. Patient Characteristics at the Time of Recurrence
eTable 3. Locations of Regional Nodal Failures in Relation to IMRT Treatment Fields Across Disease Subsites
eTable 4. Oral Cavity Regional Nodal Failures and Relationship to Neck Dissection
eTable 5. First Organs of Distant Metastasis Across HNSCC Subsites
eTable 6. Burden of Metastatic Disease Across Head and Neck Squamous Cell Carcinoma Subsites
eFigure 1. Cumulative Incidences of (A) Local, (B) Regional and (C) Distant Failure in HPV/p16+ vs HPV/p16– Oropharyngeal Squamous Cell Carcinoma
eFigure 2. Patterns of Failure Across Subsites
eFigure 3. Survival Following Locoregional Recurrence According to Receipt of Salvage Surgery
eFigure 4. Patterns of Regional Nodal Failure Across Head and Neck Squamous Cell Carcinoma Subsites
eFigure 5. Examples of Out-of-Field Superficial Anterior Cervical Node Failures in Patients With Oral Cavity Squamous Cell Carcinoma Following Postoperative Intensity Modulated Radiation Therapy
eFigure 6. Examples of Regional Failures Contralateral to the Dissected Neck in Oral Cavity Patients Treated With Postoperative IMRT
eFigure 7. Survival Following Metastasis According to Receipt of Palliative Systemic Therapy Regimen Containing Cetuximab
eFigure 8. Survival Following Distant Metastasis According to Human Papillomavirus (HPV)/p16 Status
eReferences.
References
- 1.Nutting CM, Morden JP, Harrington KJ, et al. ; PARSPORT trial management group . Parotid-sparing intensity modulated versus conventional radiotherapy in head and neck cancer (PARSPORT): a phase 3 multicentre randomised controlled trial. Lancet Oncol. 2011;12(2):127-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pfister DG. NCCN clinical practice guidelines in oncology: head and neck cancers. Version 1. https://www.nccn.org/professionals/physician_gls/pdf/head-and-neck.pdf. Accessed September 1, 2016.
- 3.Chao KS, Ozyigit G, Tran BN, Cengiz M, Dempsey JF, Low DA. Patterns of failure in patients receiving definitive and postoperative IMRT for head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2003;55(2):312-321. [DOI] [PubMed] [Google Scholar]
- 4.Mendenhall WM, Amdur RJ, Morris CG, Kirwan JM, Li JG. Intensity-modulated radiotherapy for oropharyngeal squamous cell carcinoma. Laryngoscope. 2010;120(11):2218-2222. [DOI] [PubMed] [Google Scholar]
- 5.Setton J, Caria N, Romanyshyn J, et al. . Intensity-modulated radiotherapy in the treatment of oropharyngeal cancer: an update of the Memorial Sloan-Kettering Cancer Center experience. Int J Radiat Oncol Biol Phys. 2012;82(1):291-298. [DOI] [PubMed] [Google Scholar]
- 6.Peters LJ, O’Sullivan B, Giralt J, et al. . Critical impact of radiotherapy protocol compliance and quality in the treatment of advanced head and neck cancer: results from TROG 02.02. J Clin Oncol. 2010;28(18):2996-3001. [DOI] [PubMed] [Google Scholar]
- 7.Wuthrick EJ, Zhang Q, Machtay M, et al. . Institutional clinical trial accrual volume and survival of patients with head and neck cancer. J Clin Oncol. 2015;33(2):156-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC Cancer Staging Manual and the future of TNM. Ann Surg Oncol. 2010;17(6):1471-1474. [DOI] [PubMed] [Google Scholar]
- 9.Ang KK, Harris J, Wheeler R, et al. . Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363(1):24-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dutta PR, Riaz N, McBride S, et al. . Postoperative PET/CT and target delineation before adjuvant radiotherapy in patients with oral cavity squamous cell carcinoma. Head Neck. 2016;38(suppl 1):E1285-E1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gomez DR, Zhung JE, Gomez J, et al. . Intensity-modulated radiotherapy in postoperative treatment of oral cavity cancers. Int J Radiat Oncol Biol Phys. 2009;73(4):1096-1103. [DOI] [PubMed] [Google Scholar]
- 12.Gutiontov S, Leeman J, Lok B, et al. . Cervical nodal level V can safely be omitted in the treatment of locally advanced oropharyngeal squamous cell carcinoma with definitive IMRT. Oral Oncol. 2016;58:27-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Katsoulakis E, Riaz N, Hu M, et al. . Hypopharyngeal squamous cell carcinoma: three-dimensional or intensity-modulated radiotherapy? a single institution’s experience. Laryngoscope. 2016;126(3):620-626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Damast S, Wolden S, Lee N. Marginal recurrences after selective targeting with intensity-modulated radiotherapy for oral tongue cancer. Head Neck. 2012;34(6):900-906. [DOI] [PubMed] [Google Scholar]
- 15.Boero IJ, Paravati AJ, Xu B, et al. . Importance of radiation oncologist experience among patients with head-and-neck cancer treated with intensity-modulated radiation therapy. J Clin Oncol. 2016;34(7):684-690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mochizuki T, Okumura S, Ishii G, et al. . Surgical resection for oral tongue cancer pulmonary metastases. Interact Cardiovasc Thorac Surg. 2010;11(1):56-59. [DOI] [PubMed] [Google Scholar]
- 17.Shiono S, Kawamura M, Sato T, et al. ; Metastatic Lung Tumor Study Group of Japan . Pulmonary metastasectomy for pulmonary metastases of head and neck squamous cell carcinomas. Ann Thorac Surg. 2009;88(3):856-860. [DOI] [PubMed] [Google Scholar]
