Abstract
Importance
Transdiagnostic interventions have been developed to address barriers to the dissemination of evidence-based psychological treatments, but only a few preliminary studies have compared these approaches with existing evidence-based psychological treatments.
Objective
To determine whether the Unified Protocol for Transdiagnostic Treatment of Emotional Disorders (UP) is at least as efficacious as single-disorder protocols (SDPs) in the treatment of anxiety disorders.
Design, Setting, and Participants
From June 23, 2011, to March 5, 2015, a total of 223 patients at an outpatient treatment center with a principal diagnosis of panic disorder with or without agoraphobia, generalized anxiety disorder, obsessive-compulsive disorder, or social anxiety disorder were randomly assigned by principal diagnosis to the UP, an SDP, or a waitlist control condition. Patients received up to 16 sessions of the UP or an SDP for 16 to 21 weeks. Outcomes were assessed at baseline, after treatment, and at 6-month follow-up. Analysis in this equivalence trial was based on intention to treat.
Interventions
The UP or SDPs.
Main Outcomes and Measures
Blinded evaluations of principal diagnosis clinical severity rating were used to evaluate an a priori hypothesis of equivalence between the UP and SDPs.
Results
Among the 223 patients (124 women and 99 men; mean [SD] age, 31.1 [11.0] years), 88 were randomized to receive the UP, 91 to receive an SDP, and 44 to the waitlist control condition. Patients were more likely to complete treatment with the UP than with SDPs (odds ratio, 3.11; 95% CI, 1.44-6.74). Both the UP (Cohen d, −0.93; 95% CI, −1.29 to −0.57) and SDPs (Cohen d, −1.08; 95% CI, −1.43 to −0.73) were superior to the waitlist control condition at acute outcome. Reductions in clinical severity rating from baseline to the end of treatment (β, 0.25; 95% CI, −0.26 to 0.75) and from baseline to the 6-month follow-up (β, 0.16; 95% CI, −0.39 to 0.70) indicated statistical equivalence between the UP and SDPs.
Conclusions and Relevance
The UP produces symptom reduction equivalent to criterion standard evidence-based psychological treatments for anxiety disorders with less attrition. Thus, it may be possible to use 1 protocol instead of multiple SDPs to more efficiently treat the most commonly occurring anxiety and depressive disorders.
Trial Registration
clinicaltrials.gov Identifier: NCT01243606
This randomized clinical equivalence trial examines whether the Unified Protocol is at least as efficacious as single-disorder protocols in the treatment of anxiety disorders.
Key Points
Question
Is a single transdiagnostic psychological treatment, the Unified Protocol, at least as effective as various well-established single-disorder protocols in the treatment of various anxiety disorders?
Findings
In this randomized clinical equivalence trial of 223 adults, treatment with the Unified Protocol produced reductions in symptom severity for 4 different anxiety disorders that were statistically equivalent to reductions with single-disorder protocols both at acute outcome and at 6-month follow-up.
Meaning
The Unified Protocol, a transdiagnostic intervention consisting of 5 core modules, may produce effects comparable with those of single-disorder protocols targeting individual disorders, thereby facilitating dissemination and increasing access to these treatments.
Introduction
Despite the development of robust evidence-based psychological treatments (EBPTs) for anxiety, mood, and related emotional disorders (J.R.B., H.T.B., S.S.Z., and D.H.B.; unpublished review), the effect of these interventions on public health has been limited. Two of the foremost barriers to widespread dissemination and implementation of EBPTs are the burden associated with training clinicians to competently administer different manual-based interventions for each individual anxiety, depressive, or related disorder (single-disorder protocols [SDPs]) and the criticism that these protocols lack external validity. For this reason, in a recent report the Institute of Medicine (now the National Academy of Medicine) recommended increased emphasis on further development, dissemination, and implementation of EBPTs. One approach is to develop interventions applicable to several related disorders (transdiagnostic) based on theory or empirical grounds; initial results have been promising. The Unified Protocol for Transdiagnostic Treatment of Emotional Disorders (UP) is an emotion-focused, cognitive behavioral intervention consisting of 5 core modules or components that target temperamental characteristics, particularly neuroticism and resulting emotion dysregulation, underlying all anxiety, depressive, and related disorders. By addressing shared mechanisms associated with neuroticism, specifically, negative evaluation and avoidance of intense emotional experience, this approach could simplify training efforts while also addressing concerns about generalizability to routine care settings by simultaneously accommodating comorbid emotional disorders. Such an approach may increase access to EBPTs for the most common psychiatric disorders.
After developing preliminary support for the efficacy of the UP for the treatment of anxiety and comorbid depressive disorders, it was important to determine the relative efficacy of this approach compared with well-established SDPs, which are currently first-line treatments in extant clinical practice guidelines. We hypothesized that the UP would be at least as efficacious as SDPs at acute outcome and 6 months following treatment when delivered to a heterogeneous group of patients with principal anxiety disorders and diverse comorbidities.
Study Design
Participants
A sample of 223 patients was recruited from individuals seeking treatment at the Center for Anxiety and Related Disorders at Boston University, Boston, Massachusetts. Recruitment was designed to be broadly inclusive. Individuals were eligible for the study if they were (1) assigned a principal (most interfering and severe) diagnosis of panic disorder with or without agoraphobia (PD/A), generalized anxiety disorder, obsessive-compulsive disorder (OCD), or social anxiety disorder; (2) 18 years or older; and (3) fluent in English. Following long-standing procedures in our clinical trials, individuals taking psychotropic medications at the time of enrollment were required to be stable on the same dose for at least 6 weeks prior to enrolling in the study and were requested to maintain these medications and dosages during treatment. The study was approved by the Boston University Institutional Review Board, and written informed consent was obtained prior to any research activity. The full study protocol is in Supplement 1.
Exclusion criteria consisted primarily of conditions that required prioritization for immediate or simultaneous treatment: specifically, a current diagnosis of bipolar disorder, schizophrenia, schizoaffective disorder, or organic mental disorder; current high risk of suicide; or recent (within 3 months) history of substance use disorder, with the exception of nicotine (1 patient), marijuana (0 patients), and caffeine (0 patients). Individuals were also excluded if they received at least 8 sessions of cognitive behavioral therapy within the past 5 years. Anyone receiving non–cognitive behavioral therapy focused on an emotional disorder agreed to discontinue that treatment.
Procedures
Figure 1 depicts the study design and summarizes patient flow. The study consisted of 2 phases: (1) a 16-session acute treatment (12 sessions for patients with a principal diagnosis of PD/A) or 16-week waitlist control (WLC) phase; and (2) a 6-month follow-up phase (WLC patients were not included in the follow-up phase of the study). The acute treatment phase was limited to a maximum of 21 weeks (16 weeks for patients with PD/A). If patients were unable to complete the full course of treatment during the specified treatment window, treatment was terminated and follow-up assessments were conducted.
Figure 1. Recruitment Flow Diagram .
SDP indicates single-disorder protocol; UP, Unified Protocol for Transdiagnostic Treatment of Emotional Disorders; and WLC, waitlist control.
aCompleted treatment indicates that the patient attended at least 75% of the allotted number of sessions (ie, 9 of 12 for patients with panic disorder with or without agoraphobia and 12 of 16 for patients with other principal diagnoses).
bOne patient with principal panic disorder with or without agoraphobia completed 12 sessions but was withdrawn before the posttreatment assessment (ie, completed treatment, but was not eligible for posttreatment assessment).
cOne patient was withdrawn from the study after completing the posttreatment assessment, but before the 6-month follow-up, so was no longer eligible for 6-month follow-up; however, this individual is included in the “eligible for posttreatment and 6-month follow-up” classification (n = 78).
Randomization and Blinding
After patients were deemed eligible for the study and provided consent, a research assistant who was not involved in those evaluations randomized patients by principal diagnosis (PD/A, generalized anxiety disorder, OCD, and social anxiety disorder) using a computerized block randomization with a 2:2:1 allocation ratio to the UP, SDP, and WLC study conditions, respectively. The project coordinator (T.J.F.) who was responsible for final determination of study eligibility was blinded to the randomization sequence. Patients were unaware of study hypotheses and were instructed not to reveal their randomization status to raters prior to each assessment. To further protect blinding, raters were located separately from therapists (therapists: T.J.F., H.M.-L., S.S.-Z., J.T.-H., L.R.C., J.F.B., and J.R.C.; and raters: J.R.B., M.W.G., K.H.B., A.A., H.T.B., and C.C.-R.) and a new rater was assigned in the event of an unintentional unblinding.
Interventions
The number and length of treatment sessions were based on each SDP’s recommended dose of treatment as described below. Treatment dosage for the UP was matched to the corresponding SDP of each principal diagnosis so that there were no differences between the active treatment conditions in the amount of treatment patients received.
Single-Disorder Protocols
The SDPs included: Managing Social Anxiety: A Cognitive-Behavioral Therapy Approach, second edition; Mastery of Your Anxiety and Panic, fourth edition; Mastery of Your Anxiety and Worry, second edition; and Treating Your Obsessive-Compulsive Disorder With Exposure and Response (Ritual) Prevention Therapy, second edition. As recommended by the protocol developers, patients with a principal diagnosis of social anxiety disorder, generalized anxiety disorder, or OCD received 16 sessions of treatment and patients with a principal diagnosis of PD/A received 12 sessions. Treatment sessions were approximately 50 to 60 minutes, except for patients with a principal diagnosis of OCD, for whom treatment sessions were 80 to 90 minutes.
Unified Protocol
The UP contains strategies similar to those in the SDPs, including cognitive reappraisal and exposure, but the focus is on the reactions to the experience of emotion itself, such as autonomic arousal, rather than situational factors, such as crowds. The UP consists of the following 5 core treatment modules: (1) mindful emotion awareness, (2) cognitive flexibility, (3) identifying and preventing patterns of emotion avoidance, (4) increasing awareness and tolerance of emotion-related physical sensations, and (5) interoceptive and situational emotion-focused exposures. The 5 core modules are preceded by a module focused on enhancing motivation as well as an introductory module on the adaptive nature of emotions that provides a framework for understanding emotional experiences.
Therapists and Treatment Integrity
Therapists for the study included doctoral students in clinical psychology with 2 to 4 years of experience, postdoctoral fellows with 5 to 6 years of experience, and licensed psychologists with 10 or more years of experience. Each therapist administered both types of treatment in approximately equal proportions. Initial training and certification in the treatment protocols involved procedures used in clinical trials at the Center for Anxiety and Related Disorders at Boston University over the past 20 years. Twenty percent of treatment sessions were randomly selected and rated for adherence and competence by an external team of expert raters associated with development of the specific treatments using standardized adherence ratings approved by the respective protocol developers. Treatment fidelity scores were good to excellent (mean score of a possible total of 5, UP = 4.44; SDPs = 4.09).
Assessments and Instruments
Patients were assessed for current DSM diagnoses using the Anxiety Disorders Interview Schedule (ADIS), a semistructured clinical interview that focuses on DSM diagnoses of anxiety, mood, somatic symptom disorders, and substance use disorders, with screening for other disorders. Diagnoses are assigned a dimensional clinical severity rating (CSR) on a scale from 0 (no symptoms) to 8 (extremely severe symptoms), with a rating of 4 or higher (definitely disturbing or disabling) representing the clinical threshold for DSM diagnostic criteria. Owing to the introduction of the DSM-5 partway through the trial, 168 patients (75.3%) were assigned diagnoses based on DSM-IV criteria and 55 patients (24.7%) were assigned diagnoses based on DSM-5 criteria. To standardize CSRs across these phases, an additional rating was assigned to overall PD/A symptoms for patients diagnosed according to DSM-5, despite the separation of panic disorder and agoraphobia in DSM-5. The ADIS CSR was assessed by study evaluators blinded to condition allocation and served as the primary outcome for the power analysis and a priori specification of the equivalence margin. To maintain interrater reliability throughout the trial, a study evaluator (J.R.B., M.W.G., K.H.B., A.A., H.T.B., and C.C.-R.) was randomly selected each month to rate an audiotaped assessment conducted by another evaluator; rated assessments were equally distributed across principal diagnoses and time points. With the use of criteria specified by Brown et al, interrater agreement was 98% for principal diagnosis ADIS CSR.
Clinical response was assessed using the clinician-rated Clinical Global Impression–Severity scale and Clinical Global Impression–Improvement scale. General symptoms of anxiety and depression were assessed using the clinician-rated Hamilton Anxiety Rating Scale and the Hamilton Rating Scale for Depression in accordance with the Structured Interview Guide for the Hamilton Anxiety and Depression Rating Scale. Self-reported outcomes included the Overall Anxiety Severity and Impairment Scale and Overall Depression Severity and Impairment Scale. In addition, self-reported interference in the areas of work, home management, private leisure, social leisure, and family relationships was assessed with the Work and Social Adjustment Scale. Additional clinician-rated measures were used to assess diagnosis-specific symptom outcomes; these results are presented in eTables 1-3 in Supplement 2. Patients were assessed at baseline, after every 4 treatment sessions (ie, after sessions 4, 8, and 12), after treatment (ie, after session 16), and at the 6-month follow-up.
