Key Points
Question
Do individuals who develop cognitive decline over time experience an increase in variability of standard automated perimetry results?
Findings
In this observational cohort study including 211 eyes of 115 patients, a 5-unit decline in Montreal Cognitive Assessment score was associated with an increase of 0.23 dB in the SD of residuals of standard automated perimetry mean deviation.
Meaning
Cognitive decline was associated with increased visual field variability over time in patients with glaucoma and those suspected of having the disease, suggesting screening and monitoring of cognitive dysfunction may be important in assessing visual field progression in the context of glaucoma.
Abstract
Importance
Visual field variability may impair detection of glaucoma progression over time. Despite the possible overlap between neurocognitive disorders and glaucoma in older individuals, no study has investigated the association between cognitive changes and visual field variability.
Objective
To evaluate the association between global neurocognitive impairment and visual field variability in patients diagnosed as having glaucoma or glaucoma suspects.
Design, Setting, and Participants
This prospective observational cohort study was conducted at the Visual Performance Laboratory, University of California, San Diego. The study involved 211 eyes of 115 patients followed up for a mean (SD) period of 2.5 (0.8) years, ranging from 1.2 to 4.7 years. Data were obtained during the period extending from March 2011 to April 2015, with data analysis conducted from November 2015 to May 2016.
Main Outcomes and Measures
Association between cognitive decline and visual field variability. Patients were monitored with standard automated perimetry (SAP) and had longitudinal assessment of cognitive ability using the Montreal Cognitive Assessment (MoCA). Visual field variability was estimated by the SD of the residuals of ordinary least squares linear regressions of SAP mean deviation (MD) values over time. Linear regression models were used to investigate the association between cognitive decline and visual field variability, adjusting for potentially confounding factors.
Results
Among the 115 patients, the mean (SD) age at baseline was 67.4 (10.1) years, 63 were men (54.8%), and 86 were white (74.8%). There was a statistically significant association between change in MoCA scores and visual field variability over time. In a univariable model, a 5-point decline in MoCA score was associated with an increase of 0.18 dB in the SD of residuals of SAP MD (R2 = 4.3%; 95% CI, 0.06-0.30; P = .003). In a multivariable model adjusting for baseline MoCA score, mean SAP MD, age, sex, race/ethnicity, educational level, income, and number of SAP tests, each 5-point decline in MoCA score was associated with an increase of 0.23 dB in the SD of residuals of SAP MD (95% CI, 0.11-0.35; P < .001).
Conclusions and Relevance
Cognitive decline was associated with increased visual field variability during follow-up. These findings suggest that screening and monitoring of cognitive dysfunction may be important in the assessment of visual field progression in the context of glaucoma.
This observational cohort study evaluates the association between global neurocognitive impairment and visual field variability in patients diagnosed as having glaucoma or glaucoma suspects.
Introduction
Standard automated perimetry (SAP) remains the gold standard for assessment of visual field loss in glaucoma and is still the most widely used method for detecting progressive damage in clinical practice. However, SAP may have considerable test-retest variability. As detection of progression depends on the ability to separate true change (the signal) from test-retest variability (the noise), the presence of large test-retest variability may impair detection of disease progression over time. Previous studies have demonstrated that factors, such as age and disease severity, are related to long-term variability on SAP tests. However, to our knowledge, no previous study has specifically investigated the impact of cognitive status on the variability of SAP testing. As the prevalence of glaucoma increases substantially with aging, the coexistence of the disease and impaired cognition in the same patients is not uncommon.
As SAP testing requires significant patient concentration and cooperation, it may be difficult, if not impossible, to perform the test in patients who present with major neurocognitive impairment or dementia, such as those with Alzheimer disease. However, mild cognitive impairment (MCI) is a condition that is described as having cognitive difficulties that are not severe enough to interfere with activities of daily living and so not defined as dementia. Mild cognitive impairment represents a transitional state between cognitive changes of normal aging and early dementia and is becoming increasingly recognized as a risk factor for Alzheimer disease. Indeed, studies suggest that 10% to 20% of adults aged 65 years and older may have MCI and the estimated incidence in individuals aged 65 years and older is about 1% to 4% per year. Importantly, individuals with MCI tend to progress to Alzheimer disease at a rate of approximately 10% to 15% per year. Owing to the relatively high prevalence of MCI, it is likely that many patients with glaucoma may develop MCI in the course of their follow-up. However, it is currently unknown whether the development of mild cognitive losses could interfere with SAP results over time and, as a consequence, affect the ability to monitor glaucoma progression.