- 18.Pickering CR, Zhang J, Yoo SY, et al. . Integrative genomic characterization of oral squamous cell carcinoma identifies frequent somatic drivers. Cancer Discov. 2013;3(7):770-781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Riaz N, Morris LG, Lee W, Chan TA. Unraveling the molecular genetics of head and neck cancer through genome-wide approaches. Genes Dis. 2014;1(1):75-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.India Project Team of the International Cancer Genome Consortium Mutational landscape of gingivo-buccal oral squamous cell carcinoma reveals new recurrently-mutated genes and molecular subgroups. Nat Commun. 2013;4:2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hosokawa S, Funai K, Sugiyama K, et al. . Survival outcomes after surgical resection of pulmonary metastases of head and neck tumours. J Laryngol Otol. 2016;130(3):291-295. [DOI] [PubMed] [Google Scholar]
- 22.Winter H, Meimarakis G, Hoffmann G, et al. . Does surgical resection of pulmonary metastases of head and neck cancer improve survival? Ann Surg Oncol. 2008;15(10):2915-2926. [DOI] [PubMed] [Google Scholar]
- 23.Young ER, Diakos E, Khalid-Raja M, Mehanna H. Resection of subsequent pulmonary metastases from treated head and neck squamous cell carcinoma: systematic review and meta-analysis. Clin Otolaryngol. 2015;40(3):208-218. [DOI] [PubMed] [Google Scholar]
- 24.Wedman J, Balm AJ, Hart AA, et al. . Value of resection of pulmonary metastases in head and neck cancer patients. Head Neck. 1996;18(4):311-316. [DOI] [PubMed] [Google Scholar]
- 25.Liu D, Labow DM, Dang N, et al. . Pulmonary metastasectomy for head and neck cancers. Ann Surg Oncol. 1999;6(6):572-578. [DOI] [PubMed] [Google Scholar]
- 26.Gomez DR, Blumenschein GR, Lee JJ, et al. Local consolidative therapy (LCT) to improve progression-free survival (PFS) in patients with oligometastatic non–small cell lung cancer (NSCLC) who receive induction systemic therapy (IST): results of a multi-institutional phase II randomized study. ASCO Meeting Abstracts 2016;34(15)(suppl):9004. [Google Scholar]
- 27.Baxi SS, Pinheiro LC, Patil SM, Pfister DG, Oeffinger KC, Elkin EB. Causes of death in long-term survivors of head and neck cancer. Cancer. 2014;120(10):1507-1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fakhry C, Zhang Q, Nguyen-Tan PF, et al. . Human papillomavirus and overall survival after progression of oropharyngeal squamous cell carcinoma. J Clin Oncol. 2014;32(30):3365-3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tam M, Riaz N, Kannarunimit D, et al. . Sparing bilateral neck level IB in oropharyngeal carcinoma and xerostomia outcomes. Am J Clin Oncol. 2015;38(4):343-347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Spencer CR, Gay HA, Haughey BH, et al. . Eliminating radiotherapy to the contralateral retropharyngeal and high level II lymph nodes in head and neck squamous cell carcinoma is safe and improves quality of life. Cancer. 2014;120(24):3994-4002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Daly ME, Lieskovsky Y, Pawlicki T, et al. . Evaluation of patterns of failure and subjective salivary function in patients treated with intensity modulated radiotherapy for head and neck squamous cell carcinoma. Head Neck. 2007;29(3):211-220. [DOI] [PubMed] [Google Scholar]
- 32.Dandekar V, Morgan T, Turian J, et al. . Patterns-of-failure after helical tomotherapy–based chemoradiotherapy for head and neck cancer: implications for CTV margin, elective nodal dose and bilateral parotid sparing. Oral Oncol. 2014;50(5):520-526. [DOI] [PubMed] [Google Scholar]
- 33.Villaflor VM, Melotek JM, Karrison TG, et al. . Response-adapted volume de-escalation (RAVD) in locally advanced head and neck cancer. Ann Oncol. 2016;27(5):908-913. [DOI] [PubMed] [Google Scholar]
- 34.Likhterov I, Rowe ME, Khorsandi AS, Urken ML. Oral cavity squamous cell carcinoma metastatic to central compartment (level 6) lymph nodes. Laryngoscope. 2016;126(8):1803-1805. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix. Methods
eTable 1. Baseline Characteristics
eTable 2. Patient Characteristics at the Time of Recurrence
eTable 3. Locations of Regional Nodal Failures in Relation to IMRT Treatment Fields Across Disease Subsites
eTable 4. Oral Cavity Regional Nodal Failures and Relationship to Neck Dissection
eTable 5. First Organs of Distant Metastasis Across HNSCC Subsites
eTable 6. Burden of Metastatic Disease Across Head and Neck Squamous Cell Carcinoma Subsites
eFigure 1. Cumulative Incidences of (A) Local, (B) Regional and (C) Distant Failure in HPV/p16+ vs HPV/p16– Oropharyngeal Squamous Cell Carcinoma
eFigure 2. Patterns of Failure Across Subsites
eFigure 3. Survival Following Locoregional Recurrence According to Receipt of Salvage Surgery
eFigure 4. Patterns of Regional Nodal Failure Across Head and Neck Squamous Cell Carcinoma Subsites
eFigure 5. Examples of Out-of-Field Superficial Anterior Cervical Node Failures in Patients With Oral Cavity Squamous Cell Carcinoma Following Postoperative Intensity Modulated Radiation Therapy
eFigure 6. Examples of Regional Failures Contralateral to the Dissected Neck in Oral Cavity Patients Treated With Postoperative IMRT
eFigure 7. Survival Following Metastasis According to Receipt of Palliative Systemic Therapy Regimen Containing Cetuximab
eFigure 8. Survival Following Distant Metastasis According to Human Papillomavirus (HPV)/p16 Status
eReferences.