This cross-sectional study aims to determine the association between quality of life and visual function as measured by 24-2 and 10-2 visual fields in patients with primary open-angle glaucoma.
Key Points
Question
Do patients with primary open-angle glaucoma with disproportionately diminished vision-related quality of life relative to the 24-2 visual field have undetected 10-2 visual field damage?
Findings
In this cross-sectional study of 113 patients with glaucoma, the integrated 10-2 visual field model had a stronger association with the National Eye Institute Visual Function Questionnaire than the integrated 24-2 model. Furthermore, patients with 10-2 sensitivities of outliers with the lowest quality of life relative to patients with 24-2 had the strongest association with the National Eye Institute Visual Function Questionnaire and the best fit to the data.
Meaning
These results suggest that patients with disproportionately low vision-related quality of life relative to 24-2 visual field may have damage on the central field missed by the 24-2 grid.
Abstract
Importance
Recent evidence supports the presence of macular damage (within 8° of the central field) to retinal ganglion cells and associated central visual field (VF) defects in glaucoma, even in early stages. Despite this, to our knowledge, the association of 10-2 VF damage with vision-related quality of life (QOL) has not been well studied.
Objective
To determine the association between QOL and visual function as measured by 24-2 and 10-2 VFs in patients with primary open-angle glaucoma and to test the hypothesis that patients with vision-related QOL disproportionate to their 24-2 VF status may exhibit 10-2 damage overlooked by the 24-2 test.
Design, Setting, and Participants
In this cross-sectional analysis of observational cohort study data taken from a tertiary care specialty practice, 113 patients with glaucoma with the entire range of 24-2 VF damage completed the National Eye Institute Visual Function Questionnaire (NEI VFQ-25). Data were collected from May 2014 to January 2015 and were analyzed from March 2016 to May 2016.
Interventions
Standardized binocular 24-2 and 10-2 VF sensitivities were calculated for each patient.
Main Outcomes and Measures
Association of binocular 24-2 and 10-2 VF sensitivity with Rasch-calibrated NEI VFQ-25 scores. Detection of outliers was based on Cook distance of the regression of binocular 24-2 and NEI VFQ-25 score. Outlier association with QOL was then assessed using a linear regression model, with binocular 10-2 VF sensitivity as the independent variable.
Results
Of the 113 patients, the mean (SD) age was 70.1 (10.9) years, and 51 (45.1%) were male and 71 (62.8%) were white. The composite NEI VFQ-25 score was associated with both binocular 24-2 (β = 1.95; 95% CI, 0.47-3.43; P = .01) and 10-2 (β = 2.57; 95% CI, 1.12-4.01; P = .001) sensitivities, but the 10-2 VF univariable model showed an almost 2-fold better fit to the data (R2 = 9.2% vs 4.9%). However, the binocular 10-2 sensitivities of 24-2 outliers had the strongest association with the composite NEI VFQ-25 scores (β = 2.78; 95% CI, 0.84-4.72; P = .006.) and the best fit to the data (R2 = 18.2%.)
Conclusions and Relevance
The 10-2 VF model showed a stronger association with NEI VFQ-25 score than the 24-2 VF model. Patients with disproportionately low quality of vision relative to patients with 24-2 VF damage may have damage on the central field missed by the 24-2 grid. Future prospective testing, including additional dimensions of quality of life, is indicated.
Introduction
Glaucoma is characterized by progressive structural and functional damage to the optic nerve complex and is a leading cause of irreversible visual impairment in the United States and worldwide. The disease has traditionally been thought to affect midperipheral visual function in its early stages and to gradually progress to loss of central visual function only in late stages of the disease process. Even with well-maintained central vision, patients with glaucoma are more likely to experience driving and mobility difficulties. Likewise, several studies have demonstrated an association between severity of visual field defects, as detected on standard automated perimetry (SAP) of the central 30° of vision (24-2 test), and vision-related quality of life (QOL), as measured by the National Eye Institute Visual Function Questionnaire (NEI VFQ-25). In general, studies suggest that vision-related QOL decreases as visual field loss worsens, with the greatest rate of change in the driving-related visual domain. However, despite the significance of these findings, the magnitude of the associations between visual field status and NEI VFQ-25 scores remain modest.