Sample Size Calculation
Power calculations were performed using SAS PROC POWER for the primary aims of evaluating the equivalence of the UP and SDPs and evaluating the efficacy of the UP and SDPs relative to a benchmark WLC and were based on conventional target values of power = 0.80 and α = .05. With an allocation ratio of 2:1 for active treatment to WLC groups, results of the power calculations indicated that a sample size of 91 individuals per active treatment group provided adequate power for the analyses of both equivalence and superiority.
Statistical Analysis
All analyses were conducted with Mplus 7.2 on the intent-to-treat sample that included all randomized patients (ie, 88 patients for the UP, 91 for the SDP, and 44 for the WLC condition). Missing data were accommodated using multiple imputation (10 000 imputed data sets) and robust maximum likelihood methods under a missing at random assumption. Between-condition effect sizes (Cohen d) were calculated for each condition comparison using the imputed data. P < .05 (2-sided) was considered significant.
The principal hypothesis of equivalence was evaluated using slope difference scores from latent growth models (LGMs), with treatment condition as a predictor of slope. The intercept was centered on the baseline assessment, the intermediate slope loadings were freely estimated, and the final slope loading was fixed at 1.0. Slopes therefore represented total change from baseline to the end of treatment (or follow-up) and could reflect nonlinear trajectories of change. Model fit was evaluated based on the confirmatory fit index (≥0.90). The equivalence margin of 0.75 ADIS CSR units was selected based on available meta-analytic reviews of cognitive behavioral therapy outcome studies and recommendations for selecting a priori equivalence limits. A priori calculations determined that the 0.75 ADIS CSR margin corresponded with a change of 0.61 U at the end of treatment on the Structured Interview Guide for the Hamilton Anxiety Rating Scale and 0.64 U at the end of treatment on the Structured Interview Guide for the Hamilton Depression Rating Scale, and was selected because this difference or less would not represent a clinically meaningful difference between 2 treatments that would lead us to prefer one over the other. If the entire CI for the observed mean difference between the UP and SDPs falls within the zone of equivalence (−0.75 to +0.75), the 2 treatments would be determined equivalent. To minimize inflation of type I error, comparisons among conditions were based primarily on the interpretation of CIs and effect sizes.
To compare the UP and SDPs on other outcomes and to evaluate the UP and SDPs relative to the WLC condition, a 95% CI of between-condition effect sizes from the LGM was used. Treatment response rates were evaluated by comparing the percentage of individuals in each condition who no longer met diagnostic criteria for their principal diagnosis (ie, ADIS CSR ≤3) and by calculating the relative risk effect size with 95% CIs. As an exploratory analysis, the percentage of individuals who no longer met diagnostic criteria for any emotional disorder (ie, principal or comorbid) was also examined in each condition.
Results
Sample Characteristics
Table 1 contains the demographic and baseline diagnostic characteristics of patients within and across conditions. Most of the 223 patients met the criteria for at least 1 comorbid diagnosis (188 [84.3%]) and the mean (SD) number of comorbid diagnoses was 2.3 (1.8). There were no differences in clinical severity or prevalence of comorbid disorders. The only demographic difference at baseline was that individuals assigned to the WLC condition had a higher rate of marriage than did those in the UP or SDP conditions (WLC: 18 of 44 [40.9%]; UP: 14 of 88 [15.9%]; and SDP: 15 of 91 [16.5%]; χ2 = 10.97; P = .002).
Table 1. Baseline Demographic and Diagnostic Characteristics .
| Characteristic | Valuea | |||
|---|---|---|---|---|
| Total (N = 223) |
UP (n = 88) |
SDP (n = 91) |
WLC (n = 44) |
|
| Age, mean (SD), y | 31.1 (11.0) | 31.0 (11.6) | 30.4 (10.0) | 32.7 (11.9) |
| Female sex | 124 (55.6) | 48 (54.5) | 51 (56.0) | 25 (56.8) |
| Hispanic | 17 (7.6) | 3 (3.4) | 12 (13.2) | 2 (4.5) |
| Race | ||||
| White | 186 (83.4) | 73 (83.0) | 76 (83.5) | 37 (84.1) |
| Asian | 16 (7.2) | 6 (6.8) | 6 (6.6) | 4 (9.1) |
| African American | 15 (6.7) | 8 (9.1) | 5 (5.5) | 2 (4.5) |
| Other | 6 (2.6) | 1 (1.1) | 4 (4.4) | 1 (2.3) |
| Married | 47 (21.1) | 14 (15.9) | 15 (16.5) | 18 (40.9)b |
| College degree or higher | 149 (66.8) | 50 (56.8) | 63 (69.2) | 36 (81.8) |
| Current psychotropic medication | 121 (54.3) | 47 (53.4) | 53 (58.2) | 21 (47.7) |
| Current psychotherapy | 65 (29.1) | 32 (36.4) | 22 (24.2) | 11 (25.0) |
| Previous psychiatric hospitalization | 32 (14.3) | 11 (12.5) | 15 (16.5) | 6 (13.6) |
| Principal diagnosis | ||||
| Obsessive-compulsive disorder | 44 (19.7) | 18 (20.5) | 17 (18.7) | 9 (20.5) |
| Generalized anxiety disorder | 62 (27.8) | 22 (25.0) | 27 (29.7) | 13 (29.5) |
| Panic disorder with or without agoraphobia | 59 (26.5) | 25 (28.4) | 22 (24.2) | 12 (27.3) |
| Social anxiety disorder | 58 (26.0) | 23 (26.1) | 25 (27.5) | 10 (22.7) |
| Comorbid diagnosesc | ||||
| Any | 188 (84.3) | 72 (81.8) | 78 (85.7) | 38 (86.4) |
| Obsessive-compulsive disorder | 15 (6.7) | 3 (3.4) | 10 (11.0) | 2 (4.5) |
| Generalized anxiety disorder | 40 (17.9) | 11 (12.5) | 20 (22.0) | 9 (20.5) |
| Panic disorder with or without agoraphobia | 12 (5.2) | 3 (3.4) | 5 (5.5) | 4 (9.1) |
| Social anxiety disorder | 55 (24.7) | 23 (26.1) | 20 (22.0) | 12 (27.3) |
| Major depressive disorder | 31 (13.9) | 12 (13.6) | 9 (9.9) | 10 (22.7) |
| Specific phobia | 36 (16.1) | 15 (17.0) | 14 (15.4) | 7 (15.9) |
Abbreviations: SDP, single-disorder protocol; UP, Unified Protocol for Transdiagnostic Treatment of Emotional Disorders; WLC, waitlist control.
Data are presented as number (percentage) of patients unless otherwise indicated.
Significantly different using 2-tailed t test at P < .05.
Comorbid diagnoses that were present in fewer than 20 cases are not listed separately in the table but are included in the category of any comorbid disorder and the number of comorbid disorders.
Treatment Credibility and Attrition
There were no statistically significant differences in patients’ ratings of perceived credibility or expectancy between the UP and SDP conditions as measured by ratings on the Credibility/Expectancy Questionnaire. Patients in the UP condition (77 of 88 [87.5%]) were more likely to be classified as treatment completers (ie, ≥75% of sessions completed) than were patients in the SDP condition (63 of 91 [69.2%]; odds ratio, 3.11; 95% CI, 1.44-6.74).
Equivalence
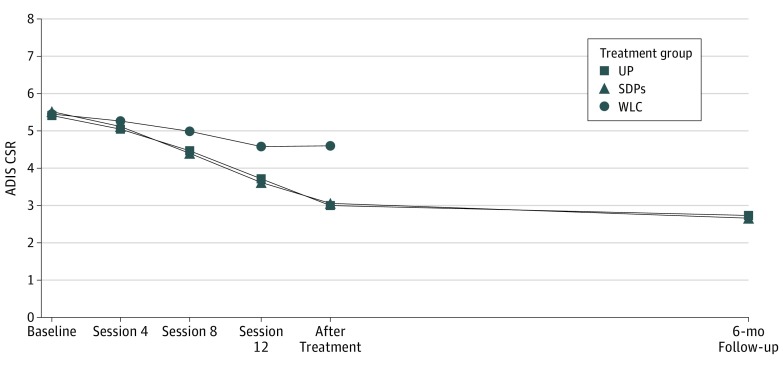
Slope difference scores and between-condition effect sizes for all outcomes from the LGM are presented in Table 2. Results of the LGM for principal diagnosis CSR were used to examine the primary research question of statistical equivalence of the UP and SDPs. The estimate of the UP vs SDPs effect on the slope of change in principal diagnosis CSR from baseline to the end of treatment was 0.25 (95% CI, −0.26 to 0.75) and the estimate from baseline to 6-month follow-up was 0.16 (95% CI, −0.39 to 0.70). The CIs for the changes in CSR fell entirely within the prespecified equivalence criteria of ±0.75 ADIS CSR units and therefore support the hypothesis of statistical equivalence of the UP vs SDPs when collapsing across diagnoses at both the end of treatment and 6-month follow-up (Figure 2). The effect size (Cohen d) difference for the slope of change in principal diagnosis from baseline to the end of treatment was 0.15 (95% CI, −0.16 to 0.46) and the effect size from baseline to 6-month follow-up was 0.10 (95% CI, −0.24 to 0.44) (Table 2).
Table 2. Slope Difference Scores and Between-Condition Effect Sizes From Growth Curve Models.
| Outcome and Visit | Slope Difference Score, Mean (95% CI)a | Effect Size, Cohen d (95% CI)a | ||||
|---|---|---|---|---|---|---|
| UP vs WLC | SDP vs WLC | UP vs SDP | UP vs WLC | SDP vs WLC | UP vs SDP | |
| Primary Clinician-Rated Outcome | ||||||
| Anxiety Disorder Interview Schedule CSR | ||||||
| Posttreatment | −1.51 (−2.14 to −0.87) | −1.75 (−2.40 to −1.10) | 0.25 (−0.26 to 0.75) | −0.93 (−1.29 to −0.57) | −1.08 (−1.43 to −0.73) | 0.15 (−0.16 to 0.46) |
| 6-mo Follow-up | NA | NA | 0.16 (−0.39 to 0.70) | NA | NA | 0.10 (−0.24 to 0.44) |
| Additional Clinician-Rated Outcomes | ||||||
| Clinical Global Impression–Severity Scale | ||||||
| Posttreatment | −1.47 (−1.96 to −0.98) | −1.40 (−1.90 to −0.91) | −0.07 (−0.46 to 0.32) | −1.36 (−1.76 to −0.97) | −1.30 (−1.70 to −0.90) | −0.06 (−0.43 to 0.30) |
| 6-mo Follow-up | NA | NA | −0.05 (−0.45 to 0.36) | NA | NA | −0.05 (−0.45 to 0.36) |
| Clinical Global Impression–Improvement Scale | ||||||
| Posttreatment | −1.20 (−1.67 to −0.73) | −1.06 (−1.53 to −0.59) | −0.14 (−0.50 to 0.22) | −1.54 (−2.18 to −0.90) | −1.36 (−2.01 to −0.71) | −0.18 (−0.64 to 0.28) |
| 6-mo Follow-up | NA | NA | −0.07 (−0.41 to 0.26) | NA | NA | −0.06 (−0.31 to 0.20) |
| Structured Interview Guide for Hamilton Anxiety Scale | ||||||
| Posttreatment | −4.90 (−7.91 to −1.88) | −6.86 (−9.83 to −3.89) | 1.96 (−0.41 to 4.34) | −0.86 (−1.37 to −0.36) | −1.21 (−1.68 to −0.74) | 0.35 (−0.07 to 0.76) |
| 6-mo Follow-up | NA | NA | 2.09 (−0.37 to 4.55) | NA | NA | 0.35 (−0.05 to 0.74) |
| Structured Interview Guide for Hamilton Depression Scale | ||||||
| Posttreatment | −3.59 (−6.09 to −1.09) | −3.53 (−6.01 to −1.04) | −0.06 (−2.05 to 1.92) | −1.20 (−2.16 to −0.24) | −1.18 (−2.12 to −0.23) | −0.02 (−0.68 to 0.64) |
| 6-mo Follow-up | NA | NA | 0.07 (−1.86 to 2.01) | NA | NA | 0.02 (−0.46 to 0.50) |
| Self-reported Outcomes | ||||||
| Overall Anxiety Severity and Impairment Scale | ||||||
| Posttreatment | −4.25 (−5.68 to −2.82) | −4.47 (−5.92 to −3.02) | 0.22 (−0.96 to 1.40) | −1.39 (−1.82 to −0.95) | −1.46 (−1.90 to −1.02) | 0.07 (−0.31 to 0.46) |
| 6-mo Follow-up | NA | NA | 0.37 (−0.81 to 1.55) | NA | NA | 0.13 (−0.28 to 0.54) |
| Overall Depression Severity and Impairment Scale | ||||||
| Posttreatment | −1.69 (−3.19 to −0.18) | −0.68 (−2.18 to 0.82) | −1.01 (−2.19 to 0.17) | −0.73 (−1.35 to −0.10) | −0.29 (−0.93 to 0.34) | −0.44 (−0.94 to 0.07) |
| 6-mo Follow-up | NA | NA | −0.55 (−1.77 to 0.67) | NA | NA | −0.20 (−0.65 to 0.25) |
| Work and Social Adjustment Scale | ||||||
| Posttreatment | −6.63 (−9.47 to −3.79) | −6.22 (−9.07 to −3.38) | −0.41 (−2.65 to 1.83) | −1.16 (−1.60 to 0.72) | −1.09 (−1.54 to −0.64) | −0.07 (−0.46 to 0.32) |
| 6-mo Follow-up | NA | NA | −0.47 (−2.77 to 1.84) | NA | NA | −0.08 (−0.50 to 0.33) |
Abbreviations: CSR, clinical severity rating for principal diagnosis obtained from Anxiety Disorder Interview Schedule; NA, not applicable; SDP, single-disorder protocol; UP, Unified Protocol for Transdiagnostic Treatment of Emotional Disorders; WLC, waitlist control.
Negative slope difference scores and effect sizes indicate that the treatment listed first was associated with a greater decrease in the outcome. Positive slope difference scores and effect sizes indicate that the treatment listed first was associated with a lesser decrease or a greater increase in the outcome. Slope difference scores and between-condition effect sizes for diagnosis-specific outcomes are reported in eTable 3 in Supplement 2.
Figure 2. Model-Based Estimates of the Principal Diagnosis ADIS CSR Score Trajectories From Baseline to 6-Month Follow-up.
Development of mean clinical severity rating for patients’ principal diagnosis in each condition throughout the study. ADIS CSR indicates clinical severity rating for principal diagnosis obtained from Anxiety Disorder Interview Schedule; SDP, single-disorder protocol; UP, Unified Protocol; and WLC, waitlist control.
Additional Clinician-Rated and Self-reported Outcomes
The imputed means and between-condition effect sizes for the primary outcome as well as other additional outcomes of interest are reported in Table 3. Consistent with hypotheses, the UP and SDPs each demonstrated superior effects to the WLC condition on both clinician-rated and self-reported outcomes of anxiety and depression based on the CIs of the effect sizes. Effect sizes for comparisons of the UP vs SDPs at the end of treatment and 6-month follow-up for all clinical outcomes were generally small and statistically nonsignificant; these findings were consistent across both the LGM-derived slope difference scores (Table 2) and effect sizes (Table 3). Change score means and within-condition effect sizes are reported in eTable 4 in Supplement 2.
Table 3. Means and Between-Condition Effect Sizes of Outcomes .
| Outcome and Visit | Mean (SD) | Effect Size, Hedges g (95% CI)a | ||||
|---|---|---|---|---|---|---|
| UP (n = 88) |
SDP (n = 91) |
WLC (n = 44) |
UP vs WLC | SDP vs WLC | UP vs SDP | |
| Primary Clinician-Rated Outcome | ||||||
| Anxiety Disorder Interview Schedule CSR | ||||||
| Baseline | 5.41 (0.76) | 5.52 (0.80) | 5.45 (0.69) | −0.05 (−0.41 to 0.31) | 0.09 (−0.27 to 0.45) | −0.14 (−0.43 to 0.16) |
| Posttreatment | 2.99 (1.84) | 3.05 (2.02) | 4.60 (1.61) | −0.91 (−1.29 to −0.53) | −0.81 (−1.19 to −0.44) | −0.03 (−0.32 to 0.26) |
| 6-mo Follow-up | 2.73 (1.71) | 2.66 (2.06) | NA | NA | NA | 0.03 (−0.26 to 0.33) |
| Additional Clinician-Rated Outcomes | ||||||
| Clinical Global Impression–Severity Scale | ||||||
| Baseline | 4.60 (0.90) | 4.74 (0.98) | 4.61 (0.71) | −0.01 (−0.38 to 0.35) | 0.13 (−0.23 to 0.49) | −0.14 (−0.44 to 0.15) |
| Posttreatment | 3.11 (1.34) | 3.15 (1.49) | 4.25 (1.15) | −0.89 (−1.26 to −0.51) | −0.79 (−1.16 to −0.42) | −0.03 (−0.32 to 0.26) |
| 6-mo Follow-up | 3.01 (1.41) | 3.01 (1.35) | NA | NA | NA | 0.00 (−0.29 to 0.29) |
| Clinical Global Impression–Improvement Scale | ||||||
| Session 1 | 3.49 (0.67) | 3.49 (0.78) | 3.74 (0.97) | −0.31 (−0.68 to 0.05) | −0.30 (−0.66 to 0.06) | 0.01 (−0.28 to 0.30) |
| Posttreatment | 2.22 (1.15) | 2.39 (1.32) | 3.38 (0.98) | −1.05 (−1.43 to −0.67) | −0.81 (−1.18 to −0.43) | −0.14 (−0.43 to 0.16) |
| 6-mo Follow-up | 2.30 (1.45) | 2.21 (1.24) | NA | NA | NA | 0.07 (−0.22 to 0.36) |
| Structured Interview Guide for Hamilton Anxiety Scale | ||||||
| Baseline | 17.06 (8.50) | 17.01 (9.51) | 16.77 (8.44) | 0.03 (−0.33 to 0.40) | 0.03 (−0.33 to 0.39) | 0.01 (−0.29 to 0.30) |
| Posttreatment | 10.38 (8.07) | 8.94 (8.08) | 14.63 (7.80) | −0.53 (−0.90 to −0.16) | −0.71 (−1.08 to −0.34) | 0.18 (−0.12 to 0.47) |
| 6-mo Follow-up | 9.94 (7.94) | 8.95 (8.49) | NA | NA | NA | 0.12 (−0.17 to 0.41) |
| Structured Interview Guide for Hamilton Depression Scale | ||||||
| Baseline | 11.55 (7.02) | 11.49 (6.30) | 11.82 (6.32) | −0.04 (−0.40 to 0.32) | −0.05 (−0.41 to 0.31) | 0.01 (−0.28 to 0.30) |
| Posttreatment | 7.21 (6.12) | 7.20 (7.10) | 10.76 (6.20) | −0.57 (−0.94 to −0.21) | −0.52 (−0.88 to −0.15) | 0.00 (−0.29 to 0.29) |
| 6−mo Follow-up | 7.57 (6.79) | 6.87 (7.04) | NA | NA | NA | 0.10 (−0.19 to 0.39) |
| Self-reported Outcomes | ||||||
| Overall Anxiety Severity and Impairment Scale | ||||||
| Baseline | 9.68 (3.81) | 10.37 (6.30) | 9.62 (3.77) | 0.02 (−0.35 to 0.38) | 0.13 (−0.23 to 0.49) | −0.13 (−0.42 to 0.16) |
| Posttreatment | 4.70 (3.18) | 4.98 (4.24) | 7.91 (4.10) | −0.91 (−1.29 to −0.53) | −0.70 (−1.07 to −0.33) | −0.07 (−0.37 to 0.22) |
| 6-mo Follow-up | 4.86 (4.03) | 4.78 (3.88) | NA | NA | NA | 0.02 (−0.27 to 0.31) |
| Overall Depression Severity and Impairment Scale | ||||||
| Baseline | 5.38 (5.14) | 5.28 (4.69) | 6.09 (5.00) | −0.14 (−0.50 to 0.22) | −0.17 (−0.53 to 0.19) | 0.02 (−0.27 to 0.31) |
| Posttreatment | 2.95 (3.82) | 3.11 (4.17) | 4.88 (5.09) | −0.45 (−0.81 to −0.08) | −0.39 (−0.76 to −0.03) | −0.04 (−0.33 to 0.25) |
| 6-mo Follow-up | 3.49 (4.39) | 2.65 (3.88) | NA | NA | NA | 0.20 (−0.09 to 0.49) |
| Work and Social Adjustment Scale | ||||||
| Baseline | 15.09 (7.36) | 15.04 (6.38) | 15.55 (6.89) | −0.06 (−0.42 to 0.30) | −0.08 (−0.44 to 0.28) | 0.01 (−0.29 to 0.30) |
| Posttreatment | 7.63 (7.61) | 7.75 (7.67) | 13.58 (7.52) | −0.78 (−1.15 to −0.41) | −0.76 (−1.13 to −0.39) | −0.02 (−0.31 to 0.28) |
| 6-mo Follow-up | 6.85 (7.07) | 6.59 (7.95) | NA | NA | NA | 0.03 (−0.26 to 0.33) |
Abbreviations: CSR, clinical severity rating for principal diagnosis obtained from Anxiety Disorder Interview Schedule; NA, not applicable; SDP, single-disorder protocol; UP, Unified Protocol for Transdiagnostic Treatment of Emotional Disorders; WLC, waitlist control.
Negative effect sizes indicate that the treatment listed first was associated with lower levels of the outcome and positive effect sizes indicate that the treatment listed first was associated with higher levels of the outcome. Means and between-condition effect sizes for diagnosis-specific outcomes are reported in eTable 1 in Supplement 2.
Treatment Response and Remission
At the end of treatment, 56 of 88 patients in the UP condition (63.6%) no longer met diagnostic criteria for their principal diagnosis compared with 52 of 91 patients (57.1%) in the SDP condition and 12 of 44 patients (27.3%) in the WLC condition. At the 6-month follow-up, these percentages increased to 70.5% (62 of 88) for the UP condition and 62.6% (57 of 91) for the SDP condition. The UP (relative risk, 2.38; 95% CI, 1.42-3.98) and SDP (relative risk, 2.15; 95%, CI 1.27-3.61) conditions were both associated with a significantly greater proportion of patients no longer meeting diagnostic criteria for their principal diagnosis than in the WLC condition.
As an exploratory analysis, we also examined the proportion of individuals who no longer met diagnostic criteria for any emotional disorder following treatment. At the end of treatment, 39 of 63 individuals in the UP condition (61.9%) no longer met diagnostic criteria for any emotional disorder compared with 27 of 57 (47.4%) in the SDP condition and 4 of 32 (12.5%) in the WLC condition. At 6-month follow-up, these percentages decreased slightly to 56.7% (34 of 60) for the UP condition and 40.7% (24 of 59) for the SDP condition.
Discussion
Results indicated treatment equivalence of the UP and 4 different SDPs on changes in severity of principal diagnosis at both the end of treatment and 6-month follow-up, with the UP evidencing significantly less attrition than the SDPs, possibly owing to the inclusion of strategies for enhancing motivation. Treatment with both the UP and SDPs was consistently associated with improved outcomes relative to the WLC condition on clinician-rated and self-reported outcomes. Also, relative to the WLC condition, patients receiving either the UP or SDPs had a greater chance of no longer meeting criteria for their principal diagnosis at the end of treatment.
These findings provide support for the utility of a parsimonious, mechanism-focused, transdiagnostic approach consisting of 5 core modules for addressing the most commonly occurring mental disorders. This study also demonstrates that patients with diverse diagnoses view a transdiagnostic approach to be as credible as SDPs, which is an important consideration given the increasing emphasis on patient preferences in the implementation of EBPTs and the finding that patients generally prefer psychosocial treatment options to other approaches.
Clinical trials are commonly criticized for failing to replicate the comorbidity and clinical complexity that clinicians encounter in real-world settings. However, inclusion criteria for this trial were liberal, including the full range of comorbid disorders, thus allowing for significant heterogeneity among patients and, consequently, greater generalizability of results. A total of 121 of 223 patients (54.3%) were taking psychotropic medications and 65 of 223 (29.1%) were currently receiving non–cognitive behavioral therapy at the intake, which was discontinued if the focus was on anxiety. In addition, most patients had received some form of previous treatment that failed to provide significant or lasting remission of symptoms.
Limitations
Results from this trial should be interpreted in the context of several limitations. Patients were generally well educated and somewhat less depressed than comparable samples, which may have augmented their ability to benefit from treatment, although previous studies have failed to observe consistent effects of educational level on treatment outcome in anxiety disorders. More important, the UP was developed at the Center for Anxiety and Related Disorders at Boston University (as were 3 of the 4 SDPs); thus, it is possible these results may not be fully generalizeable to other clinical settings.
Conclusions
Use of a single protocol designed to target temperamental factors underlying the development and maintenance of the full range of emotional disorders has implications for bridging the science-to-service gap. Training clinicians in the delivery of a single protocol that can simultaneously target commonly comorbid disorders may be more efficient and cost-effective because clinicians are adhering to core strategies that can be flexibly applied to a range of emotional experiences. Thus, a transdiagnostic approach, such as the UP, may decrease known barriers of receiving an EBPT delivered with fidelity, at an adequate dose, in a cost- and time-efficient manner.
Trial Protocol
eTable 1. Means and Between-Condition Effect Sizes for Diagnosis-Specific Outcomes
eTable 2. Change Score Means and Within-Condition Effect Sizes for Diagnosis-Specific Outcomes
eTable 3. Slope Difference Scores and Between-Condition Effect Sizes for Diagnosis-Specific Outcomes
eTable 4. Change Score Means and Within-Condition Effect Sizes of Clinician-Rated and Self-reported Outcomes
References
- 1.Barlow DH, Bullis JR, Comer JS, Ametaj AA. Evidence-based psychological treatments: an update and a way forward. Annu Rev Clin Psychol. 2013;9:1-27. [DOI] [PubMed] [Google Scholar]
- 2.Wang PS, Lane M, Olfson M, Pincus HA, Wells KB, Kessler RC. Twelve-month use of mental health services in the United States: results from the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):629-640. [DOI] [PubMed] [Google Scholar]
- 3.Weissman MM, Verdeli H, Gameroff MJ, et al. National survey of psychotherapy training in psychiatry, psychology, and social work. Arch Gen Psychiatry. 2006;63(8):925-934. [DOI] [PubMed] [Google Scholar]
- 4.Kazdin AE. Evidence-based treatment and practice: new opportunities to bridge clinical research and practice, enhance the knowledge base, and improve patient care. Am Psychol. 2008;63(3):146-159. [DOI] [PubMed] [Google Scholar]
- 5.McHugh RK, Barlow DH. The dissemination and implementation of evidence-based psychological treatments: a review of current efforts. Am Psychol. 2010;65(2):73-84. [DOI] [PubMed] [Google Scholar]
- 6.Kazdin AE, Blase SL. Rebooting psychotherapy research and practice to reduce the burden of mental illness. Perspect Psychol Sci. 2011;6(1):21-37. [DOI] [PubMed] [Google Scholar]
- 7.Institute of Medicine Psychosocial Interventions For Mental And Substance Use Disorders: A Framework For Establishing Evidence-Based Standards. Washington, DC: The National Academies Press; 2015. [PubMed] [Google Scholar]
- 8.Barlow DH, Allen LB, Choate ML. Toward a unified treatment for emotional disorders. Behav Ther. 2004;35(2):205-230. doi: 10.1016/s0005-7894(04)80036-4 [DOI] [PubMed] [Google Scholar]
- 9.Sauer-Zavala S, Gutner CA, Farchione TJ, Boettcher HT, Bullis JR, Barlow DH. Current definitions of “transdiagnostic” in treatment development: a search for consensus. Behav Ther. 2017;48(1):128-138. doi: 10.1016/j.beth.2016.09.004 [DOI] [PubMed] [Google Scholar]
- 10.Mansell W, Harvey A, Watkins E, Shafran R. Conceptual foundations of the transdiagnostic approach to CBT. J Cogn Psychother. 2009;23(1):6-19. doi: 10.1891/0889-8391.23.1.6 [DOI] [Google Scholar]
- 11.Norton PJ, Paulus DJ. Toward a unified treatment for emotional disorders: update on the science and practice. Behav Ther. 2016;47(6):854-868. [DOI] [PubMed] [Google Scholar]
- 12.Craske MG. Transdiagnostic treatment for anxiety and depression. Depress Anxiety. 2012;29(9):749-753. [DOI] [PubMed] [Google Scholar]
- 13.Norton PJ. A randomized clinical trial of transdiagnostic cognitve-behavioral treatments for anxiety disorder by comparison to relaxation training. Behav Ther. 2012;43(3):506-517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Norton PJ, Barrera TL. Transdiagnostic versus diagnosis-specific CBT for anxiety disorders: a preliminary randomized controlled noninferiority trial. Depress Anxiety. 2012;29(10):874-882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roy-Byrne P, Craske MG, Sullivan G, et al. Delivery of evidence-based treatment for multiple anxiety disorders in primary care: a randomized controlled trial. JAMA. 2010;303(19):1921-1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weisz JR, Chorpita BF, Palinkas LA, et al. ; Research Network on Youth Mental Health . Testing standard and modular designs for psychotherapy treating depression, anxiety, and conduct problems in youth: a randomized effectiveness trial. Arch Gen Psychiatry. 2012;69(3):274-282. [DOI] [PubMed] [Google Scholar]
- 17.Barlow DH, Farchione TJ, Fairholme CP, et al. Unified Protocol for Transdiagnostic Treatment of Emotional Disorders: Therapist Guide. New York, NY: Oxford University Press; 2011. [Google Scholar]
- 18.Barlow DH, Sauer-Zavala S, Carl JR, Bullis JR, Ellard KK. The nature, diagnosis, and treatment of neuroticism: back to the future. Clin Psychol Sci. 2014;2(3):344-365. doi: 10.1177/2167702613505532 [DOI] [Google Scholar]
- 19.Sauer-Zavala S, Boswell JF, Gallagher MW, Bentley KH, Ametaj A, Barlow DH. The role of negative affectivity and negative reactivity to emotions in predicting outcomes in the Unified Protocol for the transdiagnostic treatment of emotional disorders. Behav Res Ther. 2012;50(9):551-557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ellard KK, Fairholme CP, Boisseau CL, Farchione TJ, Barlow DH. Unified Protocol for the transdiagnostic treatment of emotional disorders: protocol development and initial outcome data. Cognit Behav Pract. 2010;17(1):88-101. doi: 10.1016/j.cbpra.2009.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Farchione TJ, Fairholme CP, Ellard KK, et al. Unified Protocol for transdiagnostic treatment of emotional disorders: a randomized controlled trial. Behav Ther. 2012;43(3):666-678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.National Institute for Health and Clinical Excellence [NICE] Generalised Anxiety Disorder and Panic Disorder (With or Without Agoraphobia) in Adults: Management in Primary, Secondary and Community Care. London, UK: National Institute for Health & Clinical Excellence; 2011. [PubMed] [Google Scholar]
- 23.American Psychiatric Association Practice Guideline for the Treatment of Patients With Panic Disorder. 2nd ed Washington, DC: American Psychiatric Association; 2009. [Google Scholar]
- 24.Hope DA, Heimberg RG, Turk CL. Managing Social Anxiety: A Cognitive Behavioral Therapy Approach (Workbook). New York, NY: Oxford University Press; 2010. [Google Scholar]
- 25.Hope DA, Heimberg RG, Turk CL. Managing Social Anxiety: A Cognitive Behavioral Therapy Approach (Therapist Guide). 2nd ed New York, NY: Oxford University Press; 2006. [Google Scholar]
- 26.Barlow DH, Craske MG. Mastery of Your Anxiety and Panic (Workbook). 4th ed New York, NY: Oxford University Press; 2007. [Google Scholar]
- 27.Barlow DH, Craske MG. Mastery of Your Anxiety and Panic (Therapist Guide). 4th ed New York, NY: Oxford University Press; 2007. [Google Scholar]
- 28.Craske MG, Barlow DH. Mastery of Your Anxiety and Worry (Workbook). 2nd ed New York, NY: Oxford University Press; 2006. [Google Scholar]
- 29.Zinbarg RE, Craske MG, Barlow DH. Mastery of Your Anxiety and Worry (Therapist Guide). 2nd ed New York, NY: Oxford University Press; 2006. [Google Scholar]
- 30.Yadin E, Foa EB, Lichner TK. Exposure and Response (Ritual) Prevention for Obsessive-Compulsive Disorder (Workbook). 2nd ed New York, NY: Oxford University Press; 2012. [Google Scholar]
- 31.Foa EB, Yadin E, Lichner TK. Exposure and Response (Ritual) Prevention for Obsessive-Compulsive Disorder (Therapist Guide). 2nd ed New York, NY: Oxford University Press; 2012. [Google Scholar]
- 32.Barlow DH, Ellard KK, Fairholme CP, et al. The Unified Protocol for Transdiagnostic Treatment of Emotional Disorders (Workbook). New York, NY: Oxford University Press; 2011. [Google Scholar]
- 33.Barlow DH, Gorman JM, Shear MK, Woods SW. Cognitive-behavioral therapy, imipramine, or their combination for panic disorder: a randomized controlled trial. JAMA. 2000;283(19):2529-2536. [DOI] [PubMed] [Google Scholar]
- 34.Di Nardo PA, Brown TA, Barlow DH. Anxiety Disorders Interview Schedule for DSM-IV: Lifetime Version (ADIS-IV-L). New York, NY: Oxford University Press; 1994. [Google Scholar]
- 35.Brown TA, Barlow DH. Anxiety and Related Disorders Interview Schedule for DSM-5—Lifetime Version. London, UK: Oxford University Press; 2014. [Google Scholar]
- 36.Brown TA, Di Nardo PA, Lehman CL, Campbell LA. Reliability of DSM-IV anxiety and mood disorders: implications for the classification of emotional disorders. J Abnorm Psychol. 2001;110(1):49-58. [DOI] [PubMed] [Google Scholar]
- 37.Guy W. ECDEU Assessment Manual for Psychopharmacology. Rockville, MD: US Dept of Health Education & Welfare; 1976. [Google Scholar]
- 38.Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol. 1959;32(1):50-55. [DOI] [PubMed] [Google Scholar]
- 39.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shear MK, Vander Bilt J, Rucci P, et al. Reliability and validity of a structured interview guide for the Hamilton Anxiety Rating Scale (SIGH-A). Depress Anxiety. 2001;13(4):166-178. [PubMed] [Google Scholar]
- 41.Williams JB. A structured interview guide for the Hamilton Depression Rating Scale. Arch Gen Psychiatry. 1988;45(8):742-747. [DOI] [PubMed] [Google Scholar]
- 42.Norman SB, Cissell SH, Means-Christensen AJ, Stein MB. Development and validation of an Overall Anxiety Severity and Impairment Scale (OASIS). Depress Anxiety. 2006;23(4):245-249. [DOI] [PubMed] [Google Scholar]
- 43.Bentley KH, Gallagher MW, Carl JR, Barlow DH. Development and validation of the Overall Depression Severity and Impairment Scale. Psychol Assess. 2014;26(3):815-830. [DOI] [PubMed] [Google Scholar]
- 44.Mundt JC, Marks IM, Shear MK, Greist JH. The Work and Social Adjustment Scale: a simple measure of impairment in functioning. Br J Psychiatry. 2002;180:461-464. [DOI] [PubMed] [Google Scholar]
- 45.Marks IM, Connolly J, Hallam RS. Psychiatric nurse as therapist. Br Med J. 1973;3(5872):156-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.SAS [computer program]. Cary, NC: SAS Institute Inc; 2003.
- 47.Mplus User’s Guide [computer program]. Los Angeles, CA: Muthén & Muthén; 1998-2012.
- 48.Hofmann SG, Smits JA. Cognitive-behavioral therapy for adult anxiety disorders: a meta-analysis of randomized placebo-controlled trials. J Clin Psychiatry. 2008;69(4):621-632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wiens BL. Choosing an equivalence limit for noninferiority or equivalence studies. Control Clin Trials. 2002;23(1):2-14. [DOI] [PubMed] [Google Scholar]
- 50.Devilly GJ, Borkovec TD. Psychometric properties of the credibility/expectancy questionnaire. J Behav Ther Exp Psychiatry. 2000;31(2):73-86. [DOI] [PubMed] [Google Scholar]
- 51.Montori VM, Brito JP, Murad MH. The optimal practice of evidence-based medicine: incorporating patient preferences in practice guidelines. JAMA. 2013;310(23):2503-2504. [DOI] [PubMed] [Google Scholar]
- 52.McHugh RK, Whitton SW, Peckham AD, Welge JA, Otto MW. Patient preference for psychological vs pharmacologic treatment of psychiatric disorders: a meta-analytic review. J Clin Psychiatry. 2013;74(6):595-602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lilienfeld SO, Ritschel LA, Lynn SJ, Cautin RL, Latzman RD. Why many clinical psychologists are resistant to evidence-based practice: root causes and constructive remedies. Clin Psychol Rev. 2013;33(7):883-900. [DOI] [PubMed] [Google Scholar]
- 54.Rothwell PM. Factors that can affect the external validity of randomised controlled trials. PLoS Clin Trials. 2006;1(1):e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rothwell PM. External validity of randomised controlled trials: “to whom do the results of this trial apply?” Lancet. 2005;365(9453):82-93. [DOI] [PubMed] [Google Scholar]
- 56.Schneider RL, Arch JJ, Wolitzky-Taylor KB. The state of personalized treatment for anxiety disorders: a systematic review of treatment moderators. Clin Psychol Rev. 2015;38:39-54. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eTable 1. Means and Between-Condition Effect Sizes for Diagnosis-Specific Outcomes
eTable 2. Change Score Means and Within-Condition Effect Sizes for Diagnosis-Specific Outcomes
eTable 3. Slope Difference Scores and Between-Condition Effect Sizes for Diagnosis-Specific Outcomes
eTable 4. Change Score Means and Within-Condition Effect Sizes of Clinician-Rated and Self-reported Outcomes