In the current study, we hypothesized that patients who develop cognitive decline over time will show an increase in the variability of SAP results. We investigated our hypothesis by following a cohort of patients with glaucoma and those suspected of having the disease with SAP testing, as well as with one of the most sensitive neuropsychological test screening instruments designed to assess overall cognitive status, the Montreal Cognitive Assessment (MoCA) test. We then investigated the association between change in MoCA results and SAP variability over time.
Methods
Participants in this study were drawn from a prospective longitudinal study designed to evaluate functional impairment in glaucoma conducted at the Visual Performance Laboratory of the University of California–San Diego. The institutional review board at the University of California–San Diego approved the methods, and written informed consent was obtained from all participants. The study adhered to the laws of the Health Insurance Portability and Accountability Act, and all study methods complied with the Declaration of Helsinki guidelines for human subject research.
All participants underwent a comprehensive ophthalmologic examination including review of medical history, visual acuity, slitlamp biomicroscopy, intraocular pressure measurement using Goldmann applanation tonometry, corneal pachymetry, gonioscopy, dilated fundoscopy examination using a 78-diopter lens, stereoscopic optic disc photography (Kowa Nonmyd WX3D; Kowa Optimed Inc), and SAP using the 24-2 Swedish Interactive Threshold Algorithm standard of the Humphrey Field Analyzer II model 750i (Carl Zeiss Meditec Inc). Only patients with open angles on gonioscopy were included. Patients with coexisting retinal disease, uveitis, amblyopia, or nonglaucomatous optic disc neuropathy were excluded from the study.
The study included patients diagnosed as having glaucoma and those suspected of having the disease. Eyes were classified as glaucomatous if they had 2 or more repeatable glaucomatous visual field defects at baseline, defined as a pattern SD with P < .05, or a Glaucoma Hemifield Test result outside normal limits, and evidence of glaucomatous optic neuropathy based on masked assessment of stereophotographs. Eyes were classified as glaucoma suspects if they had a history of elevated intraocular pressure (>21 mm Hg) and/or suspicious or glaucomatous appearance of the optic nerve, but normal and reliable visual field results at baseline. If both eyes from the same patient were eligible for the study, both eyes were included in the analysis and statistical procedures were used to take into account the correlation between measurements within the same patient. Sociodemographic questionnaires were also administered to patients at the time of the baseline. These questionnaires contained a survey about demographics, educational level, and income.
Standard Automated Perimetry
Monocular SAP was performed using the 24-2 Swedish Interactive Threshold Algorithm standard test at all visits during the follow-up period. Only reliable visual field tests (≤33% fixation losses and ≤15% false-positive errors) were included. In addition, visual fields were reviewed and excluded in the presence of artifacts, such as eyelid or rim artifacts, learning effect, or abnormalities that could indicate diseases other than glaucoma, such as homonymous hemianopia.
Montreal Cognitive Assessment Test
The presence of cognitive impairment was evaluated using the MoCA test, a cognitive screening tool developed to detect mild cognitive dysfunction. The test has previously been shown to have adequate psychometric properties as a screening instrument for the detection of MCI. Unlike many other standard screening instruments currently in use (eg, Mini-Mental State Examination), the MoCA assesses several different cognitive domains: attention and concentration, executive functions, memory, language, visuoconstructional skills, conceptual thinking, calculations, and orientation (eFigure in the Supplement). A trained technician administered the MoCA test in approximately 10 to 15 minutes. The total possible score is 30 points, and a score of 26 or greater is considered normal.
For inclusion in the study, patients were required to have completed a baseline and follow-up MoCA test over a minimum period of 1 year. Change in cognitive scores was assessed by calculating the difference between MoCA scores at the last follow-up visit from those at baseline. Therefore, a decreased score indicated cognitive decline during follow-up. In addition, participants were required to have had at least 4 visual fields during the corresponding period. Data for this study were obtained during the period extending from March 2011 to April 2015, with data analysis conducted from November 2015 to May 2016. During follow-up, each patient was treated at the discretion of the attending ophthalmologist.