Recent evidence supports the presence of macular damage (within 8° of the central field) to retinal ganglion cells and associated central visual field defects in glaucoma, even in early stages. In general, such damage is best detected using strategies that concentrate test points at 2° increments in the central field (10-2 visual field strategy), as the 24-2 strategy does not sufficiently sample the central field and corresponding macular function. Moreover, approximately 50% of all retinal ganglion cells are within the macular region, and the phenomenon of magnification throughout the visual pathways leads to more than 90% of the primary visual cortex being within the central 10° of the visual field.
Interestingly, previous studies investigating the association between NEI VFQ-25 scores and glaucomatous visual field loss using a threshold testing algorithm have been limited to measuring loss of visual function using the 24-2 and 30-2 testing strategy. Because the disease affects both central and midperipheral vision, assessment of visual function using only 24-2 and/or 30-2 visual fields may underestimate the extent, location, and implication of glaucomatous visual field loss. That is, it is likely that patients with visual field damage in the central 10° would have greater impairment in visual function than those with preserved central visual field.
The purpose of this study was to investigate and compare the association between NEI VFQ-25 scores and visual field status as measured by the 10-2 and 24-2 visual field tests. We also tested the hypothesis that patients with vision-related QOL disproportionate to their 24-2 visual field status may exhibit 10-2 damage overlooked by the 24-2 test.
Methods
Patients with glaucoma were enrolled in a prospective study from May 2014 through January 2015 at the Department of Ophthalmology at the Columbia University Medical Center. The Columbia University Medical Center Institutional Review Board approved the study methods, and the study was compliant with Health Insurance Portability and Accountability Act regulations. Written informed consent was obtained from all participants, and all methods adhered to the tenets of the Declaration of Helsinki. Cross-sectional data analysis was performed from March 2016 through May 2016.
All patients underwent a comprehensive ophthalmologic examination, which included a review of medical history, best-corrected visual acuity, slitlamp biomicroscopy, intraocular pressure, gonioscopy, dilated ophthalmoscopic examination, and SAP using the Swedish interactive threshold algorithm standard 24-2 and 10-2 algorithms (Carl Zeiss Meditec). Patients were included if they had primary open-angle glaucoma with perimetric damage in at least 1 eye as determined by a glaucoma specialist, the cognitive ability to complete a reliable questionnaire, and a visual acuity of 20/30 or better in both eyes. Patients were excluded if they had any ocular or systemic disease that could affect the optic nerve or visual field or if they could not perform a reliable visual field, defined as less than 33% fixation losses or false-negative errors and less than 15% false-positive errors.
Patients were classified as having glaucoma based on characteristic optic nerve damage on stereophotographs and a repeatable abnormal SAP in at least 1 eye on 2 consecutive 24-2 fields. Abnormal SAP was defined as a pattern standard deviation with P < .05 and/or a glaucoma hemifield test outside normal limits. A patient was considered eligible if there was 24-2 visual field loss in at least 1 eye.
SAP: Monocular and Binocular Visual Fields
For each patient, we defined the better or worse eye based on the 24-2 mean deviation value. In 1 patient whose mean deviation values were equal, the eye with the worse pattern standard deviation was deemed worse. Estimates of binocular mean sensitivity were calculated from the total deviation plots of both eyes according to the best-location algorithm described by Nelson-Quigg et al. With this approach, the binocular visual field is a composite of the most sensitive of the 2 visual field locations for each eye.
Vision-Related QOL Questionnaires
Interviewers administered the NEI VFQ-25 survey on the same day that the visual field tests were performed. The NEI VFQ-25 evaluates vision-related QOL. This questionnaire was designed to assess the dimensions of self-reported vision that are relevant for patients with chronic eye diseases. The NEI VFQ-25 consists of 25 questions representing 11 subscales plus an additional single-item general health-rating question. The subscales are general vision, near and distance vision activities, ocular pain, vision-related social function, vision-related role function, vision-related mental health, vision-related dependency, driving difficulties, color vision, and peripheral vision. Each subscale consists of 1 to 4 items.
Statistical Analyses
For comparison purposes, the binocular 24-2 and 10-2 sensitivities were standardized by subtracting their means and dividing by their SDs. Rasch analysis was performed to obtain final estimates of person-measures, which can be used to express where each person falls on a scale representing the degree of impairment, as measured by the NEI VFQ-25. These linear Rasch-calibrated scores were computed and used for subsequent parametric statistical analyses. Rasch analysis was performed using Andrich rating-scale models to obtain the estimates of the required ability of each item, perceived ability of each patient, and the thresholds for each response category. Rasch analysis locates item difficulty and person ability on a logit (log odds) scale, which was linearly rescaled to range from 0 to 100. Person and item measures were examined for fit to the Rasch model using infit and outfit item fit statistics. Univariable and multivariable linear regression analyses using ordinary least squares were conducted to determine the contribution of each of the binocular fields in determining the variation in each domain of Rasch-calibrated NEI VFQ-25 scores seen among patients with glaucoma. The following 5 linear regression analyses were conducted: 2 univariable regression models of the entire cohort of 113 patients using 24-2 and 10-2 algorithms, a composite 10-2 and 24-2 multivariable regression model of the entire cohort, a univariable 24-2 model for the subgroup of 107 patients without advanced binocular 10-2 visual field damage, and a univariable 10-2 model for the subgroup of 35 24-2 outliers.
A 24-2 univariable regression analysis was performed in the subgroup of 107 patients without advanced macular damage on 10-2 visual fields. For the purpose of this analysis, 10-2 visual field damage was defined as having advanced loss if the binocular 10-2 mean sensitivity (or average of threshold sensitivities) was less than 25 dB. This value was selected because 25 dB was associated with 1% of the normative data for mean and pattern standard deviation (n = 6). It should be noted that this is a conservative estimate and was an effort to dichotomize patients with glaucoma based on those patients with and without advanced macular damage.
In addition, scatterplots were used to evaluate the association between NEI VFQ-25 score and 24-2 visual fields. We defined outliers based on the Cook distance of the regression of the model NEI VFQ-25 vs binocular 24-2 sensitivity. The 35 outliers were selected because they were in the range below the fitted line of 4/n, which corresponded to NEI VFQ-25 values disproportionally low relative to the binocular 24-2 sensitivity. Comparisons between the outlier group and the overall cohort were made using a 2-tailed t test for continuous variables and a χ2 test for categorical variables. Outlier association with QOL was then assessed using a univariable regression model, with 10-2 sensitivity results as the independent variable.
Overall fit was assessed using the root-mean-square error (RMSE), Akaike information criterion (AIC) and Bayesian information criterion (BIC). Statistical analyses were performed using Winsteps version 3.81.0 (Winsteps) and Stata version 14 (StataCorp). All P values were 2-tailed, and significance was set at P < .05.
Results
The study included 226 eyes of 113 patients with glaucomatous visual field loss on standard 24-2 Swedish interactive threshold algorithm visual field tests. Patient, eye, and visual field details are listed in Table 1.
