This multicenter cohort study examines the incidence of atrophic lesions of the retinal pigment epithelium in patients with Stargardt disease as determined by fundus autofluorescence imaging.
Key Points
Question
What is the incidence of atrophic lesions in patients with Stargardt disease?
Findings
In a multicenter cohort study of 217 patients, the median time to develop a definitely decreased autofluorescence lesion was 4.9 years among the eyes with questionably decreased autofluorescence but no definitely decreased autofluorescence at the baseline visit. Among eyes without questionably decreased autofluorescence, the median time to develop such a lesion was 6.3 years.
Meaning
These results suggest that the incidence of atrophic lesions may serve as an outcome measure for trials of sufficient duration in Stargardt disease.
Abstract
Importance
Outcome measures that are sensitive to disease progression are needed as clinical end points for future treatment trials in Stargardt disease.
Objective
To examine the incidence of atrophic lesions of the retinal pigment epithelium in patients with Stargardt disease as determined by fundus autofluorescence imaging.
Design, Setting, and Participants
In this retrospective multicenter cohort study, 217 patients 6 years and older at baseline at tertiary referral centers in Europe, the United States, and the United Kingdom who were harboring disease-causing variants in the adenosine triphosphate (ATP)–binding cassette subfamily A member 4 (ABCA4) gene and who met the following criteria were enrolled: (1) at least 1 well-demarcated area of atrophy with a minimum diameter of 300 µm, with the total area of all atrophic lesions being less than or equal to 12 mm2 in at least 1 eye at the most recent visit, and (2) fundus autofluorescence images for at least 2 visits with a minimum of 6 months between at least 2 visits. Data were collected between August 22, 2013, and December 12, 2014. Data analysis was performed from March 15, 2015, through January 31, 2017.
Exposures
Images were evaluated by staff at a central reading center. Areas of definitely decreased autofluorescence (DDAF) and questionably decreased autofluorescence (QDAF) were outlined and quantified. Lesion-free survival rates were estimated using Kaplan-Meier survival curves.
Main Outcomes and Measures
Incidence of atrophic lesions as determined by fundus autofluorescence.
Results
The 217 patients (mean [SD] age, 21.8 [13.3] years; 127 female [57.5%]; 148 white [68.2%]) contributed 390 eyes for which the mean (SD) follow-up time was 3.9 (1.6) years (range, 0.7-12.1 years). Among eyes without DDAF at first visit, the median time to develop a DDAF lesion was 4.9 years (95% CI, 4.3-5.6 years). Among eyes without QDAF, the median time to develop a QDAF lesion was 6.3 years (95% CI, 5.6-9.7 years). Eyes with a lesion of DDAF at the first visit were less likely to develop a QDAF lesion compared with eyes without a lesion of DDAF (hazard ratio, 0.19; 95% CI, 0.05-0.70; P = .01).
Conclusions and Relevance
An estimated 50% of the eyes without DDAF at first visit will develop the lesion in less than 5 years, suggesting that incidence of DDAF could serve as an outcome measure for treatment trials.
Introduction
The most common juvenile macular degeneration is Stargardt macular dystrophy (Stargardt disease 1 [STGD1]; OMIM: 248200), with a prevalence of 10 to 12.5 per 100 000 persons; it is inherited as an autosomal-recessive trait attributable to disease-causing mutations in the adenosine triophosphate (ATP)–binding cassette subfamily A member 4 (ABCA4) (OMIM 601691) gene. Although there is currently no treatment approved by the US Food and Drug Administration, several treatment approaches, including gene augmentation, stem cell therapy, and pharmacotherapy, are in early clinical trials. Measurement of enlargement of geographic atrophy is being used as a primary outcome measure in current trials. Use of fundus autofluorescence (FAF), as opposed to the traditional color fundus photography, provides the advantages of better distinction and delineation between dead and nonfunctioning retinal pigment epithelium and living but depigmented retinal pigment epithelium. The concept that atrophic areas in geographic atrophy have a markedly reduced autofluorescent signal, thereby indicating and reflecting loss of retinal pigment epithelium cells, is widely accepted and also considered as a potential surrogate end point by the US Food and Drug Administration. The incidence of new lesions as defined using FAF in eyes at risk for developing these lesions has not been well studied.
The Progression of Atrophy Secondary to Stargardt Disease (ProgStar) retrospective study (NCT01977846) is an ideal study to address the incidence of new FAF lesions because the entry criteria allowed eyes with no lesion at the first baseline visit and the observational period is longer than in the prospective study. The purpose of this article is to report the incidence of atrophic lesions in the retrospective ProgStar study as determined by FAF.
