Key Points
Question
Can in vivo confocal microscopy detect axonal loss in patients with multiple sclerosis?
Findings
In this cross-sectional comparative study of 57 patients with multiple sclerosis and 30 healthy individuals, in vivo corneal confocal microscopy demonstrated reduced corneal nerve measures and increased dendritic cell density in patients with multiple sclerosis.
Meaning
Corneal confocal microscopy may be used as an imaging biomarker for identifying axonal loss in patients with multiple sclerosis.
Abstract
Importance
Multiple sclerosis (MS) is characterized by demyelination, axonal degeneration, and inflammation. Corneal confocal microscopy has been used to identify axonal degeneration in several peripheral neuropathies.
Objective
To assess corneal subbasal nerve plexus morphologic features, corneal dendritic cell (DC) density, and peripapillary retinal nerve fiber layer (RNFL) thickness in patients with MS.
Design, Setting, and Participants
This single-center, cross-sectional comparative study was conducted at a tertiary referral university hospital between May 27, 2016, and January 30, 2017. Fifty-seven consecutive patients with relapsing-remitting MS and 30 healthy, age-matched control participants were enrolled in the study. Corneal subbasal nerve plexus measures and DC density were quantified in images acquired with the laser scanning in vivo corneal confocal microscope, and peripapillary RNFL thickness was measured with spectral-domain optical coherence tomography.
Main Outcomes and Measures
Corneal nerve fiber density, nerve branch density, nerve fiber length, DC density, peripapillary RNFL thickness, and association with the severity of neurologic disability as assessed by the Kurtzke Expanded Disability Status Scale (score range, 0-10; higher scores indicate greater disability) and Multiple Sclerosis Severity Score (score range, 0.01-9.99; higher scores indicate greater severity).
Results
Of the 57 participants with MS, 42 (74%) were female and the mean (SD) age was 35.4 (8.9) years; of the 30 healthy controls, 19 (63%) were female and the mean (SD) age was 34.8 (10.2) years. Corneal nerve fiber density (mean [SE] difference, −6.78 [2.14] fibers/mm2; 95% CI, −11.04 to −2.52; P = .002), nerve branch density (mean [SE] difference, −17.94 [5.45] branches/mm2; 95% CI, −28.77 to −7.10; P = .001), nerve fiber length (mean [SE] difference, −3.03 [0.89] mm/mm2; 95% CI, −4.81 to −1.25; P = .001), and the mean peripapillary RNFL thickness (mean [SE] difference, −17.06 [3.14] μm; 95% CI, −23.29 to −10.82; P < .001) were reduced in patients with MS compared with healthy controls. The DC density was increased (median [interquartile range], 27.7 [12.4-66.8] vs 17.3 [0-28.2] cells/mm2; P = .03), independent of a patient’s history of optic neuritis. Nerve fiber density and RNFL thickness showed inverse associations with the Expanded Disability Status Scale (ρ = −0.295; P = .03 for nerve fiber density and ρ = −0.374; P = .004 for RNFL thickness) and the Multiple Sclerosis Severity Score (R = −0.354; P = .007 for nerve fiber density and R = −0.283; P = .03 for RNFL thickness), whereas other study measures did not.
Conclusions and Relevance
These data suggest that corneal confocal microscopy demonstrates axonal loss and increased DC density in patients with MS. Additional longitudinal studies are needed to confirm the use of corneal confocal microscopy as an imaging biomarker in patients with MS.
This cross-sectional study examines the use of in vivo corneal confocal microscopy to identify axonal degeneration in patients with multiple sclerosis.
Introduction
Multiple sclerosis (MS) is a progressive, chronic inflammatory disease characterized by demyelination and axonal degeneration. It primarily affects patients aged 20 to 40 years and is the leading cause of neurologic disability in young adults. Optic neuritis is the most common ocular manifestation and may be the initial presentation in 20% of patients with MS, while many others will develop optic neuritis at some point during the course of the disease. Many studies with optical coherence tomography (OCT) have demonstrated a reduction in thickness of the retinal nerve fiber layer (RNFL) in patients with MS. Indeed, it has been suggested that retrograde axonal degeneration may underlie RNFL thinning even without acute optic neuritis.
The cornea is one of the most densely innervated tissues in the human body, receiving sensory innervation from the trigeminal nerve. Similar to OCT, which can quantify the unmyelinated retinal nerve fibers, in vivo corneal confocal microscopy (CCM) is a noninvasive imaging technique that allows detailed quantification of the corneal subbasal nerve plexus. In addition, CCM allows visualization of dendritic cells (DCs), which are increased in inflammatory and immune processes. In a recent study of 26 patients with MS, Mikolajczak and colleagues reported a reduction in corneal nerve fibers and no difference in DC density, indicating that CCM could potentially be used to image axonal degeneration in patients with MS. To our knowledge, these findings have not been published in the ophthalmological literature.
For this study, we undertook detailed quantification of the corneal subbasal nerve plexus, DC density, and peripapillary RNFL thickness in patients with relapsing-remitting MS, compared the quantification with that in healthy control individuals, and correlated these measures with the severity of neurologic disability.
Methods
Fifty-seven eyes of 57 patients with relapsing-remitting MS and 30 eyes of 30 healthy individuals were enrolled in this cross-sectional study undertaken at a single tertiary referral university hospital between May 27, 2016, and January 30, 2017. The patients were diagnosed with MS according to the revised McDonald criteria, based on clinical and radiologic findings. A complete neurologic examination was performed, and physical disability score was assessed using the Kurtzke Expanded Disability Status Scale (EDSS) for each patient. The Multiple Sclerosis Severity Score (MSSS) was calculated from the disease duration and EDSS. Scores for the EDSS range from 0 to 10, and a higher score indicates greater disability; scores for the MSSS range from 0.01 to 9.99, and a higher score indicates greater severity. The presence of previous episodes of optic neuritis as reported by the neurologist or the patient was also recorded. Exclusion criteria were a history of optic neuritis within 6 months of the study, any other neurologic disorders, diabetes, previous ocular trauma or surgery, and glaucoma or contact lens use. The study was approved by the institutional review board of Necmettin Erbakan University and adhered to the tenets of the Declaration of Helsinki. Written informed consent was obtained from all participants after a detailed explanation of the nature of the study.
Of the 57 patients with MS, 8 (14%) had a history of bilateral optic neuritis, 20 (35%) had a history of unilateral optic neuritis, and 29 (51%) had not experienced optic neuritis. Only the right eyes were assessed if the patient had bilateral optic neuritis or no optic neuritis in either eye. The eyes with previous optic neuritis (right eye of 8 patients and left eye of 12 patients) were assessed in patients with a history of unilateral optic neuritis. Thus, 2 subgroups were created, including 28 patients with optic neuritis and 29 patients without optic neuritis. For healthy controls, only the right eyes were included.
All participants underwent a complete ophthalmologic evaluation, including visual acuity testing, anterior segment biomicroscopy, intraocular pressure assessment, and fundus examination. The peripapillary RNFL thickness measurements were obtained using spectral-domain OCT (Heidelberg Engineering). The OCT scanning circle (diameter, 3.4 mm) was manually positioned at the center of the optic disc, and the average peripapillary RNFL thickness was recorded in micrometers.
Laser scanning CCM (Rostock Cornea Module/Heidelberg Retina Tomograph lll; Heidelberg Engineering) was performed on all participants. The full thickness of the central cornea was scanned using the section mode. The total duration of CCM examination was approximately 2 minutes per eye. Three to 5 high-quality subbasal nerve plexus images were analyzed from each participant, and the mean of these results was considered. Automated CCMetrics software, version 2.0 (University of Manchester) was used to quantify the nerve fibers. Three measures were quantified: (1) corneal nerve fiber density, the total number of major nerves per square millimeter; (2) nerve branch density, the number of branches emanating from major nerve trunks per square millimeter; and (3) nerve fiber length, the total length of all nerve fibers and branches (in millimeters per square millimeter). The same image frames were used to quantify DC density. The number of highly reflective cells with dendriform structures were counted manually, and the density was derived as the number of cells in the area of frame assessed per square millimeters. All the image analyses were performed by a single masked observer (G.B.).