Table 1. Patient and Eye Characteristics of Cohort.
| Characteristic | No. (%) |
|---|---|
| Age, mean (SD), y | 70.1 (10.9) |
| Male | 51 (45.1) |
| Race | |
| White | 71 (62.8) |
| African American | 41 (36.3) |
| Asian/other | 1 (0.9) |
| Worse eye 24-2 average MD, dB | −6.08 (7.2) |
| Range, dB | −28.74 to 2.13 |
| Worse eye 10-2 average MD, dB | −4.98 (6.1) |
| Range, dB | −26.9 to 2.41 |
| Better eye 24-2 average MD, dB | −1.50 (3.9) |
| Range, dB | −22.48 to 3.38 |
| Better eye 10-2 average MD, dB | 1.16 (2.7) |
| Range, dB | −14.3 to 3.42 |
| Binocular 24-2 mean sensitivity, dB | 28.14 (3.94) |
| Range, dB | 7.52 to 33.38 |
| Binocular 10-2 mean sensitivity, dB | 29.12 (2.54) |
| Range, dB | 15.7 to 33.42 |
| Mean composite Rasch-calibrated NEI VFQ-25 score | 89.23 (8.1) |
| Range | 64.17 to 99.46 |
Abbreviations: MD, mean deviation; NEI VFQ-25, National Eye Institute Visual Function Questionnaire.
The results for the univariable regression analyses (Table 2) suggest that the 10-2 visual field has a stronger association with QOL than the 24-2 visual field among the entire cohort of 113 patients with glaucoma. First, each standardized 1-dB increase in binocular 24-2 sensitivity was associated with a 1.95-fold improvement in NEI VFQ-25 Rasch score (95% CI, 0.47-3.43; P = .01), whereas each 1-dB increase in 10-2 was associated with a 2.57-fold improvement in NEI VFQ-25 Rasch score (95% CI, 1.12- 4.01; P = .001). Second, the RMSE, AIC, and BIC statistics were lower for the 10-2 model, suggesting the 10-2 visual field has a stronger association with QOL than 24-2 visual fields overall. Third, while binocular 24-2 fields explained 4.9% of the variance in composite NEI VFQ-25 scores, binocular 10-2 fields explained almost 2-fold of the variance (R2 = 9.3%).
Table 2. Goodness-of-Fit Statistics of the Different Linear Models Using the Composite National Eye Institute Visual Function Questionnaire Score as Outcome Variable.
| Models | RMSE | AIC | BIC | Standardized β (95% CI) | P Value |
|---|---|---|---|---|---|
| Binocular 24-2 MS (entire cohort; 113 patients) | 7.91 | 790.34 | 795.79 | 1.95 (0.47-3.43) | .01 |
| Binocular 10-2 MS (entire cohort; 113 patients) | 7.73 | 785.10 | 790.56 | 2.57 (1.12-4.01) | .001 |
| Bincoular 24-2 MS (patients without significant macular damage on 10-2 VF; 107 patients) | 7.73 | 743.45 | 748.79 | 2.39 (0.29-4.48) | .03 |
| Binocular 10-2 MS (outliers; 35 patients) | 6.80 | 235.54 | 238.65 | 2.78 (0.84-4.72) | .006 |
Abbreviations: AIC, Akaike information criterion; BIC, Bayesian information criterion; MS, mean sensitivity; RMSE, root-mean-square error; VF, visual field.
By extension, the composite 10-2 and 24-2 multivariable regression model of the entire cohort further suggests that the 10-2 visual field has a stronger association with QOL. First, to test for possible collinearity in the model, the variance inflation factor was calculated and found to be 3.00, suggesting that collinearity was not an issue. Next, when both 10-2 and 24-2 visual fields were included in the model, the association of QOL with binocular 24-2 visual field was no longer observed (P = .89), whereas QOL remained associated with the binocular 10-2 visual field (β = 2.70; 95% CI, 0.36-5.04; P = .02) (Table 3).
Table 3. Multivariable Linear Models Using the Composite National Eye Institute Visual Function Questionnaire Score as Outcome Variable.
| Predictors | Standardized β (95% CI) | P Value |
|---|---|---|
| Binocular 24-2 MS | −0.16 (−2.50 to 2.17) | .89 |
| Binocular 10-2 MS | 2.70 (0.36 to 5.04) | .02 |
Abbreviation: MS, mean sensitivity.
The univariable 24-2 model for the subgroup of the 107 patients without advanced macular damage on 10-2 visual fields found lower RMSE, AIC, and BIC statistics than the 24-2 model for the entire cohort (Table 2). This suggests that while 10-2 showed a stronger association with QOL among all patients with glaucoma sampled, the correlation of 24-2 and NEI VFQ-25 score is improved when tested in the subgroup of patients without advanced functional macular loss.
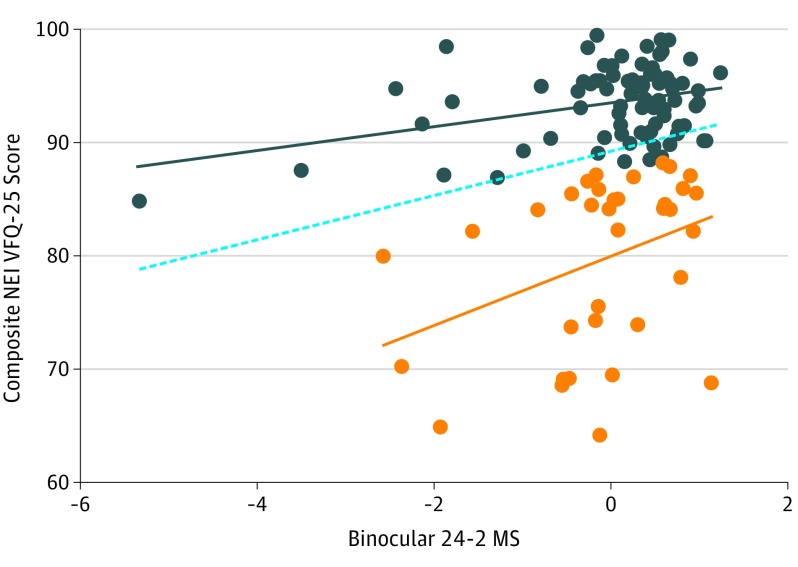
The binocular 10-2 fields of outliers from the 24-2 model (Figure) had a strong association (P = .006) with the composite NEI VFQ-25 scores. As seen in the Figure, there are 35 outliers on the scatterplot of 24-2 visual fields vs NEI VFQ-25 scores. These 35 outliers had disproportionately low NEI VFQ-25 scores relative to 24-2 visual field mean sensitivities. No differences were found between the overall cohort and the outlier subgroup with regard to age (mean [SD] age, 69.0 [9.6]; P = .59), sex (19 of 35 were male; P = .44), race/ethnicity (10 of 35 were white; P = .84), or average mean deviation of the 24-2 field (worse eye, −7.6 [SD, 8.1] dB; P = .28; better eye, −1.9 [SD, 3.5] dB; P = .66; binocular testing, 28.5 [SD, 3.9] dB; P = .57). These 35 outliers were then assessed with a univariable regression model that used the binocular 10-2 visual field results as the independent variable and composite NEI VFQ-25 scores as the outcome variable. The RMSE, AIC, and BIC statistics were lowest in this 10-2 model, suggesting that the strongest association between visual field and QOL was 10-2 visual field damage among the 24-2 outliers (Table 2). Among the outliers, the 10-2 visual field explains the largest variation (R2 = 18%) of NEI VFQ-25 scores. In this group, each 1-dB increase in the binocular 10-2 sensitivity was associated with a 2.78-fold improvement of NEI VFQ-25 Rasch score (95% CI, 0.84-4.72; P = .006).
Figure. Scatterplot of Rasch-Adjusted National Eye Institute Visual Function Questionnaire (NEI VFQ-25) Scores vs Standardized Binocular 24-2 Mean Sensitivity (MS).
Outliers with disproportionately poor scores relative to the 24-2 are shown in orange. The remainder of the patients are seen in blue. Best-fitting lines are shown for the outliers (orange line), the rest of the patients (blue line), and the entire sample (dotted line).
Discussion
Our results suggest that patient-reported QOL outcomes appear to be associated with 10-2 visual field defects resulting from glaucomatous damage to the macula. In fact, based on the fit of the first 2 models and the multivariable regression, 10-2 visual fields appear to have a stronger association with QOL than the 24-2 visual fields, even in early disease. This finding is in agreement with previous investigations that found the central points on the 24-2 visual field had the greatest effect on NEI VFQ-25 scores. Our last 2 regression equations further support the role of the 10-2 visual field in QOL. First, by removing the patients with the most advanced 10-2 damage, we were able to improve the fit of the 24-2 visual field compared with the overall cohort. Second, the strongest association among all the models was found between the 10-2 visual field damage of the outliers and NEI VFQ-25 score, suggesting that patients with disproportionately low QOL relative to 24-2 visual field damage may have undetected macular damage.
Our results have important clinical implications for monitoring patients with glaucoma. Most ophthalmologists traditionally think of performing 10-2 visual fields only in advanced disease, when a patient’s island of vision has grown so constricted that the 24-2 visual field no longer provides much useful information. However, there is a growing body of evidence that macular damage can occur early in the glaucomatous process; it can be both diffuse and/or local and can be missed by 24-2 visual fields, in which test points are separated by 6°. In fact, macular damage seen on 10-2 visual fields appears to occur almost as frequently as defects seen on 24-2 visual fields in patients with early glaucoma. Further, based on optical coherence tomography results, it is clear that the 24-2 test pattern inadequately samples this damage. Now, it is clear that the 10-2 visual field is an important determinant of vision-related QOL across the disease spectrum. Thus, the present study further supports the need to monitor visual function using 10-2 visual fields in earlier stages of glaucoma and/or modifying the 24-2 test pattern to include some of the 10-2 points.
Frequently in clinical practice, patients with glaucoma with visual complaints inconsistent with 24-2 visual field damage are evaluated for coexistent ocular morbidities, such as dry eye or cataract. Our results suggest that at least some of these visual complaints stem from glaucomatous damage to the macula and not from other causes. In particular, the outlier group was intended to reflect patients with glaucoma with disproportionately greater visual complaints than would be expected based on the 24-2 visual field. We found that among all visual field models, the 10-2 visual field when tested in the outlier group explained the most variation in NEI VFQ-25 scores. This supports the idea that macular glaucoma as measured by the 10-2 visual field is a strong, frequently unmeasured, explanatory variable in vision-related QOL.
Limitations
There are several limitations to our current study. Our results suggest that the binocular 24-2 visual fields alone explain only 4.9% of the NEI VFQ-25 score when measured in all patients in the sample. We did not include other covariates known to affect QOL in the models, such as socioeconomic status or education level. The purpose here was solely the comparison between 24-2 and 10-2 visual fields, and it is a fair assumption that the aforementioned covariates will not differ between the 2 tests. Other studies evaluating the association of 24-2 visual fields with NEI VFQ-25 scores have found similar R2 values, with results largely dependent on the methodology, particularly the use of calibrated scores, the selection of monocular or integrated visual fields, and the range of disease severity included in the analysis. However, it is possible that one of the unmeasured covariates is associated with both macular glaucoma and NEI VFQ-25 score, which would result in confounding of the study results. Future studies should consider a full multivariable model. Second, we did not have a group of patients with functional loss limited to macular glaucomatous damage as detected by 10-2 visual field. Future research in this area is warranted. Furthermore, we also did not consider the effect of dry eye, cataract, or other media opacities on the NEI VFQ-25 score; however, we have no reason to believe that diminished visual acuity would differentially effect either the 10-2 or 24-2 visual field more than the other. Third, recent studies have shown that a history of rapid binocular visual field loss is associated with poorer NEI VFQ-25 scores. Future efforts should target longitudinal changes in both 10-2 and 24-2 visual fields. Lastly, we included patients’ binocular visual fields derived from their monocular tests as an assessment of visual field loss. Historically, many reports have used the better-seeing eye as a measure of visual field loss, but recent reports suggest that an integrated visual field that considers the degree of overlapping visual field loss between the 2 eyes may be a better measure. However, this remains an area of ongoing research and thus could be prone to error.
