Key Points
Question
Does collaborative care for opioid and alcohol use disorders increase treatment use and self-reported abstinence compared with usual primary care?
Findings
Results from this randomized clinical trial found that, relative to usual care, the collaborative care intervention increased both the proportion of primary care patients receiving evidence-based treatment for opioid and alcohol use disorders and the number achieving abstinence from opioids or alcohol use at 6 months.
Meaning
Effective treatment for opioid and alcohol use disorders can be integrated into primary care using a collaborative care intervention and results in improved patient outcomes.
This randomized clinical trial examines whether collaborative care for patients with opioid and/or alcohol use disorders improves the delivery of evidence-based treatments and increases self-reported abstinence compared with usual primary care.
Abstract
Importance
Primary care offers an important and underutilized setting to deliver treatment for opioid and/or alcohol use disorders (OAUD). Collaborative care (CC) is effective but has not been tested for OAUD.
Objective
To determine whether CC for OAUD improves delivery of evidence-based treatments for OAUD and increases self-reported abstinence compared with usual primary care.
Design, Setting, and Participants
A randomized clinical trial of 377 primary care patients with OAUD was conducted in 2 clinics in a federally qualified health center. Participants were recruited from June 3, 2014, to January 15, 2016, and followed for 6 months.
Interventions
Of the 377 participants, 187 were randomized to CC and 190 were randomized to usual care; 77 (20.4%) of the participants were female, of whom 39 (20.9%) were randomized to CC and 38 (20.0%) were randomized to UC. The mean (SD) age of all respondents at baseline was 42 (12.0) years, 41(11.7) years for the CC group, and 43 (12.2) yearsfor the UC group. Collaborative care was a system-level intervention, designed to increase the delivery of either a 6-session brief psychotherapy treatment and/or medication-assisted treatment with either sublingual buprenorphine/naloxone for opioid use disorders or long-acting injectable naltrexone for alcohol use disorders. Usual care participants were told that the clinic provided OAUD treatment and given a number for appointment scheduling and list of community referrals.
Main Outcomes and Measures
The primary outcomes were use of any evidence-based treatment for OAUD and self-reported abstinence from opioids or alcohol at 6 months. The secondary outcomes included the Healthcare Effectiveness Data and Information Set (HEDIS) initiation and engagement measures, abstinence from other substances, heavy drinking, health-related quality of life, and consequences from OAUD.
Results
At 6 months, the proportion of participants who received any OAUD treatment was higher in the CC group compared with usual care (73 [39.0%] vs 32 [16.8%]; logistic model adjusted OR, 3.97; 95% CI, 2.32-6.79; P < .001). A higher proportion of CC participants reported abstinence from opioids or alcohol at 6 months (32.8% vs 22.3%); after linear probability model adjustment for covariates (β = 0.12; 95% CI, 0.01-0.23; P = .03). In secondary analyses, the proportion meeting the HEDIS initiation and engagement measures was also higher among CC participants (initiation, 31.6% vs 13.7%; adjusted OR, 3.54; 95% CI, 2.02-6.20; P < .001; engagement, 15.5% vs 4.2%; adjusted OR, 5.89; 95% CI, 2.43-14.32; P < .001) as was abstinence from opioids, cocaine, methamphetamines, marijuana, and any alcohol (26.3% vs 15.6%; effect estimate, β = 0.13; 95% CI, 0.03-0.23; P = .01).
Conclusions and Relevance
Among adults with OAUD in primary care, the SUMMIT collaborative care intervention resulted in significantly more access to treatment and abstinence from alcohol and drugs at 6 months, than usual care.
Trial Registration
clinicaltrials.gov Identifier: NCT01810159
Introduction
Mortality rates are rising from increases in drug overdoses, suicides, and alcohol-related liver disease, yet substance use disorders (SUDs) continue to be underidentified and undertreated. The consequences of this unmet need are great, including increased risk of disease, injury, disability and death, and large social and health care costs. Opioid and alcohol use disorders (OAUD) are of particular concern owing to their high rates of morbidity and mortality and the increasing prevalence of prescription opioid misuse. Research supports the effectiveness of treatment for OAUD but few individuals receive treatment. Addressing this need has numerous potential benefits.
Although treatment in specialty settings is important for individuals with severe dependence, limited availability and stigma mean that specialty care alone is insufficient to address treatment needs. Primary care offers an important and underutilized setting for OAUD treatment. Recent federal legislation has increased coverage for SUD treatment and the prevalence of OAUD is high in primary care. Opioid and alcohol use disorders have standards for care and, unlike other SUDs, can be treated with medications, making them appropriate for treatment in primary care.
Collaborative care (CC) is an effective strategy for increasing the delivery of evidence-based treatment and improving outcomes, but, to our knowledge, has not been tested for OAUD. A previous trial failed to find an effect of chronic care management on SUD outcomes. Collaborative care is based on principles of the chronic care model and involves integrating behavioral health into primary care. We conducted a randomized clinical trial to determine whether a community health clinic-based CC intervention would improve patient use of evidence-based treatments for OAUD and increase self-reported abstinence. We hypothesized that patients randomized to the CC condition would have: (1) increased use of a 6-session brief psychotherapy treatment (BT) based on motivational interviewing and cognitive behavioral therapy approaches and/or medication-assisted treatment (MAT) with either sublingual buprenorphine/naloxone (BUP/NX) for opioid use disorders or long-acting injectable naltrexone (XR-NTX) for alcohol use disorders; and (2) increased past-30-day abstinence from opioids or alcohol at 6 months.
Methods
Study Design
The Substance Use Motivation and Medication Integrated Treatment (SUMMIT) study was a randomized clinical trial comparing the effect of CC vs usual care on primary care-based OAUD treatment utilization and self-reported abstinence. The study was approved by the RAND institutional review board on April 26, 2012. See the Supplement for the Trial Protocol.
Participants
We partnered with a multisite Federally Qualified Health Center (FQHC) located in Los Angeles, California that provides on-site behavioral health care for depression and anxiety disorders. Of 114 512 patients seen each year, 58% are of Hispanic origin, and 11% are African-American; clinicians include internists, family practitioners, physician assistants, and nurse practitioners. Participants were recruited between June 3, 2014 and January 15, 2016 from the FQHC’s 2 largest clinical sites. During the study’s design, a power analysis determined that a sample of 400 participants would allow estimation of small to medium effect sizes.
All patients attending a primary care visit with a clinician were screened by a medical assistant for substance use in the past 3 months using a 3-question screener based on the National Institute on Drug Abuse (NIDA) quick screen. Consenting patients who screened positive for risky use were referred for assessment by the research team. Inclusion criteria were (1) age 18 years or older; (2) probable OAUD diagnosis based on the NIDA-modified Alcohol, Smoking and Substance Involvement Screening Test (ASSIST); (3) English or Spanish-speaking; (4) willing to switch therapists if already receiving therapy at the clinic. Exclusion criteria were (1) marked functional impairment from bipolar disorder or schizophrenia; (2) current abstinence from alcohol and/or opioids in the previous 30 days; and (3) current substance use treatment. Participants gave written informed consent and were compensated for research activities ($5 for eligibility screener, $50 for baseline assessment, $50 for follow-up assessment).
Assessment at Baseline
The baseline interview assessed demographics; homeless status; Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition) diagnosis of alcohol, heroin, and prescription opioid abuse or dependence using the Comprehensive International Diagnostic Interview (CIDI), version 3.0, past 30-day use of alcohol and opioids using the Timeline Follow-back; 30-day use of methamphetamines, cocaine, marijuana, and other drugs; typical number of drinks per drinking day in past 12 months; heavy drinking days (defined as 4 or more ethanol drinks in a day for women and 5 or more for men); consequences of alcohol or opioid use using the Short Inventory of Problems Alcohol and Drugs (SIP-AD; range 0-15); depression symptoms using the Patient Health Questionnaire-8 (PHQ-8; range 0-24); health-related quality of life using the SF-12 Health Survey (SF-12 mental component summary [MCS] and physical component summary [PCS] scores; range of 0-100); emergency department or overnight hospital stay in past 90 days; and self-reported receipt of lifetime and past year SUD treatment.
Randomization Procedures
We used an R software (version 3, R Project) random number generator to randomly assign eligible participants to either CC or usual care. We used a concealed randomization protocol where neither participant nor researcher was aware of the randomization until after the baseline interview. None of the participants or clinicians was blinded to treatment allocation after randomization.
Participant Assessment at Follow-up
A follow-up assessment using a subset of questions from baseline was conducted by telephone 6 months after the baseline interview. Interviewers were blinded to treatment allocation. The last participant follow-up interview was conducted in September, 2016.
Collaborative Care and Usual Care
The CC intervention included a population-based management approach, measurement-based care, and integration of addiction expertise through a RAND-based clinical psychologist affiliated with the Motivational Interviewing Network of Trainers. Care coordinators met with patients who had positive screening results and who were randomized to CC to assess motivation and encourage patients to meet with a therapist for evaluation and treatment planning. All CC patients were entered into a registry that tracked treatment progress and prompted care coordinators to reach out to patients with missed visits. Care coordinators conducted regular assessments of substance use; results were entered into the registry and reviewed during team meetings. Participants in usual care were told by the research team that the clinic provided OAUD treatment, and given a number for appointment scheduling and list of community referrals. They did not receive any additional outreach or contact.
The 2 clinical sites employed 7 therapists who had counselling or social work masters’ degrees. None had expertise in addiction treatment. All therapists received a 1-hour overview of the BT manuals; 5 were randomized to the CC condition and received an additional 2 days of BT training. Care coordinators had a high school degree and received 2 days of motivational interviewing training; each had worked at the clinic for more than 15 years. Clinicians were not randomized; all 28 were offered MAT training; 24 received XR-NTX training and 18 received training on BUP/NX (7 nonphysicians were not eligible to be waivered and 3 physicians were not trained). Twelve clinicians received their Drug Enforcement Agency waiver to prescribe BUP/NX. Patients who wanted MAT but whose clinician had not been trained or who were ineligible were referred to a waivered clinician. All clinicians were employed by the FQHC; pharmacotherapy consultation was provided by a board-certified addiction medicine physician affiliated with a local academic medical center. Weekly team meetings and group supervision for the care coordinators and therapists in the CC condition were led by the clinical psychologist. All BT sessions were audiotaped and uploaded to a secure site for review during clinical supervision.
Outcomes
There were 2 primary outcomes: use of any evidence-based OAUD treatment (BT or MAT) during the 6-month study period, and self-reported 30-day abstinence from all opioids or any alcohol at 6 months. The BT and MAT visit data were obtained from electronic medical record administrative files; XR-NTX use data were obtained from a pharmacy log; and BUP/NX use data were obtained from medical chart review of electronic medical record notes; we were unable to confirm whether patients filled the BUP/NX prescriptions. Visit data from administrative files were cross-referenced with medical chart review data for all patients and BT audio files for accuracy. Secondary outcomes included Healthcare Effectiveness Data and Information Set (HEDIS) initiation and engagement measures, 30-day abstinence from any alcohol and all drugs (including opioids, methamphetamine, cocaine, and marijuana), consequences from opioid and alcohol use, health-related quality of life, and any heavy drinking days.
Statistical Analysis
We conducted descriptive analyses to test the balance between CC and usual care group characteristics at baseline using a χ2 test for categorical variables and a t test for continuous variables. Multivariable analyses controlled for characteristics that significantly differed between the 2 groups at an α level of 0.20.
We conducted all evaluations as intent-to-treat analyses. We conducted multivariable logistic regression to test the hypothesis that CC increased utilization of OAUD treatment. All patient outcome analyses were weighted to represent the sample of patients recruited through randomization, using a raking algorithm in SAS (RAkinge). Because only patients who were currently using alcohol and/or opioids at baseline qualified for the study, for our primary outcome of abstinence from all opioids and alcohol, as well as 2 other secondary abstinence outcomes, we conducted a fixed effects linear probability model comparing patients in the UC and CC at 6 months after baseline, controlling for clinic enrollment site and relevant covariates, including age, race, diagnosis of heroin use disorder at baseline and clinic enrollment site. To test the relationship between CC and the remaining patient measures at baseline, we used fixed effects difference-in-difference regression models, controlling for time, clinic enrollment site, and relevant covariates. We conducted multivariable linear regression to model continuous outcomes, and both linear probability and logistic regression models for the categorical outcomes. Results from the logistic regression and linear probability models were similar, and for ease of interpretation, we present results from the linear probability models only.
Few missing values were observed for all variables except the SF-12 MCS and PCS measures. Owing to a programming error, approximately 200 respondents were given incorrect answer choices for 4 of the 12 component questions used to calculate the MCS and PCS. We therefore imputed 5 sets of plausible values, with the stipulation that the distributions of the imputed variables remain similar to the observed data. Outcome model results were aggregated across these multiple imputed data sets using standard procedures.
Results
Enrollment and Follow-up
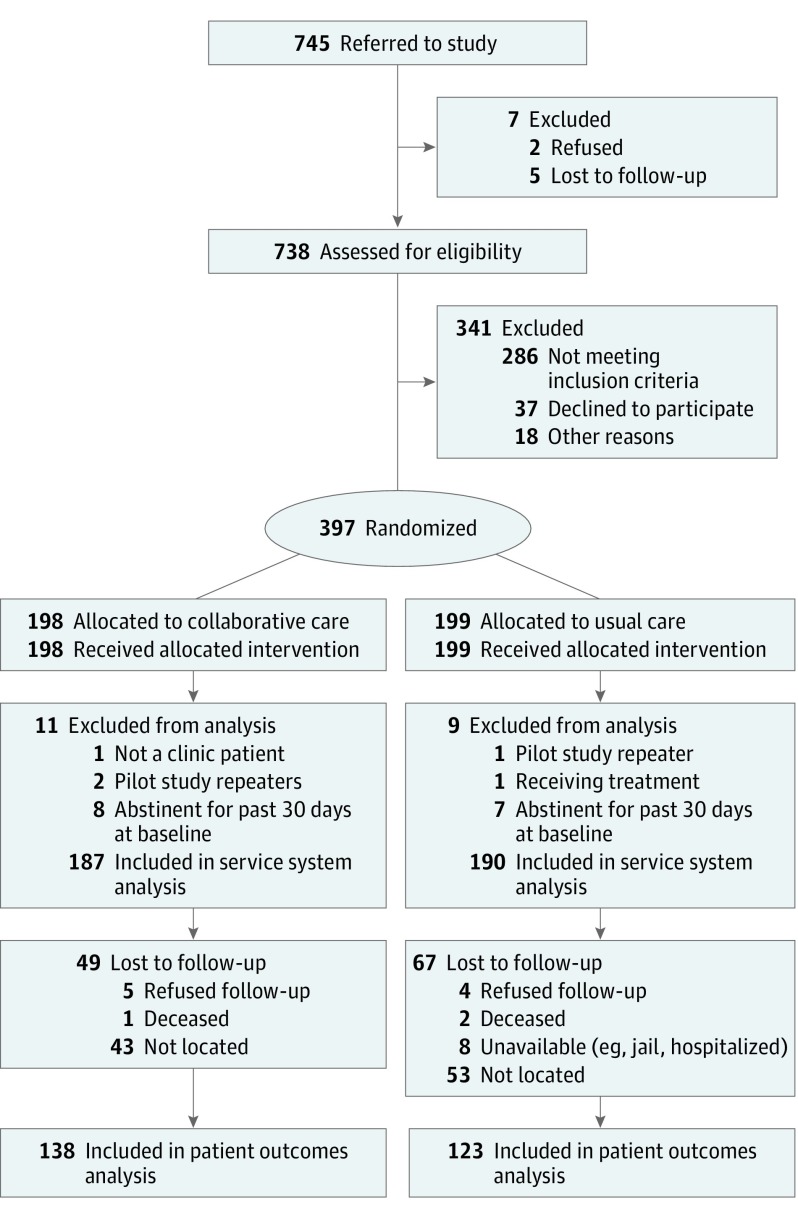
There were 15 723 unique adult primary care visits in the study period. Based on a predetermined sample of weekly screening audits, 94% to 98% of the visits were screened (approximately 15 000) by the medical assistants, 5% were eligible for referral, and 745 were referred to the study. Of those, 738 were assessed for eligibility (Figure), 452 were eligible, and 397 were randomized. Postrandomization, 20 were excluded after reporting either being in treatment or being abstinent in the 30 days prior to the assessment, leaving 377 in the analysis of outcomes (187 in CC and 190 in UC). Of these, 261 (69%) were interviewed at 6 months and included in analysis of patient outcomes. The 2 groups were comparable with respect to observed characteristics at baseline, with no differences at P < .05 (Table 1): 203 (54%) had only alcohol abuse or dependence, 115 (31%) abused or were dependent on heroin with or without cooccurring alcohol or prescription opioid abuse or dependence, and 59 (16%) had prescription opioid abuse or dependence with or without cooccurring alcohol abuse or dependence. One hundred eighty six (49.3%) were homeless.
Figure. CONSORT Diagram.
Table 1. Characteristics of Study Participants at Baseline and 6 Months.
| Characteristics | Baseline | 6-Month Follow-up | ||||
|---|---|---|---|---|---|---|
| Overall (n = 377) |
Treatment (n = 187) |
Control (n = 190) |
Overall (n = 261) |
Treatment (n = 138) |
Control (n = 123) |
|
| Age, mean (SD), y | 42 (12.0) | 41 (11.7) | 43 (12.2) | 42 (12.1) | 41 (12.2) | 43 (12.1) |
| Female, No. (%) | 77 (20.4) | 39 (20.9) | 38 (20.0) | 63 (24.1) | 31 (22.5) | 32 (26.0) |
| Race, No. (%) | ||||||
| White | 165 (43.8) | 79 (42.2) | 86 (45.3) | 107 (41.0) | 52 (37.7) | 55 (44.7) |
| Black | 50 (13.3) | 24 (12.8) | 26 (13.7) | 43 (16.5) | 19 (13.8) | 24 (19.5) |
| American Indian/Alaska Native | 5 (1.3) | 2 (1.1) | 3 (1.6) | 3 (1.1) | 2 (1.4) | 1 (0.8) |
| Native Hawaiian/Pacific Islander | 2 (0.5) | 2 (1.1) | 0 | 0 | 0 | 0 |
| Asian | 3 (0.8) | 1 (0.5) | 2 (1.1) | 3 (1.1) | 1 (0.7) | 2 (1.6) |
| Other | 101 (26.8) | 48 (25.7) | 53 (27.9) | 70 (26.8) | 39 (28.3) | 31 (25.2) |
| Multiple | 51 (13.5) | 31 (16.6) | 20 (10.5) | 35 (13.4) | 25 (18.1) | 10 (8.1) |
| Hispanic origin | 117 (31.0) | 56 (29.9) | 61 (32.1) | 81 (31.0) | 43 (31.0) | 38 (31.0) |
| Education, No. (%) | ||||||
| Less than high school | 105 (27.9) | 52 (27.8) | 53 (27.9) | 68 (26.1) | 36 (26.1) | 32 (26.0) |
| High school graduate/GED | 117 (31.0) | 54 (28.9) | 63 (33.2) | 78 (29.9) | 39 (28.3) | 39 (31.7) |
| More than high school | 155 (41.1) | 81 (43.3) | 74 (38.9) | 115 (44.1) | 63 (45.7) | 52 (42.3) |
| Homeless, No. (%)a | 186 (49.3) | 88 (47.1) | 98 (51.6) | 77 (29.5) | 38 (27.5) | 39 (31.7) |
| PHQ-8 depressive symptoms score, mean (SD) | 12 (6.4) | 11 (6.5) | 12 (6.2) | 9 (6.3) | 8 (6.2) | 9 (6.4) |
| SF-12 MCS mental health-related quality-of-life score, mean (SD) | 40 (11.0) | 40 (10.8) | 39 (10.9) | 42 (11.8) | 43 (11.7) | 42 (11.8) |
| SF-12 PCS physical health-related quality-of-life score, mean (SD)b | 47 (10.3) | 48 (9.9) | 47 (10.2) | 48 (10.5) | 49 (10.0) | 46 (10.8) |
| CIDI diagnosis, No. (%) | ||||||
| Only alcohol abuse or dependence | 203 (53.8) | 104 (56.0) | 99 (52.0) | 149 (57.1) | 79 (57.2) | 70 (56.9) |
| Heroin abuse or dependence, with or without cooccurring alcohol or prescription opioid abuse or dependence | 115 (30.5) | 51 (27.0) | 64 (34.0) | 70 (26.8) | 34 (24.6) | 36 (29.3) |
| Prescription opioid abuse or dependence, with or without co-occurring alcohol abuse or dependence | 59 (15.6) | 32 (17.1) | 27 (14.2) | 42 (16.1) | 25 (18.1) | 17 (13.8) |
| Any alcohol use, past 30 days, No. (%) | 355 (94.2) | 174 (93.0) | 181 (95.3) | 189 (72.4) | 94 (68.1) | 95 (77.2) |
| Any heroin use, past 30 days, No. (%) | 69 (18.3) | 31 (16.6) | 38 (20.0) | 22 (8.4) | 9 (6.5) | 13 (10.6) |
| Any prescription opioid use, past 30 days, No. (%) | 88 (23.3) | 42 (22.5) | 46 (24.2) | 22 (8.4) | 14 (10.1) | 8 (6.5) |
| Any methamphetamine use, past 30 days, No. (%) | 96 (25.5) | 46 (24.6) | 50 (26.3) | 36 (13.8) | 13 (9.4) | 23 (18.7) |
| Any cocaine use, past 30 days, No. (%) | 62 (16.4) | 35 (18.7) | 27 (14.2) | 32 (12.3) | 18 (13.0) | 14 (11.4) |
| Typical drinks per day, past 12 moonths, median, (IQR)c | 6 (3-10) | 6 (3-10) | 6 (4-11) | |||
| Years of heroin use, median, (IQR)d | 4 (2-10) | 4 (2-10) | 5 (2-11) | |||
| Ever hospitalized for alcohol or opioid use, No. (%) | 101 (26.8) | 46 (24.6) | 55 (28.9) | |||
| Emergency department visit or hospital stay, past 90 days, No. (%) | 139 (36.9) | 72 (38.5) | 67 (35.3) | 55 (21.1) | 27 (19.6) | 28 (22.8) |
| Substance use treatment, past 12 months, No. (%) | 50 (13.3) | 21 (11.2) | 29 (15.3) | |||
| Discussed substance use with a professional, lifetime, No. (%) | 219 (58.1) | 107 (57.2) | 112 (58.9) | |||
| Short Inventory of Problems-Alcohol and Drugs score, past 3 months, median (IQR) | 11 (5-14) | 10 (5-14) | 11 (6-14) | 5 (0-11) | 6 (0-11) | 5 (0-12) |
| Arrested owing to drinking, lifetime, median (IQR) | 3 (1-6) | 3 (1-5) | 3 (1-7) | |||
Abbreviations: CC, collaborative care; GED, general education development; IQR, interquartile range; MCS, mental health composite scale; PHQ-8, patient health questionnaire depression scale; PCS, physical health composite scale; SF-12, 12-item short form survey.
Participant considered homeless if spent previous night sleeping outside, in a shelter, in an abandoned building, or lacks a regular place to stay.
P < .05 between treatment and control group at follow-up.
Limited to those who reported having used alcohol in the past (n = 366).
Limited to those who reported having used heroin in the past (n = 149).
Delivery of Collaborative Care Intervention
Of 187 individuals randomized to CC, 184 were entered into the registry, 171 (93%) met with the care coordinator, 143 (76%) scheduled an appointment with a CC therapist, 65 (45%) kept the appointment (number derived from patient registry and includes individuals subsequently excluded from the service system outcome analysis), and 37 had at least 1 additional psychotherapy session. Sixteen of the 24 clinicians who were trained prescribed XR-NTX and 11 of the 12 waivered prescribers prescribed BUP/NX; overall 17 of 28 (61%) prescribed MAT.
Treatment Utilization Outcomes
Treatment utilization models were adjusted for age, race, heroin abuse or dependence, and clinic enrollment site. At 6 months, the proportion of participants who had received any evidence-based OAUD treatment was higher in the CC group compared with usual care (39.0% vs 16.8%; adjusted OR, 3.97; 95% CI, 2.32-6.79; P < .001) (Table 2). In secondary analyses, the proportion of participants receiving any BT, but not the proportion receiving any MAT, was higher in the CC group compared with usual care (35.8% vs 10.5%; adjusted OR, 6.22; 95% CI, 3.36-11.52; P < .001; 13.4% vs 12,6%; adjusted OR, 1.23; 95% CI, 0.64-2.38). The proportion meeting the HEDIS initiation and engagement measures was also higher in the CC group than in usual care (initiation, 31.6% vs 13.7%; adjusted OR, 3.54; 95% CI, 2.02-6.20; P < .001; engagement, 15.5% vs 4.2%; adjusted OR, 5.89; 95% CI, 2.43-14.32; P < .001).
Table 2. Effects of Collaborative Care on OAUD Treatment Utilization and Patient Outcomes.
| Treatment Utilization Outcomes | No. (%) | Odds Ratioa (95% CI) | P Value | |
|---|---|---|---|---|
| CC (n = 187) | Usual Care (n = 190) | |||
| Primary outcome | ||||
| Patient received any evidence-based treatment (BT or MAT) | 73 (39.0) | 32 (16.8) | 3.97 (2.3-6.8) | <.001 |
| Secondary outcomes | ||||
| Patient received any BT | 67 (35.8) | 20 (10.5) | 6.22 (3.4-11.5) | <.001 |
| Patient received any medication assisted treatment | 25 (13.4) | 24 (12.6) | 1.23 (0.6-2.4) | .53 |
| HEDIS Initiation | 59 (31.6) | 26 (13.7) | 3.54 (2.0-6.2) | <.001 |
| HEDIS Engagement | 29 (15.5) | 8 (4.2) | 5.89 (2.4-14.3) | <.001 |
Abbreviations: CC, collaborative care; HEDIS, Healthcare Effectiveness Data and Information Set.
Multivariable logistic regression controlling for age, race, diagnosis of heroin use disorder at baseline, and clinic enrollment site.
Participant Outcomes
At 6 months, the proportion of participants abstinent from all opioids or any alcohol was 10.5 percentage points higher in the CC group compared with usual care (32.8 vs 22.3 percentage points) (Table 3). After adjustment for covariates, abstinence remained higher by 12 percentage points in the CC group (effect estimate, β = 0.12; 95% CI, 0.01-0.23; P = .03). Only 1 of the 7 secondary outcomes—abstinence from opioids, cocaine, methamphetamines, marijuana, and any alcohol—showed a significant improvement in the CC group, (26.3% vs 15.6%; effect estimate, β = 0.13; 95% CI, 0.03-0.23; P = .01). Patient outcome models were adjusted for age, race, receipt of SUD treatment in the previous 12 months, and clinic enrollment site.
Table 3. Participant Outcomesa.
| Outcome | Baseline, %b | 6-Month Follow-up,c % | Effect Estimated (95% CI) | P Value | |||
|---|---|---|---|---|---|---|---|
| CC (n = 187) |
Usual Care (n = 190) |
CC (n = 138) |
Usual Care (n = 123) |
||||
| Primary outcome | |||||||
| Abstinence from all opioids or any alcohol, past 30 days | NA | NA | 32.8 | 22.3 | 0.12 (0.01 to 0.23) | .03 | |
| Secondary outcomes | |||||||
| Short inventory of problems-alcohol and drugs score, mean (SD) | 9.1 (4.9) | 9.6 (4.8) | 7.0 (5.9) | 6.2 (5.5) | 1.55 (−0.21 to 3.31) | .08 | |
| Abstinence from opioids, any alcohol, cocaine, methamphetamines, and marijuana, past 30 days | NA | NA | 26.3 | 15.6 | 0.13 (0.03 to 0.23) | .01 | |
| Heavy drinking, past 30 days | 79.1 | 82.6 | 53.9 | 56.2 | 0.01 (−0.14 to 0.16) | .91 | |
| Abstinence from all opioids, past 30 days | 72.0 | 67.9 | 88.7 | 79.9 | 0.07 (−0.07 to 0.22) | .33 | |
| Abstinence from all opioids or no heavy drinkinge | NA | NA | 42.7 | 40.9 | 0.04 (−0.09 to 0.17) | .50 | |
| Mental health-related quality-of-life (MCS score), mean (SD) | 40.1 (10.8) | 39.5 (10.9) | 41.0 (12.4) | 40.8 (12.2) | −1.61 (−5.61 to 2.39) | .43 | |
| Physical health-related quality-of-life (PCS score), mean (SD) | 47.6 (9.9) | 47.2 (10.2) | 48.1 (11.53) | 46.7 (10.8) | 1.49 (−2.05 to 5.03) | .41 | |
Abbreviations: BT, brief treatment; CC, collaborative care; HEDIS, Healthcare Effectiveness Data and Information Set; MAT, medication-assisted treatment; NA, not applicable.
Reported numbers are not raw data but rather weighted numbers that do not necessarily represent whole numbers of patients.
Patients who abstained from alcohol, heroin, and prescription opioids in the 30 days prior to baseline were excluded from the study
All 6-month results are weighted to be analogous to baseline sample.
Parameter estimates from a linear probability model controlling for age, race, receipt of substance abuse treatment in the 12 months prior to baseline, and clinic enrollment site.
Patients who abstained from opioids or did not report any heavy drinking in the 30 days prior to baseline were excluded from this analysis.
Discussion
We found that a CC intervention implemented in a multisite FQHC increased both the proportion of primary care patients receiving evidence-based treatment for OAUD, and the number achieving self-reported abstinence from opioids or alcohol use at 6 months, compared with usual care. Among individuals with SUDs, abstinence is linked to a decreased likelihood of relapse compared with nonproblem use and better long-term outcomes. In secondary analyses, CC increased the proportion of patients meeting the HEDIS initiation and engagement measures, the proportion receiving psychotherapy, and the number reporting abstinence from alcohol and other drugs. The HEDIS initiation and engagement measures have been associated with lower mortality, fewer arrests, and improvements in employment and drug and alcohol use. Collaborative Care did not increase use of MAT, reduce levels of heavy drinking, significantly improve health-related quality of life, or reduce problems from drugs and alcohol. Our results suggest that a CC intervention for adults with OAUD in community health clinics is feasible and effective.
Opioid and/or alcohol use disorders are 2 of the most prevalent and problematic SUDs with high unmet need. Among our sample both severity and unmet need were high, with over one-quarter having been hospitalized for their OAUD use; only 209 (58.1%) had previously discussed substance use with a professional; and only 50 (13.3%) had received any treatment during the prior 12 months. In addition, with nearly half the sample homeless at the time of enrollment, our study included a population with special challenges to engage and treat successfully.
To our knowledge, this is the first study to implement and test CC for OAUD in a community clinic setting. Our intervention differed from previous studies to increase treatment for OAUD in several important ways, which may have contributed to our results. We recruited participants when they presented to their regular clinician, rather than through residential detoxification services, advertisements, or community referrals. Treatment for OAUD was delivered by participants’ primary care clinician or by behavioral health professionals integrated with the clinic rather than through a specialty addiction medicine clinic or specialty care. Using participants’ usual care clinicians may have increased participant motivation or decreased stigma, leading to better outcomes. It may have also reduced barriers to treatment utilization.
Although results favored CC, receipt of evidence-based treatment was still relatively low (73 [39%]) and only 25 (13%) of CC participants received MAT. It is not clear whether the low rate of MAT is related to clinician supply or patient demand. Of the 28 clinicians at the 2 clinics, 17 (61%) prescribed MAT, suggesting that clinicians integrated MAT treatment into their practice and the organization had increased its capacity to provide MAT. However, clinicians did not regularly participate in the team meetings attended by the care coordinators, therapists, and addiction specialists, citing a lack of protected time. The low rates of treatment and MAT specifically may also be owing to patients not identifying as having an OAUD, since most accessed primary care for reasons perceived as unrelated to substance use. Population-based data suggest that over 90% of individuals with substance use disorders do not perceive a need for treatment. Given this, BT may have been seen as a more acceptable initial treatment option.
Strengths and Limitations
Our study has several strengths. The study population was ethnically and racially diverse, and the FQHC served a low-income population. Existing staff were trained to deliver the intervention and treatment. This approach supports the intervention’s feasibility and long-term sustainability, and its transportability to other similar FQHCs. Screening at every visit and use of the registry facilitated population-based management; regular symptom assessment facilitated measurement-based care. It is notable that in both groups we observed increased abstinence, which may speak to the power of the primary care relationship.
There were also limitations. The study took place at an FQHC with integrated behavioral health. Thus, results may not generalize to other types of settings. By design, the clinics undertook a year-long effort to prepare to provide OAUD treatment before the trial, which may limit dissemination. However, to the extent that this preparation phase may have biased results, it would be toward the null hypothesis. We assessed patient outcomes by self-report, because biological tests are inadequate for detecting 30-day opioid and alcohol use. Although we used validated tools, social desirability bias may have influenced CC participants to report abstinence more than participants receiving usual care. We had differential retention, and individuals lost to follow-up may have had worse outcomes than those for whom we have outcome data. There was also no follow-up after 6 months, and we do not know if the effect was sustained. Finally, patient-level vs clinic-level randomization may have increased the likelihood of spillover, but to the extent that this occurred it would have weakened statistical power to detect differences between groups.
Conclusions
Among adults with OAUDs seen in primary care, the SUMMIT intervention resulted in significantly more evidence-based treatment and abstinence from alcohol and drugs at 6 months, compared with usual care. These findings suggest that treatment for OAUDs can be integrated into primary care, and that primary care-based treatment is effective for OAUDs.
Trial Protocol
References
- 1.Case A, Deaton A Mortality and morbidity in the 21st century (conference version). BPEA. 2017;March. https://www.brookings.edu/wp-content/uploads/2017/03/6_casedeaton.pdf. Accessed June 20, 2017. [DOI] [PMC free article] [PubMed]
- 2.Blanco C, Iza M, Rodríguez-Fernández JM, Baca-García E, Wang S, Olfson M. Probability and predictors of treatment-seeking for substance use disorders in the U.S. Drug Alcohol Depend. 2015;149:136-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blanco C, Iza M, Schwartz RP, Rafful C, Wang S, Olfson M. Probability and predictors of treatment-seeking for prescription opioid use disorders: a national study. Drug Alcohol Depend. 2013;131(1-2):143-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Compton WM, Thomas YF, Stinson FS, Grant BF. Prevalence, correlates, disability, and comorbidity of DSM-IV drug abuse and dependence in the United States: results from the national epidemiologic survey on alcohol and related conditions. Arch Gen Psychiatry. 2007;64(5):566-576. [DOI] [PubMed] [Google Scholar]
- 5.Grella CE, Karno MP, Warda US, Moore AA, Niv N. Perceptions of need and help received for substance dependence in a national probability survey. Psychiatr Serv. 2009;60(8):1068-1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Substance Abuse and Mental Health Services Administration Results From the 2013 National Survey on Drug Use and Health: Summary of National Findings, NSDUH Series H-46, HHS publication no. (SMA) 14-4863. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2014. [Google Scholar]
- 7.Bouchery EE, Harwood HJ, Sacks JJ, Simon CJ, Brewer RD. Economic costs of excessive alcohol consumption in the U.S., 2006. Am J Prev Med. 2011;41(5):516-524. [DOI] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention Excessive drinking costs US $223.5 billion. CDC features 2014; 705-709. 2016; http://www.cdc.gov/features/alcoholconsumption/. Accessed June 16, 2016.
- 9.Mokdad AH, Marks JS, Stroup DF, Gerberding JL. Actual causes of death in the United States, 2000. JAMA. 2004;291(10):1238-1245. [DOI] [PubMed] [Google Scholar]
- 10.National Drug Intelligence Center The Economic Impact of Illicit Drug Use on American Society. Washington, DC: United States Department of Justice; 2011. [Google Scholar]
- 11.Degenhardt L, Bucello C, Mathers B, et al. . Mortality among regular or dependent users of heroin and other opioids: a systematic review and meta-analysis of cohort studies. Addiction. 2011;106(1):32-51. [DOI] [PubMed] [Google Scholar]
- 12.Roerecke M, Rehm J. Alcohol use disorders and mortality: a systematic review and meta-analysis. Addiction. 2013;108(9):1562-1578. [DOI] [PubMed] [Google Scholar]
- 13.Ronan MV, Herzig SJ. Hospitalizations related to opioid abuse/dependence and associated serious infections increased sharply, 2002–12. Health Aff (Millwood). 2016;35(5):832-837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Substance Abuse and Mental Health Services Administration Emergency department data. Undated. http://www.samhsa.gov/data/emergency-department-data-dawn. Accessed June 28, 2016.
- 15.Substance Abuse and Mental Health Services Administration Prescription drug use and misuse in the United States: results from the 2015 national survey on drug use and health. 2016; https://www.samhsa.gov/data/sites/default/files/NSDUH-FFR2-2015/NSDUH-FFR2-2015.htm. Accessed March 3, 2017.
- 16.US Department of Veteran Affairs, Department of Defense (VA/DOD) VA/DoD clinical practice guideline for the management of substance use disorders. Version 3.0. 2015. http://www.healthquality.va.gov/guidelines/MH/sud/VADoDSUDCPGRevised22216.pdf. Accessed January 10, 2017.
- 17.Jonas DE, Amick HR, Feltner C, et al. . Pharmacotherapy for adults with alcohol use disorders in outpatient settings: a systematic review and meta-analysis. JAMA. 2014;311(18):1889-1900. [DOI] [PubMed] [Google Scholar]
- 18.Kaner EF, Beyer F, Dickinson HO, et al. . Effectiveness of brief alcohol interventions in primary care populations. Cochrane Database Syst Rev. 2007;(2):CD004148. [DOI] [PubMed] [Google Scholar]
- 19.Schackman BR, Leff JA, Polsky D, Moore BA, Fiellin DA. Cost-effectiveness of long-term outpatient buprenorphine-naloxone treatment for opioid dependence in primary care. J Gen Intern Med. 2012;27(6):669-676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smedslund G, Berg RC, Hammerstrøm KT, et al. . Motivational interviewing for substance abuse. Cochrane Database Syst Rev. 2011;(5):CD008063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murthy VH. Surgeon General’s report on alcohol, drugs, and health. JAMA. 2017;317(2):133-134. [DOI] [PubMed] [Google Scholar]
- 22.Appel PW, Ellison AA, Jansky HK, Oldak R. Barriers to enrollment in drug abuse treatment and suggestions for reducing them: opinions of drug injecting street outreach clients and other system stakeholders. Am J Drug Alcohol Abuse. 2004;30(1):129-153. [DOI] [PubMed] [Google Scholar]
- 23.Cunningham JA, Sobell LC, Sobell MB, Agrawal S, Toneatto T. Barriers to treatment: why alcohol and drug abusers delay or never seek treatment. Addict Behav. 1993;18(3):347-353. [DOI] [PubMed] [Google Scholar]
- 24.Grant BF. Barriers to alcoholism treatment: reasons for not seeking treatment in a general population sample. J Stud Alcohol. 1997;58(4):365-371. [DOI] [PubMed] [Google Scholar]
- 25.Beronio K, Po R, Skopec L Affordable Care Act will expand mental health and substance use disorder benefits and parity protections for 62 million Americans. ASPE Research Brief. 2013. http://aspe.hhs.gov/health/reports/2013/mental/rb_mental.cfm. Accessed February 20, 2013.
- 26.Buck JA. The looming expansion and transformation of public substance abuse treatment under the Affordable Care Act. Health Aff (Millwood). 2011;30(8):1402-1410. [DOI] [PubMed] [Google Scholar]
- 27.Cherpitel CJ, Ye Y. Trends in alcohol- and drug-related emergency department and primary care visits: data from four U.S. national surveys (1995-2010). J Stud Alcohol Drugs. 2012;73(3):454-458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pilowsky DJ, Wu LT. Screening for alcohol and drug use disorders among adults in primary care: a review. Subst Abuse Rehabil. 2012;3(1):25-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Archer J, Bower P, Gilbody S, et al. . Collaborative care for depression and anxiety problems. Cochrane Database Syst Rev. 2012;10:CD006525. [DOI] [PubMed] [Google Scholar]
- 30.Saitz R, Cheng DM, Winter M, et al. . Chronic care management for dependence on alcohol and other drugs: the AHEAD randomized trial. JAMA. 2013;310(11):1156-1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bodenheimer T, Wagner EH, Grumbach K. Improving primary care for patients with chronic illness: the chronic care model, Part 2. JAMA. 2002;288(15):1909-1914. [DOI] [PubMed] [Google Scholar]
- 32.Bodenheimer T, Wagner EH, Grumbach K. Improving primary care for patients with chronic illness. JAMA. 2002;288(14):1775-1779. [DOI] [PubMed] [Google Scholar]
- 33.Ober AJ, Watkins KE, Lamp K, et al. . SUMMIT Study Protocol: Step-by-Step Procedures for Providing Screening, Brief Intervention, and Treatment Services to Primary Care Patients With Opioid or Alcohol Use Disorders (TL-219-NIDA). Santa Monica, CA: RAND Corporation; 2017. [Google Scholar]
- 34.Osilla KC, D’Amico EJ, Lind M, Ober AJ, Watkins KE Brief treatment for substance use disorders: a guide for behavioral health providers (TL-147-NIDA). 2016. http://www.rand.org/pubs/tools/TL147.html. Accessed June 16, 2016.
- 35.Heinzerling KG, Ober AJ, Lamp K, De Vries D, Watkins KE SUMMIT: procedures for medication-assisted treatment of alcohol or opioid dependence in primary care (TL-148-NIDA). 2016; http://www.rand.org/pubs/tools/TL148.html. Accessed June 16, 2016.
- 36.Venice Family Clinic Venice Family Clinic. 2017. http://www.venicefamilyclinic.org/. Accessed June 20, 2017.
- 37.National Institute on Drug Abuse NIDA drug screening tool: NIDA-modified ASSIST (NM ASSIST). Undated. https://www.drugabuse.gov/nmassist/. Accessed March 20, 2017.
- 38.Arbuckle R, Frye MA, Brecher M, et al. . The psychometric validation of the Sheehan Disability Scale (SDS) in patients with bipolar disorder. Psychiatry Res. 2009;165(1-2):163-174. [DOI] [PubMed] [Google Scholar]
- 39.Luciano JV, Bertsch J, Salvador-Carulla L, et al. . Factor structure, internal consistency and construct validity of the Sheehan Disability Scale in a Spanish primary care sample. J Eval Clin Pract. 2010;16(5):895-901. [DOI] [PubMed] [Google Scholar]
- 40.Forman RF, Svikis D, Montoya ID, Blaine J. Selection of a substance use disorder diagnostic instrument by the National Drug Abuse Treatment Clinical Trials Network. J Subst Abuse Treat. 2004;27(1):1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Haro JM, Arbabzadeh-Bouchez S, Brugha TS, et al. . Concordance of the composite international diagnostic interview Version 3.0 (CIDI 3.0) with standardized clinical assessments in the WHO world mental health surveys. Int J Methods Psychiatr Res. 2006;15(4):167-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sobell LC, Sobell MB. Timeline follow-back: a technique for assessing self-reported alcohol consumption In: Litten RZ, Allen JP, eds. Measuring Alcohol Consumption: Psychosocial and Biochemical Methods. Totowa, NJ: Humana Press; 1992:41-72. [Google Scholar]
- 43.Alterman AI, Cacciola JS, Ivey MA, Habing B, Lynch KG. Reliability and validity of the alcohol short index of problems and a newly constructed drug short index of problems. J Stud Alcohol Drugs. 2009;70(2):304-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Blanchard KA, Morgenstern J, Morgan TJ, Lobouvie EW, Bux DA. Assessing consequences of substance use: psychometric properties of the inventory of drug use consequences. Psychol Addict Behav. 2003;17(4):328-331. [DOI] [PubMed] [Google Scholar]
- 45.Gelaye B, Tadesse MG, Williams MA, Fann JR, Vander Stoep A, Andrew Zhou XH. Assessing validity of a depression screening instrument in the absence of a gold standard. Ann Epidemiol. 2014;24(7):527-531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606-613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gandek B, Ware JE, Aaronson NK, et al. . Cross-validation of item selection and scoring for the SF-12 Health Survey in nine countries: results from the IQOLA Project. International Quality of Life Assessment. J Clin Epidemiol. 1998;51(11):1171-1178. [DOI] [PubMed] [Google Scholar]
- 48.Ware J Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34(3):220-233. [DOI] [PubMed] [Google Scholar]
- 49.Watkins KE, Ober AJ, Lamp K, et al. . Implementing the chronic care model for opioid and alcohol use disorders in primary care. Progress in Community Health Partnerships: Research, Education, and Action. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.National Quality Measures Clearinghouse Initiation of alcohol and other drug (AOD) treatment: percentage of patients who initiate treatment through an inpatient AOD admission, outpatient visit, intensive outpatient service or partial hospitalization within 14 days of the diagnosis. 2014. https://www.qualitymeasures.ahrq.gov/summaries/summary/48854/initiation-of-alcohol-and-other-drug-aod-treatment-percentage-of-patients-who-initiate-treatment-through-an-inpatient-aod-admission-outpatient-visit-intensive-outpatient-service-or-partial-hospitalization-within-14-days-of-the-diagnosis. Accessed March 3, 2017.
- 51.National Quality Measures Clearinghouse Engagement of alcohol and other drug (AOD) treatment: percentage of members who initiated treatment and who had two or more additional services with a diagnosis of AOD within 30 days of the initiation visit. 2015. https://www.qualitymeasures.ahrq.gov/summaries/summary/49778. Accessed March 3, 2017.
- 52.Bishop YMM, Fienberg SE, Holland PW. Discrete Multivariate Analysis: Theory and Practice. Cambridge, MA: MIT Press; 1975. [Google Scholar]
- 53.Deming WE. Statistical Adjustment of Data. New York, NY: John Wiley & Sons; 1943. [Google Scholar]
- 54.Izrael D, Hoaglin DC, Battaglia MP A SAS macro for balancing a weighted sample, Paper 275. Twenty-Fifth Annual SAS Users Group International Conference; 2000; Indianapolis, IN. [Google Scholar]
- 55.Schafer JL. Analysis of incomplete multivariate data. London, UK: Chapman & Hall; 1997. [Google Scholar]
- 56.Schafer JL, Graham JW. Missing data: our view of the state of the art. Psychol Methods. 2002;7(2):147-177. [PubMed] [Google Scholar]
- 57.Ilgen MA, Wilbourne PL, Moos BS, Moos RH. Problem-free drinking over 16 years among individuals with alcohol use disorders. Drug Alcohol Depend. 2008;92(1-3):116-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Maisto SA, Clifford PR, Stout RL, Davis CM. Moderate drinking in the first year after treatment as a predictor of three-year outcomes. J Stud Alcohol Drugs. 2007;68(3):419-427. [DOI] [PubMed] [Google Scholar]
- 59.Mertens JR, Kline-Simon AH, Delucchi KL, Moore C, Weisner CM. Ten-year stability of remission in private alcohol and drug outpatient treatment: non-problem users versus abstainers. Drug Alcohol Depend. 2012;125(1-2):67-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kline-Simon AH, Falk DE, Litten RZ, et al. . Posttreatment low-risk drinking as a predictor of future drinking and problem outcomes among individuals with alcohol use disorders. Alcohol Clin Exp Res. 2013;37(s1)(suppl 1):E373-E380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kline-Simon AH, Litten RZ, Weisner CM, Falk DE. Posttreatment low-risk drinking as a predictor of future drinking and problem outcomes among individuals with alcohol use disorders: a 9-year follow-up. Alcohol Clin Exp Res. 2017;41(3):653-658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dunigan R, Acevedo A, Campbell K, et al. . Engagement in outpatient substance abuse treatment and employment outcomes. J Behav Health Serv Res. 2014;41(1):20-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Garnick DW, Horgan CM, Acevedo A, et al. . Criminal justice outcomes after engagement in outpatient substance abuse treatment. J Subst Abuse Treat. 2014;46(3):295-305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Harris AH, Humphreys K, Bowe T, Tiet Q, Finney JW. Does meeting the HEDIS substance abuse treatment engagement criterion predict patient outcomes? J Behav Health Serv Res. 2010;37(1):25-39. [DOI] [PubMed] [Google Scholar]
- 65.Paddock SM, Hepner KA, Hudson TJ, et al. . Association between process-based quality indicators and mortality for patients with substance use disorders. J Stud Alcohol Drugs. 2017;78:588-596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Buchholz JR, Malte CA, Calsyn DA, et al. . Associations of housing status with substance abuse treatment and service use outcomes among veterans. Psychiatr Serv. 2010;61(7):698-706. [DOI] [PubMed] [Google Scholar]
- 67.Kertesz SG, Crouch K, Milby JB, Cusimano RE, Schumacher JE. Housing first for homeless persons with active addiction: are we overreaching? Milbank Q. 2009;87(2):495-534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Upshur C, Weinreb L, Bharel M, Reed G, Frisard C. A randomized control trial of a chronic care intervention for homeless women with alcohol use problems. J Subst Abuse Treat. 2015;51:19-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Park-Lee E, Lipari RN, Hedden SL, Copello EAP, Kroutil LA. Receipt of Services for Substance Use and Mental Health Issues Among Adults: Results From the 2015 National Survey on Drug Use and Health. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2016. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol