Abstract
Importance
Although borderline personality disorder (BPD)—one of the most common, burdensome, and costly psychiatric conditions—is characterized by repeated interpersonal conflict and instable relationships, the neurobiological mechanism of social interactive deficits remains poorly understood.
Objective
To apply recent advancements in the investigation of 2-person human social interaction to investigate interaction difficulties among people with BPD.
Design, Setting, and Participants
Cross-brain information flow in BPD was examined from May 25, 2012, to December 4, 2015, in pairs of participants studied in 2 linked functional magnetic resonance imaging scanners in a university setting. Participants performed a joint attention task. Each pair included a healthy control individual (HC) and either a patient currently fulfilling DSM-IV criteria for BPD (cBPD) (n = 23), a patient in remission for 2 years or more (rBPD) (n = 17), or a second HC (n = 20). Groups were matched for age and educational level.
Main Outcomes and Measures
A measure of cross-brain neural coupling was computed following previously published work to indicate synchronized flow between right temporoparietal junction networks (previously shown to host neural coupling abilities in health). This measure is derived from an independent component analysis contrasting the time courses of components between pairs of truly interacting participants compared with bootstrapped control pairs.
Results
In the sample including 23 women with cBPD (mean [SD] age, 26.8 [5.7] years), 17 women with rBPD (mean [SD] age, 28.5 [4.3] years), and 80 HCs (mean [SD] age, 24.0 [3.4] years]) investigated as dyads, neural coupling was found to be associated with disorder state (η2 = 0.17; P = .007): while HC-HC pairs showed synchronized neural responses, cBPD-HC pairs exhibited significantly lower neural coupling just above permutation-based data levels (η2 = 0.16; P = .009). No difference was found between neural coupling in rBPD-HC and HC-HC pairs. The neural coupling in patients was significantly associated with childhood adversity (T = 2.3; P = .03).
Conclusions and Relevance
This study provides a neural correlate for a core diagnostic and clinical feature of BPD. Results indicate that hyperscanning may deliver state-associated biomarkers for clinical social neuroscience. In addition, at least some neural deficits of BPD may be more reversible than is currently assumed for personality disorders.
This cohort study applies recent advancements in the investigation of 2-person human social interaction to examine interaction difficulties among people with borderline personality disorder.
Key Points
Questions
What are the neurobiological mechanisms associated with social interaction deficits in borderline personality disorder and how do they change during the course of the disorder?
Findings
In this cohort study using 2-person neuroimaging, neural coupling between participants’ brains during interaction was significantly lower between healthy controls and patients with borderline personality disorder than between pairs of healthy controls. No differences were observed between the coupling of patients with borderline personality disorder in remission and controls.
Meaning
Social neural deficits of borderline personality disorder can be measured in 2-person interaction and may be more reversible than is currently assumed for personality disorders, illustrating how social neuroscience can provide novel biomarkers of previously understudied symptom domains in psychiatry.
Introduction
Social impairments are a hallmark of many psychiatric disorders and key contributors to patients’ distress and difficulties in work and in their personal lives. One example is borderline personality disorder (BPD), a condition closely linked with neglect or trauma during childhood, in which dysregulation of emotions and interpersonal relationships are defining features. Patients with BPD repeatedly encounter interpersonal conflicts, unstable relationships, and intense emotional distress. Successful social interactions, cooperation, and interpersonal trust are impeded by a negative social evaluation bias, feelings of exclusion, and a heightened sensitivity to social rejection, and are often challenged by attention deficits. Pharmacotherapy remains largely aimed at treating affective symptoms, but tailored psychotherapeutic interventions often achieve a long-term clinical remission. Still, classification as a personality disorder defines an enduring and stable condition.
At the neural level, results of functional magnetic resonance imaging (fMRI) studies point to a dysregulation of the amygdala, including a failure to habituate to affective stimuli, and a hyperreactivity of the insular cortex. Limbic dysregulation is accompanied by lower engagement of prefrontal regions, which may underlie patients’ difficulties in regulating emotional states. However, identification of the neural mechanisms underlying disturbed social functioning beyond affective dysregulation remains elusive, not least because of the challenges of studying naturalistic social interactions using neuroimaging. One pioneering study used fMRI to identify functional brain abnormalities, while studies using virtual participants to induce social experiences found hyperactivity of the medial prefrontal and anterior cingulate cortex in patients with BPD. Nevertheless, the neural mechanisms of disturbed social interaction and the association with clinical state are currently unclear. Here, we used advances in studying immediate 2-person social interaction involving BPD during fMRI-hyperscanning, where multiple individuals simultaneously undergo neuroimaging. A recent study reported an advanced hardware setup using fMRI-hyperscanning that allows for interaction-based paradigms with a partner scan site.