Statistical analysis was performed using SPSS, version 17.0 software (IBM). Basic descriptive statistics were calculated on all the data gathered and are reported as the mean (SD or SE) or median (interquartile range [IQR]), as appropriate. Unpaired, 2-tailed Pearson χ2 test was used to compare categorical variables. A normal distribution of continuous variables was confirmed with the Kolmogorov-Smirnov test. The independent samples t test for normally distributed data and Mann-Whitney test for nonnormally distributed data were used to compare the measures between the patients and healthy controls. A 1-way analysis of variance test followed by the Tukey test or Kruskal-Wallis test and Mann-Whitney test with Bonferroni adjustment were used to compare the measures among subgroups according to patient history of optic neuritis. The association of disease severity with other study measures was determined using the Pearson correlation coefficient for normally distributed data and the Spearman correlation coefficient for nonnormally distributed data. A 2-sided P < .05 was considered statistically significant. After Bonferroni adjustment, P = .017 (equal to .05/3) was considered as a significant level.
Results
The main characteristics of the participants are given in Table 1. The mean (SD) age of the 57 participants with relapsing-remitting multiple sclerosis was 35.4 (8.9) years, and 42 (74%) of them were female. The mean (SD) age of the 30 healthy, age-matched controls was 34.8 (10.2) years, and 19 (63%) of them were female. At the time of this study, 47 of the 57 patients (82%) were receiving a disease-modifying treatment. There were no differences between patients with MS and healthy controls in terms of age (mean [SE] difference, 0.64 [2.12]; 95% CI, −3.57 to 4.85; P = .76) and sex (women vs men with MS: 42 vs 15; healthy control women vs men: 19 vs 11; P = .32). No differences were observed between the optic neuritis and non-optic neuritis subgroups in terms of age (mean [SE] difference, 0.68 [2.38]; 95% CI, −4.10 to 5.47; P = .78), sex (women vs men with optic neuritis, 22 vs 6; women vs men without optic neuritis: 20 vs 9; P = .41), disease duration (mean [SE] difference, 0.36 [1.22]; 95% CI, −2.07 to 2.81; P = .76), EDSS (mean [SD], 2.64 [0.93] vs 3.01 [1.31]; P = .60), and MSSS (mean [SE] difference, −0.65 [0.44]; 95% CI, −1.55 to 0.23; P = .15). None of the patients reported a history of trigeminal neuralgia.
Table 1. Baseline Characteristics of Study Participants.
| Characteristic | Healthy Control Participant (n = 30) |
Patients With MS | ||
|---|---|---|---|---|
| Total (n = 57) |
With ON (n = 28) |
Without ON (n = 29) |
||
| Age, mean (SD), y | 34.8 (10.2) | 35.4 (8.9) | 35.8 (8.9) | 35.1 (9.1) |
| Sex, No. female/male | 19/11 | 42/15 | 22/6 | 20/9 |
| Disease duration, mean (SD), y | NA | 7.47 (4.57) | 7.66 (4.02) | 7.29 (5.11) |
| EDSS score, mean (SD) | NA | 2.83 (1.15) | 2.64 (0.93) | 3.01 (1.31) |
| MSSS, mean (SD) | NA | 4.47 (1.70) | 4.14 (1.75) | 4.79 (1.61) |
Abbreviations: EDSS, Expanded Disability Status Scale; MS, multiple sclerosis; MSSS, Multiple Sclerosis Severity Score; NA, not applicable; ON, optic neuritis.
There were reductions in the mean nerve fiber density (mean [SE] difference, −6.78 [2.14] fibers/mm2; 95% CI, −11.04 to −2.52; P = .002), nerve branch density (mean [SE] difference, −17.94 [5.45] branches/mm2; 95% CI, −28.77 to −7.10; P = .001), nerve fiber length (mean [SE] difference, −3.03 [0.89] mm/mm2; 95% CI, −4.81 to −1.25; P = .001), and peripapillary RNFL thickness (mean [SE] difference, −17.06 [3.14] μm; 95% CI, −23.29 to −10.82; P < .001) between patients with MS compared with healthy controls (Table 2 and Figure). Dendritic cell density was higher in patients with MS (median [interquartile range], 27.7 [12.4-66.8] vs 17.3 [0-28.2] cells/mm2; P = .03). In subgroup analysis, RNFL thickness was reduced in patients with optic neuritis compared with patients without optic neuritis, but no difference was observed in the CCM measures. When compared with the control group, both the optic neuritis and non–optic neuritis subgroups showed reductions in the average nerve fiber density, nerve branch density, nerve fiber length, and RNFL thickness; however, DC density did not demonstrate a difference.
Table 2. Corneal Confocal Microscopic Measures and Peripapillary RNFL Thickness in Patients With MS and Healthy Control Participants.
| Measure | Healthy Control Participants (n = 30) |
Patients With MS | P Value | |||||
|---|---|---|---|---|---|---|---|---|
| Total (n = 57) |
With ON (n = 28) |
Without ON (n = 29) |
Patients With MS vs Healthy Control | Patients With MS, With ON vs Without ON | Patients With MS, Without ON vs Healthy Control | Patients With MS, With ON vs Healthy Control | ||
| Nerve fiber density, mean (SD), fibers/mm2 | 33.5 (7.9) | 26.7 (10.2) | 27.4 (10.7) | 26.1 (9.9) | .002a | .85b | .01b | .045b |
| Nerve branch density, mean (SD), branches/mm2 | 54.9 (30.2) | 37.1 (20.3) | 38.6 (21.1) | 35.6 (19.8) | .001a | .88b | .008b | .03b |
| Nerve fiber length, mean (SD), mm/mm2 | 19.2 (3.7) | 16.1 (4.1) | 16.6 (4.4) | 15.7 (3.8) | .001a | .71b | .004b | .04b |
| RNFL thickness, mean (SD), µm | 102.2 (10.8) | 85.2 (15.2) | 79.5 (14.6) | 90.6 (14.1) | <.001a | .006b | .003b | <.001b |
| DC density, median (IQR), cells/mm2 | 17.3 (0-28.2) |
27.7 (12.4-66.8) |
30.5 (12.9-78.3) |
26.7 (0-62.3) |
.03c | .31d | .17d | .02d |
Abbreviations: DC, dendritic cells; IQR, interquartile range; MS, multiple sclerosis; ON, optic neuritis; RNFL, retinal nerve fiber layer.
Independent-samples t test.
One-way analysis of variance test followed by Tukey test.
Mann-Whitney test.
Kruskal-Wallis test followed by Mann-Whitney test with Bonferroni adjustment. (P < .017 [equal to .05/3] is considered statistically significant.)
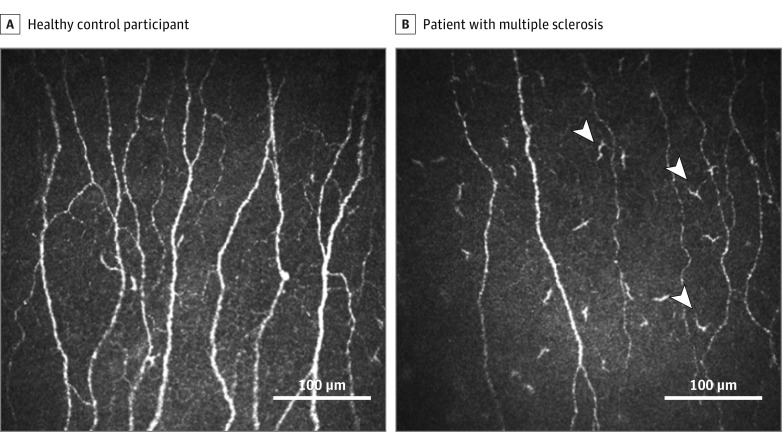
Figure. Representative Corneal Confocal Microscopic Images of the Central Cornea.
Subbasal nerve plexus in a healthy control participant (A) and in a patient with multiple sclerosis (B). The white arrowheads indicate the hyperreflective cells with dendriform structures.
Of the 57 patients with MS, 47 (82%) were receiving disease-modifying agents for the treatment of MS: 17 (30%) were receiving fingolimod, 13 (23%) were receiving interferon beta, 11 (19%) were receiving azathioprine, 5 (9%) were receiving glatiramer acetate, and 1 (2%) was receiving teriflunomide; 10 patients (18%) were not receiving any disease-modifying treatment. (Percentages in the preceding sentence may not total 100% because of rounding.) There were no differences between the treatment groups in any of the study measures.
Correlations
Inverse correlations were found between EDSS and nerve fiber density (ρ = −0.295; P = .03), EDSS and RNFL (ρ = −0.374; P = .004), MSSS and nerve fiber density (R = −0.354; P = .007), and MSSS and RNFL (R = −0.283; P = .03). No correlations were observed between the disease severity scores and nerve branch density or nerve fiber length. There was no correlation between DC density and neurologic disability, CCM, or the OCT measures.
Discussion
Axonal loss is considered to be a key marker for diagnosing and assessing disease progression in patients with MS. Indeed, OCT has been used to quantify retinal axonal degeneration in numerous studies showing thinning of the RNFL in patients with MS, even at the very early stages, and in patients without a history of optic neuritis. A recent longitudinal study of 45 patients with relapsing-remitting MS has reported a progressive reduction of the temporal RNFL thickness in eyes without optic neuritis. Our results are consistent with those of previous studies, showing a reduction in the peripapillary RNFL thickness in patients with relapsing-remitting MS and in the optic neuritis and non–optic neuritis subgroups compared with healthy controls, but patients with a recent (<6 months’) history of optic neuritis were excluded. Peripapillary RNFL thickness was also inversely associated with EDSS and MSSS scores, confirming some but not other studies. These conflicting findings may be attributed to differing study populations with varying disease severity, ongoing treatment, proportion of patients with optic neuritis, and different OCT devices used.
According to studies by Behbehani and colleagues and Albrecht and colleagues, RNFL thickness was associated with EDSS only in eyes without previous optic neuritis. Although the occurrence of optic neuritis and associated inflammation can result in a temporary increase in RNFL, it is followed by further thinning, enabling the use of OCT as an imaging end point in longitudinal studies and in clinical trials of patients with MS. However, recent reports advocating OCT measurement of RNFL thickness as a useful biomarker for disease progression have focused on eyes without optic neuritis among patients with MS.
Corneal confocal microscopy is a rapid, noninvasive technique that quantifies axons with good sensitivity and specificity. We have established corneal nerve fiber density as a measure of degeneration and corneal nerve branch density as a measure of regeneration that may increase the utility of CCM as an imaging end point in clinical trials of novel agents in MS. Furthermore, trigeminal nerve involvement, which may affect CCM assessment, has been reported in only 2.9% of patients with MS. DeSouza and colleagues have demonstrated abnormal tissue microstructure in the trigeminal nerves of patients with trigeminal neuralgia. Our study did not include participants with a history of trigeminal neuralgia, but it is possible that the presence of trigeminal nerve involvement may affect corneal innervation. To date, there is only 1 recent report demonstrating the utility of CCM in detecting axonal degeneration in patients with MS. Mikolajczak and colleagues reported a reduction in corneal nerve fiber length in patients with MS, which was associated with clinical disability but not the presence of a history of trigeminal symptoms. We now confirm these findings in a larger cohort of patients with MS and demonstrate a reduction in additional morphometric corneal nerve measures. To our knowledge, our study constitutes the first such report in the ophthalmologic literature. Furthermore, nerve fiber density showed an inverse correlation with EDSS and MSSS but no association with optic neuritis, suggesting that CCM may be a promising new imaging modality to diagnose and evaluate the progress of MS without being influenced by the occurrence of optic neuritis.
Knier and colleagues have demonstrated that the retinal inner nuclear layer is thinner in patients with MS receiving disease-modifying agents. In our study, 82% of the patients were receiving disease-modifying medications, which could also have had an effect on the corneal subbasal nerve plexus. Therefore, further investigations in untreated patients are warranted to eliminate the possible effects of treatment on corneal nerves.
In addition, we have demonstrated an increase in DC density in patients with MS, which was not associated with disease severity or the degree of corneal or retinal axonal loss or the past occurrence of optic neuritis. Dendritic cells migrate and accumulate in the central cornea in a number of inflammatory conditions. A previous study has shown that DC density is dynamic and initially may be increased in patients with mild diabetic neuropathy but then decreases in patients with more severe neuropathy. A recent study has demonstrated the loss of corneal nerve fibers and an increase in DC density in patients with chronic inflammatory demyelinating neuropathy. Given that inflammation is a key part of the pathogenesis of MS, an increase in DC density may also be expected. Mikolajczak and colleagues reported no increase in DC density in patients with MS, suggesting that the assessment of DC density at a single time point may not be a reliable marker of ongoing inflammation. Further longitudinal studies are required to better understand the utility of quantifying DC density and dynamics, particularly the occurrence of optic neuritis and the effect of concomitant therapy in MS because, despite ongoing treatment, DC density was higher in this cohort.
Limitations
A limitation of this study is the cross-sectional nature of the study design, which prevents us from drawing any firm conclusion regarding the natural history of axonal degeneration in patients with MS.
