This randomized clinical trial tests the effectiveness of a psychosocial intervention to improve early adherence among primary care patients aged 55 years and older receiving newly initiated antidepressants for depression.
Key Points
Question
Can a brief psychosocial intervention (ie, Treatment Initiation and Participation Program) targeting medication barriers improve antidepressant adherence among middle-aged and older adults with a newly initiated depression treatment by primary care physicians?
Findings
In this randomized clinical trial, the Treatment Initiation and Participation Program participants were 3 times more likely to be at least 80% adherent to their antidepressant pharmacotherapy at both 6 and 12 weeks. The Treatment Initiation and Participation Program did not have a sustained effect on depression; however, participants who reported 80% adherence at both 6 and 12 weeks showed greater improvement in depression severity by 24 weeks.
Meaning
The Treatment Initiation and Participation Program improved early adherence at weeks 6 and 12 combined and participants showed an early reduction in depressive symptoms at 6 weeks; participants in the Treatment Initiation and Participation Program and treatment as usual groups who were adherent at weeks 6 and 12 had a greater improvement in depressive symptoms.
Abstract
Importance
Nonadherence to antidepressant medication is common and leads to poor outcomes. Early nonadherence is especially problematic.
Objective
To test the effectiveness of a psychosocial intervention to improve early adherence among older patients whose primary care physician newly initiated an antidepressant for depression.
Design, Setting, and Participants
The Treatment Initiation and Participation Program (TIP) was offered in a 2-site randomized clinical effectiveness study between January 2011 and December 2014 at primary care practices in New York, New York, and Ann Arbor, Michigan. Analyses began in February 2016. All participants were middle-aged and older adults (aged ≥55 years) who received newly initiated depression treatment by their primary care physician and recruited within 10 days of their prescription. Analyses were intention-to-treat.
Interventions
Participants were randomly assigned to the intervention (TIP) or treatment as usual. Participants in the TIP group identified and addressed barriers to adherence, including stigma, misconceptions, and fears about treatment, before developing a personalized adherence strategy. The Treatment Initiation and Participation Program was delivered in three 30-minute contacts scheduled during a 6-week period just after the antidepressant was prescribed.
Main Outcomes and Measures
The primary outcome was self-reported adherence on the Brief Medication Questionnaire, with adequate early adherence defined as taking 80% or more of the prescribed doses at 6 and 12 weeks. The secondary outcome was depression severity.
Results
In total, 231 middle-aged and older adults (167 women [72.3%] and 64 men [27.7%]) without significant cognitive impairment were randomly assigned to the TIP intervention (n = 115) or treatment as usual (n = 116). Participants had a mean (SD) age of 67.3 (8.4) years. Participants in the TIP group were 5 times more likely to be adherent at 6 weeks (odds ratio, 5.54; 95% CI, 2.57 to 11.96; χ21 = 19.05; P < .001) and 3 times more likely to be adherent at both 6 and 12 weeks (odds ratio, 3.27; 95% CI, 1.73 to 6.17; χ21 = 13.34; P < .001). Participants in the TIP group showed a significant earlier reduction (24.9%) in depressive symptoms (95% CI, 13.9 to 35.9; t337 = 4.46; adjusted P < .001). In both groups, participants who were 80% adherent at weeks 6 and 12 had a 15% greater improvement in depressive symptoms from baseline over the course of treatment (95% CI, −0.2 to −30; t369 = 1.93; P = .051).
Conclusions and Relevance
The Treatment Initiation and Participation Program is an effective intervention to improve early adherence to pharmacotherapy. Improved adherence can promote improvement in depression.
Trial Registration
clinicaltrials.gov Identifier: NCT01301859
Introduction
Nonadherence to antidepressant therapy remains a major challenge to quality depression care. Rates of nonadherence among older adults range from 29% to 40% in the United States. Most depression management is provided in primary care, and higher rates of antidepressant nonadherence are documented in primary care compared with psychiatry sites. Although nonadherence is problematic throughout treatment, the first 6 weeks of treatment is a particularly critical period to promote adherence, with increased risks of treatment dropout, relapse, medication discontinuation, vulnerability to suicide, and greater economic burden among those who show early nonadherence to antidepressants. There is evidence that early adequate antidepressant dosing and good adherence are both associated with recovery from depression and may improve the long-term outcomes of patients with depression.
Key risk factors for nonadherence include age, comorbid conditions, beliefs about treatment, and concerns over adverse events. In the elderly population, additional risk factors may include patient variables such as the lack of a medication routine, retaining discontinued medications, combining prescriptions, and multiple storage locations. Medication adherence problems also increase with the total number of drugs prescribed. The average older American adult takes 3 prescription and 4 over-the-counter medications daily, and those with depression typically take more medications. Even in successful clinical intervention studies for older adults with depression, nonadherence is a challenge.