Methods
The study was conducted according to the International Conference on Harmonisation Good Clinical Practice Guidelines, the applicable regulatory requirements, the current Declaration of Helsinki, and the Health Insurance Portability and Accountability Act. Ethics committee approval was granted by the Western Institutional Review Board, the local institutional review boards (Cleveland Clinic Institutional Review Board; the University of Texas Southwestern Institutional Review Board; the Greater Baltimore Medical Center Institutional Review Board; the Johns Hopkins University, School of Medicine, Office of Human Subjects Research Institutional Review Boards; University of Pennsylvania, Office of Regulatory Affairs, Institutional Review Board; University of Utah Institutional Review Board; National Health Service Health Research Authority, London–Queen Square Research Ethics Committee; French National Commission of Data Processing and Liberties; Ethics Committee of the Medical Faculty of the Eberhard-Karls University and of the University Hospitals, Tübingen), and the Human Research Protection Office of the US Army Medical Research and Materiel Command before enrollment of the first patient. However, all except one local institutional review board (French National Commission of Data Processing and Liberties) exempted the centers from an informed consent process; at this institution, written informed consent was obtained from participants before enrollment.
The design, inclusion and exclusion criteria, and the participants in the retrospective ProgStar study have been described in detail previously. Briefly summarized for the purpose of this article, the key inclusion criteria required the presence of the following: (1) at least 2 pathogenic mutations in the ABCA4 gene (presence of 1 mutation was accepted in case the clinical phenotype was typical for Stargardt disease; ie, with flecks on the level of the retinal pigment epithelium); (2) the same examination modality was available for at least 2 visits for FAF obtained with an angiograph instrument (eg, Heidelberg Retina Angiograph 2 [HRA2], Heidelberg Engineering), spectral domain optical coherence tomograph (SD-OCT) obtained with an expandable diagnostic imaging platform (Spectralis, Heidelberg Engineering), and/or microperimetry at least 6 months apart; (3) 1 well-defined atrophic lesion in at least 1 eye at the most recent visit that measured at least 300 µm in diameter as determined by the site’s principal investigator; however, the area of all lesions together must have been less than or equal to 12 mm2 (corresponding to no more than 5 disc areas); (4) sufficient quality of images; and (5) minimal age of 6 years at the most recent visit. Participants in the retrospective study could have had 2, 3, or 4 visits. The observational period between 2 single visits was between 6 and 60 months.
Patient medical records were reviewed by the participating clinical sites to gather data such as age at enrollment, sex, race, visual acuity, findings derived from ophthalmologic examination, and vitamin A supplementation and sent to the data coordinating center (Dana Center for Preventive Ophthalmology, Wilmer Eye Institute, Johns Hopkins University, Baltimore, Maryland). FAF image obtainment with a Heidelberg Engineering device was required; infrared reflectance images obtained with such a device (eg, HRA2 or Heidelberg Spectralis HRA+OCT [excitation light, 815 nm]) were also accepted.
Deidentified FAF images of eligible patients were sent by the sites to the central reading center (Doheny Imaging Reading Center, David Geffen School of Medicine at UCLA [University of California, Los Angeles]), where they were evaluated for focus and clarity. The FAF imaging grading protocols included qualitative and quantitative factors.
Qualitative Grading Factors
An even background FAF is characterized by a smooth distribution of FAF with a decrease at the fovea based on the presence of macular pigment, in contrast to an irregular background FAF with the presence of mottled, speckled, or reticular pattern of FAF. Irregular background FAF was graded independent of discrete areas of abnormal FAF.
Qualitative grading factors included absence or presence of flecks in and outside the arcades. Uniformity of the background FAF was graded based on 2 categories proposed by Fujinami et al: a homogeneous background signal was defined as an even distribution of background autofluorescence and a heterogeneous background signal was characterized by widespread small foci of increased or reduced autofluorescence.
Quantitative Grading Factors
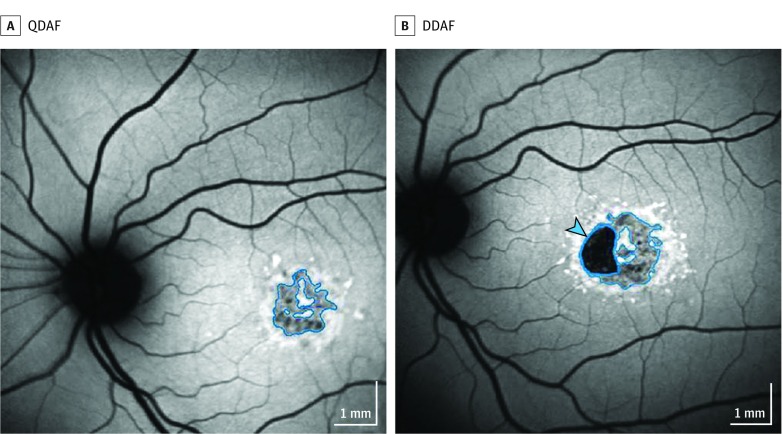
The grading scheme for quantitative assessment of different types of decreased areas of autofluorescence was published previously. Qualitative measures of darkness and lesion border determined distinct lesion types (Figure 1): definitely decreased autofluorescence (DDAF) and questionably decreased autofluorescence (QDAF). Reference points for gray levels were the background and the optic disc and nerve head, respectively; the periphery of an image often revealed a normal retina with undisturbed areas and a homogeneous background. The optic nerve disc and head were set as the reference point to determine the 100% degree of blackness. For all types, the pattern of decreased areas of autofluorescence had to appear as different (darker) from the background gray level and the lesion diameter had to be 125 μm or larger (approximately equal to the diameter of a vein originating from the optic disc) according to grading protocols. The distinct types of decreased areas of autofluorescence had differences in the black level: if an area of interest was more than 90% black (where the optic disc was defined as being 100% black), this was defined as DDAF, whereas an area of interest with black levels ranging from 50% to 90% was defined as QDAF.
Figure 1. Different Decreased Autofluorescence Categories Used for Grading of Fundus Autofluorescence Images.
A, For areas with levels between 50% and 90% of darkness in reference to the optic nerve head, the term questionably decreased autofluorescence (QDAF) is used. B, For lesions with at least 90% darkness, the term definitely decreased autofluorescence is used (DDAF) (blue arrowhead).
The size of the areas was semiautomatically evaluated using the RegionFinder module of the Heidelberg Eye Explorer (Heidelberg Engineering) review software. The previously described conventions were used: application of shadow correction when the FAF images were unevenly or inadequately illuminated; each noncontiguous area of decreased autofluorescence has been quantified separately if needed; algorithm growth power was adjusted and refined manually until the region fully captured the area of decreased FAF; and manual line, circle, or free-hand constraints were used as needed to distinguish lesion boundaries and exclude vascular structures. The sum of all areas of respective decreased areas of autofluorescence (within each subtype) was calculated in cases of presence of multifocal lesions.
The FAF images were evaluated independently and objectively. Each image type was initially assessed with masking from information from other image types or visits. The reading center allowed graders to eventually reference all available image types from that visit date if desired and available; in ambiguous cases, graders were allowed to reference the grading decisions from the patient’s previous visits to guide grading of the images from the current visit. At least 1 of the graders was a senior-level grader. All cases of discordant initial answers underwent adjudication, and the final answer was determined by a reading center investigator.
In 8 visits of 7 patients (16 eye visits), the method using the RegionFinder tool could not be applied because of image size constraints. In these patients, grading was performed using a planimetric grading software program (GRADOR), custom built for use by the reading center, because very good concordance with the Heidelberg RegionFinder was previously reported. Only grades determined by the reading center were included in the analyses.
Statistical Analysis
The incidence of an outcome was defined as the first visit in which the lesion appeared in eyes that did not have the lesion at their baseline visit (but eyes could have other lesions). Outcomes were the appearance of flecks, heterogeneous background, QDAF, and DDAF. The median time to event and cumulative incidence to 3 and 5 years were calculated using Kaplan-Meier product-limit survival estimates. To adjust for baseline characteristics that possibly influenced incidence, multivariate models were constructed for outcomes using Cox proportional hazard regression models; robust sandwich covariance estimates were used to correct SEs to account for correlation between eyes of the same patient. Statistical analyses were conducted using SAS statistical software, version 9.3 (SAS Institute Inc), and R project for statistical computing, version 2.15.1 (Institute for Statistics and Mathematics).
Characteristics associated with the presence of DDAF and QDAF lesions were examined cross-sectionally at baseline. Logistic regression models were created to test for significant associations. The generalized estimated equation approach was used to account for the correlation between eyes of the same patient. Age adjusted odds ratios are presented. We present 2-sided age-adjusted P values. The CIs and P values are based on the empirical SE estimates from the generalized estimating equation model.
Results
A total of 251 patients with pathogenic mutations in the ABCA4 gene were enrolled at 9 clinical centers in Europe, the United Kingdom, and the United States between August 2, 2013, and December 12, 2014; these patients were seen for visits at the participating sites between August 14, 2001, and December 9, 2014. Data analysis was performed from March 15, 2015, through January 31, 2017. The FAF images for at least 2 visits were available in 217 patients (mean [SD] age, 21.8 [13.3] years; 127 female [57.5%]; 148 white [68.2%]), of whom 173 patients (79.7%) were enrolled with both eyes and 44 patients (20.3%) with 1 eye, for a total of 390 study eyes. Table 1 summarizes the patient characteristics at the first visit. The mean (SD) follow-up time was 3.9 (1.6) years (range, 0.7-12.1 years; median follow-up, 3.6 years; interquartile range, 2.7-5.0 years).
Table 1. Demographic Characteristics of Participants at First Visit With Fundus Autofluorescence Images of Sufficient Quality in at Least 2 Study Visitsa .
| Characteristics | Finding (N = 217) |
|---|---|
| Age at first visit, mean (SD), y | 29.9 (14.7) |
| Age at first visit | |
| <18 y | 53 (24.4) |
| 18-29 y | 70 (32.3) |
| ≥30 y | 94 (43.3) |
| Age at onset of symptoms, mean (SD), yb | 21.8 (13.3) |
| Age at onset of symptoms | |
| <18 y | 94 (43.3) |
| 18-29 y | 48 (22.1) |
| ≥30 y | 43 (19.8) |
| Unknown | 32 (14.7) |
| Female sex | 127 (57.5) |
| Racec | |
| White/Middle Eastern | 148 (68.2) |
| Black | 9 (4.2) |
| Asian/Indian | 8 (3.7) |
| Other | 4 (1.8) |
| Several | 2 (0.9) |
| Unknown | 46 (21.2) |
| No. of disease-causing mutationsd | |
| 1 Pathogenic variant | 37 (17.3) |
| ≥2 Pathogenic variants | 178 (82.7) |
| No. of eyes enrolled per participant | |
| 2 | 173 (79.7) |
| 1 | 44 (20.3) |
| Total No. of eyes | 390 |
| No. of visits (eye level) | |
| 2 | 155 (39.7) |
| 3 | 175 (44.9) |
| 4 | 60 (15.4) |
| Follow-up time, mean (SD) [range], y | 3.9 (1.6) [0.7-12.1] |
Data are presented as number (percentage) of participants unless otherwise indicated.
Age at onset not available for 32 participants.
Race was extracted from medical record review.
Characterization not available for 2 participants.
The presence and absence of the qualitative grading parameters flecks and background heterogeneity for eyes with DDAF and/or QDAF lesions at the first visit are summarized in Table 2. Nearly all eyes with an area of DDAF also had flecks. The presence of DDAF lesions was negatively associated with the presence of QDAF lesions. The QDAF lesions were present in 85% of the eyes without DDAF compared with 39% of the eyes with DDAF (age-adjusted odds ratio, 0.10; 95% CI, 0.05-0.3; P<.001) (Table 3). Only 10 eyes at baseline had neither DDAF nor QDAF.
Table 2. Baseline Associations With DDAF and QDAF Lesions.
| Characteristic | No. of Eyes | DDAF Lesion Present | QDAF Lesion Present | ||||
|---|---|---|---|---|---|---|---|
| No. (%) | Age Adjusted | No. (%) | Age Adjusted | ||||
| OR (95% CI) | P Valuea | OR (95% CI) | P Valuea | ||||
| Demographic Feature | |||||||
| Age group, y | |||||||
| <18 | 94 | 19 (20.2) | 1 [Reference] | <.001b | 85 (90.4) | 1 [Reference] | .008b |
| 18-29 | 125 | 60 (48.0) | 3.6 (1.6-8.2) | 110 (88.0) | 0.8 (0.3-2.4) | ||
| ≥30 | 171 | 105 (61.4) | 6.3 (2.9-13.6) | 126 (73.7) | 0.3 (0.1-0.8) | ||
| Age at onset, yc | |||||||
| <18 | 163 | 66 (40.5) | 19.1 (3.8-95.4) | <.001b | 134 (82.2) | 0.24 (0.05-1.2) | .07b |
| 18-29 | 87 | 42 (48.3) | 5.4 (1.5-19.3) | 78 (89.7) | 1.03 (0.3-3.9) | ||
| ≥30 | 81 | 49 (60.5) | 1 [Reference] | 61 (75.3) | 1 [Reference] | ||
| Sex | |||||||
| Male | 161 | 96 (41.9) | 1 [Reference] | .13 | 126 (78.3) | 1 [Reference] | .28 |
| Female | 229 | 88 (54.7) | 1.6 (0.9-2.7) | 195 (85.2) | 1.5 (0.7-3.1) | ||
| Fundus Autofluorescence Factors | |||||||
| Flecks | |||||||
| Absent | 57 | 2 (3.1) | 1 [Reference] | <.001 | 53 (93.0) | 1 [Reference] | .57 |
| Present | 333 | 182 (54.7) | 20.0 (2.8-148.0) | 268 (80.5) | 0.7 (0.2-2.6) | ||
| Background signal | <.001 | .003 | |||||
| Homogeneous | 263 | 92 (35.0) | 1 [Reference] | 231 (87.8) | 1 [Reference] | ||
| Heterogeneous | 127 | 92 (74.2) | 5.0 (2.4-10.1) | 90 (70.9) | 0.3 (0.1-0.7) | ||
| QDAF | |||||||
| Absent | 69 | 59 (85.5) | 1 [Reference] | <.001 | NA | NA | NA |
| Present | 321 | 125 (38.9) | 0.1 (0.05-0.3) | NA | NA | ||
| DDAF | |||||||
| Absent | 206 | NA | NA | NA | 196 (90.2) | 1 [Reference] | <.001 |
| Present | 184 | NA | NA | 125 (67.9) | 0.1 (0.05-0.3) | ||
Abbreviations: DDAF, definitely decreased autofluorescence; NA, not applicable; OR, odds ratio; QDAF, questionably decreased autofluorescence.
Adjusted for the correlation between eyes of the same participant using generalized estimating equations.
Age-adjusted test for trend.
Missing for 59 eyes.
Table 3. Incidence of Qualitative FAF Grading Factors and the Types of DDAF and QDAF Lesions.
| Variable | No. (%) Present at First Visit (N = 390) | Incidencea | |||
|---|---|---|---|---|---|
| Eyes at Risk | Cumulative | Time, Median (95% CI), y | |||
| Year 3 | Year 5 | ||||
| Qualitative FAF grading factor | |||||
| Flecks | 333 (85.4) | 57 | 37.0 | 50.0 | 4.9 (2.9-6.1) |
| Heterogeneous background | 127 (32.6) | 263 | 6.4 | 30.0 | 8.0 (8.0-12.0) |
| Lesion type | |||||
| DDAF | 184 (47.2) | 206 | 24.0 | 51.6 | 4.9 (4.3-5.6) |
| QDAF | 321 (82.3) | 69 | 7.9 | 28.6 | 6.3 (5.6-9.7) |
| Any | 380 (97.4) | 10 | 50.0 | 90.0 | 3.2 (1.3-4.2) |
Abbreviations: DDAF, definitely decreased autofluorescence; FAF, fundus autofluorescence; QDAF, questionably decreased autofluorescence.
From product-limit survival estimates.
Incidence of Qualitative Factors
At the first visit, 57 (14.6%) of the 390 eyes did not have any flecks. The estimated cumulative incidence to 3.0 years was 37%, and the median time to develop flecks was 4.9 years (95% CI, 2.9-6.1 years). A total of 263 eyes (67.4%) had a homogeneous background at the first visit, the estimated 3-year cumulative incidence of a heterogeneous background in eyes without the feature at first visit was 6.4%, and the median time to develop a heterogeneous background was estimated to be 8.0 years (95% CI, 8.0-12.0 years) (Table 3). All eyes with a heterogeneous background at the first visit also had a heterogeneous background at the most recent visit.
Incidence of DDAF Lesions
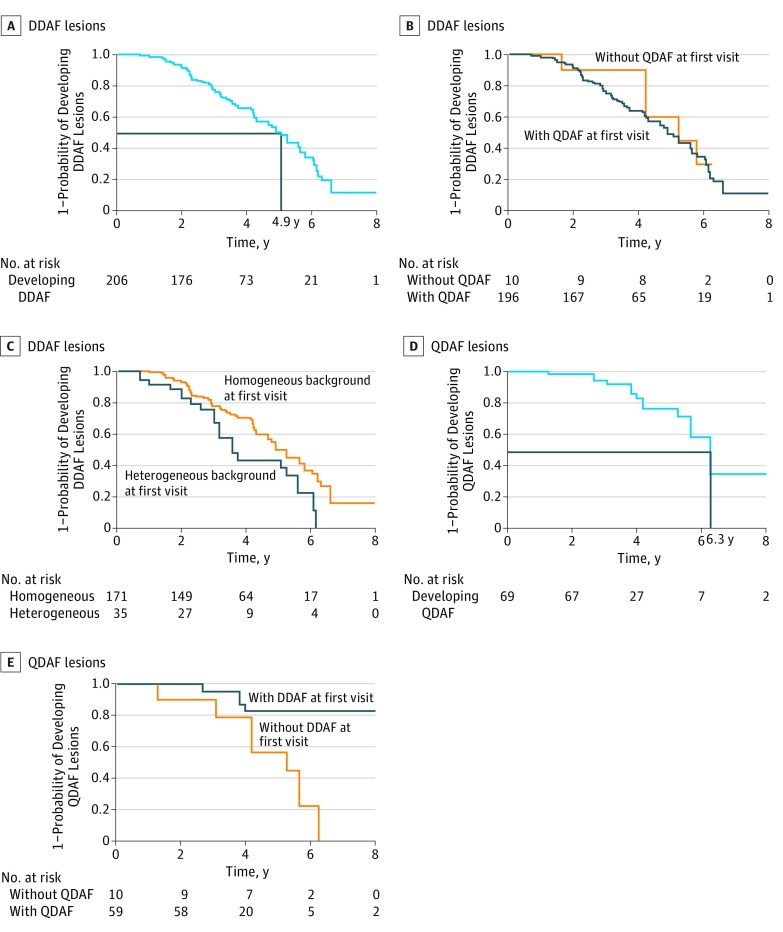
At the first visit, 206 eyes (52.8% of all eyes) did not have any area of DDAF, although all but 10 eyes had QDAF. The median time to develop DDAF lesions was 4.9 years (95% CI, 4.3-5.6 years), and by 3.0 years, 24% had developed such a lesion (Figure 2). There was no increased probability of developing a lesion of DDAF when there was baseline QDAF at the first visit (Figure 2). There was also no difference in the probability of developing such a lesion depending on the presence or absence of flecks (hazard ratio [HR], 1.14; 95% CI, 0.72-1.83; P = .57); however, compared with eyes with homogeneous background, eyes with heterogeneous background at the first visit were more likely to develop DDAF lesions (median time, 3.6 years for eyes with a heterogeneous background and 4.9 years for eyes with a homogeneous background; HR, 1.90; 95% CI, 1.17-3.06; P = .009). However, after adjusting for age at onset, the HR decreased to 1.54 (95% CI, 0.84-2.83; P = .16).
Figure 2. Probability of Developing Lesions of Definitely Decreased Autofluorescence (DDAF) and Questionably Decreased Autofluorescence (QDAF).
A, A total of 206 eyes at risk for developing DDAF lesions (black lines indicate median time to develop a DDAF lesion). B, A total of 196 eyes with QDAF lesions at risk for developing DDAF lesions. C, A total of 171 eyes with homogeneous background and 35 eyes with heterogeneous background at risk for developing DDAF lesions. D, A total of 69 eyes at risk for developing QDAF lesions (black lines indicate median time to develop at QDAF lesion). E, A total of 59 with DDAF lesions and 10 eyes without DDAF lesions at risk for developing QDAF lesions.
Incidence of QDAF Lesions
The number of eyes at risk of developing QDAF lesions was 69, with all but 10 eyes having DDAF. Median time to develop QDAF lesions was 6.3 years (95% CI, 5.6-9.7) (Figure 2). There was no difference in the probability of developing QDAF lesions between eyes with and without flecks at the first visit (HR, 0.72; 95% CI, 0.11-4.69; P = .70) or between the eyes with a heterogeneous or homogeneous background (HR, 0.39; 95% CI, 0.07-2.45; P = .39). However, eyes with a DDAF lesion at the first visit were less likely to develop a QDAF lesion compared with eyes without a DDAF lesion at first visit (HR, 0.19; 95% CI, 0.05-0.70; P = .01).
Discussion
The findings in this report add valuable information about the natural course of Stargardt disease. A previous study correlating DDAF and QDAF as determined by SD-OCT found a difference in the layers between the external limiting membrane and the inner boundary of the choroid, indicating a relative reduction in thickness for these layers in DDAF, and a difference for all the layers, indicating a relative decrease in preserved areas for all the layers in DDAF. This finding suggests that QDAF may be considered as a transition state between healthy retina and later stages of Stargardt disease, thus proposing Stargardt disease progression.
Because the median time for the development of DDAF lesions was 4.93 years in our cohort, this may be a suitable outcome measure in studies with longer follow-up. Similarly, the incidence of QDAF lesions provided further insight into the progression of Stargardt disease. We observed 2 major groups of patients with DDAF lesions at baseline: one with solely DDAF lesions and one with DDAF and (nearly always surrounding) QDAF lesions. The lower likelihood for the development of QDAF lesions in eyes with the presence of DDAF lesions at the first visit vs eyes without such lesions may implicate that DDAF lesions expand and increase in size over time without the development of new QDAF lesions.
Limitations
Outcome measures for forthcoming treatment trials on Stargardt disease are needed. At present, FAF imaging is widely applied in inherited retinal dystrophy clinics and has been used as an outcome measure in clinical trials for age-related macular degeneration, and areas of decreased autofluorescence have a correlation with morphofunctional outcomes in eyes affected by Stargardt disease. Previous results from single-center observational studies in Stargardt disease have reported the growth of atrophic lesions, corresponding to lesions defined here as DDAF. The growth rate of atrophic lesions is also the primary outcome in the multicenter ProgStar studies; however, these studies were designed in an exploratory way to get a deeper understanding of the natural course of Stargardt disease and investigate additional potential outcome measures for this phenotypically heterogeneous disease. Thus, additional evaluation of QDAF lesions and qualitative grading factors were included in the grading protocols. The development of new lesions was included in the study protocol of the retrospective study by requiring the presence of a well-defined lesion of atrophy at only the most recent visit (in contrast to the prospective study in which the presence of an atrophic lesion at baseline was an inclusion criterion). As described previously, the variability of clinical phenotypes was challenging for the ProgStar studies, but some eyes did not fulfill this requirement when graded by reading center criteria because it is well known that Stargardt disease may manifest with different patterns of reduced autofluorescence and clear, discernible atrophic lesions still may have poorly demarcated borders. The design may have led to some skews. First, the lesion size at the most recent visit was restricted to have a minimum width of 300 µm and a maximum area of 5 standard disc areas (corresponding to 12 mm2) in at least one eye, and thus larger lesions were eliminated. Second, the requirement that a lesion needed to be present at the most recent visit may bias the incidence estimates toward higher values because eyes with slower incidence rates were likely to have been excluded. Third, the number of evaluated visits could be as low as 2 visits; furthermore, the observational period was variable between 2 and 15 years.
The retrospective study design also has inherent limitations. In addition to the requirement for a minimum lesion size at the most recent visit, we required at least 2 visits with FAF gradable images at least 24 months apart, although there could be interim FAF images as well. Of the original enrollment in the retrospective study of 251 patients, 34 did not have 2 FAF images and were not included. In addition, there may be patients at the sites who were not followed up and would not have been enrolled in our study. To the extent that such patients had incidence rates different from the ones that we determined, our estimates could be greater or smaller. Another limitation based on the retrospective design can be issues with image quality. Some images may have poor illumination or exposure, which affects the subjective darkness-level assessment (and the software’s attempt at a shadow correction).
To our knowledge, the incidence of atrophic lesions in Stargardt disease has not been reported previously, which may be explained by the wide range of phenotypical appearances and disease severity from early or childhood-onset to late-onset disease in Stargardt disease. We also acknowledge that the data provided in this report represent an incidence based on a cohort recruited according to the aforementioned inclusion criteria and may therefore not be applicable in routine clinical practice. There are other risk factors, such as smoking, that may promote a faster progression of eye diseases, including macular degeneration; however, recording of smoking history was insufficient in this retrospective arm of the ProgStar studies and was only performed in the prospective study. Similarly, we faced problems when extracting patient medical records on vitamin A supplementation because this was not consistently registered in patient medical records in the past and therefore was only available for a few patients, especially those participating in the prospective study.
Conclusions
An estimated 50% of the eyes without DDAF at first visit will develop the lesion in less than 5 years, suggesting that incidence of DDAF could serve as an outcome measure for treatment trials. The prospective ProgStar study will evaluate further the incidence, particularly of DDAF lesions, during a 2-year period and elucidate the structural (as determined by SD-OCT) and functional (as determined by microperimetry) consequences.
References
- 1.Tanna P, Strauss RW, Fujinami K, Michaelides M. Stargardt disease: clinical features, molecular genetics, animal models and therapeutic options. Br J Ophthalmol. 2017;101(1):25-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allikmets R, Singh N, Sun H, et al. . A photoreceptor cell-specific ATP-binding transporter gene (ABCR) is mutated in recessive Stargardt macular dystrophy. Nat Genet. 1997;15(3):236-246. [DOI] [PubMed] [Google Scholar]
- 3.Strauss RW, Ho A, Muñoz B, et al. ; Progression of Stargardt Disease Study Group . The natural history of the Progression of Atrophy Secondary to Stargardt Disease (ProgStar) studies: design and baseline characteristics: ProgStar report No. 1. Ophthalmology. 2016;123(4):817-828. [DOI] [PubMed] [Google Scholar]
- 4.Scholl HP, Strauss RW, Singh MS, et al. . Emerging therapies for inherited retinal degeneration. Sci Transl Med. 2016;8(368):368rv6. [DOI] [PubMed] [Google Scholar]
- 5.Csaky KG, Richman EA, Ferris FL III. Report from the NEI/FDA Ophthalmic Clinical Trial Design and Endpoints Symposium. Invest Ophthalmol Vis Sci. 2008;49(2):479-489. [DOI] [PubMed] [Google Scholar]
- 6.Bearelly S, Cousins SW. Fundus autofluorescence imaging in age-related macular degeneration and geographic atrophy. Adv Exp Med Biol. 2010;664:395-402. [DOI] [PubMed] [Google Scholar]
- 7.World Medical Association World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 8.Fujinami K, Lois N, Mukherjee R, et al. . A longitudinal study of Stargardt disease: quantitative assessment of fundus autofluorescence, progression, and genotype correlations. Invest Ophthalmol Vis Sci. 2013;54(13):8181-8190. [DOI] [PubMed] [Google Scholar]
- 9.Kuehlewein L, Hariri AH, Ho A, et al. . Comparison of manual and semiautomated fundus autofluorescence analysis of macular atrophy in Stargardt disease phenotype. Retina. 2016;36(6):1216-1221. [DOI] [PubMed] [Google Scholar]
- 10.Strauss RW, Muñoz B, Jha A, et al. . Comparison of short-wavelength reduced-illuminance and conventional autofluorescence imaging in Stargardt macular dystrophy. Am J Ophthalmol. 2016;168:269-278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schmitz-Valckenberg S, Brinkmann CK, Alten F, et al. . Semiautomated image processing method for identification and quantification of geographic atrophy in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2011;52(10):7640-7646. [DOI] [PubMed] [Google Scholar]
- 12.Ho A, Kuehlewein L, Hariri A, et al. the ProgStar Research Group. Quantitative characteristics of spectral-domain optical coherence tomography (SDOCT) in corresponding areas of decreased autofluorescence in patients with Stargardt disease. Poster presented at: Annual Meeting of the Association for Research in Vision and Ophthalmology; May 3-7, 2015; Denver, Colorado. Poster 5924-A0095. [Google Scholar]
- 13.Schmitz-Valckenberg S, Sahel JA, Danis R, et al. . Natural history of geographic atrophy progression secondary to age-related macular degeneration (Geographic Atrophy Progression Study). Ophthalmology. 2016;123(2):361-368. [DOI] [PubMed] [Google Scholar]
- 14.Parodi MB, Iacono P, Triolo G, et al. . Morpho-functional correlation of fundus autofluorescence in Stargardt disease. Br J Ophthalmol. 2015;99(10):1354-1359. [DOI] [PubMed] [Google Scholar]
- 15.Chen B, Tosha C, Gorin MB, Nusinowitz S. Analysis of autofluorescent retinal images and measurement of atrophic lesion growth in Stargardt disease. Exp Eye Res. 2010;91(2):143-152. [DOI] [PubMed] [Google Scholar]
- 16.McBain VA, Townend J, Lois N. Progression of retinal pigment epithelial atrophy in Stargardt disease. Am J Ophthalmol. 2012;154(1):146-154. [DOI] [PubMed] [Google Scholar]
- 17.Fujinami K, Zernant J, Chana RK, et al. . Clinical and molecular characteristics of childhood-onset Stargardt disease. Ophthalmology. 2015;122(2):326-334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lambertus S, van Huet RA, Bax NM, et al. . Early-onset stargardt disease: phenotypic and genotypic characteristics. Ophthalmology. 2015;122(2):335-344. [DOI] [PubMed] [Google Scholar]
- 19.Westeneng-van Haaften SC, Boon CJ, Cremers FP, Hoefsloot LH, den Hollander AI, Hoyng CB. Clinical and genetic characteristics of late-onset Stargardt’s disease. Ophthalmology. 2012;119(6):1199-1210. [DOI] [PubMed] [Google Scholar]
- 20.Galor A, Lee DJ. Effects of smoking on ocular health. Curr Opin Ophthalmol. 2011;22(6):477-482. [DOI] [PubMed] [Google Scholar]
- 21.Strauss RW, Munoz B, Wolfson Y, et al. . Assessment of estimated retinal atrophy progression in Stargardt macular dystrophy using spectral-domain optical coherence tomography. Br J Ophthalmol. 2016;100(7):956-962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cideciyan AV, Swider M, Aleman TS, et al. . Macular function in macular degenerations: repeatability of microperimetry as a potential outcome measure for ABCA4-associated retinopathy trials. Invest Ophthalmol Vis Sci. 2012;53(2):841-852. [DOI] [PMC free article] [PubMed] [Google Scholar]