In a previous study of cross-brain coupling within pairs of healthy volunteers during real-time social interaction, a distinct association was identified between both partners’ brain systems, occurring exclusively during immediate social contact and among real interaction pairs (ie, individuals with immediate face-to-face interaction). The neural coupling developed specifically among their right temporoparietal junctions (rTPJs). This region is selective to social stimuli, is closely functionally connected with all other social brain regions, and is assumed to host the key theory of mind ability of mental state inference, thus mediating interpersonal interactions. Activation deficits in the TPJ had been reported for BPD, and may have also been a component of activation cluster differences reported for superior temporal sulcus and gyrus in several studies.
In the present study, we assessed the value of this approach to examine neural coupling and interaction deficits in patients with BPD in relation to clinical state (Figure 1). We hypothesized that cross-brain information flow would be impaired and persist in patients with BPD in remission. Adverse childhood experiences are one of the most widely acknowledged risk factors for BPD. Following frequent reports on early-life traumatization evoking reductions in brain volume alongside neurochemical and functional alterations, which are believed to facilitate later neurocognitive and affective deficits as well as disorder development, we expected an association between neural coupling and early traumatization.
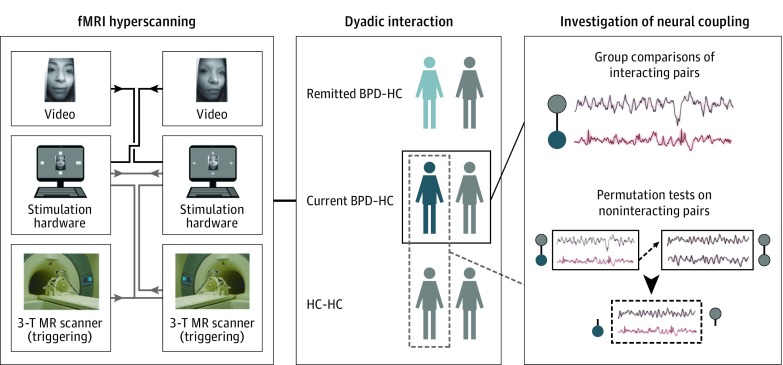
Figure 1. Experimental Setup and Data Analysis.
Participants underwent functional magnetic resonance (MR) imaging (fMRI) hyperscanning in pairs consisting of a healthy control (HC; gray) and a patient with acute borderline personality disorder (BPD; dark red), an HC and a patient with BPD in remission (light red), or an HC and another HC. Analysis focused on between-group differences in cross-brain coupling during social interaction. Distinctiveness of coupling parameters to truly interacting pairs was demonstrated by permutation tests comparing coupling in randomly assigned noninteracting pairs. Parts of the figure are reprinted from Bilek et al with permission from the National Academy of Sciences.
Methods
Participants
From May 25, 2012, to December 4, 2015, we examined 120 females in dyadic pairs (mean [SD] age, 25.2 [4.4] years; mean [SD] educational level, 12.4 [1.2] years). Participants underwent thorough screening by trained masters-degreed or medical-degreed personnel using the Structured Clinical Interview for DSM-IV for BPD and the International Personality Disorder Examination for antisocial personality disorder and avoidant personality disorder (see Table 1 for participant data, and Table 2 for pairwise data). The study was approved by the Ethics Committee of Heidelberg University. Participants provided written informed consent (see the eAppendix in the Supplement for details on recruitment and diagnostic reliability).