Although older adults face structural barriers (eg, costs of medications, distance from home to physician’s office), often negative attitudes are the most important factors affecting adherence. Perceived stigma predicts poorer medication adherence and treatment discontinuation among elderly people with depression Older adults worry about having a diagnosis of depression, especially older adults of African descent. Low perceived symptom severity is associated with poorer adherence, and even when distress is acknowledged, many older adults feel they should not need mental health help. In a community-based study of older adults, when perceived costs outweighed perceived benefits, nonadherent behaviors were more likely.
The Treatment Initiation and Participation Program (TIP) is a brief psychosocial intervention designed to improve adherence to pharmacotherapy for patients with depression. The Treatment Initiation and Participation Program is informed by the theory of reasoned action and targets individual-level, modifiable factors such as psychological barriers (eg, stigma, self-efficacy), fears about antidepressants, misattributions about causes of depression, and the lack of an adherence strategy. The program helps patients address barriers, identify treatment benefits, and feel empowered to manage their medication regimen and communicate with the physician effectively.
For this 2-site randomized clinical effectiveness trial of TIP, we targeted the critical period of early adherence among middle-aged and older adults with a newly initiated antidepressant treatment for depression by their primary care physician (PCP). We hypothesized TIP participants would be more likely to be at least 80% adherent to their antidepressant medication at both 6 and 12 weeks after the prescription was provided compared with participants who received treatment as usual. In addition, we hypothesized that TIP participants would have a greater reduction of depressive symptoms compared with participants receiving treatment as usual. Finally, we explored whether greater adherence in the TIP and treatment as usual groups was associated with decreased depression at 24 weeks.
Methods
Study Design
The trial protocol (available in the Supplement) was approved by the institutional review boards at Weill Cornell Medicine and the University of Michigan. Potential participants were identified at the participating primary care sites through daily electronic medical record system reports on antidepressant prescriptions. Trained staff reviewed each medical record to verify inclusion eligibility (eg, a newly prescribed antidepressant for treatment of depression) prior to contacting patients. To capture early adherence decisions among a wide range of patients, all eligible patients were cold-called by staff to gauge their interest in the study within 10 days of their prescription date. Patients who expressed interest were met in person by research staff for a more in-depth discussion of the study, written informed consent, and baseline assessment. The meetings were held at either the primary care office or in nearby research offices. Participants who met eligibility criteria during the baseline assessment were randomized 1 to 1 (stratified by site) to receive either the TIP intervention in addition to the antidepressant monitoring provided by their PCP or treatment monitoring as usual provided by the PCP. The TIP sessions were delivered within the first 6 weeks of pharmacotherapy. Research assessments were collected at 6, 12, and 24 weeks to evaluate the impact of TIP on adherence and depressive symptoms. Participants in both groups were compensated $40 for the longer baseline and $25 for each of the 3 follow-up research visits. The 2 site principal investigators (J.A.S. and H.C.K.) oversaw the conduct and data analysis of the trial and data with an annual review by an independent data and safety monitoring committee.
Participants
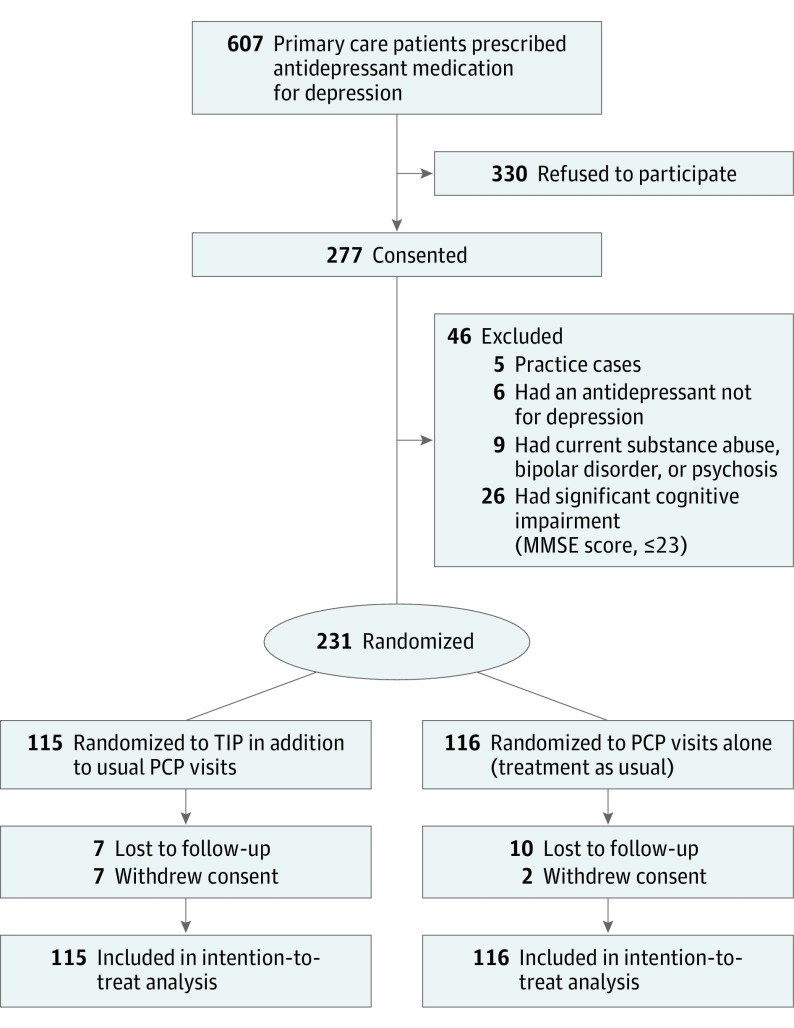
Participants were consecutively recruited beginning in January 2011 through December 2014, and analyses began in February 2016 (Figure). Patients 55 years and older were eligible; this age cutoff was designed to include middle-aged adults who might be more similar to older adults in terms of medical burden or socioeconomic disadvantage. All patients had been newly prescribed an antidepressant by their PCP specifically for depressive symptoms. Given the high rates of prescription of antidepressants among older adults, this target population was chosen to test the effectiveness of TIP among patients typically treated by PCPs according to guidelines from the National Institute for Health and Care Excellence. Patients were excluded if they had (1) active suicidality; (2) current substance abuse, bipolar disorder, or psychosis; (3) significant cognitive impairment (Mini-Mental State Examination score ≤23); (4) terminal illness or current chemotherapy; or (5) an inability to communicate in English.
Figure. CONSORT Diagram of Participant Flow in Randomized Clinical Trial of Treatment Initiation and Participation Program (TIP).
MMSE indicates Mini-Mental State Examination; PCP, primary care physician.
TIP Intervention
The TIP intervention included 5 steps: (1) review symptoms and antidepressant regimen and conduct a barriers assessment; (2) define a personal goal that could be achieved with adherence; (3) provide education about depression and antidepressant therapy; (4) collaborate to address barriers to treatment participation; and (5) create an adherence strategy and empower the older adult to talk directly with the PCP about treatment. The study counselor documented each TIP intervention meeting on a contact sheet that helped structure and standardize the TIP intervention. For this study, the TIP intervention was delivered in three 30-minute meetings during the first 6 weeks of pharmacotherapy. The 3-session format was designed to allow for an alliance between the TIP counselor and participants while ensuring brevity and decreasing potential dependency.
Intervention Fidelity
The intervention was delivered by 2 master’s-level social workers trained in the TIP protocol by 1 of us (J.A.S.) during a full-day training, with 6 months of weekly supervision. Treatment Initiation and Participation Program competency certification was based on audiotape ratings of practice cases by the principal investigator. After certification and the initial 6 months, TIP supervision was conducted bimonthly. Two randomly selected audiotapes from each counselor were reviewed quarterly to evaluate drift. If there were indications of drift, corrective interventions were integrated into supervision.
Measures
Research assessments were conducted at baseline and at 6, 12, and 24 weeks by trained research assistants blinded to group assignment. The structured clinical interview from DSM-IV was administered to confirm a diagnosis of depression. The 24-item Hamilton Depression Rating Scale (HAM-D) was used to assess the severity of depressive symptoms. The use of services and number of medical visits were recorded at baseline and at 12 and 24 weeks using the Cornell Services Index.
Adherence and Depression Outcomes
Early adherence was measured using the Brief Medication Questionnaire, which is a self-report measure validated against an electronic bottle cap data (MEMS Cap; AARDEX Group Ltd) and used extensively with older adults. Adequate antidepressant adherence was defined as 80% adherence to assure that most doses are taken to achieve a biological effect (eg, therapeutic level). For this project, the goal was a sustained period of early adherence defined as self-reported 80% adherence or greater at both the 6- and 12-week assessments. Self-reported adherence was selected both to capture acknowledged nonadherence and to minimize the effects of the adherence measure (such as MEMs Caps) on participants’ behavior.
Depression outcomes were measured based on percentage reductions in depressive symptoms on the HAM-D. Because the inclusion criteria were PCP initiation of a new treatment of depression with antidepressant medication, rather than an official diagnosis, depression severity was expected to be heterogeneous.
Statistical Analysis
A sample size of 130 participants per treatment arm was planned to have 90% power to detect a 70% (TIP) vs 50% (treatment as usual) adherence rate (≥80%) at weeks 6 and 12 for a 2-sided test at α = .05. Sociodemographic, functional, or clinical differences were evaluated using t and χ2 tests comparisons. In separate logistic regression models, we examined group differences on achieving 80% adherence to antidepressants at week 6 and week 12 and a combined measure of adherence at both 6 and 12 weeks. Percentage improvements in depressive symptoms (HAM-D scores) from baseline to weeks 6, 12, and 24 were modeled in an intent-to-treat mixed-effects regression model with a participant-specific random intercept and fixed effects for study site, treatment, time, and treatment by time interaction. To examine the effect of adherence on depressive symptoms, a similar mixed-effects regression model was used, which included additional fixed effects of adherence, adherence by treatment, adherence by time, and adherence by treatment by time interactions. As none of the interactions were significantly different from 0, they were dropped from the model. These analyses were repeated in the subgroup of participants (n = 175) with mild-to-moderate depressive symptoms recorded during the baseline (HAM-D score, ≥10). Post hoc tests were conducted using the least-square means, and P values were adjusted for multiple comparisons using Bonferroni-Holm step-down procedure. All tests conducted used a 2-sided alternative at a significance level of α = .05, and all statistical analyses were conducted using SAS software, version 9.4 (SAS Institute Inc).
Results
Sample Characteristics
To target early adherence, we identified patients with a newly prescribed antidepressant for depression (no antidepressant in the past 3 months). Of the 607 patients who met initial study inclusion criteria, 277 (45.6%) consented and 231 were randomized to receive either the TIP intervention in addition to their antidepressant and PCP care (115 [49.8%]) or treatment as usual with the PCP (116 [50.2%]) (Figure). All participants were recruited from either Weill Cornell Internal Medical Associates in New York, New York (114 [49.4%]), or Michigan Medicine outpatient primary care clinics in Ann Arbor (117 [50.6%]). Primary outcome data were collected at 6 weeks from 211 participants (91.3%); 200 at 12 weeks (86.6%); and 199 at 24 weeks (86.1%). There were no significant differences in retention rates by treatment group, sex, race/ethnicity, age, site, or education level (Table 1).
Table 1. Characteristics of Patients in TIP and Treatment as Usual Groups.
| Characteristic | No. (%) | P Value | ||
|---|---|---|---|---|
| Total (N = 231) |
TIP (n = 115) |
Treatment as Usual (n = 116) |
||
| Age, mean (SD), y | 67.3 (8.4) | 67.6 (8.9) | 67.0 (8.0) | .59 |
| Female | 167 (72.3) | 81 (70.4) | 86 (74.1) | .53 |
| Hispanic | 31 (13.4) | 13 (11.3) | 18 (15.5) | .35 |
| Race/ethnicity | ||||
| Black | 46 (19.9) | 23 (20) | 23 (19.8) | .51 |
| White | 163 (70.6) | 78 (67.8) | 85 (73.3) | |
| Asian | 9 (3.9) | 5 (4.4) | 4 (3.4) | |
| Other | 13 (5.6) | 9 (7.8) | 4 (3.4) | |
| Marital status | ||||
| Single | 30 (13.0) | 15 (13.0) | 15 (12.9) | .21 |
| Married | 98 (42.4) | 55 (47.8) | 43 (37.1) | |
| Divorced/separated | 65 (28.1) | 26 (22.6) | 39 (33.6) | |
| Widowed | 38 (16.5) | 19 (16.5) | 19 (16.4) | |
| Living alone | 77 (33.3) | 35 (30.4) | 42 (36.2) | .35 |
| Education level, mean (SD), y | 14.6 (3.0) | 14.8 (2.9) | 14.4 (3.1) | .31 |
| Employment status | ||||
| Full- or part-time | 56 (24.2) | 30 (26.1) | 26 (22.4) | .92 |
| Unemployed | 27 (11.7) | 11 (9.6) | 16 (13.8) | |
| Retired | 127 (55.0) | 64 (55.7) | 63 (54.3) | |
| On disability | 19 (8.2) | 9 (8.6) | 11 (9.5) | |
| Baseline HAM-D score, mean (SD) | 17.8 (9.5) | 17.9 (9.6) | 17.6 (9.6) | .93 |
| SCID diagnosis | ||||
| Major depression | 100 (43.3) | 51 (44.3) | 49 (42.2) | .40 |
| Minor depression | 63 (27.3) | 27 (23.5) | 36 (31.0) | |
| Did not meet criteria | 68 (29.4) | 37 (32.2) | 31 (26.7) | |
| MMSE score, mean (SD) | 27.9 (2.0) | 28.0 (2.1) | 27.8 (2.0) | .63 |
| Psychiatric hospitalization | 0 | 0 | 0 | NA |
Abbreviations: HAM-D, Hamilton Depression Rating Scale; MMSE, Mini-Mental State Examination; NA, not applicable; SCID, severe combined immunodeficiency; TIP, Treatment Initiation and Participation Program.
Reliability and Fidelity
Cross-site interrater reliability was calculated based on audiotapes of participants’ assessments compared with a criterion standard rater (clinical psychologist) for the structured clinical interview and HAM-D depression severity ratings. Raters maintained good reliability throughout the study (Spearman r = 0.81-0.95). Treatment Initiation and Participation Program intervention delivery was monitored by the study principal investigator (J.A.S.) who listened to 18 intervention audiotapes provided by the TIP counselors to assure continued competency and offered ongoing supervision. Of 116 participants, 111 (95.6%) who were referred to the TIP intervention completed all 3 TIP meetings.
Impact of the TIP Intervention on Adherence
Rates of nonadherence were higher among participants in the treatment as usual group throughout the study period. Participants in the TIP group were 5 times more likely to be adherent to their antidepressant at week 6 (odds ratio [OR], 5.54; 95% CI, 2.57 to 11.96; χ21 = 19.05; P < .001), and nearly 3 times more likely at week 12 after controlling for study site (OR, 2.84; 95% CI, 1.47 to 5.50; χ21 = 9.60; P = .002) compared with participants in the treatment as usual group. A combined measure of 80% adherence at 6 and 12 weeks showed that TIP participants as a group were 3 times more likely to be adherent at both 6 and 12 weeks combined (OR, 3.27; 95% CI, 1.73 to 6.17; χ21 = 13.34; P < .001) (Table 2). The TIP effect on the combined adherence measure was also higher than treatment as usual (OR, 2.2; 95% CI, 1.1 to 4.3; χ21 = .8; P = .03) within the subgroup of individuals with mild-to-moderate baseline depression (HAM-D score, ≥10).
Table 2. Logistic Regression Predicting Adherence Based on Site and TIP Intervention.
| Adherence at Follow-up, wk | AIC | TIP | Treatment as Usual | ||||
|---|---|---|---|---|---|---|---|
| OR (95%CI) | χ2 | P Value | OR (95%CI) | χ2 | P Value | ||
| 6 | 204.76 | 5.54 (2.57-11.96) | 19.05 | <.001 | 3.48 (1.70-7.13) | 11.60 | .001 |
| 12 | 231.28 | 2.84 (1.47-5.50) | 9.60 | .002 | 2.82 (1.47-5.41) | 9.78 | .002 |
| 24 | 228.06 | 1.49 (0.78-2.85) | 1.48 | .22 | 2.71 (1.41-5.24) | 8.85 | .003 |
| 6 and 12 | 244.18 | 3.27 (1.73-6.17) | 13.34 | <.001 | 3.30 (1.76-6.19) | 13.80 | <.001 |
| 6, 12, and 24 | 248.38 | 2.79 (1.50-5.19) | 10.50 | .001 | 3.90 (2.10-7.25) | 18.58 | <.001 |
Abbreviations: AIC, Akaike information criterion; OR, odds ratio; TIP, Treatment Initiation and Participation Program.
Impact of TIP on Depression Severity
Improvement in depressive symptoms from baseline changed significantly with time in both TIP and treatment as usual groups after adjusting for study site (time: F2,377 = 5.05, P = .01). The group rates of symptom improvement for the total sample did not differ significantly over time (time by treatment: F2,377 = 1.03, P = .36). However, TIP participants showed a significant improvement (24.9%) in depressive symptoms at 6 weeks (95% CI, 13.9 to 35.9; t337 = 4.46; adjusted P < .001), whereas treatment as usual participants showed a less robust, nonsignificant improvement (10.7%) (95% CI, −0.01 to 21.4; t337 = 1.96; adjusted P = .05). Participants in the TIP group showed additional improvements in depressive symptoms that were not statistically significant over the course of the study. Treatment as usual participants showed a 12% improvement at 12 weeks (95% CI, 2 to 23; t337 = 2.32; adjusted P = .04), with no additional reduction in depressive symptoms. In a post hoc analysis of participants with mild-to-moderate depression severity (HAM-D score, ≥10) at the baseline assessment, both TIP and treatment as usual participants improved over the course of the study with no significant differences between groups. However, at 6 weeks, TIP participants had improved by 30.2% from baseline (95% CI, 21.7 to 38.8), whereas among treatment as usual participants, the improvement was less robust (23.8%) (95% CI, 15.5 to 32.1). Improvement in depressive symptoms, measured as percentage change from baseline, did not change significantly as a function of 80% adherent status (at weeks 6 and 12) and treatment arm (time by treatment by adherence interaction: F2,363 = 2.28, P = .01). However, participants in both groups who were 80% adherent at weeks 6 and 12 had a 15% greater improvement in depressive symptoms over the course of treatment (95% CI, −0.2 to 30; t369 = 1.93; P = .051) after controlling for study site.
Discussion
In this community-based effectiveness trial among adults 55 years and older who had been prescribed pharmacotherapy for depression in primary care, patients who participated in TIP were more likely to be adherent to their medication than participants who received treatment as usual. The 5-fold increase in adherence during the first 6 weeks of care supports the clinical usefulness of TIP to improve early adherence. Higher adherence among TIP participants was further sustained throughout the first 3 months of care. Although TIP did not significantly improve overall depression, the TIP group showed a significant early reduction in depressive symptoms. These findings were replicated in the participants with mild-to-moderate depression severity at baseline.
To our knowledge, few prior interventions have effectively improved adherence among adults with depression, despite its importance in quality care and potential to reduce the personal and societal costs of depressive disorders. Research has documented that rates of antidepressant prescriptions in primary care are increasing, and antidepressant medication adherence is associated with decreased mortality and a lower likelihood of suicidal ideation at 1-year follow-up. In a 2015 cluster randomized trial, shared decision making was found to be useful in selecting an antidepressant and improving patient satisfaction but was not effective in improving adherence. Programs focused on increasing patient activation to maximize participation and engagement in care have had limited impact on retention. In 2014, the most recent update to the Cochrane review of adherence research concluded that additional interventions for adherence are needed given its bearing on treatment outcomes. To our knowledge, TIP is the only brief intervention that targets early adherence specifically by working directly with the patient to reduce barriers, increase perceived benefits, and promote a personalized antidepressant adherence strategy. This patient-centered approach may help to address the informal cost-benefit analysis resulting in meaningful increases in adherence. These findings also support the relationship between early adherence and depression improvement.
In the present study, we did not find a significant impact of TIP on depression compared with treatment as usual over the course of the study. There was an early depression response among the TIP group, but both groups improved over time. There is emerging evidence of the differential impact of antidepressant medications on depression trajectory based on symptom clusters as well as the benefit of looking beyond symptom severity to predict treatment response. Targeted treatments and improved adherence could improve depression outcomes as adequate dosing remains a challenge for depression treatment especially in primary care, which continues to be the largest provider of mental health services for older adults. Recent research has documented that depression remains poorly managed in primary care, especially when compared with other chronic diseases.
Limitations
There are limitations to this study. First, we cannot account for factors outside of the primary care setting that may affect adherence. Second, this study uses a self-report measure of adherence. Although the adherence measure has been validated, is reliable, and does not increase attention to adherence or serve as an intervention in itself, we recognize that different measures (eg, electronic monitoring, pill counts, self-report) yield different information. Individuals who deny nonadherence or are unintentionally nonadherent could have been missed. In addition, by focusing on acknowledged adherence, we could not include adults with cognitive impairments who may have higher rates of unintentional nonadherence. Third, as a new intervention, we did not use an independent rater to cross-validate intervention fidelity. Finally, by focusing on early adherence, we recruited only patients whose physicians diagnosed them as having depression and who could be contacted within 10 days of their prescription to capture early decisions. Although this criterion is stringent for community-based clinical research, it misses those patients with depression who refused to accept a prescription. We chose to exclude patients with treatment for refractory depression from an earlier antidepressant trial as their views of medication, barriers to adherence, and overall adherence strategies may differ. Future implementation of TIP will target all patients in primary care to increase its usefulness.
Conclusions
This 2-site randomized clinical trial evaluated the effectiveness of TIP on early adherence among adults prescribed antidepressant therapy for depression in primary care. The Treatment Initiation and Participation Program uses strategies to identify barriers to adherence and develop a personalized adherence strategy. The TIP intervention was more effective in helping patients achieve adequate adherence during the critical early adherence period. Adequate antidepressant adherence was of 6-month depression outcomes. Given the high rates of depression, nonadherence, and use of primary care as a mental health service, wide-scale implementation of a targeted adherence intervention, such as TIP, could have a meaningful impact on public health. In addition, future work can extend TIP to other patient groups with depression (eg, patients with treatment for refractory depression from an earlier antidepressant trial) or patients with comorbid clinical conditions (eg, cognitive impairment).
Trial protocol.
References
- 1.Kales HC, Kavanagh J, Chiang C, et al. Predictors of antidepressant nonadherence among older veterans with depression. Psychiatr Serv. 2016;67(7):728-734. [DOI] [PubMed] [Google Scholar]
- 2.Kales HC, Nease DE Jr, Sirey JA, et al. Racial differences in adherence to antidepressant treatment in later life. Am J Geriatr Psychiatry. 2013;21(10):999-1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Olfson M, Kroenke K, Wang S, Blanco C. Trends in office-based mental health care provided by psychiatrists and primary care physicians. J Clin Psychiatry. 2014;75(3):247-253. [DOI] [PubMed] [Google Scholar]
- 4.Samples H, Mojtabai R. Antidepressant self-discontinuation: results from the collaborative psychiatric epidemiology surveys. Psychiatr Serv. 2015;66(5):455-462. [DOI] [PubMed] [Google Scholar]
- 5.Rossom RC, Shortreed S, Coleman KJ, et al. Antidepressant adherence across diverse populations and healthcare settings. Depress Anxiety. 2016;33(8):765-774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Melfi CA, Chawla AJ, Croghan TW, Hanna MP, Kennedy S, Sredl K. The effects of adherence to antidepressant treatment guidelines on relapse and recurrence of depression. Arch Gen Psychiatry. 1998;55(12):1128-1132. [DOI] [PubMed] [Google Scholar]
- 7.Sirey JA, Bruce ML, Alexopoulos GS, Perlick DA, Friedman SJ, Meyers BS. Stigma as a barrier to recovery: perceived stigma and patient-rated severity of illness as predictors of antidepressant drug adherence. Psychiatr Serv. 2001;52(12):1615-1620. [DOI] [PubMed] [Google Scholar]
- 8.Sirey JA, Bruce ML, Alexopoulos GS, et al. Perceived stigma as a predictor of treatment discontinuation in young and older outpatients with depression. Am J Psychiatry. 2001;158(3):479-481. [DOI] [PubMed] [Google Scholar]
- 9.Valenstein M, Kim HM, Ganoczy D, et al. Higher-risk periods for suicide among VA patients receiving depression treatment: prioritizing suicide prevention efforts. J Affect Disord. 2009;112(1-3):50-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pompili M, Venturini P, Palermo M, et al. Mood disorders medications: predictors of nonadherence: review of the current literature. Expert Rev Neurother. 2013;13(7):809-825. [DOI] [PubMed] [Google Scholar]
- 11.Datto CJ, Thompson R, Horowitz D, Disbot M, Bogner H, Katz IR. Do clinician and patient adherence predict outcome in a depression disease management program? J Clin Outcomes Manage. 2003;10(2):79-85. [Google Scholar]
- 12.Meyers BS, Sirey JA, Bruce M, et al. Predictors of early recovery from major depression among persons admitted to community-based clinics: an observational study. Arch Gen Psychiatry. 2002;59(8):729-735. [DOI] [PubMed] [Google Scholar]
- 13.Sorensen L, Stokes JA, Purdie DM, Woodward M, Roberts MS. Medication management at home: medication-related risk factors associated with poor health outcomes. Age Ageing. 2005;34(6):626-632. [DOI] [PubMed] [Google Scholar]
- 14.Barat I, Andreasen F, Damsgaard EM. Drug therapy in the elderly: what doctors believe and patients actually do. Br J Clin Pharmacol. 2001;51(6):615-622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lotrich FE, Pollock BG. Aging and clinical pharmacology: implications for antidepressants. J Clin Pharmacol. 2005;45(10):1106-1122. [DOI] [PubMed] [Google Scholar]
- 16.Bogner HR, Lin JY, Morales KH. Patterns of early adherence to the antidepressant citalopram among older primary care patients: the PROSPECT study. Int J Psychiatry Med. 2006;36(1):103-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Andrade LH, Alonso J, Mneimneh Z, et al. Barriers to mental health treatment: results from the WHO World Mental Health surveys. Psychol Med. 2014;44(6):1303-1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ayalon L, Areán PA, Alvidrez J. Adherence to antidepressant medications in black and Latino elderly patients. Am J Geriatr Psychiatry. 2005;13(7):572-580. [DOI] [PubMed] [Google Scholar]
- 19.Conner KO, Copeland VC, Grote NK, et al. Mental health treatment seeking among older adults with depression: the impact of stigma and race. Am J Geriatr Psychiatry. 2010;18(6):531-543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sirey JA, Franklin AJ, McKenzie SE, Ghosh S, Raue PJ. Race, stigma, and mental health referrals among clients of aging services who screened positive for depression. Psychiatr Serv. 2014;65(4):537-540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spoont MR, Nelson DB, Murdoch M, et al. Impact of treatment beliefs and social network encouragement on initiation of care by VA service users with PTSD. Psychiatr Serv. 2014;65(5):654-662. [DOI] [PubMed] [Google Scholar]
- 22.Brenes GA, Danhauer SC, Lyles MF, Hogan PE, Miller ME. Barriers to mental health treatment in rural older adults. Am J Geriatr Psychiatry. 2015;23(11):1172-1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sirey JA, Greenfield A, Weinberger MI, Bruce ML. Medication beliefs and self-reported adherence among community-dwelling older adults. Clin Ther. 2013;35(2):153-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sirey JA, Bruce ML, Alexopoulos GS. The Treatment Initiation Program: an intervention to improve depression outcomes in older adults. Am J Psychiatry. 2005;162(1):184-186. [DOI] [PubMed] [Google Scholar]
- 25.Zivin K, Kales HC. Adherence to depression treatment in older adults: a narrative review. Drugs Aging. 2008;25(7):559-571. [DOI] [PubMed] [Google Scholar]
- 26.Sirey JA, Bruce ML, Kales HC. Improving antidepressant adherence and depression outcomes in primary care: the Treatment Initiation and Participation (TIP) program. Am J Geriatr Psychiatry. 2010;18(6):554-562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moon M, Guo J, McSorley VE Is 65 the best cutoff for defining “older Americans?” American Institutes for Research. Published January 30, 2015. http://www.air.org/resource/65-best-cutoff-defining-older-americans. Accessed August 11, 2017.
- 28.World Health Organization World Report on Ageing and Health 2015. Geneva, Switzerland: World Health Organization, 2015. [Google Scholar]
- 29.Mojtabai R. Diagnosing depression in older adults in primary care. N Engl J Med. 2014;370(13):1180-1182. [DOI] [PubMed] [Google Scholar]
- 30.Goldberg D. The “NICE Guideline” on the treatment of depression. Epidemiol Psichiatr Soc. 2006;15(1):11-15. [PubMed] [Google Scholar]
- 31.Spitzer RL, Gibbon M, Williams JB. Structured Clinical Interview for Axis I DSM-IV Disorders (SCID). Washington, DC: American Psychiatric Association Press, Inc; 1995. [Google Scholar]
- 32.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23(1):56-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sirey JA, Meyers BS, Teresi JA, et al. The Cornell Service Index as a measure of health service use. Psychiatr Serv. 2005;56(12):1564-1569. [DOI] [PubMed] [Google Scholar]
- 34.Svarstad BL, Chewning BA, Sleath BL, Claesson C. The Brief Medication Questionnaire: a tool for screening patient adherence and barriers to adherence. Patient Educ Couns. 1999;37(2):113-124. [DOI] [PubMed] [Google Scholar]
- 35.Rickles NM, Svarstad BL. Relationships between multiple self-reported nonadherence measures and pharmacy records. Res Social Adm Pharm. 2007;3(4):363-377. [DOI] [PubMed] [Google Scholar]
- 36.Brook OH, van Hout HP, Stalman WA, de Haan M. Nontricyclic antidepressants: predictors of nonadherence. J Clin Psychopharmacol. 2006;26(6):643-647. [DOI] [PubMed] [Google Scholar]
- 37.Krivoy A, Balicer RD, Feldman B, et al. Adherence to antidepressant therapy and mortality rates in ischaemic heart disease: cohort study. Br J Psychiatry. 2015;206(4):297-301. [DOI] [PubMed] [Google Scholar]
- 38.Krivoy A, Balicer RD, Feldman B, et al. Adherence to antidepressants is associated with lower mortality: a 4-year population-based cohort study. J Clin Psychiatry. 2016;77(5):e566-e572. [DOI] [PubMed] [Google Scholar]
- 39.Henein F, Prabhakar D, Peterson EL, Williams LK, Ahmedani BK. A prospective study of antidepressant adherence and suicidal ideation among adults. Prim Care Companion CNS Disord. 2016;18(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.LeBlanc A, Herrin J, Williams MD, et al. Shared decision making for antidepressants in primary care: a cluster randomized trial. JAMA Intern Med. 2015;175(11):1761-1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alegría M, Carson N, Flores M, et al. Activation, self-management, engagement, and retention in behavioral health care: a randomized clinical trial of the DECIDE intervention. JAMA Psychiatry. 2014;71(5):557-565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nieuwlaat R, Wilczynski N, Navarro T, et al. Interventions for enhancing medication adherence. Cochrane Database Syst Rev. 2014;(11):CD000011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chekroud AM, Gueorguieva R, Krumholz HM, Trivedi MH, Krystal JH, McCarthy G. Reevaluating the efficacy and predictability of antidepressant treatments: a symptom clustering approach. JAMA Psychiatry. 2017;74(4):370-378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Smagula SF, Butters MA, Anderson SJ, et al. Antidepressant response trajectories and associated clinical prognostic factors among older adults. JAMA Psychiatry. 2015;72(10):1021-1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thornicroft G, Chatterji S, Evans-Lacko S, et al. Undertreatment of people with major depressive disorder in 21 countries. Br J Psychiatry. 2017;210(2):119-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Olfson M, Blanco C, Marcus SC. Treatment of adult depression in the United States. JAMA Intern Med. 2016;176(10):1482-1491. [DOI] [PubMed] [Google Scholar]
- 47.Bishop TF, Ramsay PP, Casalino LP, Bao Y, Pincus HA, Shortell SM. Care management processes used less often for depression than for other chronic conditions in US primary care practices. Health Aff (Millwood). 2016;35(3):394-400. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial protocol.