Key Points
Question
Can diagnostic criteria be defined for idiopathic orbital inflammation, which is a disorder diagnosed by exclusion?
Findings
Using a modified Delphi consensus process, 35 international experts on orbital diseases agreed on a set of clinical and radiologic criteria for the diagnosis of idiopathic orbital inflammation, supported by tissue biopsy results for the nonmyositic construct and by a prompt clinical response to a trial with systemic corticosteroids for the myositic construct.
Meaning
Although untested in clinical practice, criteria specific for the diagnosis of idiopathic orbital inflammation are defined and include tissue biopsy for lesions not confined to the extraocular muscles.
Abstract
Importance
Current practice to diagnose idiopathic orbital inflammation (IOI) is inconsistent, leading to frequent misdiagnosis of other orbital entities, including cancer. By specifying criteria, diagnosis of orbital inflammation will be improved.
Objective
To define a set of criteria specific for the diagnosis of IOI.
Design, Setting, and Participants
A 3-round modified Delphi process with an expert panel was conducted from June 8, 2015, to January 25, 2016. Fifty-three orbital scientist experts, identified through membership in the Orbital Society, were invited to participate in on online survey and they scored, using 5-point Likert scales, items that are eligible as diagnostic criteria from the literature and from personal experience. The items were clustered around the anatomic subtypes of IOI: idiopathic dacryoadenitis and idiopathic orbital fat inflammation (2 nonmyositic IOIs), and idiopathic orbital myositis (myositic IOI). Items with dissensus were rescored in the second round, and all items with consensus (median, ≥4; interquartile range, ≤1) were ranked by importance in the third round.
Main Outcomes and Measures
Consensus on items to be included in the criteria.
Results
Of the 53 experts invited to participate, a multinational panel of 35 (66%) individuals with a mean (SD) years of experience of 31 (11) years were included. Consensus was achieved on 7 of 14 clinical and radiologic items and 5 of 7 pathologic items related to diagnosis of nonmyositic IOI, and 11 of 14 clinical and radiologic items and 1 of 5 pathologic items for myositic IOI. There was agreement among panelists to focus on surgical tissue biopsy results in the diagnosis of nonmyositic IOI and on a trial with systemic corticosteroids in myositic IOI. Panelists agreed that a maximum number of 30 IgG4-positive plasma cells per high-power field in the orbital tissue is compatible with the diagnosis of IOI.
Conclusions and Relevance
An international panel of experts endorsed consensus diagnostic criteria of IOI. These criteria define a level of exclusion suggested for diagnosis and include tissue biopsy for lesions not confined to the extraocular muscles. This consensus is a step toward developing guidelines for the management of IOI, which needs to be followed by validation studies of the criteria.
This study defines consensus criteria for diagnosis of idiopathic orbital inflammation using a survey of members of the Expert Panel of the Orbital Society.
Introduction
Originally known as orbital pseudotumor, idiopathic orbital inflammation (IOI) is characterized by an enlarged orbital structure or mass consisting of nonspecific inflammation of unknown cause. However, making a diagnosis of IOI may be difficult, owing to variability of its clinical phenotype, unknown etiopathogenesis, and lack of robust distinguishing features. Moreover, the term idiopathic orbital inflammation is often improperly applied as a working diagnosis of orbital inflammation syndrome or unexplained orbital mass until a specific disease is identified.
Despite IOI being reportedly the most prevalent orbital mass, there is surprisingly little guidance available in the literature on the process of reaching its diagnosis. Current practice of identifying patients with IOI is pragmatic and empirical and varies significantly between and within countries. Diagnostic strategies range from narrowing the differential diagnosis with laboratory workup and systemic screening to identification through corticosteroid response and tissue biopsy. The most divergent views are held on the discriminative value of a corticosteroid trial and biopsy. In the absence of valid and consistent criteria, patients are often incorrectly given the diagnosis of IOI and, as a result, do not receive appropriate or timely treatment.