Statistical Analysis
Ordinary least squares linear regression models of SAP mean deviation (MD) values over time were fit to the sequence of visual field tests for each eye and residuals were obtained. Visual field variability was estimated through the SD of the residuals of SAP MD from ordinary least squares linear regression. Linear regression models were then used to investigate the association between cognitive decline and variability on visual fields. Initially, a univariable model was used to evaluate the association between change in MoCA questionnaire scores and SD of the residuals of SAP MD over time. Subsequently, the association was studied in a multivariable model adjusting for potentially confounding factors such as disease severity (mean SAP MD during the follow-up period), age, sex, race/ethnicity, educational level, income, and number of SAP tests. Socioeconomic variables were categorized for inclusion in the multivariable models as educational level (at least high school degree [yes/no]) and income (<$25 000/year [yes/no]). As the association between SAP MD variability and visual field sensitivity is nonlinear, it was modeled using restricted cubic splines. Cubic splines are piecewise-polynomial line segments joined together at knots. The final number of knots was determined by fitting models with different number of knots and evaluating the best-fitting model by cross-validation. Generalized estimating equations were used to adjust for the correlations of multiple observations from the same patient.
All statistical analyses were performed using commercially available software (Stata version 14; StataCorp LP). The α level (type I error) was set at .05.
Results
The study included 211 eyes of 115 patients followed up for a mean (SD) period of 2.5 (0.8) years (range, 1.2-4.7 years). Eighty-seven individuals (75.6%) had a diagnosis of glaucoma and 28 (24.4%) were considered glaucoma suspects. Table 1 shows the demographic and clinical characteristics of the included patients. The mean (SD) age at baseline was 67.4 (10.1) years. There were 63 men (54.8%) and 52 women (45.2%) and 86 were white (74.8%) and 29 were African American (25.2%). The baseline mean (SD) MoCA score was 28.3 (1.9) points, ranging from 23 to 30 points. At baseline, the mean (SD) SAP MD and pattern SD were −2.9 (5.8) dB and 3.6 (3.3) dB, respectively. However, there was a wide range of MD values of visual fields included in the study, ranging from −28.8 to 2.6 dB. A total of 106 patients (92.2%) had at least a high school degree and 9 (7.8%) had not completed high school. Twenty-one patients (18.3%) had an income of less than $25 000 per year.
Table 1. Baseline Demographic and Clinical Characteristics of 211 Eyes/115 Patients Included in the Study.
| Characteristic | No. (%) |
|---|---|
| Age, mean (SD), y | 67.4 (10.1) |
| Male | 63 (54.8) |
| African American | 29 (25.2) |
| MoCA score, mean (SD), points | 28.3 (1.9) |
| SAP 24-2, mean (SD), dB | |
| MD | −2.9 (5.8) |
| PSD | 3.6 (3.3) |
| Educational level, at least high school degree | 106 (92.2) |
| Income <$25 000/y | 21 (18.3) |
Abbreviations: MD, mean deviation; MoCA, Montreal Cognitive Assessment; PSD, pattern SD; SAP, standard automated perimetry.
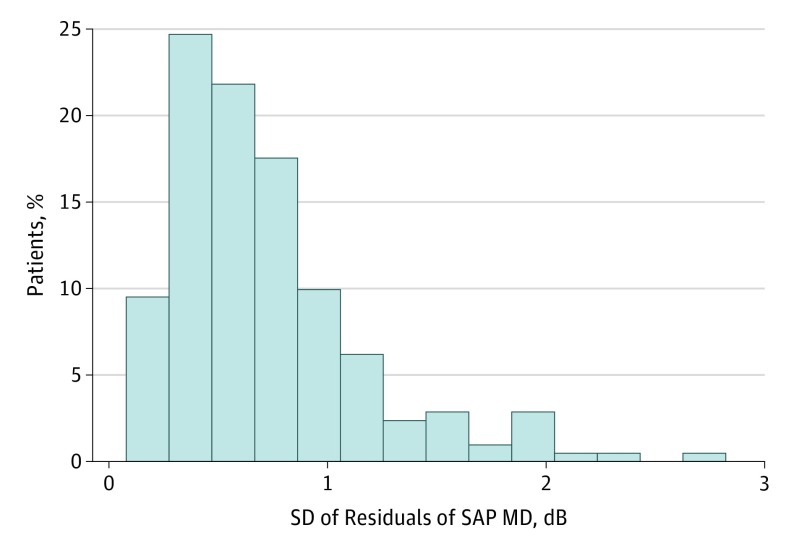
The total number of 24-2 SAP Swedish Interactive Threshold Algorithm tests during follow-up was 1725. A total of 232 visual fields (13.4%) were reviewed and excluded owing to the presence of artifacts and 35 (2.0%) were considered unreliable (≤33% fixation losses and ≤15% false-positive errors) and also excluded. The number of SAP visual tests included in the analyses was 1458, with a median of 6 tests per eye (interquartile range, 5-10). The mean (SD) change in MoCA score was −0.1 (2.5) points (range, −9 to 4 points). The mean (SD) SD of residuals in SAP MD was 0.71 (0.45) dB (range, 0.08-2.82 dB). The SD of residuals of SAP MD was correlated between both eyes (R2 = 31.9%; 95% CI, 0.42-0.78; P < .001). Figure 1 shows a histogram of the SD of the residuals of SAP MD over time.
Figure 1. Distribution of Variability in Standard Automated Perimetry (SAP) Over Time.
Histogram of the SD of residuals of SAP mean deviation (MD) over time.
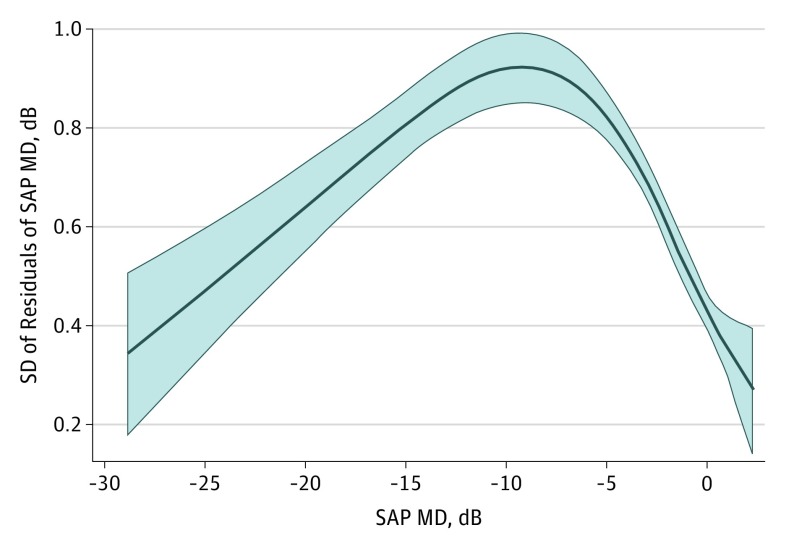
Table 2 shows the results of univariable models investigating factors associated with variability in SAP MD. There was a statistically significant association between change in MoCA scores and variability in SAP MD. A 5-point decline in MoCA score was associated with an increase of 0.18 dB in the variability of SAP MD during the follow-up period (R2 = 4.3%; 95% CI, 0.06-0.30; P = .003). Baseline MoCA score was not statistically significantly associated with variability in SAP MD in the univariable model (0.11 dB; 95% CI, −0.05 to 0.28; P = .18). Disease severity (mean SAP MD) was associated with increased variability, as modeled by restricted cubic splines. Figure 2 illustrates this association showing an increase in variability with worsening disease, which reaches a peak and then declines. Age, sex, educational level, and income were not statistically significantly associated with variability in SAP MD in the univariable models. However, race/ethnicity and number of SAP tests were statistically significantly associated with variability in SAP MD in the univariable models (Table 2).
Table 2. Results of the Univariable and Multivariable Regression Models Investigating the Association Between Variability on Visual Field Over Time and Cognitive Changea.
| Characteristic | Univariable Model | Multivariable Model | ||
|---|---|---|---|---|
| Coefficient (95% CI) | P Value | Coefficient (95% CI) | P Value | |
| MoCA score, per 5 points lower | ||||
| Change | 0.18 (0.06 to 0.30) | .003 | 0.23 (0.11 to 0.35) | <.001 |
| Baseline | 0.11 (−0.05 to 0.28) | .18 | 0.20 (0.02 to 0.38) | .03 |
| SAP MD 24-2, per 1 dB lower | ||||
| Spline 1 | −0.03 (−0.06 to −0.01) | <.001b | −0.04 (−0.06 to −0.02) | <.001b |
| Spline 2 | 0.07 (0.03 to 0.10) | 0.07 (0.04 to 0.10) | ||
| Spline 3 | −0.47 (−2.11 to 1.18) | −1.08 (−2.73 to 0.57) | ||
| Age, per 1 decade older | 0.01 (−0.05 to 0.07) | .72 | −0.05 (−0.11 to 0.01) | .10 |
| Male | 0.12 (−0.00 to 0.24) | .05 | 0.07 (−0.04 to 0.19) | .20 |
| African American | 0.15 (0.01 to 0.29) | .04 | −0.00 (−0.16 to 0.16) | .98 |
| Educational level, at least high school degree | −0.02 (−0.24 to 0.21) | .87 | −0.08 (−0.30 to 0.13) | .45 |
| Income <$25 000/y | 0.08 (−0.08 to 0.24) | .31 | 0.04 (−0.11 to 0.18) | .62 |
| No. of SAP tests, per 1 additional test | 0.03 (0.01 to 0.06) | .002 | 0.02 (0.00 to 0.04) | .02 |
Abbreviations: MD, mean deviation; MoCA, Montreal Cognitive Assessment; SAP, standard automated perimetry.
Visual field variability was measured by the SD of residuals of SAP MD and cognitive change was assessed by change in scores of the MoCA test.
Joint Wald test P Value.
Figure 2. Nonlinear Association Between Disease Severity and Visual Field Variability.
Graph illustrating the nonlinear association between the mean standard automated perimetry (SAP) mean deviation (MD) and SD of residuals of SAP MD. Variability increases with worsening disease severity until a peak is reached.
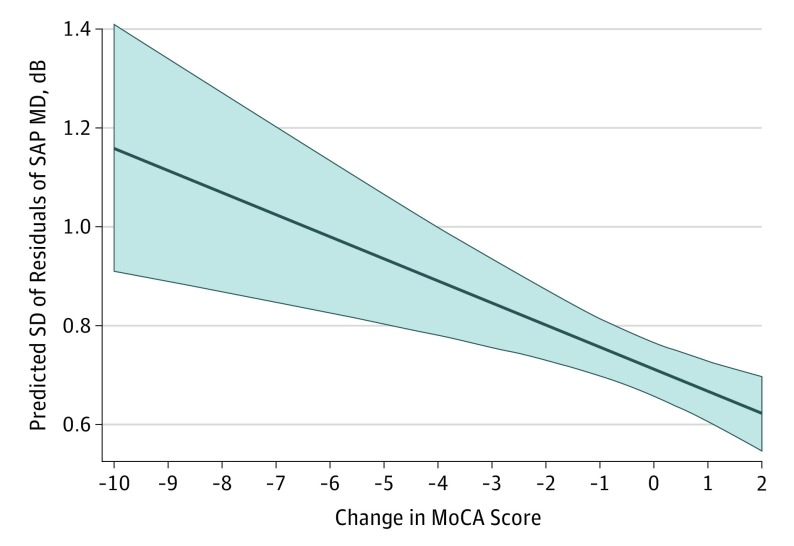
In a multivariable model adjusting for baseline MoCA score, mean SAP MD, age, sex, race/ethnicity, educational level, income, and number of SAP tests, each 5-point decline in MoCA score was associated with an increase of 0.23 dB in the SD of residuals of SAP MD during the follow-up (95% CI, 0.11-0.35; P < .001) (Table 2 and Figure 3).
Figure 3. Predicted Values of Visual Field Variability for Different Amounts of Cognitive Change.
Predicted values and 95% CIs (shaded region) of the SD of residuals of standard automated perimetry (SAP) mean deviation (MD) for different amounts of change in Montreal Cognitive Assessment (MoCA) score. Predictive values and 95% CIs are adjusted for age, sex, race/ethnicity, disease severity, number of visual field tests over time, baseline MoCA score, and income and educational levels.
There were statistically significant associations between change in MoCA scores and reliability indexes for the visual fields included in the analyses. Each 5-point decline in MoCA score was associated with an increase of 1.4% in fixation losses (P = .001), 0.5% in false-positive errors (P = .008), and 1.6% in false-negative errors (P < .001). However, the exclusion of visual fields deemed unreliable according to cutoffs for reliability indices did not affect the magnitude of the association between cognitive decline and visual field variability. If the 35 visual fields considered unreliable were included in the analysis, a 5-point decline in MoCA score was associated with an increase of 0.22 dB in the SD of residuals of SAP MD in the multivariable model (P = .001).
Discussion
In the current study, global cognitive decline as measured by the MoCA test was associated with visual field variability in patients with glaucoma and those suspected of having the disease followed up over time. A 5-point decline in MoCA scores over time was associated with an increase of 0.18 dB in the SD of the residuals of SAP MD, indicating increased visual field variability (95% CI, 0.06-0.30; P = .003). The association between MoCA scores and visual field variability was still present even after adjusting for several potential confounding factors. In the multivariable model adjusting for baseline MoCA score, disease severity, age, sex, race/ethnicity, educational level, income, and number of SAP tests, each 5-point decline in MoCA score was associated with a change of 0.23 dB in the SD of residuals of SAP MD during the follow-up period (95% CI, 0.11-0.35; P < .001).
The association between cognitive changes and visual field variability found in our study seems clinically relevant. Indeed, a 0.23-dB increase in the SD of the residuals for a 5-point decline in MoCA scores corresponds to approximately a 30% increase in variability, considering the mean SD of residuals of 0.71 dB found in our population. A previous study using computer simulations of MD variability over time has suggested that the variability of SAP must be reduced by approximately 20% for a clinically appreciable improvement in detection of visual field change. Therefore, using a similar reasoning, we can conclude that an increase of 30% in variability would likely result in a clinically appreciable worsening in the ability to detect visual field change over time. An increase in visual field variability can delay detection of true disease progression, potentially resulting in irreversible visual function loss. In addition, variability may also result in an increase in the number of visual field sequences being declared as having progressed when in fact no true change has occurred. These false-positives may lead to unnecessary escalation of treatment with potential adverse effects to patients. It is interesting to observe that although all visual field tests included in the analyses were considered reliable based on standard cutoffs for reliability indices, there were still statistically significant associations between cognitive change and reliability indices. Cognitive decline was associated with an increase in fixation losses and false-positive and false-negative errors, although the magnitudes of the associations were generally relatively small. The decrease in reliability may explain, at least in part, the association between visual field variability and cognitive decline found in our study.
Most patients in our study did not have evidence of cognitive impairment at baseline. The mean MoCA score at baseline was 28.3, although 10 patients (8.7%) demonstrated a MoCA score less than 26. However, even after the relatively short period of follow-up of the study, the number of patients with MoCA scores less than 26 at the last visit almost doubled to 19 patients (16.5%). Seventeen patients (14.8%) experienced a decline in MoCA score greater than 2 points. Considering the increasing prevalence of MCI with aging, it is expected that even greater changes in MoCA scores would be found over time during longer follow-up periods. As glaucoma is a relatively slowly progressive disease, which generally requires lifetime monitoring, a significant number of patients could have impactful changes on visual field variability as a result of cognitive decline over time.
Our results suggest that in the presence of increasing visual field variability over time, clinicians should suspect of the presence of possible decline in cognitive functioning as well. Importantly, such findings should prompt referral for investigation of a cognitive disorder such as MCI or dementia. In the presence of a significant increase in visual field variability as a result of cognitive decline, clinicians may need to increase the frequency of testing to obtain more precise estimates of indices of change over time. Alternatively, it may be necessary to increase the reliance on other tests for assessment of progression, such as structural imaging of the optic nerve, nerve fiber layer, or macular areas. Approaches that combine structural and functional measurements could also potentially be helpful, by improving the accuracy and precision of estimates of functional change by borrowing information from structural testing and vice versa.
For the purposes of this study, our assessment of cognitive ability was limited to the MoCA test, which is a brief cognitive screening tool. Although there are several other measures available for assessing cognitive abilities in patients suspected of having MCI or dementia, a previous investigation has demonstrated that MoCA scores have desirable psychometric properties in the screening for cognitive impairment, especially in mild forms. A study by Krishnan and colleagues explored the utility of MoCA scores in the detection of cognitive change over time in a community population sample. The MoCA was administered twice at approximately 3.5 years apart to a group of 139 participants. The results showed that when accounting for age and education, the MCI group showed a decline of 1.7 points over time, while cognitively intact participants remained stable.
Strengths and Limitations
The strengths of the study included a large well-characterized sample, careful screening, and comprehensive testing of the eye, as well as sensitive methods for data analysis. However, our study had limitations that are important to note. For example, we used only 1 neuropsychological test that is considered to be a global cognitive screening tool and it has limitations (eg, test-retest variability and scores tend to be higher in highly educated individuals with more cognitive reserve). Additionally, the mean follow-up time was relatively short, considering the long-term evolution of both glaucoma and MCI. Despite that, we found statistically significant and relevant associations between longitudinal changes in cognitive scores and visual field variability. Nevertheless, studies with longer follow-up and use of more refined and sensitive tests to more comprehensively assess neuropsychological function will be important to provide improved and possibly more reliable estimates of these important associations.
Conclusions
Our findings suggest that cognitive decline was associated with increased visual field variability in patients with glaucoma and those suspected of having the disease followed up over time. Screening and monitoring of cognitive dysfunction in patients with glaucoma may be important for the assessment of visual field progression over time.
eFigure. Montreal Cognitive Assessment (MoCA) Test
References
- 1.Flammer J, Drance SM, Zulauf M. Differential light threshold: short- and long-term fluctuation in patients with glaucoma, normal controls, and patients with suspected glaucoma. Arch Ophthalmol. 1984;102(5):704-706. [DOI] [PubMed] [Google Scholar]
- 2.Flammer J, Drance SM, Fankhauser F, Augustiny L. Differential light threshold in automated static perimetry: factors influencing short-term fluctuation. Arch Ophthalmol. 1984;102(6):876-879. [DOI] [PubMed] [Google Scholar]
- 3.Flammer J, Drance SM, Augustiny L, Funkhouser A. Quantification of glaucomatous visual field defects with automated perimetry. Invest Ophthalmol Vis Sci. 1985;26(2):176-181. [PubMed] [Google Scholar]
- 4.Heijl A, Lindgren G, Olsson J. Normal variability of static perimetric threshold values across the central visual field. Arch Ophthalmol. 1987;105(11):1544-1549. [DOI] [PubMed] [Google Scholar]
- 5.Flammer J, Drance SM, Schulzer M. Covariates of the long-term fluctuation of the differential light threshold. Arch Ophthalmol. 1984;102(6):880-882. [DOI] [PubMed] [Google Scholar]
- 6.Katz J, Sommer A. A longitudinal study of the age-adjusted variability of automated visual fields. Arch Ophthalmol. 1987;105(8):1083-1086. [DOI] [PubMed] [Google Scholar]
- 7.Heijl A, Lindgren A, Lindgren G. Test-retest variability in glaucomatous visual fields. Am J Ophthalmol. 1989;108(2):130-135. [DOI] [PubMed] [Google Scholar]
- 8.Matsuura M, Hirasawa K, Murata H, Asaoka R. The relationship between visual acuity and the reproducibility of visual field measurements in glaucoma patients. Invest Ophthalmol Vis Sci. 2015;56(9):5630-5635. [DOI] [PubMed] [Google Scholar]
- 9.Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56(3):303-308. [DOI] [PubMed] [Google Scholar]
- 10.Petersen RC, Stevens JC, Ganguli M, Tangalos EG, Cummings JL, DeKosky ST. Practice parameter: early detection of dementia: mild cognitive impairment (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2001;56(9):1133-1142. [DOI] [PubMed] [Google Scholar]
- 11.Morris JC, Storandt M, Miller JP, et al. . Mild cognitive impairment represents early-stage Alzheimer disease. Arch Neurol. 2001;58(3):397-405. [DOI] [PubMed] [Google Scholar]
- 12.Grundman M, Petersen RC, Ferris SH, et al. ; Alzheimer’s Disease Cooperative Study . Mild cognitive impairment can be distinguished from Alzheimer disease and normal aging for clinical trials. Arch Neurol. 2004;61(1):59-66. [DOI] [PubMed] [Google Scholar]
- 13.Hänninen T, Hallikainen M, Tuomainen S, Vanhanen M, Soininen H. Prevalence of mild cognitive impairment: a population-based study in elderly subjects. Acta Neurol Scand. 2002;106(3):148-154. [DOI] [PubMed] [Google Scholar]
- 14.Larrieu S, Letenneur L, Orgogozo JM, et al. . Incidence and outcome of mild cognitive impairment in a population-based prospective cohort. Neurology. 2002;59(10):1594-1599. [DOI] [PubMed] [Google Scholar]
- 15.Manly JJ, Tang MX, Schupf N, Stern Y, Vonsattel JP, Mayeux R. Frequency and course of mild cognitive impairment in a multiethnic community. Ann Neurol. 2008;63(4):494-506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ravaglia G, Forti P, Montesi F, et al. . Mild cognitive impairment: epidemiology and dementia risk in an elderly Italian population. J Am Geriatr Soc. 2008;56(1):51-58. [DOI] [PubMed] [Google Scholar]
- 17.Plassman BL, Langa KM, Fisher GG, et al. . Prevalence of cognitive impairment without dementia in the United States. Ann Intern Med. 2008;148(6):427-434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Plassman BL, Langa KM, McCammon RJ, et al. . Incidence of dementia and cognitive impairment, not dementia in the United States. Ann Neurol. 2011;70(3):418-426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Solfrizzi V, Panza F, Colacicco AM, et al. ; Italian Longitudinal Study on Aging Working Group . Vascular risk factors, incidence of MCI, and rates of progression to dementia. Neurology. 2004;63(10):1882-1891. [DOI] [PubMed] [Google Scholar]
- 20.Maioli F, Coveri M, Pagni P, et al. . Conversion of mild cognitive impairment to dementia in elderly subjects: a preliminary study in a memory and cognitive disorder unit. Arch Gerontol Geriatr. 2007;44(suppl 1):233-241. [DOI] [PubMed] [Google Scholar]
- 21.Storandt M, Grant EA, Miller JP, Morris JC. Rates of progression in mild cognitive impairment and early Alzheimer’s disease. Neurology. 2002;59(7):1034-1041. [DOI] [PubMed] [Google Scholar]
- 22.Gabryelewicz T, Styczynska M, Luczywek E, et al. . The rate of conversion of mild cognitive impairment to dementia: predictive role of depression. Int J Geriatr Psychiatry. 2007;22(6):563-567. [DOI] [PubMed] [Google Scholar]
- 23.Hoops S, Nazem S, Siderowf AD, et al. . Validity of the MoCA and MMSE in the detection of MCI and dementia in Parkinson disease. Neurology. 2009;73(21):1738-1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Russell RA, Crabb DP, Malik R, Garway-Heath DF. The relationship between variability and sensitivity in large-scale longitudinal visual field data. Invest Ophthalmol Vis Sci. 2012;53(10):5985-5990. [DOI] [PubMed] [Google Scholar]
- 25.Buse A, Lim J. Cubic splines as a special case of restricted least squares. J Am Stat Assoc. 1977;72(357):64-68. doi: 10.1080/01621459.1977.10479907 [DOI] [Google Scholar]
- 26.Smith PL. Splines as a useful and convenient statistical tool. Am Stat. 1979;33(2):57-62. doi: 10.1080/00031305.1979.10482661 [DOI] [Google Scholar]
- 27.Turpin A, McKendrick AM. What reduction in standard automated perimetry variability would improve the detection of visual field progression? Invest Ophthalmol Vis Sci. 2011;52(6):3237-3245. [DOI] [PubMed] [Google Scholar]
- 28.Medeiros FA, Leite MT, Zangwill LM, Weinreb RN. Combining structural and functional measurements to improve detection of glaucoma progression using Bayesian hierarchical models. Invest Ophthalmol Vis Sci. 2011;52(8):5794-5803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Medeiros FA, Zangwill LM, Girkin CA, Liebmann JM, Weinreb RN. Combining structural and functional measurements to improve estimates of rates of glaucomatous progression. Am J Ophthalmol. 2012;153(6):1197-1205.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krishnan K, Rossetti H, Hynan LS, et al. . Changes in Montreal Cognitive Assessment scores over time [published online June 18, 2016]. Assessment. doi: 10.1177/1073191116654217 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure. Montreal Cognitive Assessment (MoCA) Test