Table 1. Demographic Data .
| Characteristic | cBPD | rBPD | HC | P Value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Independent-Samples t Test | ANOVA/χ2 | |||||||||
| Value | No. | Value | No. | Value | No. | cBPD × rBPD | cBPD × HC | rBPD × HC | ||
| Demographic data | ||||||||||
| Age, mean (SD), y | 26.8 (5.7) | 23 | 28.5 (4.3) | 17 | 24.0 (3.4) | 80 | .30 | .02 | <.001 | .004a |
| Educational level, mean (SD), y | 11.8 (1.4) | 23 | 12.5 (1.4) | 17 | 12.6 (1.0) | 80 | .15 | .02 | .70 | .32a |
| Relationship status (No. living in a married or unmarried relationship) | 9 | 23 | 9 | 17 | 22 | 80 | NA | NA | NA | .25a |
| BMI, mean (SD) | 25.4 (5.5) | 23 | 27.6 (6.5) | 17 | 22.2 (3.0) | 80 | .25 | .01 | .004 | <.001a |
| Intelligence, mean (SD), T-score | 54.3 (3.2) | 23 | 54.7 (3.2) | 15 | 55.3 (3.3) | 33 | .73 | .25 | .52 | .50a |
| Clinical interviews | ||||||||||
| BPD symptoms: IPDE, mean (SD), total score | 6.3 (1.2) | 23 | 1.0 (1.5) | 17 | 0.03 (0.2) | 33 | <.001 | <.001 | .02 | NA |
| BPD Symptom: ZAN, mean (SD), total score | 11.3 (5.0) | 22 | 3.3 (2.7) | 17 | 0.4 (1.5) | 32 | <.001 | <.001 | <.001 | NA |
| GAF score, mean (SD) | 52.0 (9.7) | 21 | 69.4 (7.8) | 17 | 90.4 (6.6) | 33 | <.001 | <.001 | <.001 | NA |
| Self-report | ||||||||||
| BPD symptoms: BSL, mean (SD), total score | 1.9 (0.8) | 22 | 0.5 (0.5) | 17 | 0.2 (0.4) | 36 | <.001 | <.001 | .03 | NA |
| NSSI (self-injury), mean (SD), total score | 22.1 (16.0) | 22 | 0.4 (1.0) | 16 | 0.0 (0.0) | 33 | <.001 | <.001 | .11 | NA |
| FDS (dissociation), mean (SD), total score | 21.0 (11.8) | 22 | 8.3 (6.2) | 17 | 2.5 (1.8) | 36 | <.001 | <.001 | .002 | NA |
| BIS (impulsivity), mean (SD), total score | 78.1 (15.6) | 22 | 69.9 (9.4) | 17 | 55.2 (7.0) | 36 | .07 | <.001 | <.001 | NA |
| DERS (emotional regulation), mean (SD), total score | 119.2 (22.6) | 22 | 80.8 (28.5) | 17 | 52.8 (10.4) | 32 | <.001 | <.001 | .001 | NA |
| CTQ, mean (SD), total score | 60.3 (20.5) | 22 | 52.3 (15.4) | 15 | 30.0 (7.3) | 80 | .21 | <.001 | <.001 | NA |
| BDI II (depression), mean (SD), total score | 27.0 (9.0) | 22 | 8.3 (7.3) | 17 | 2.2 (3.3) | 32 | <.001 | <.001 | .004 | NA |
| Suicide attempts (if occurred), mean (SD), No. | 1.9 (0.8) | 9 | 1.7 (1.1) | 7 | NA | NA | .72 | NA | NA | NA |
| Age at first suicide attempt (if occurred), mean (SD), y | 17.7 (5.5) | 9 | 16.1 (3.8 | 8 | NA | NA | .52 | NA | NA | NA |
| Axis I disorders (DSM-IV criteria, SCID I) | ||||||||||
| Any current diagnosis, No. | 21 | 23 | 7 | 17 | 0 | 80 | NA | NA | NA | .001b |
| Any lifetime diagnosis, No. | 22 | 23 | 17 | 17 | 0 | 80 | NA | NA | NA | .38b |
| Axis II disorders (DSM-IV criteria, IPDE) | ||||||||||
| Current | ||||||||||
| Antisocial personality disorder, No. | 1 | 20 | 0 | 16 | NA | NA | NA | .43b | ||
| Avoidant personality disorder, No. | 6 | 23 | 0 | 17 | NA | NA | NA | .004b | ||
| Lifetime | ||||||||||
| Antisocial personality disorder, No. | 1 | 21 | 0 | 17 | NA | NA | NA | .27b | ||
| Avoidant personality disorder, No. | 4 | 22 | 3 | 17 | NA | NA | NA | .83b | ||
| Treatment history | ||||||||||
| Age at first treatment, mean (SD), y | 17.8 (6.8) | 18 | 16.3 (4.7) | 16 | NA | NA | .46 | NA | NA | NA |
| Age at first inpatient treatment, mean (SD), y | 21.9 (5.4) | 15 | 19.3 (5.2) | 15 | NA | NA | .19 | NA | NA | NA |