To date, IOI is a diagnosis of exclusion, but exclusion of competing diseases is a broad criterion and relies on the expertise of the clinician. With this background, a consensus-establishing exercise was performed that combines scientific literature with expert opinion by using a Delphi process. The purpose was to define a set of criteria to assist clinicians worldwide to safely diagnose IOI and avoid misdiagnosis of other orbital entities. The study constitutes the initiative on defining criteria specific for the diagnosis of IOI.
Methods
The study was conducted between June 8, 2015, and January 25, 2016, using the modified Delphi technique, involving 3 consecutive rounds of electronically developed, email-distributed surveys (Survey Monkey). The panelists were experts in orbital disease management, identified through membership in the Orbital Society, which is a North American–based multinational association of orbital scientists, of whom all 54 active and emeritus members except the moderator (I.M.) were invited to participate. They were informed of the purpose and number of rounds for the study in cover letters sent with the questionnaires. For each round, 2 reminder invitations were sent to the nonresponders at an interval of 6 weeks. The study adhered to the tenets of the Declaration of Helsinki and was considered exempt from institutional review board and ethical committee oversight at the University Hospitals Leuven, Leuven, Belgium, since it concerned diagnostic strategies in an educational setting without the use of patient data.
The sequence of the Delphi rounds is depicted in Figure 1. Each round had questions formulated to gauge the diagnostic steps among the experts, clustered around the main anatomic subgroups: idiopathic dacryoadenitis and idiopathic orbital fat inflammation (2 nonmyositic IOIs) and idiopathic orbital myositis (myositic IOI). The candidate diagnostic items were assembled from the literature dealing with large series and, to reduce presentation bias, questions in the second and third rounds were presented in random order.
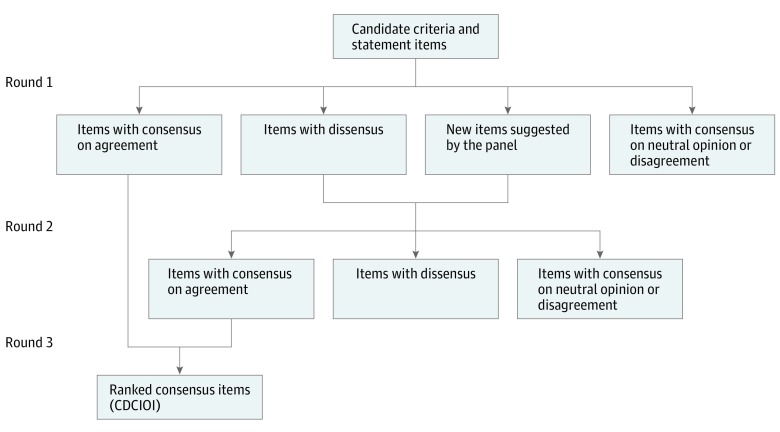
Figure 1. Flow Diagram of 3-Round Delphi Consensus Method.
CDCIOI indicates consensus diagnostic criteria of idiopathic orbital inflammation.
The participants were asked to rate each item on a 5-point Likert scale, with a rating of 1 for strongly disagree, 2, disagree; 3, neither agree nor disagree; 4, agree; and 5, strongly agree. Participants were also invited to provide comments and suggest new items. The consensus index was defined by the interquartile range (IQR), which measures the dispersion of the median by taking the difference between the 25th and the 75th percentiles. The threshold for consensus was set a priori before the data were analyzed: items were considered to reflect consensus on agreement for an IQR of 1 or less with a median of 4 or more. Items with an IQR of more than 1 indicate a dissensus (ie, polarized opinions) and were reassessed in the second round (Figure 1). Items with consensus on neutral opinion (median, 2.5-3.5) or on disagreement (median, <2.5) were excluded from the subsequent round. In the final round, items that had received an agreement consensus score in the 2 preceding rounds were presented to the participants, and they were asked to rank the items for each of the 3 anatomic subgroups, from most important to least important, by a forced-choice methodology.
Except for the moderator, the participants and their responses remained anonymous. A structured data summary from each round was made available to the participants, along with their individual responses, which allowed them to change their rating in the next round in light of the group’s opinion. The study design, progress, and results were presented by the moderator at the closed annual meetings of the Orbital Society, in Capri, Italy, September 2014 (before the first round), in Boston, Massachusetts, October 2015 (following the first round), and in San Francisco, California, May 2016 (following the third round).
Results
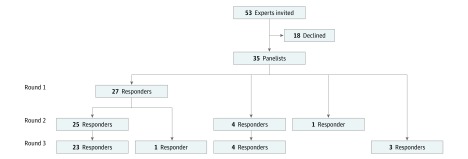
The Delphi panel included 35 (66%) ophthalmologists (22 from North America (20 from the United States and 2 from Canada), 6 from Europe, 3 from Australia, 2 from South America, 1 from East Asia, and 1 from Southeast Asia, with a mean (SD) years of experience in diagnosis and management of orbital diseases of 31 (11) years. Of these ophthalmologists, 27 (77%) completed the first round, 30 (86%) completed the second round, and 31 (89%) completed the third round; 23 (66%) of the panelists participated in all 3 rounds (Figure 2). At the first round, experts were asked their approximate annual number of new patients with the diagnosis of IOI: 9 initiated care for 4 to 9 patients, 12 for 10 to 19 patients, and 6 for 20 to 45 patients.
Figure 2. Development Process for the Expert Panel.
Many panelists responded in all 3 Delphi rounds, and some in 2 rounds or in 1 round only.
The questionnaire of the first Delphi round included 14 diagnosis-related items each for idiopathic dacryoadenitis and idiopathic orbital myositis and 12 items for idiopathic orbital fat inflammation, along with questions regarding the diagnostic steps (eTable 1 and eFigure in the Supplement). The second Delphi round comprised rerating 5 diagnostic steps and 8 diagnostic criteria with dissensus, and rating 5 new criteria voiced by the panel during the first iteration (eTable 2 in the Supplement). A few questions regarding specific histopathologic and immunohistochemical features were not answered by all of the participants, with the lowest response numbers of 21 (74%), 23 (92%), and 26 (84%), respectively, in each of the 3 rounds. The experts agreed with the following 2 statements:
1. The most accurate assessment of nonmyositic IOI is based on clinical indicators, magnetic resonance imaging (MRI), or computed tomography (CT) studies, selected normal laboratory findings, and incisional biopsy.
2. The most accurate assessment of myositic IOI is based on clinical indicators, MRI or CT studies, selected normal laboratory findings, and corticosteroid responsiveness.
For the third round, the 23 consensus diagnostic criteria of IOI (CDCIOI) were ranked in importance (Table).
Table. Consensus Diagnostic Criteria of IOI Ranked in Importance: Round 3 of Delphi Consensus Method.
| Criteriona | Mean Sum Rank, %b |
|---|---|
| In the case of diffuse, contrast-enhanced orbital mass (for lacrimal gland, diffuse swelling; for orbital fat, irregular margins), the clinical and radiologic imaging indicators of nonmyositic IOI include, ranked in importance | |
| Orbital pain | 82 |
| Acute or subacute onset | 73 |
| Intact orbital bone | 59 |
| No history of orbital-related systemic disease | 54 |
| Unilateral | 53 |
| Normal adjacent paranasal sinus | 47 |
| Age between 10 and 75 y | 38 |
| With the pathologic indicators, ranked in importance | |
| Lymphoplasmacytic infiltrate (may include neutrophils, eosinophils, few histiocytes, and macrophages) | 84 |
| Absence of granulomatous inflammation and/or vasculitis | 65 |
| Absence of necrosis | 57 |
| Plasma cell IgG4-positivity of ≤30 cells/HPF, or IgG4+/IgG ratio ≤40% | 50 |
| Fibrosis | 44 |
| In the case of diffuse, contrast-enhanced extraocular muscle enlargement, the clinical and radiologic imaging indicators of myositic IOI include, ranked in importance | |
| Painful eye movement | 79 |
| Orbital pain | 76 |
| Enlarged muscle tendon | 70 |
| Acute or subacute onset | 65 |
| Limited eye movement | 59 |
| Unilateral | 54 |
| Intact orbital bone | 53 |
| No history of thyroid disease | 48 |
| Normal-sized supraorbital and infraorbital nerve | 42 |
| Normal adjacent paranasal sinus | 39 |
| Age between 10 and 60 y | 36 |
Abbreviations: HPF, high-power field; IOI, idiopathic orbital inflammation.