Conclusions
This study represents the largest and most detailed CCM study to date that shows reductions in a range of corneal subbasal nerve measures, increased DC density, and reduced peripapillary RNFL thickness in patients with MS. It also demonstrates an association between nerve fiber density and RNFL thickness and neurologic disability. Its findings strongly encourage further evaluation of CCM as an imaging method for diagnosing and assessing disease progression and potentially regression in clinical trials of patients with MS.
References
- 1.Compston A, Coles A. Multiple sclerosis. Lancet. 2008;372(9648):1502-1517. [DOI] [PubMed] [Google Scholar]
- 2.Arnold AC. Evolving management of optic neuritis and multiple sclerosis. Am J Ophthalmol. 2005;139(6):1101-1108. [DOI] [PubMed] [Google Scholar]
- 3.Pulicken M, Gordon-Lipkin E, Balcer LJ, Frohman E, Cutter G, Calabresi PA. Optical coherence tomography and disease subtype in multiple sclerosis. Neurology. 2007;69(22):2085-2092. [DOI] [PubMed] [Google Scholar]
- 4.Henderson AP, Trip SA, Schlottmann PG, et al. . An investigation of the retinal nerve fibre layer in progressive multiple sclerosis using optical coherence tomography. Brain. 2008;131(pt 1):277-287. [DOI] [PubMed] [Google Scholar]
- 5.Iester M, Cioli F, Uccelli A, et al. . Retinal nerve fibre layer measurements and optic nerve head analysis in multiple sclerosis patients. Eye (Lond). 2009;23(2):407-412. [DOI] [PubMed] [Google Scholar]
- 6.Siepman TA, Bettink-Remeijer MW, Hintzen RQ. Retinal nerve fiber layer thickness in subgroups of multiple sclerosis, measured by optical coherence tomography and scanning laser polarimetry. J Neurol. 2010;257(10):1654-1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Behbehani R, Al-Hassan AA, Al-Khars A, Sriraman D, Alroughani R. Retinal nerve fiber layer thickness and neurologic disability in relapsing-remitting multiple sclerosis. J Neurol Sci. 2015;359(1-2):305-308. [DOI] [PubMed] [Google Scholar]
- 8.Albrecht P, Fröhlich R, Hartung HP, Kieseier BC, Methner A. Optical coherence tomography measures axonal loss in multiple sclerosis independently of optic neuritis. J Neurol. 2007;254(11):1595-1596. [DOI] [PubMed] [Google Scholar]
- 9.Klistorner A, Garrick R, Barnett MH, et al. . Axonal loss in non-optic neuritis eyes of patients with multiple sclerosis linked to delayed visual evoked potential. Neurology. 2013;80(3):242-245. [DOI] [PubMed] [Google Scholar]
- 10.Graham EC, You Y, Yiannikas C, et al. . Progressive loss of retinal ganglion cells and axons in nonoptic neuritis eyes in multiple sclerosis: a longitudinal optical coherence tomography study. Invest Ophthalmol Vis Sci. 2016;57(4):2311-2317. [DOI] [PubMed] [Google Scholar]
- 11.Tavakoli M, Quattrini C, Abbott C, et al. . Corneal confocal microscopy: a novel noninvasive test to diagnose and stratify the severity of human diabetic neuropathy. Diabetes Care. 2010;33(8):1792-1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tavakoli M, Boulton AJ, Efron N, Malik RA. Increased Langerhan cell density and corneal nerve damage in diabetic patients: role of immune mechanisms in human diabetic neuropathy. Cont Lens Anterior Eye. 2011;34(1):7-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mayer WJ, Mackert MJ, Kranebitter N, et al. . Distribution of antigen presenting cells in the human cornea: correlation of in vivo confocal microscopy and immunohistochemistry in different pathologic entities. Curr Eye Res. 2012;37(11):1012-1018. [DOI] [PubMed] [Google Scholar]
- 14.Mikolajczak J, Zimmermann H, Kheirkhah A, et al. . Patients with multiple sclerosis demonstrate reduced subbasal corneal nerve fibre density [published online November 1, 2016]. Mult Scler. doi: 10.1177/1352458516677590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Polman CH, Reingold SC, Banwell B, et al. . Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol. 2011;69(2):292-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roxburgh RH, Seaman SR, Masterman T, et al. . Multiple Sclerosis Severity Score: using disability and disease duration to rate disease severity. Neurology. 2005;64(7):1144-1151. [DOI] [PubMed] [Google Scholar]
- 17.World Medical Association World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. [DOI] [PubMed] [Google Scholar]
- 18.Dabbah MA, Graham J, Petropoulos IN, Tavakoli M, Malik RA. Automatic analysis of diabetic peripheral neuropathy using multi-scale quantitative morphology of nerve fibres in corneal confocal microscopy imaging. Med Image Anal. 2011;15(5):738-747. [DOI] [PubMed] [Google Scholar]
- 19.Malik RA, Kallinikos P, Abbott CA, et al. . Corneal confocal microscopy: a non-invasive surrogate of nerve fibre damage and repair in diabetic patients. Diabetologia. 2003;46(5):683-688. [DOI] [PubMed] [Google Scholar]
- 20.Zhivov A, Stave J, Vollmar B, Guthoff R. In vivo confocal microscopic evaluation of Langerhans cell density and distribution in the normal human corneal epithelium. Graefes Arch Clin Exp Ophthalmol. 2005;243(10):1056-1061. [DOI] [PubMed] [Google Scholar]
- 21.Gelfand JM, Goodin DS, Boscardin WJ, Nolan R, Cuneo A, Green AJ. Retinal axonal loss begins early in the course of multiple sclerosis and is similar between progressive phenotypes. PLoS One. 2012;7(5):e36847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maghzi AH, Graves J, Revirajan N, et al. . Retinal axonal loss in very early stages of multiple sclerosis. Eur J Neurol. 2015;22(7):1138-1141. [DOI] [PubMed] [Google Scholar]
- 23.Knier B, Berthele A, Buck D, et al. . Optical coherence tomography indicates disease activity prior to clinical onset of central nervous system demyelination. Mult Scler. 2016;22(7):893-900. [DOI] [PubMed] [Google Scholar]
- 24.Toledo J, Sepulcre J, Salinas-Alaman A, et al. . Retinal nerve fiber layer atrophy is associated with physical and cognitive disability in multiple sclerosis. Mult Scler. 2008;14(7):906-912. [DOI] [PubMed] [Google Scholar]
- 25.Garcia-Martin E, Polo V, Larrosa JM, et al. . Retinal layer segmentation in patients with multiple sclerosis using spectral domain optical coherence tomography. Ophthalmology. 2014;121(2):573-579. [DOI] [PubMed] [Google Scholar]
- 26.Naismith RT, Tutlam NT, Xu J, et al. . Optical coherence tomography is less sensitive than visual evoked potentials in optic neuritis. Neurology. 2009;73(1):46-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Esen E, Sizmaz S, Balal M, et al. . Evaluation of the innermost retinal layers and visual evoked potentials in patients with multiple sclerosis. Curr Eye Res. 2016;41(10):1353-1358. [DOI] [PubMed] [Google Scholar]
- 28.Martinez-Lapiscina EH, Arnow S, Wilson JA, et al. ; IMSVISUAL consortium . Retinal thickness measured with optical coherence tomography and risk of disability worsening in multiple sclerosis: a cohort study. Lancet Neurol. 2016;15(6):574-584. [DOI] [PubMed] [Google Scholar]
- 29.da Silva CJ, da Rocha AJ, Mendes MF, Maia AC Jr, Braga FT, Tilbery CP. Trigeminal involvement in multiple sclerosis: magnetic resonance imaging findings with clinical correlation in a series of patients. Mult Scler. 2005;11(3):282-285. [DOI] [PubMed] [Google Scholar]
- 30.DeSouza DD, Hodaie M, Davis KD. Abnormal trigeminal nerve microstructure and brain white matter in idiopathic trigeminal neuralgia. Pain. 2014;155(1):37-44. [DOI] [PubMed] [Google Scholar]
- 31.Knier B, Schmidt P, Aly L, et al. . Retinal inner nuclear layer volume reflects response to immunotherapy in multiple sclerosis. [published online August 30, 2016]. Brain. 2016;aww219. [DOI] [PubMed] [Google Scholar]
- 32.Cruzat A, Witkin D, Baniasadi N, et al. . Inflammation and the nervous system: the connection in the cornea in patients with infectious keratitis. Invest Ophthalmol Vis Sci. 2011;52(8):5136-5143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stettner M, Hinrichs L, Guthoff R, et al. . Corneal confocal microscopy in chronic inflammatory demyelinating polyneuropathy. Ann Clin Transl Neurol. 2015;3(2):88-100. [DOI] [PMC free article] [PubMed] [Google Scholar]