Conclusions
In conclusion, these data suggest that 10-2 visual fields show a stronger association with NEI VFQ-25 score than do 24-2 visual fields. Patients with disproportionately poor NEI VFQ-25 scores, despite relatively good binocular 24-2 visual fields, may have undetected damage on 10-2 visual field testing.
References
- 1.Friedman DS, Wolfs RC, O’Colmain BJ, et al. ; Eye Diseases Prevalence Research Group . Prevalence of open-angle glaucoma among adults in the United States. Arch Ophthalmol. 2004;122(4):532-538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90(3):262-267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tham YC, Li X, Wong TY, Quigley HA, Aung T, Cheng CY. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology. 2014;121(11):2081-2090. [DOI] [PubMed] [Google Scholar]
- 4.Ramulu PY, West SK, Munoz B, Jampel HD, Friedman DS. Driving cessation and driving limitation in glaucoma: the Salisbury Eye Evaluation Project. Ophthalmology. 2009;116(10):1846-1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Janz NK, Musch DC, Gillespie BW, Wren PA, Niziol LM; Collaborative Initial Glaucoma Treatment Study (CIGTS) Investigators . Evaluating clinical change and visual function concerns in drivers and nondrivers with glaucoma. Invest Ophthalmol Vis Sci. 2009;50(4):1718-1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McGwin G Jr, Xie A, Mays A, et al. Visual field defects and the risk of motor vehicle collisions among patients with glaucoma. Invest Ophthalmol Vis Sci. 2005;46(12):4437-4441. [DOI] [PubMed] [Google Scholar]
- 7.McKean-Cowdin R, Wang Y, Wu J, Azen SP, Varma R; Los Angeles Latino Eye Study Group . Impact of visual field loss on health-related quality of life in glaucoma: the Los Angeles Latino Eye Study. Ophthalmology. 2008;115(6):941-948.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hood DC, Raza AS, de Moraes CG, Liebmann JM, Ritch R. Glaucomatous damage of the macula. Prog Retin Eye Res. 2013;32:1-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ehrlich AC, Raza AS, Ritch R, Hood DC. Modifying the conventional visual field test pattern to improve the detection of early glaucomatous defects in the central 10°. Transl Vis Sci Technol. 2014;3(6):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qiu A, Rosenau BJ, Greenberg AS, et al. Estimating linear cortical magnification in human primary visual cortex via dynamic programming. Neuroimage. 2006;31(1):125-138. [DOI] [PubMed] [Google Scholar]
- 11.Schiefer U, Papageorgiou E, Sample PA, et al. Spatial pattern of glaucomatous visual field loss obtained with regionally condensed stimulus arrangements. Invest Ophthalmol Vis Sci. 2010;51(11):5685-5689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Curcio CA, Allen KA. Topography of ganglion cells in human retina. J Comp Neurol. 1990;300(1):5-25. [DOI] [PubMed] [Google Scholar]
- 13.Hyman LG, Komaroff E, Heijl A, Bengtsson B, Leske MC; Early Manifest Glaucoma Trial Group . Treatment and vision-related quality of life in the early manifest glaucoma trial. Ophthalmology. 2005;112(9):1505-1513. [DOI] [PubMed] [Google Scholar]
- 14.Gutierrez P, Wilson MR, Johnson C, et al. Influence of glaucomatous visual field loss on health-related quality of life. Arch Ophthalmol. 1997;115(6):777-784. [DOI] [PubMed] [Google Scholar]
- 15.van Gestel A, Webers CA, Beckers HJ, et al. The relationship between visual field loss in glaucoma and health-related quality-of-life. Eye (Lond). 2010;24(12):1759-1769. [DOI] [PubMed] [Google Scholar]
- 16.Sawada H, Yoshino T, Fukuchi T, Abe H. Assessment of the vision-specific quality of life using clustered visual field in glaucoma patients. J Glaucoma. 2014;23(2):81-87. [DOI] [PubMed] [Google Scholar]
- 17.Gillespie BW, Musch DC, Niziol LM, Janz NK. Estimating minimally important differences for two vision-specific quality of life measures. Invest Ophthalmol Vis Sci. 2014;55(7):4206-4212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nelson-Quigg JM, Cello K, Johnson CA. Predicting binocular visual field sensitivity from monocular visual field results. Invest Ophthalmol Vis Sci. 2000;41(8):2212-2221. [PubMed] [Google Scholar]
- 19.Mangione CM, Lee PP, Gutierrez PR, Spritzer K, Berry S, Hays RD; National Eye Institute Visual Function Questionnaire Field Test Investigators . Development of the 25-item National Eye Institute Visual Function Questionnaire. Arch Ophthalmol. 2001;119(7):1050-1058. [DOI] [PubMed] [Google Scholar]
- 20.Abe RY, Diniz-Filho A, Costa VP, Gracitelli CP, Baig S, Medeiros FA. The impact of location of progressive visual field loss on longitudinal changes in quality of life of patients with glaucoma. Ophthalmology. 2016;123(3):552-557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Traynis I, De Moraes CG, Raza AS, Liebmann JM, Ritch R, Hood DC. Prevalence and nature of early glaucomatous defects in the central 10° of the visual field. JAMA Ophthalmol. 2014;132(3):291-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park SC, Kung Y, Su D, et al. Parafoveal scotoma progression in glaucoma: humphrey 10-2 versus 24-2 visual field analysis. Ophthalmology. 2013;120(8):1546-1550. [DOI] [PubMed] [Google Scholar]
- 23.Hood DC, Slobodnick A, Raza AS, de Moraes CG, Teng CC, Ritch R. Early glaucoma involves both deep local, and shallow widespread, retinal nerve fiber damage of the macular region. Invest Ophthalmol Vis Sci. 2014;55(2):632-649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grillo LM, Wang DL, Ramachandran R, et al. The 24-2 visual field test misses central macular damage confirmed by the 10-2 visual field test and optical coherence tomography. Transl Vis Sci Technol. 2016;5(2):15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ekici F, Loh R, Waisbourd M, et al. Relationships between measures of the ability to perform vision-related activities, vision-related quality of life, and clinical findings in patients with glaucoma. JAMA Ophthalmol. 2015;133(12):1377-1385. [DOI] [PubMed] [Google Scholar]
- 26.Sun Y, Lin C, Waisbourd M, et al. The impact of visual field clusters on performance-based measures and vision-related quality of life in patients with glaucoma. Am J Ophthalmol. 2016;163:45-52. [DOI] [PubMed] [Google Scholar]
- 27.Peters D, Heijl A, Brenner L, Bengtsson B. Visual impairment and vision-related quality of life in the Early Manifest Glaucoma Trial after 20 years of follow-up. Acta Ophthalmol. 2015;93(8):745-752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Friedman DS, Freeman E, Munoz B, Jampel HD, West SK. Glaucoma and mobility performance: the Salisbury Eye Evaluation Project. Ophthalmology. 2007;114(12):2232-2237. [DOI] [PubMed] [Google Scholar]
- 29.Sumi I, Shirato S, Matsumoto S, Araie M. The relationship between visual disability and visual field in patients with glaucoma. Ophthalmology. 2003;110(2):332-339. [DOI] [PubMed] [Google Scholar]
- 30.Arora KS, Boland MV, Friedman DS, Jefferys JL, West SK, Ramulu PY. The relationship between better-eye and integrated visual field mean deviation and visual disability. Ophthalmology. 2013;120(12):2476-2484. [DOI] [PMC free article] [PubMed] [Google Scholar]