Consensus items are ranked according to their importance (where 1 is least important and 7, 5, or 11, most important in the groups containing those numbers of items).
Higher percentages indicate a higher mean rank (100% as maximum) within the group of 7, 5, or 11 items.
Clinical Indicators
Clinical presentation of IOI varies with the orbital tissue involved, degree of inflammation and associated fibrosis, and the mass effect. Eyelid swelling, redness, ptosis, proptosis, limited ocular motility, corneal dryness, and visual dysfunction are possible symptoms but are, in terms of the source of the disease, largely nonspecific and therefore are not included in the CDCIOI.
Orbital Pain
Pain is a chief diagnostic criterion for IOI. Myositic IOI is most commonly associated with pain, which is exacerbated on eye movement, particularly on gaze away from the action of the involved muscle. On average, 70% of patients with nonmyositic IOI present with pain, which varies from discomfort and tenderness to severe pain referred to the periorbital region.
Acute or Subacute Onset
The onset of presentation is typically acute (within days) or subacute (within weeks). The rate of progression of IOI is slow.
No History of Orbital-Related Systemic Disease
The diagnosis of IOI is unlikely to include a known history of systemic disease, such as sarcoidosis, granulomatosis with polyangiitis, Sjögren syndrome, IgG4–related disease (IgG4-RD), lymphoproliferative and histiocytic disorders, xanthogranulomatous disease, or metastatic disease. This exclusion criterion of IOI is less stringent for thyroid disease since the comorbidity of nonmyositic IOI with thyroid eye disease is possible. It is not clear whether myositic IOI with coexisting Crohn disease or ulcerative colitis represents a distinct entity or whether the systemic disease is simply an epiphenomenon. Idiopathic orbital inflammation may arise during or after pregnancy, but a causal association has not been established. In the absence of clinical evidence of systemic disease, an internal medicine workup is not routinely performed, but can be useful in selected cases.
Unilaterality
Idiopathic orbital inflammation is usually unilateral, but bilateral disease, either simultaneously or sequentially, occurs with an incidence of 8% to 20% in nonmyositic IOI and 14% in myositic IOI. For patients with bilateral IOI, the possibility of contributing systemic disease should be considered, which involves new evaluation (eg, biopsy) of the contralateral orbit if affected at a later date.
Age Between 10 and 75 Years
Although patient age has no absolute limit with regards to IOI, younger (<10 years) and older (>75 years for nonmyositic and >60 years for myositic IOI) patients are more likely to be classified as having another disease. Idiopathic orbital inflammation is slightly more prevalent in females, and there are no racial differences.
MRI or CT Studies
Computed tomography is as useful as MRI in the evaluation of IOI. Idiopathic orbital inflammation lesions enhance with contrast and, on MRI, may display bright, fat-suppressed T2-weighted image signals depending on the amount of tissue edema. Lacrimal gland enlargement is diffuse and well defined and typically involves the orbital and palpebral lobe. The swelling occasionally extends to the upper eyelid, surrounding orbital fat, or lateral or superior rectus muscle. Figure 3, not shown as part of the survey, presents a case example of idiopathic dacryoadenitis. Orbital fat IOI, particularly the sclerosing variant, diffusely involves several orbital structures, has ill-defined borders, and is usually located in the superolateral orbit. Extension may occur through the orbital fissures into the cavernous sinus, the anterior and middle cranial fossa, or infratemporal fossa. Bony or mucosal changes of the adjacent paranasal sinuses are unusual features and raise the specter of other disease, such as granulomatosis with polyangiitis, lymphoproliferative disease, cancer, or IgG4-RD. Myositic IOI features relatively diffuse and smooth enlargement of 1 or more extraocular muscles, with the horizontal recti being most commonly affected. Involvement of the anterior tendon is a suggestive, but not mandatory, feature of myositic IOI. When other orbital structures are involved, it is considered orbital fat IOI.