| Inpatient treatment, mean (SD), No. | 2.0 (1.6) | 16 | 2.3 (1.7) | 12 | NA | NA | .60 | NA | NA | NA |
| Outpatient treatment, mean (SD), No, | 1.9 (1.7) | 16 | 2.0 (1.0) | 12 | NA | NA | .91 | NA | NA | NA |
| Total PT duration, mean (SD), mo | 49.6 (43.9) | 16 | 41.3 (23.2) | 12 | NA | NA | .53 | NA | NA | NA |
| Behavioral PT, No. of participants | 11 | 16 | 11 | 12 | NA | NA | NA | NA | NA | .14b |
| Depth-oriented PT, No. of participants | 6 | 16 | 3 | 12 | NA | NA | NA | NA | NA | .48b |
| Psychoanalysis, No. of participants | 0 | 16 | 1 | 12 | NA | NA | NA | NA | NA | NA |
| DBT, No. of participants | 9 | 16 | 9 | 12 | NA | NA | NA | NA | NA | .31b |
| Skills training, No. of participants | 10 | 16 | 8 | 12 | NA | NA | NA | NA | NA | .82b |
| Current SSRI treatment, No. | 5 | 23 | 0 | 17 | NA | NA | NA | NA | NA | NA |
| Liftime SSRI treatment, No. | 8 | 16 | 5 | 12 | NA | NA | NA | NA | NA | .66b |
| Lifetime other medication, No. | 7 | 16 | 6 | 12 | NA | NA | NA | NA | NA | .74b |
Abbreviations: ANOVA, univariate analysis of variance; BDI II, Beck Depression Inventory; BIS, Barratt Impulsivity Scale; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); BPD, borderline personality disorder; BSL, Borderline Symptom List; cBPD, current BPD; CTQ, Childhood Trauma Questionnaire; DBT, dialectical-behavioral therapy; DERS, Difficulties in Emotion Regulation Scale; FDS, questionnaire on dissociative symptoms; GAF, Global Assessment of Functioning; HC, healthy control; IPDE, international personality disorder examination; NA, not applicable; NSSI, nonsuicidal self-injurious behavior within the past 12 months; PT, psychotherapy; rBPD, remitted BPD; SCID-I, Structured Clinical Interview for DSM-IV Axis I Disorders; SSRI, selective serotonin reuptake inhibitor; ZAN, Zanarini Rating Scale for Borderline Personality Disorder.
Analysis of variance for all participant groups.
Compared with the χ2 test on cBPD × rBPD.
Table 2. Pairwise Demographic Data.
| Characteristic | cBPD-HC Pairs | rBPD-HC Pairs | HC-HC Pairs | P Value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Independent-Samples t Test | ANOVA | |||||||||
| Value | No. | Value | No. | Value | No. | cBPD-HC × rBPD-HC | cBPD-HC × HC-HC | rBPD-HC × HC-HC | ||
| Pair age, mean (SD), y | 25.1 (3.3) | 23 | 27.0 (3.3) | 17 | 23.7 (1.5) | 20 | .08 | .08 | .001 | .003 |
| Pair educational level, mean (SD), y | 12.4 (0.7) | 23 | 12.3 (1.0) | 17 | 12.7 (0.7) | 20 | .78 | .19 | .21 | .35 |
| Within-pair age difference, mean (SD), y | 4.6 (4.9) | 23 | 5.6 (3.5) | 17 | 4.0 (3.9) | 20 | .49 | .66 | .20 | .52 |
| Within-pair educational level difference, mean (SD), y | 1.4 (1.2) | 23 | 1.4 (1.4) | 17 | 0.7 (1.3) | 20 | .96 | .61 | .11 | .13 |
| Pair CTQ score, mean (SD) | 92.8 (19.6) | 22 | 84.9 (17.5) | 15 | 56.2 (4.1) | 20 | .22 | <.001 | <.001 | <.001 |
Abbreviations: ANOVA, univariate analysis of variance; cBPD, current borderline personality disorder; CTQ, Childhood Trauma Questionnaire; HC, healthy control; rBPD, remitted borderline personality disorder.
Volunteers were excluded for meeting exclusion criteria for MRI measurements, history of head trauma, neurologic illness, current pregnancy, or current alcohol abuse or other drug use. