This cohort study examines the association of evidence-based care processes in routine care for Staphylococcus aureus bacteremia with mortality at Veterans Health Administration hospitals.
Key Points
Questions
Have outcomes for patients with Staphylococcus aureus bacteremia improved, and how have changes in use of evidence-based care processes affected outcomes?
Findings
In this cohort study of 36 868 patients with S aureus bacteremia at 124 Veterans Health Administration hospitals from January 1, 2003, through December 31, 2014, all-cause 30-day mortality decreased over time from 25.7% in 2003 to 16.5% in 2014, whereas use of appropriate antibiotic therapy, echocardiography, and infectious diseases consultation increased. An estimated 57.3% decrease in mortality could be attributed to increased use of evidence-based care processes.
Meaning
Increasing application of evidence-based care may improve survival among patients with S aureus bacteremia in routine health care settings.
Abstract
Importance
Staphylococcus aureus bacteremia is common and frequently associated with poor outcomes. Evidence indicates that specific care processes are associated with improved outcomes for patients with S aureus bacteremia, including appropriate antibiotic prescribing, use of echocardiography to identify endocarditis, and consultation with infectious diseases (ID) specialists. Whether use of these care processes has increased in routine care for S aureus bacteremia or whether use of these processes has led to large-scale improvements in survival is unknown.
Objective
To examine the association of evidence-based care processes in routine care for S aureus bacteremia with mortality.
Design, Setting, and Participants
This retrospective observational cohort study examined all patients admitted to Veterans Health Administration (VHA) acute care hospitals who had a first episode of S aureus bacteremia from January 1, 2003, through December 31, 2014.
Exposures
Use of appropriate antibiotic therapy, echocardiography, and ID consultation.
Main Outcomes and Measures
Thirty-day all-cause mortality.
Results
Analyses included 36 868 patients in 124 hospitals (mean [SD] age, 66.4 [12.5] years; 36 036 [97.7%] male), including 19 325 (52.4%) with infection due to methicillin-resistant S aureus and 17 543 (47.6%) with infection due to methicillin-susceptible S aureus. Risk-adjusted mortality decreased from 23.5% (95% CI, 23.3%-23.8%) in 2003 to 18.2% (95% CI, 17.9%-18.5%) in 2014. Rates of appropriate antibiotic prescribing increased from 2467 (66.4%) to 1991 (78.9%), echocardiography from 1256 (33.8%) to 1837 (72.8%), and ID consultation from 1390 (37.4%) to 1717 (68.0%). After adjustment for patient characteristics, cohort year, and other care processes, receipt of care processes was associated with lower mortality, with adjusted odds ratios of 0.74 (95% CI, 0.68-0.79) for appropriate antibiotics, 0.73 (95% CI, 0.68-0.78) for echocardiography, and 0.61 (95% CI, 0.56-0.65) for ID consultation. Mortality decreased progressively as the number of care processes that a patient received increased (adjusted odds ratio for all 3 processes compared with none, 0.33; 95% CI, 0.30-0.36). An estimated 57.3% (95% CI, 48.4%-69.9%) of the decrease in mortality between 2003 and 2014 could be attributed to increased use of these evidence-based care processes.
Conclusions and Relevance
Mortality associated with S aureus bacteremia decreased significantly in VHA hospitals, and a substantial portion of the decreasing mortality may have been attributable to increased use of evidence-based care processes. The experience in VHA hospitals demonstrates that increasing application of these care processes may improve survival among patients with S aureus bacteremia in routine health care settings.
Introduction
Staphylococcus aureus bacteremia (SAB) is common and frequently associated with poor outcomes. The estimated annual incidence of SAB in the United States and Europe is 5 to 40 cases per 100 000 population and has been increasing in recent decades because of increasing exposure to health care.1,2,3,4 The SAB case fatality rate in the preantibiotic era was 80% and has improved to 20% to 30% in previous reports.4,5,6
Largely observational evidence indicates that specific care processes are associated with improved outcomes for patients with SAB, including early source control,7,8 use of echocardiography to identify endocarditis,7,9 appropriate antibiotic prescribing,8,10 and consultation with infectious diseases (ID) specialists to guide management.11,12 Treatment guidelines and published expert opinions call for increased use of these evidence-based care processes for patients with SAB.4,7,8,13,14 However, whether there have been widespread increases in use of these evidence-based care processes for patients with SAB or whether use of these processes has led to large-scale decreases in SAB-associated mortality is unknown.
Large studies of SAB outcomes have previously been difficult to perform in part because the administrative claims data typically used to perform outcomes studies do not accurately identify patients with SAB,15,16,17 and few large health care databases have included microbiology results. The Veterans Health Administration (VHA), the largest integrated health care system in the United States, has recently aggregated microbiology results nationally. We examined national VHA microbiology data and other clinical and administrative data to explore trends in the use of evidence-based care processes and mortality among patients with SAB in 124 hospitals between 2003 and 2014. Our aim was to estimate the extent to which increasing use of evidence-based care processes may have contributed to improving survival among patients with SAB.
Methods
The institutional review board of the University of Iowa and the Research & Development Committee of the Iowa City Veterans Affairs Health Care System approved this study and waived informed consent for this retrospective cohort. All data were deidentified.
Study Population and Data Source
We conducted a retrospective observational cohort study of all patients with a blood culture positive for S aureus during admission to acute care units at 124 VHA hospitals in the 48 continental United States, the District of Columbia, and Puerto Rico from January 1, 2003, through December 31, 2014. We obtained data through the Veterans Affairs Informatics and Computing Infrastructure, which includes data extracted from VHA’s integrated electronic medical record. The methods used to extract microbiology results from electronic medical records have been described previously.18 We also extracted antimicrobial susceptibility data to classify isolates as methicillin-resistant S aureus (MRSA) or methicillin-susceptible S aureus (MSSA). If a patient had more than 1 episode of SAB during the study period, we included only the first episode in the analysis.
Outcomes
Our primary outcome measure was all-cause 30-day mortality after the first blood culture positive for S aureus. Dates of death were obtained from the VHA Vital Status File, which combines mortality information from multiple VHA and non-VHA sources and tracks patients even if they leave the VHA system. The Vital Status File has excellent agreement with the National Death Index.19
Variable Definitions
For each patient, we defined time of bacteremia onset as the time of the first sample obtainment for which the blood culture result was positive and classified places of acquisition as community acquired (CA), health care associated (HCA), or hospital onset (HO) according to previously published definitions.20,21 The episode was considered to be HO bacteremia when the patient had been in the hospital for 48 hours or longer at the time of the first positive blood culture result. Among episodes that did not meet the criteria for HO bacteremia, HCA bacteremia was defined based on the following: (1) admission to an acute care facility within 90 days before the bacteremia episode, (2) residence in a nursing home or rehabilitation facility, (3) receipt of renal replacement therapy, or (4) receipt of wound care or specialized nursing care in an outpatient setting or at home in the 30 days before the onset of bacteremia. If the patient did not meet any of the above criteria, the episode was classified as CA bacteremia.
Additional patient characteristics were identified using diagnosis codes, laboratory tests, and vital signs. Specifically, laboratory test results and vital signs within 24 hours before and after the first positive blood culture result were collected for each patient and categorized according to previously described methods.22 Comorbidities were assessed using diagnosis codes based on the methods of Elixhauser et al23 and the algorithm proposed by Quan et al.24 Patient-level data were complete for all patients with the exception of vital signs, body mass index, and laboratory values. These data were missing in less than 8% of patients with the exception of serum albumin (missing in 31.8%) and bilirubin levels (missing in 31.3%).
Information pertaining to antimicrobial administration, echocardiography use, and ID consultation for each patient was extracted from the Veterans Affairs Informatics and Computing Infrastructure to create a series of care quality process measures. We defined appropriate antimicrobial therapy as any administration of recommended intravenous antimicrobial therapy (ie, vancomycin or daptomycin for MRSA or penicillinase-resistant penicillins or first-generation cephalosporins for MSSA) between the first positive blood culture result and discharge.8,13,14,25 We defined echocardiography use as completion of echocardiography (transthoracic or transesophageal echocardiography) between the first positive blood culture result and discharge and ID consultation as a completed consultation order or documentation from ID consultation services in the electronic medical records between the first positive blood culture result and discharge. We manually reviewed titles of consultation orders and inpatient notes to identify ID consultations. Specific details of findings and recommendations from ID consultation notes and echocardiography reports were not available.
Statistical Analysis
First, we compared characteristics of patients across the number of evidence-based care processes received using χ2 tests for categorical measures and 1-way analysis of variance or Kruskal-Wallis tests for continuous measures. We used Markov chain Monte Carlo imputation to estimate values for missing variables. Crude mortality rates and use rates for each evidence-based care process (ie, appropriate antibiotic therapy, echocardiography use, and ID consultation) were summarized by year, and statistical significance of trends were assessed using Cochrane-Armitage test for trend.
Second, we fit a patient-level, multivariable logistic regression model to predict all-cause 30-day mortality while carefully avoiding overfitting. Patient characteristics were considered to be candidate variables for the risk adjustment model if they were possibly related (P < .25) to mortality in bivariable analyses and missingness before imputation was less than 10%. This model included a random intercept for hospitals to account for the grouping of patients within hospitals. Detailed description of the model-building process is available in the eMethods in the Supplement. The risk adjustment model for 30-day mortality included patient age, body mass index, place of bacteremia acquisition, methicillin susceptibility of the S aureus isolate, 13 comorbidities, 4 vital signs, and 5 laboratory results. The model had excellent discrimination with a C statistic of 0.809.26 Model validation revealed nearly identical receiver operating characteristic curves for the training and validation data sets (eFigure 1 in the Supplement), which suggests good prediction capability without overfitting.
We used this risk adjustment model to assess trends in risk-standardized 30-day mortality rates over time. We assessed patient-level associations between receipt of individual evidence-based care processes and mortality by adding variables that indicated patients who received each of the 3 care processes to the risk adjustment model one at a time. We then included all 3 care processes in 1 model simultaneously after assessing possible collinearity between care processes using φ statistics for correlations between variables and variance inflation factors. Models for associations between receipt of care processes and mortality also included cohort year as a series of fixed effects. In addition, we created indicator variables for all possible combinations of care processes that a patient could receive and determined associations between mortality and each of the 8 possible combinations of care processes. This method allowed us to assess associations between mortality and receipt of specific combinations of care processes. We also evaluated for potential differential effects over time using stratified analyses that split the cohort into four 3-year periods (2003-2005, 2006-2008, 2009-2011, and 2012-2014).
In addition, we conducted subgroup analyses for MSSA, MRSA, and place of acquisition to determine whether mortality trends and associations with care processes were stable across subgroups. Because patients who died early might not have had time to receive evidence-based care processes, we performed sensitivity analyses for associations between care processes and mortality, excluding patients who did not survive 2 or more days after the onset of bacteremia.
Finally, we used the method of recycled predictions as described in the eMethods in the Supplement to compute the marginal effects of increases in the number of care processes on mortality to estimate the fraction of the change in mortality that may be attributed to changing use of care processes over time.27,28 All hypothesis tests were 2-sided, with a significance level of P < .05. Statistical analyses were performed using SAS software, version 9.4 (SAS Institute Inc).
Results
Study Population
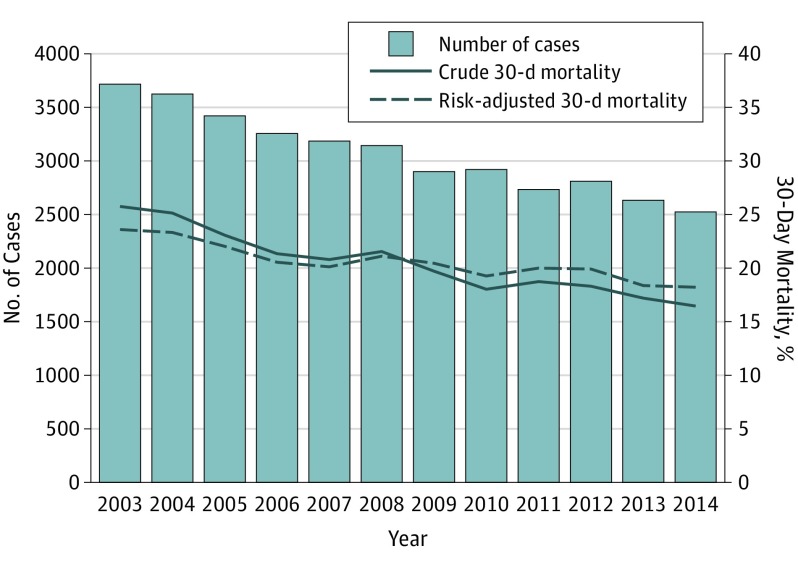
Analyses included 36 868 patients in 124 hospitals (mean [SD] age, 66.4 [12.5] years; range of mean age by year, 65.4-66.9 years; 36 036 [97.7%] male), including 19 325 (52.4%) with MRSA infection and 17 543 (47.6%) with MSSA infection. The number of cases decreased from 3717 in 2003 to 2524 in 2014 (Figure 1) principally because of decreases in the incidence of HCA and HO bacteremia (HCA bacteremia: 1726 in 2003 to 1293 in 2014; HO bacteremia: 1318 in 2003 to 557 in 2014), whereas the frequency of CA bacteremia was stable between 2003 and 2014 (673 in 2003 to 674 in 2014). Overall crude mortality was 20.9%, which improved from 25.7% in 2003 to 16.5% in 2014 (P < .001 for trend).
Figure 1. Trends in the Incidence of Staphylococcus aureus Bacteremia and All-Cause 30-Day Mortality, 2003-2014.
Table 1 summarizes the patient characteristics. Approximately half of patients with SAB had HCA bacteremia (19 271 [52.3%]), whereas 8253 (22.4%) had CA bacteremia and 9344 (25.3%) had HO bacteremia. The median number of Elixhauser comorbidities was 8 (interquartile range, 5-10), with 34 324 patients (93.1%) having at least 1 cardiopulmonary comorbidity, 11 392 (30.9%) with malignant tumors, and 23 411 (63.5%) with at least 1 mental health comorbidity. Table 2 summarizes the model used for patient-level risk adjustments. Risk-adjusted mortality decreased from 23.5% (95% CI, 23.3%-23.8%) in 2003 to 18.2% (95% CI, 17.9%-18.5%) in 2014 (Figure 1). Similar mortality trends were observed for MRSA, MSSA, and all places of acquisition (ie, HCA, HO, and CA) (eFigure 2 and eFigure 3 in the Supplement).
Table 1. Patient Characteristics by Number of Evidence-Based Care Processes Receiveda.
| Characteristic | All 3 Care
Processes (N = 10 608) |
2 of 3 Care
Processes (n = 10 086) |
1 of 3 Care
Processes (n = 9646) |
No Care
Process (n = 6528) |
P Value |
|---|---|---|---|---|---|
| Death within 30 d | 1221 (11.5) | 1806 (17.9) | 2593 (26.9) | 2069 (31.7) | <.001 |
| Male | 10 359 (97.7) | 9850 (97.7) | 9459 (98.1) | 6368 (97.5) | .10 |
| Age, mean (SD), y | 65.0 (12.1) | 66.2 (12.6) | 67.5 (12.6) | 67.3 (12.8) | <.001 |
| BMI, mean (SD) | 28.1 (7.1) | 27.6 (7.2) | 27.2 (7.4) | 27.2 (7.2) | <.001 |
| MRSA infection | 5905 (55.7) | 5820 (57.7) | 5694 (59.0) | 1906 (29.2) | <.001 |
| Place of acquisition | <.001 | ||||
| Community acquired | 2812 (26.5) | 2121 (21.0) | 1827 (18.9) | 1493 (22.9) | |
| Health care associated | 5770 (54.4) | 5540 (54.9) | 4905 (50.9) | 3056 (46.8) | |
| Hospital onset | 2026 (19.1) | 2425 (24.0) | 2914 (30.2) | 1979 (30.3) | |
| Dialysis and renal replacement therapy | 1113 (10.5) | 1114 (11.0) | 1085 (11.2) | 650 (10.0) | .04 |
| Elixhauser comorbidities | |||||
| Congestive heart failure | 3769 (35.5) | 3625 (35.9) | 3652 (37.9) | 2363 (36.2) | .004 |
| Cardiac arrhythmias | 4847 (45.7) | 4537 (45.0) | 4446 (46.1) | 3027 (46.4) | .28 |
| Hypertension | 8990 (84.7) | 8576 (85.0) | 8168 (84.7) | 5469 (83.8) | .17 |
| Paralysis | 1030 (9.7) | 1028 (10.2) | 1037 (10.8) | 608 (9.3) | .01 |
| Diabetes | 5973 (56.3) | 5653 (56.0) | 5193 (53.8) | 3423 (52.4) | <.001 |
| Renal failure | 3822 (36.0) | 3574 (35.4) | 3398 (35.2) | 2218 (34.0) | .06 |
| Liver disease | 2686 (25.3) | 2323 (23.0) | 2024 (21.0) | 1429 (21.9) | <.001 |
| Metastatic cancer | 735 (6.9) | 877 (8.7) | 967 (10.0) | 776 (11.9) | <.001 |
| Solid tumor without metastasis | 2652 (25.0) | 2609 (26.7) | 2778 (28.8) | 2103 (32.2) | <.001 |
| Coagulopathy | 2060 (19.4) | 1871 (18.6) | 1771 (18.4) | 1197 (18.3) | .17 |
| Fluid and electrolyte disorders | 5930 (55.9) | 5864 (58.1) | 5694 (59.0) | 3746 (57.4) | <.001 |
| Drug abuse | 2801 (26.4) | 2379 (23.6) | 1979 (20.5) | 1341 (20.5) | <.001 |
| Alcoholism | 3313 (31.2) | 2900 (28.8) | 2597 (26.9) | 1763 (27.0) | <.001 |
| Vital signs at time of bacteremia onset, mean (SD) | |||||
| Temperature, °C | 37.4 (1.3) | 37.3 (1.3) | 37.2 (1.3) | 37.1 (1.3) | <.001 |
| Blood pressure, mm Hg | 84.5 (22.0) | 83.1 (22.1) | 81.5 (22.3) | 81.4 (22.7) | <.001 |
| Pulse, /min | 101.3 (21.9) | 101.2 (22.5) | 102.2 (23.0) | 101.9 (23.6) | .009 |
| Respiration, /min | 20.9 (5.6) | 21.2 (6.0) | 21.8 (6.6) | 22.1 (6.9) | <.001 |
| Laboratory values at time of bacteremia onset, median (IQR) | |||||
| White blood cell count, /µL | 12 800 (9100-17 400) | 12 700 (9100-17 400) | 12 500 (8800-17 400) | 12 200 (8400-17 000) | <.001 |
| Serum sodium level, mEq/L | 134.0 (131.0-137.0) | 134.0 (132.0-138.0) | 135.0 (132.0-139.0) | 135.0 (132.0-139.0) | <.001 |
| Blood urea nitrogen level, mg/dL | 25.1 (17.0-43.0) | 26.0 (17.0-45.0) | 28.0 (18.0-46.6) | 28.0 (18.0-48.1) | <.001 |
| Serum creatinine level, mg/dL | 1.4 (1.0-2.5) | 1.5 (1.0-2.6) | 1.5 (1.0-2.7) | 1.5 (1.0-2.8) | .004 |
| Serum glucose level, mg/dL | 146.0 (108.0-235.0) | 144.0 (107.0-230.0) | 142.0 (105.0-227.0) | 140.0 (104.0-224.0) | <.001 |
Abbreviations; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); IQR, interquartile range; MRSA, methicillin-resistant Staphylococcus aureus.
SI conversion factors: To convert white blood cell count to ×109/L, multiply by 0.001; sodium level to millimoles per liter, multiply by 1; blood urea nitrogen level to millimoles per liter, multiply by 0.357; creatinine level to micromoles per liter, multiply by 88.4; and glucose level to millimoles per liter, multiply by 0.0555.
Data are presented as number (percentage) of patients unless otherwise indicated.
Table 2. Multivariable Logistic Regression Model to Predict 30-Day Mortality for Risk Adjustment.
| Variable | Adjusted OR (95% CI) | P Value |
|---|---|---|
| Age, y | ||
| <45 | 1 [Reference] | NA |
| 45-54 | 1.74 (1.31-2.31) | <.001 |
| 55-64 | 2.34 (1.78-3.07) | <.001 |
| 65-74 | 2.92 (2.22-3.86) | <.001 |
| 75-84 | 4.30 (3.26-5.67) | <.001 |
| ≥85 | 6.49 (4.87-8.63) | <.001 |
| BMI | ||
| Underweight (<18.5) | 1.38 (1.24-1.53) | <.001 |
| Normal (18.5-24.9) | 1 [Reference] | NA |
| Overweight (25.0-29.9) | 0.83 (0.78-0.90) | <.001 |
| Obese (≥30.0) | 0.74 (0.69-0.81) | <.001 |
| Year (as ordinal variable) | 0.96 (0.95-0.97) | <.001 |
| Place of acquisition | ||
| Community acquired | 1 [Reference] | NA |
| Health care associated | 0.57 (0.53-0.62) | <.001 |
| Hospital onset | 1.21 (1.11-1.31) | <.001 |
| MRSA infection | 1.24 (1.17-1.32) | <.001 |
| Elixhauser comorbidities | ||
| Congestive heart failure | 1.26 (1.18-1.35) | <.001 |
| Cardiac arrhythmia | 1.21 (1.13-1.29) | <.001 |
| Hypertension | 0.83 (0.76-0.91) | <.001 |
| Paralysis | 0.82 (0.74-0.91) | <.001 |
| Diabetes | 0.86 (0.80-0.92) | <.001 |
| Renal failurea | 0.96 (0.84-1.10) | .59 |
| Liver disease | 1.56 (1.45-1.69) | <.001 |
| Metastatic tumor | 2.04 (1.85-2.25) | <.001 |
| Solid tumor without metastasis | 1.15 (1.07-1.23) | <.001 |
| Coagulopathy | 1.40 (1.30-1.50) | <.001 |
| Fluid and electrolyte disorders | 1.14 (1.07-1.21) | <.001 |
| Alcoholism | 1.16 (1.07-1.25) | <.001 |
| Drug abuse | 0.88 (0.81-0.96) | .003 |
| Temperature, °C | ||
| <33.0 | 3.70 (1.83-7.48) | <.001 |
| 33.0-33.4 | 3.28 (1.47-7.32) | .004 |
| 33.5-33.9 | 2.78 (1.56-4.95) | <.001 |
| 34.0-34.9 | 2.11 (1.72-2.59) | <.001 |
| 35.0-35.9 | 1.28 (1.19-1.37) | <.001 |
| 36.0-39.9 | 1 [Reference] | NA |
| ≥40.0 | 0.69 (0.57-0.84) | <.001 |
| Blood pressure, mean, mm Hg | ||
| <40 | 2.32 (1.64-3.26) | <.001 |
| 40-59 | 1.22 (1.09-1.37) | <.001 |
| 60-69 | 0.86 (0.78-0.96) | .006 |
| 70-79 | 0.80 (0.72-0.89) | <.001 |
| 80-99 | 1 [Reference] | NA |
| 100-119 | 0.67 (0.60-0.75) | <.001 |
| 120-129 | 0.59 (0.50-0.70) | <.001 |
| 130-139 | 0.58 (0.45-0.74) | <.001 |
| ≥140 | 0.54 (0.37-0.79) | .001 |
| Pulse, /min | ||
| <40 | 0.91 (0.47-1.75) | .77 |
| 40-49 | 1.03 (0.79-1.38) | .84 |
| 50-99 | 1 [Reference] | NA |
| 100-109 | 1.12 (1.03-1.21) | .007 |
| 110-119 | 1.17 (1.07-1.27) | <.001 |
| 120-139 | 1.50 (1.38-1.63) | <.001 |
| 140-154 | 2.13 (1.85-2.45) | <.001 |
| ≥155 | 1.78 (1.42-2.22) | <.001 |
| Respiration, /min | ||
| <6 | 1.81 (0.68-4.85) | .24 |
| 6-11 | 1.61 (1.33-1.96) | <.001 |
| 12-13 | 1.35 (1.16-1.57) | <.001 |
| 14-24 | 1 [Reference] | NA |
| 25-34 | 1.70 (1.58-1.83) | <.001 |
| 35-39 | 2.57 (2.20-3.00) | <.001 |
| 40-49 | 3.09 (2.61-3.65) | <.001 |
| ≥50 | 2.40 (1.60-3.59) | <.001 |
| White blood cell count, /µL | ||
| <1000 | 2.54 (1.99-3.26) | <.001 |
| 1000-2999 | 2.12 (1.76-2.56) | <.001 |
| 3000-19 999 | 1 [Reference] | NA |
| 20 000-24 999 | 1.23 (1.12-1.35) | <.001 |
| ≥25 000 | 1.27 (1.14-1.41) | <.001 |
| Serum sodium level, mEq/L | ||
| <120 | 1.38 (1.05-1.82) | .02 |
| 120-134 | 0.87 (0.82-0.92) | <.001 |
| 135-154 | 1 [Reference] | NA |
| ≥155 | 1.56 (1.25-1.96) | <.01 |
| Blood urea nitrogen level, mg/dL | ||
| <17 | 1 [Reference] | NA |
| 17-19 | 1.36 (1.17-1.58) | <.001 |
| 20-39 | 2.20 (1.98-2.44) | <.001 |
| 40-79 | 4.06 (3.60-4.57) | <.001 |
| ≥80 | 6.82 (5.89-7.90) | <.001 |
| Serum creatinine level, mg/dL | ||
| <0.5 | 1.76 (1.41-2.21) | <.001 |
| 0.5-1.4 | 1 [Reference] | NA |
| 1.5-1.94 | 1.05 (0.94-1.17) | .37 |
| ≥1.95 | 1.19 (1.08-1.32) | <.001 |
| Serum glucose level, mg/dL | ||
| <40 | 2.55 (1.93-3.35) | <.001 |
| 40-59 | 1.94 (1.72-2.18) | <.001 |
| 60-199 | 1 [Reference] | NA |
| 200-349 | 1.23 (1.14-1.32) | <.001 |
| ≥350 | 1.15 (1.03-1.28) | .01 |
Abbreviations; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); MRSA, methicillin-resistant Staphylococcus aureus; NA, not applicable; OR, odds ratio.
SI conversion factors: See Table 1 footnote.
Included in the model because of significant interaction with serum creatinine level.
Trends in Use of Evidence-Based Care Processes and Association With Mortality Trends
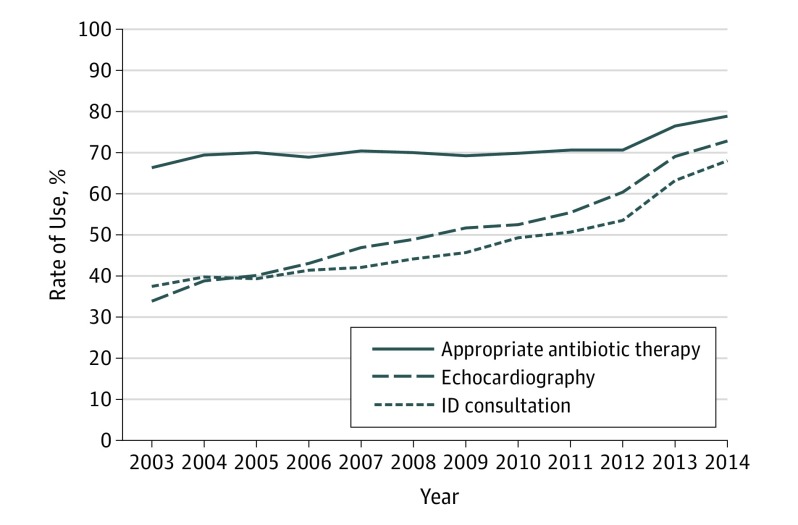
Overall, 19 608 patients (53.2%) with SAB had ID consultation during the index hospital stay. The number of patients with ID consultation increased from 1390 (37.4%) in 2003 to 1717 (68.0%) in 2014 (P < .001 for trend) (Figure 2). Echocardiography was performed for 18 345 patients with SAB (49.8%), including transesophageal echocardiography for 5522 (15.0%), transthoracic echocardiography for 10 769 (29.2%), and undocumented type of echocardiography for 2054 (5.6%). The number of patients who had echocardiography increased from 1256 (33.8%) in 2003 to 1837 (72.8%) in 2014 (P for trend <.001) (Figure 2 and eFigure 4 in the Supplement). Overall, 26 037 patients (70.6%) received appropriate antibiotic therapy for SAB before discharge. Among patients with MRSA bacteremia, 16 890 (87.4%) received vancomycin or daptomycin, whereas 9140 patients with MSSA bacteremia (52.1%) received penicillinase-resistant penicillins or first-generation cephalosporins. Among patients with MSSA infection who did not receive penicillinase-resistant penicillins or first-generation cephalosporins, 6050 (72.0%) received vancomycin or daptomycin. The proportion of patients who received appropriate antibiotic therapy increased from 66.4% (n = 2467) in 2003 to 78.9% (n = 1991) in 2014 (P < .001 for trend) (Figure 2). The proportion of appropriate therapy increased in the MRSA group (79.5% [n = 1685] in 2003 to 94.3% [n = 1037] in 2014; P < .001 for trend) and MSSA group (48.9% [n = 781] in 2003 to 66.9% [n = 953] in 2014; P for trend <.001). A mild to moderate correlation was found between use of each pair of care processes (φ coefficient for ID consultation and echocardiography, 0.37; ID consultation and appropriate therapy, 0.29; and echocardiography and appropriate therapy, 0.28). The number of patients who received all 3 of these care processes increased from 597 (16.1%) in 2003 to 1317 (52.2%) in 2014 (P < .001 for trend) (eFigure 5 in the Supplement). In 2014, 533 patients (21.1%) did not receive appropriate antibiotic therapy, 687 (27.2%) did not have an echocardiogram to evaluate for endocarditis, 807 (32.0%) did not have ID consultation during their hospital stay, and 223 (8.8%) received none of the care processes.
Figure 2. Trends in Rates of Use of Evidence-Based Care Process, 2003-2014.
ID indicates infectious diseases.
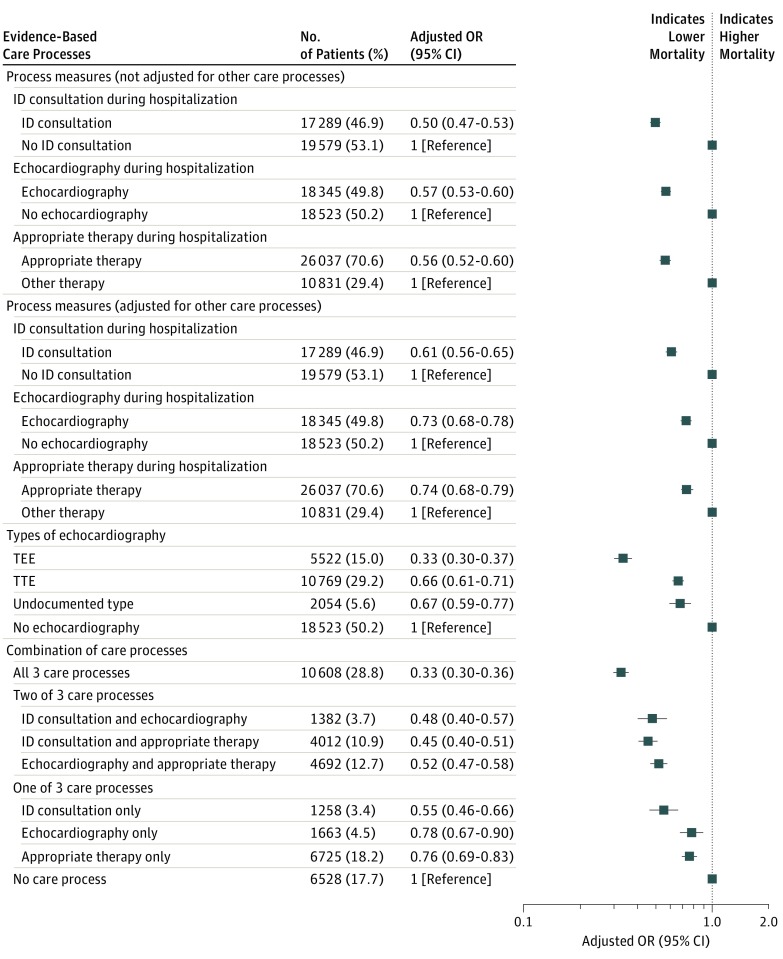
The use of echocardiography, ID consultation, and appropriate therapy were associated with lower 30-day mortality after adjustment for patient characteristics and other care processes, with risk-adjusted odds ratios (ORs) of 0.74 (95% CI, 0.68-0.79) for appropriate antibiotics, 0.73 (95% CI, 0.68-0.78) for echocardiography, and 0.61 (95% CI, 0.56-0.65) for ID consultation (Figure 3). In addition, there was a dose-response relationship between the number of evidence-based care processes that a patient received and mortality (Figure 3), which occurred regardless of the specific combinations of care processes received. The risk-adjusted OR for mortality was 0.33 (95% CI, 0.30-0.37) for patients receiving all 3 care processes compared with patients receiving no process.
Figure 3. Associations Between Receipt of Evidence-Based Care Processes and 30-Day Mortality.
Care process indicator variables were included individually in risk adjustment models with all variables in Table 2. All variables in Table 2 and 3 care processes were entered into the model simultaneously. Error bars indicate 95% CIs. ID indicates infectious diseases; MRSA, methicillin-resistant Staphylococcus aureus; MSSA, methicillin-susceptible Staphylococcus aureus; OR, odds ratio; TEE, transesophageal echocardiography; and TTE, transthoracic echocardiography.
Appropriate antibiotic therapy was associated with lower 30-day mortality in subgroup analyses of patients infected with MRSA (risk-adjusted OR, 0.53; 95% CI, 0.48-0.59) and those infected with MSSA (risk-adjusted OR, 0.57; 95% CI, 0.52-0.63) (eFigure 6 and eFigure 7 in the Supplement). The association between receipt of care processes and mortality was generally stable across cohort years and by place of bacteremia onset (eFigure 8 and eFigure 9 in the Supplement). Sensitivity analyses with elimination of patients who did not survive for 2 or more calendar days after blood culture was performed also demonstrated associations between evidence-based care processes and lower 30-day mortality (eFigure 9 in the Supplement). Using recycled predictions across cohort years and patient-level analyses, we estimated that 57.3% (95% CI, 48.4%-69.9%) of the decrease in risk-adjusted mortality between 2003 and 2014 could be attributed to increased use of the 3 evidence-based care processes.
Discussion
In the national VHA health care system, we observed lower risk-adjusted mortality among patients with SAB when they received evidence-based care processes, including appropriate antibiotic therapy, echocardiography, and consultation with ID specialists. Moreover, there was evidence of a dose-response relationship between the number of care processes that a patient received and mortality. On the basis of data from this cohort, we estimated that 57.3% of the decrease in risk-adjusted mortality among patients with SAB between 2003 and 2014 could be attributed to increased use of these evidence-based care processes.
These results from 124 hospitals suggest that advances in care are driving decreases in mortality among patients with SAB, a common and serious infection. By 2014, crude mortality among patients with SAB in the VHA was 16.5%, which is lower than described in most published cohorts.1,2,3,4,29 It is plausible that increasing use of appropriate antibiotic therapy and echocardiography contributed to decreasing mortality through more rapid clearance of infection and earlier identification of endocarditis. In addition, associations between echocardiography use and mortality could occur if echocardiography was also a marker for receipt of other beneficial procedures, such as control of infectious sources through removal of infected devices or abscess drainage. The mechanisms that link ID consultation to decreasing mortality are less obvious, but based on prior studies,4,11,12 ID physicians likely optimize antibiotic prescribing and facilitate earlier identification and control of infectious sources.
Our results also indicate that opportunity remains for improvement in SAB outcomes through even greater use of evidence-based care processes. Although the use of evidence-based processes substantially improved during the study period, approximately half (47.8%) of patients did not receive all 3 processes in 2014. Assuming that some patients have contraindications to echocardiography and allergies to antibiotics, substantial opportunity for improvement in these care processes still likely remains. Evidence from a prior study29 indicates that quality improvement programs can increase use of evidence-based care processes and improve outcomes for patients with SAB. In 12 hospitals in Spain, implementation of a quality improvement bundle, including use of appropriate antibiotic therapy, echocardiography, and early source control (eg, catheter removal, abscess drainage), led to a 5.6% absolute decrease (22.3% in the preintervention period to 16.7% in the postintervention period) in 30-day mortality. Given this evidence and the high prevalence of SAB, hospitals that do not routinely apply these evidence-based practices should prioritize quality improvement programs that address SAB management. In addition, our results support widespread tracking of quality measures based on these care processes to guide SAB quality improvement programs.
Our observation of decreasing mortality among patients with SAB must be considered in the context of prior studies30,31 that describe decreasing mortality among VHA patients hospitalized with other conditions, such as congestive heart failure and acute myocardial infarction. This finding raises the question of whether there may be secular trends in the VHA populations that are associated with a generalized improvement in hospital outcomes over time. We adjusted for factors potentially driving secular trends by adjusting at the patient level for cohort year and a wide range of patient characteristics, including demographic features, comorbidities, site of bacteremia onset, MRSA vs MSSA infection, and illness severity based on vital signs and laboratory studies. When models were stratified by year, the associations between care processes and mortality were generally constant throughout the cohort years and were stronger in later years of the cohort. Models for associations between care processes and mortality also adjusted for cohort year to account for secular trends not captured by measured patient characteristics. Prior studies30,31 that described decreasing mortality rates in VHA hospitals did not examine changes in specific care processes that may be driving decreases in mortality. Our study of SAB could help explain the decreasing risk-adjusted mortality among VHA patients through the use of disease-specific, evidence-based care processes.
This large cohort study also adds to the prior literature on risk factors for mortality among patients with SAB. Results were generally similar to those previously reported in smaller cohorts, with higher mortality associated with increasing age, infection with MRSA compared with MSSA, selected comorbidities (eg, congestive heart failure, malignant tumors, and liver disease), and patient vital signs and laboratory values that indicate increased illness severity at bacteremia onset.
The incidences of HCA and HO bacteremia decreased in VHA hospitals between 2003 and 2014, whereas the incidence of CA bacteremia was stable. It is plausible that the decrease in HCA and HO infections was influenced by the VHA’s bundled intervention to reduce HCA MRSA infections, which began in 2007.32 In contrast, one would not expect the interventions in the MRSA bundle to reduce rates of CA bacteremia.
Limitations
This study had limitations. As in any observational study, there is potential for unmeasured confounding. To fully explain our results, confounding factors would need to have affected associations between year of cohort entry and mortality, year of cohort entry and use of evidence-based care processes, and use of evidence-based care processes and mortality.33,34 We also relied on administrative data for diagnoses of comorbidities, which may be inaccurate and allow residual confounding.35,36 In addition, we did not have detailed clinical data on sources of bacteremia, whether these sources were controlled, or presence of all sites of infection. Finally, most patients with SAB in VHA hospitals were men, limiting the generalizability of these findings to other populations.
Conclusions
We observed substantial increases in the use of evidence-based care processes for patients with SAB between 2003 and 2014 that were associated with a marked decrease in mortality. There is a need for continued implementation of quality improvement initiatives to increase the adoption of these evidence-based care processes for patients with SAB, supported by quality measures that reflect use of these processes.
eMethods. Statistical Appendix
eFigure 1. Receiver Operating Characteristic Curves for Training and Validation Data sets
eFigure 2. Crude and Risk-Adjusted All-Cause 30-Day Mortality Rates of MRSA and MSSA
eFigure 3. Risk-Adjusted All-Cause 30-Day Mortality Rates by Place of Acquisition Risk
eFigure 4. Utilization of Echocardiography by Type of Procedure
eFigure 5. Number of Care Processes Received by Year
eFigure 6. Subgroup Analyses for Receipt of Care Processes and All-Cause 30-Day Mortality for Patients With MRSA Bacteremia
eFigure 7. Subgroup Analyses for Receipt of Care Processes and All-Cause 30-Day Mortality for Patients With MSSA Bacteremia
eFigure 8. Subgroup Analyses for Receipt of Care Processes and All-Cause 30-Day Mortality by Quarters of Study Period
eFigure 9. Subgroup Analyses for Receipt of Care Processes and All-Cause 30-Day Mortality for Patients Who Survived for Two or More Days and by Places of Acquisition
References
- 1.Laupland KB, Lyytikäinen O, Søgaard M, et al. ; International Bacteremia Surveillance Collaborative . The changing epidemiology of Staphylococcus aureus bloodstream infection: a multinational population-based surveillance study. Clin Microbiol Infect. 2013;19(5):465-471. [DOI] [PubMed] [Google Scholar]
- 2.Frimodt-Møller N, Espersen F, Skinhøj P, Rosdahl VT. Epidemiology of Staphylococcus aureus bacteremia in Denmark from 1957 to 1990. Clin Microbiol Infect. 1997;3(3):297-305. [DOI] [PubMed] [Google Scholar]
- 3.Mejer N, Westh H, Schønheyder HC, et al. ; Danish Staphylococcal Bacteraemia Study Group . Stable incidence and continued improvement in short term mortality of Staphylococcus aureus bacteraemia between 1995 and 2008. BMC Infect Dis. 2012;12:260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tong SY, Davis JS, Eichenberger E, Holland TL, Fowler VG Jr. Staphylococcus aureus infections: epidemiology, pathophysiology, clinical manifestations, and management. Clin Microbiol Rev. 2015;28(3):603-661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tom S, Galbraith JC, Valiquette L, et al. ; International Bacteraemia Surveillance Collaborative . Case fatality ratio and mortality rate trends of community-onset Staphylococcus aureus bacteraemia. Clin Microbiol Infect. 2014;20(10):O630-O632. [DOI] [PubMed] [Google Scholar]
- 6.Benfield T, Espersen F, Frimodt-Møller N, et al. Increasing incidence but decreasing in-hospital mortality of adult Staphylococcus aureus bacteraemia between 1981 and 2000. Clin Microbiol Infect. 2007;13(3):257-263. [DOI] [PubMed] [Google Scholar]
- 7.Holland TL, Arnold C, Fowler VG Jr. Clinical management of Staphylococcus aureus bacteremia: a review. JAMA. 2014;312(13):1330-1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu C, Bayer A, Cosgrove SE, et al. ; Infectious Diseases Society of America . Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis. 2011;52(3):e18-e55. [DOI] [PubMed] [Google Scholar]
- 9.Rasmussen RV, Høst U, Arpi M, et al. Prevalence of infective endocarditis in patients with Staphylococcus aureus bacteraemia: the value of screening with echocardiography. Eur J Echocardiogr. 2011;12(6):414-420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Calain P, Krause KH, Vaudaux P, et al. Early termination of a prospective, randomized trial comparing teicoplanin and flucloxacillin for treating severe staphylococcal infections. J Infect Dis. 1987;155(2):187-191. [DOI] [PubMed] [Google Scholar]
- 11.Paulsen J, Solligård E, Damås JK, DeWan A, Åsvold BO, Bracken MB. The impact of infectious disease specialist consultation for Staphylococcus aureus bloodstream infections: a systematic review. Open Forum Infect Dis. 2016;3(2):ofw048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vogel M, Schmitz RP, Hagel S, et al. Infectious disease consultation for Staphylococcus aureus bacteremia: a systematic review and meta-analysis. J Infect. 2016;72(1):19-28. [DOI] [PubMed] [Google Scholar]
- 13.Baddour LM, Wilson WR, Bayer AS, et al. ; American Heart Association Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease of the Council on Cardiovascular Disease in the Young, Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and Stroke Council . Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the American Heart Association. Circulation. 2015;132(15):1435-1486. [DOI] [PubMed] [Google Scholar]
- 14.Habib G, Lancellotti P, Antunes MJ, et al. ; Document Reviewers . 2015 ESC guidelines for the management of infective endocarditis: the Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC): endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur Heart J. 2015;36(44):3075-3128. [DOI] [PubMed] [Google Scholar]
- 15.Goto M, Ohl ME, Schweizer ML, Perencevich EN. Accuracy of administrative code data for the surveillance of healthcare-associated infections: a systematic review and meta-analysis. Clin Infect Dis. 2014;58(5):688-696. [DOI] [PubMed] [Google Scholar]
- 16.Schweizer ML, Eber MR, Laxminarayan R, et al. Validity of ICD-9-CM coding for identifying incident methicillin-resistant Staphylococcus aureus (MRSA) infections: is MRSA infection coded as a chronic disease? Infect Control Hosp Epidemiol. 2011;32(2):148-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schaefer MK, Ellingson K, Conover C, et al. Evaluation of International Classification of Diseases, Ninth Revision, Clinical Modification codes for reporting methicillin-resistant Staphylococcus aureus infections at a hospital in Illinois. Infect Control Hosp Epidemiol. 2010;31(5):463-468. [DOI] [PubMed] [Google Scholar]
- 18.Jones M, DuVall SL, Spuhl J, Samore MH, Nielson C, Rubin M. Identification of methicillin-resistant Staphylococcus aureus within the nation’s Veterans Affairs medical centers using natural language processing. BMC Med Inform Decis Mak. 2012;12:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sohn MW, Arnold N, Maynard C, Hynes DM. Accuracy and completeness of mortality data in the Department of Veterans Affairs. Popul Health Metr. 2006;4:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garner JS, Jarvis WR, Emori TG, Horan TC, Hughes JM. CDC definitions for nosocomial infections, 1988. Am J Infect Control. 1988;16(3):128-140. [DOI] [PubMed] [Google Scholar]
- 21.Friedman ND, Kaye KS, Stout JE, et al. Health care–associated bloodstream infections in adults: a reason to change the accepted definition of community-acquired infections. Ann Intern Med. 2002;137(10):791-797. [DOI] [PubMed] [Google Scholar]
- 22.Knaus WA, Wagner DP, Draper EA, et al. The APACHE III prognostic system: risk prediction of hospital mortality for critically ill hospitalized adults. Chest. 1991;100(6):1619-1636. [DOI] [PubMed] [Google Scholar]
- 23.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8-27. [DOI] [PubMed] [Google Scholar]
- 24.Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130-1139. [DOI] [PubMed] [Google Scholar]
- 25.McDanel JS, Perencevich EN, Diekema DJ, et al. Comparative effectiveness of beta-lactams versus vancomycin for treatment of methicillin-susceptible Staphylococcus aureus bloodstream infections among 122 hospitals. Clin Infect Dis. 2015;61(3):361-367. [DOI] [PubMed] [Google Scholar]
- 26.Hosmer DW, Lemeshow S, Sturdivant RX. Applied Logistic Regression. 3rd ed New York, NY: Wiley; 2013. [Google Scholar]
- 27.Graubard BI, Korn EL. Predictive margins with survey data. Biometrics. 1999;55(2):652-659. [DOI] [PubMed] [Google Scholar]
- 28.Li Z, Mahendra G Using “recycled predictions” for computing marginal effects. Paper presented at: SAS Global Forum 2010 Conference; April 14, 2010; Seattle, Washington. [Google Scholar]
- 29.López-Cortés LE, Del Toro MD, Gálvez-Acebal J, et al. ; REIPI/SAB Group . Impact of an evidence-based bundle intervention in the quality-of-care management and outcome of Staphylococcus aureus bacteremia. Clin Infect Dis. 2013;57(9):1225-1233. [DOI] [PubMed] [Google Scholar]
- 30.Fihn SD, Vaughan-Sarrazin M, Lowy E, et al. Declining mortality following acute myocardial infarction in the Department of Veterans Affairs health care system. BMC Cardiovasc Disord. 2009;9(1):44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heidenreich PA, Sahay A, Kapoor JR, Pham MX, Massie B. Divergent trends in survival and readmission following a hospitalization for heart failure in the Veterans Affairs health care system 2002 to 2006. J Am Coll Cardiol. 2010;56(5):362-368. [DOI] [PubMed] [Google Scholar]
- 32.Jain R, Kralovic SM, Evans ME, et al. Veterans Affairs initiative to prevent methicillin-resistant Staphylococcus aureus infections. N Engl J Med. 2011;364(15):1419-1430. [DOI] [PubMed] [Google Scholar]
- 33.VanderWeele TJ. Mediation analysis: a practitioner’s guide. Annu Rev Public Health. 2016;37(1):17-32. [DOI] [PubMed] [Google Scholar]
- 34.VanderWeele TJ, Vansteelandt S. Mediation analysis with multiple mediators. Epidemiol Methods. 2014;2(1):95-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lix LM, Quail J, Fadahunsi O, Teare GF. Predictive performance of comorbidity measures in administrative databases for diabetes cohorts. BMC Health Serv Res. 2013;13(1):340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Quail JM, Lix LM, Osman BA, Teare GF. Comparing comorbidity measures for predicting mortality and hospitalization in three population-based cohorts. BMC Health Serv Res. 2011;11:146. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. Statistical Appendix
eFigure 1. Receiver Operating Characteristic Curves for Training and Validation Data sets
eFigure 2. Crude and Risk-Adjusted All-Cause 30-Day Mortality Rates of MRSA and MSSA
eFigure 3. Risk-Adjusted All-Cause 30-Day Mortality Rates by Place of Acquisition Risk
eFigure 4. Utilization of Echocardiography by Type of Procedure
eFigure 5. Number of Care Processes Received by Year
eFigure 6. Subgroup Analyses for Receipt of Care Processes and All-Cause 30-Day Mortality for Patients With MRSA Bacteremia
eFigure 7. Subgroup Analyses for Receipt of Care Processes and All-Cause 30-Day Mortality for Patients With MSSA Bacteremia
eFigure 8. Subgroup Analyses for Receipt of Care Processes and All-Cause 30-Day Mortality by Quarters of Study Period
eFigure 9. Subgroup Analyses for Receipt of Care Processes and All-Cause 30-Day Mortality for Patients Who Survived for Two or More Days and by Places of Acquisition