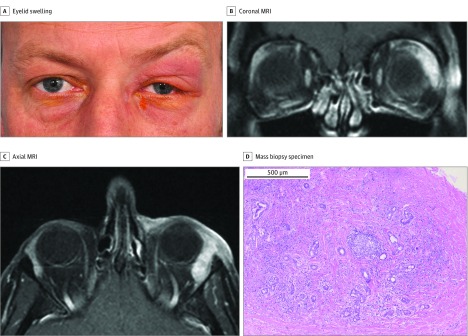
Figure 3. Idiopathic Dacryoadenitis.
A, A man in his early 40s presented with a 1-month history of tender left upper eyelid swelling. B, Coronal magnetic resonance imaging (MRI). C, Axial MRI. The MRIs demonstrated an idiopathic orbital inflammation (IOI) pattern of a well-defined, enlarged lacrimal gland with homogeneous contrast enhancement. D, Surgical biopsy specimen of the lacrimal gland mass showed IOI features with lymphoplasmacytic infiltration focally organized into a lymphoid follicle with a germinal center. Fibrosis extends into the lacrimal gland with some preserved lacrimal gland (hematoxylin-eosin, original magnification x50).
Selected Normal Laboratory Findings
The laboratory evaluation of IOI includes routine biochemistry, inflammatory markers, and a comprehensive autoimmune panel:
1. white blood cell count with differential, platelet count, calcium, and liver function tests;
2. erythrocyte sedimentation rate and C-reactive protein;
3. proteinase-3 antineutrophil cytoplasmic antibody subtype, IgG4, angiotensin-converting enzyme, and lysozyme;
4a. when the lacrimal gland is solely involved, anti-Ro (Sjögren syndrome-A), anti-La (Sjögren syndrome-B), rheumatoid factor, anti-cyclic citrullinated peptide antibody, anticitrullinated protein antibody, and antinuclear antibody titer and pattern; and
4b. when extraocular muscles are solely involved, triiodothyronine, thyroxine, thyroid-stimulating hormone, and thyroid-stimulating hormone receptor antibody.
Given that inflammatory markers and autoantibodies are commonly negative or absent in limited (eg, orbit only) or mild systemic disease, laboratory findings within the reference ranges yield little diagnostic value. In the evaluation of IgG4-RD, elevated levels of serum IgG4 confer a likelihood of both false-negative and false-positive results. There is evidence that, although currently unavailable for routine use, the plasmablast count may be a new predictive biomarker for IgG4-RD.
Pathologic Indicators
The diagnosis of nonmyositic IOI requires pathologic examination of adequate (ie, large) and representative (ie, from various involved sites and depths) tissue specimens, obtained by open biopsy. Because IOIs are usually firm tumors, the diagnostic yield from fine-needle aspiration biopsy is low and the complete pathologic picture is not gained.
Histopathologic evaluation of IOI shows a nonspecific, polymorphous infiltrate of small, well-differentiated lymphocytes (predominantly T cells), plasma cells, and neutrophil and eosinophil granulocytes (Figure 3). Other findings include the presence of histiocytes and macrophages. The infiltrate can be focally organized in lymphoid follicles with reactive germinal centers. Connective tissue is often increased with fibrosis. Fibrosis can be disproportionally prominent and the inflammatory infiltrate paucicellular, which is suggestive of the sclerosing subtype of IOI. In idiopathic dacryoadenitis, varied degrees of lacrimal gland destruction, marked by atrophy of the acini and ducts, can be seen. The presence of granulomas, granulomatous inflammation, vasculitis, or necrosis generally excludes the diagnosis of IOI.
The immunohistochemical assessment of plasma cells for IgG4 positivity is required to exclude IgG4-RD. Tissue plasma cell IgG4 positivity is not a prominent finding in IOI. A ratio of IgG4-positive to total IgG-positive plasma cells of 40% or less, or a count of 30 or fewer IgG4-positive plasma cells per high-power field (HPF) is considered compatible with a diagnosis of IOI in that the strict criteria for IgG4-RD were not met.
Corticosteroid Trial
A corticosteroid response is defined as a dramatic improvement of signs and symptoms within 48 hours after administration of systemic prednisolone, 1 mg/kg/d. Idiopathic orbital myositis is a distinct form of IOI characterized by such a response. In contrast, with nonmyositic IOI, the diagnostic corticosteroid trial has poor specificity and low positive predictive value. A poor response should not be confused with a rebound after tapering of the corticosteroids.
Discussion
This Delphi study demonstrates a consensus among 35 international orbital experts on the level of exclusion required for diagnosis of IOI, using selected criteria tailored to the anatomic subtypes. Although other studies have reviewed IOI, to our knowledge, none has sourced such a large group of experts to gauge a consensus on diagnostic criteria. The panelists agreed that diagnostic confirmation of nonmyositic IOI is based on tissue biopsy findings and, of myositic IOI, on a trial with systemic corticosteroids. This diagnostic approach differs from common current practice, in which the corticosteroid trial is used for all anatomic subgroups and biopsies are performed only in cases without initial response to corticosteroids, or in cases that relapse on tapering or discontinuing use of the drug.
Tissue biopsies are a critical diagnostic option in IOI. This approach, however, should be tailored to individual risks. Given concern for surgical morbidity, mass lesions confined to the orbital apex or around the optic nerve may confer a higher threshold for biopsy. On the contrary, a lower threshold is encouraged when myositis presents or progresses atypically or there is a history of primary cancer, owing to the likelihood of other disease. The role of screening serology testing in patients with suspected IOI is limited, as inflammatory markers are not diagnostic and reflect inflammation regardless of the cause of the disease. An autoimmune panel can be deceptively normal because autoantibodies and other biomarkers often correlate only with the burden of systemic disease.
This study has salient implications for the deeply entrenched clinical practice of the empirical corticosteroid trial for the diagnosis of IOI. Its perceived accuracy ceases with evidence of a corticosteroid response in other orbital conditions of autoimmune or autoimmunelike, infectious, or malignant causes. Moreover, diagnosis based on the response ignores predominantly fibrotic nonmyositic IOI lesions, which are characterized by a poor corticosteroid response. The trial was considered of diagnostic value in myositic IOI because most cases with typical features respond well to corticosteroids.
There are a number of practical considerations and caveats when applying the CDCIOI. First, the criteria represent the acute stage of a disease spectrum and are less accurate when used at different time points, such as at a very early stage or late end-stage disease, or following treatment. Previous surgical treatment or recent administration of corticosteroids or other immunosuppressants may alter the clinical presentation, radiologic profile, and pathologic features. Second, optimal care requires interpretation in the context of the individual patient and ongoing follow-up. The sole finding of nonspecific tissue inflammation is not sufficient to make a diagnosis of IOI and, when pathologic analysis is inconclusive, biopsy from other sites of the mass should be considered. Follow-up with serial examinations is required to monitor for alternative diagnoses, and the diagnosis of IOI should be revised when new evidence of a distinct disease arises. Third, the CDCIOI do not assess the severity or activity of IOI that is relevant to treatment decisions but is beyond the scope of this study.
Recognition of IgG4-RD as a possible alternative to IOI has increased over the past decade, since the histopathologic appearance of IgG4-RD resembles that of IOI. The pathologic differentiation is based on the absolute and relative number of tissue IgG4-positive plasma cells. Positive IgG4 stains, however, are common in various orbital inflammatory lesions unrelated to IgG4-RD. The panelists agreed that the maximum absolute number of IgG4-positive cells compatible with a diagnosis of IOI—in the context of clinical, radiologic, and pathologic indicators—is 30 cells/HPF for any orbital tissue. We recognize that this number correlates with the pathology literature and may represent perceived knowledge. However, the panelists also have experience with diagnosing and treating orbital IgG4-RD.
In the future, the validity of the CDCIOI could be evaluated using patient-driven data. These data could include studies for face validity (Are the criteria credible? Are the criteria sensible?), convergent construct validity (Do the criteria identify patients with the clinical construct?), and divergent construct validity (Do the criteria not identify patients with other diseases, eg, the IOI-mimics?). Cluster analysis could be performed to reduce the number of criteria to a more practical level for clinical guidelines purposes. After validation, the CDCIOI may aid and serve as a template for clinical trial design, development of practical guidelines, and, ultimately, routine clinical management.
Limitations
There are several limitations to consider in this study. We recognize that not all panelists participated in all rounds; however, this is consistent with previous Delphi studies. We sought recruitment only through online surveys, which lacks the encouragement of a face-to-face approach. However, this allowed anonymity and time constraints required for a Delphi exercise. Although the survey was designed by the moderator and may have imposed preconceptions, each survey was carefully formulated to include all possible items, and the experts were encouraged to provide feedback and comment on each item and add new items where required. Furthermore, there was overrepresentation of panelists from North America, but there are no known variations in the prevalence or presentation of IOI across ethnic groups.
Conclusions
The CDCIOI likely will require amendment as new evidence emerges on etiopathogenesis and biology, as new pathologic markers and biomarkers become available, and as sophisticated genomic and immunohistochemical studies lead to identification of new discrete entities. Continued efforts should be made to update the criteria, which may improve diagnosis, and hence the clinical outcome, of IOI.
eTable 1. Questionnaire and Scores of the First Delphi Round
eTable 2. Questionnaire and Scores of the Second Delphi Round
eFigure. The Initial Diagnostic Steps (With or Without Serology) Among Experts (N = 27), for Suspected IOI in the Lacrimal Gland, Muscle, or Orbital Fat
References
- 1.Mombaerts I, Goldschmeding R, Schlingemann RO, Koornneef L. What is orbital pseudotumor? Surv Ophthalmol. 1996;41(1):66-78. [DOI] [PubMed] [Google Scholar]
- 2.Kahana A, Elner VM. The meaning of diagnoses in orbital disease. Ophthal Plast Reconstr Surg. 2013;29(5):347-348. [DOI] [PubMed] [Google Scholar]
- 3.Ben Simon GJ, Yoon MK, Atul J, Nakra T, McCann JD, Goldberg RA. Clinical manifestations of orbital mass lesions at the Jules Stein Eye Institute, 1999-2003. Ophthalmic Surg Lasers Imaging. 2006;37(1):25-32. [PubMed] [Google Scholar]
- 4.Harris GJ. Idiopathic orbital inflammation: a pathogenetic construct and treatment strategy: the 2005 ASOPRS Foundation Lecture. Ophthal Plast Reconstr Surg. 2006;22(2):79-86. [DOI] [PubMed] [Google Scholar]
- 5.Dagi Glass LR, Freitag SK. Orbital inflammation: corticosteroids first. Surv Ophthalmol. 2016;61(5):670-673. [DOI] [PubMed] [Google Scholar]
- 6.Mombaerts I, Rose GE, Garrity JA. Orbital inflammation: biopsy first. Surv Ophthalmol. 2016;61(5):664-669. [DOI] [PubMed] [Google Scholar]
- 7.Mombaerts I, Schlingemann RO, Goldschmeding R, Koornneef L. Are systemic corticosteroids useful in the management of orbital pseudotumors? Ophthalmology. 1996;103(3):521-528. [DOI] [PubMed] [Google Scholar]
- 8.Holey EA, Feeley JL, Dixon J, Whittaker VJ. An exploration of the use of simple statistics to measure consensus and stability in Delphi studies. BMC Med Res Methodol. 2007;7:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World Medical Association World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. [DOI] [PubMed] [Google Scholar]
- 10.Swamy BN, McCluskey P, Nemet A, et al. . Idiopathic orbital inflammatory syndrome: clinical features and treatment outcomes. Br J Ophthalmol. 2007;91(12):1667-1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Andrew NH, Kearney D, Sladden N, et al. . Idiopathic dacryoadenitis: clinical features, histopathology, and treatment outcomes. Am J Ophthalmol. 2016;163:148-53.e1. [DOI] [PubMed] [Google Scholar]
- 12.Mombaerts I, Cameron JD, Chanlalit W, Garrity JA. Surgical debulking for idiopathic dacryoadenitis: a diagnosis and a cure. Ophthalmology. 2014;121(2):603-609. [DOI] [PubMed] [Google Scholar]
- 13.Mannor GE, Rose GE, Moseley IF, Wright JE. Outcome of orbital myositis: clinical features associated with recurrence. Ophthalmology. 1997;104(3):409-413. [DOI] [PubMed] [Google Scholar]
- 14.Mombaerts I, Koornneef L. Current status in the treatment of orbital myositis. Ophthalmology. 1997;104(3):402-408. [DOI] [PubMed] [Google Scholar]
- 15.Hsuan JD, Selva D, McNab AA, Sullivan TJ, Saeed P, O’Donnell BA. Idiopathic sclerosing orbital inflammation. Arch Ophthalmol. 2006;124(9):1244-1250. [DOI] [PubMed] [Google Scholar]
- 16.Deshpande V, Zen Y, Chan JK, et al. . Consensus statement on the pathology of IgG4-related disease. Mod Pathol. 2012;25(9):1181-1192. [DOI] [PubMed] [Google Scholar]
- 17.von der Gracht HA. Consensus measurement in Delphi studies: review and implications for future quality assurance. Technol Forecast Soc Change. 2012;79:1525-1536. [Google Scholar]
- 18.Bijlsma WR, Kalmann R. Idiopathic orbital inflammation and Graves ophthalmopathy. Arch Ophthalmol. 2010;128(1):131-132. [DOI] [PubMed] [Google Scholar]
- 19.Garrity JA, Coleman AW, Matteson EL, Eggenberger ER, Waitzman DM. Treatment of recalcitrant idiopathic orbital inflammation (chronic orbital myositis) with infliximab. Am J Ophthalmol. 2004;138(6):925-930. [DOI] [PubMed] [Google Scholar]
- 20.Bennion J, Harris MA, Sivak-Callcott JA, Nguyen J. Bilateral diffuse orbital myositis in a patient with relapsing ulcerative colitis. Ophthal Plast Reconstr Surg. 2012;28(5):e119-e120. [DOI] [PubMed] [Google Scholar]
- 21.Jakobiec FA, Syed ZA, Stagner AM, et al. . Orbital inflammation in pregnant women. Am J Ophthalmol. 2016;166:91-102. [DOI] [PubMed] [Google Scholar]
- 22.Pemberton JD, Fay A. Idiopathic sclerosing orbital inflammation: a review of demographics, clinical presentation, imaging, pathology, treatment, and outcome. Ophthal Plast Reconstr Surg. 2012;28(1):79-83. [DOI] [PubMed] [Google Scholar]
- 23.Tang SX, Lim RP, Al-Dahmash S, et al. . Bilateral lacrimal gland disease: clinical features of 97 cases. Ophthalmology. 2014;121(10):2040-2046. [DOI] [PubMed] [Google Scholar]
- 24.Zborowska B, Ghabrial R, Selva D, McCluskey P. Idiopathic orbital inflammation with extraorbital extension: case series and review. Eye (Lond). 2006;20(1):107-113. [DOI] [PubMed] [Google Scholar]
- 25.Mahr MA, Salomao DR, Garrity JA. Inflammatory orbital pseudotumor with extension beyond the orbit. Am J Ophthalmol. 2004;138(3):396-400. [DOI] [PubMed] [Google Scholar]
- 26.Wallace ZS, Deshpande V, Stone JH. Ophthalmic manifestations of IgG4-related disease: single-center experience and literature review. Semin Arthritis Rheum. 2014;43(6):806-817. [DOI] [PubMed] [Google Scholar]
- 27.Wallace ZS, Mattoo H, Carruthers M, et al. . Plasmablasts as a biomarker for IgG4-related disease, independent of serum IgG4 concentrations. Ann Rheum Dis. 2015;74(1):190-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rootman J, McCarthy M, White V, Harris G, Kennerdell J. Idiopathic sclerosing inflammation of the orbit: a distinct clinicopathologic entity. Ophthalmology. 1994;101(3):570-584. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Questionnaire and Scores of the First Delphi Round
eTable 2. Questionnaire and Scores of the Second Delphi Round
eFigure. The Initial Diagnostic Steps (With or Without Serology) Among Experts (N = 27), for Suspected IOI in the Lacrimal Gland, Muscle, or Orbital Fat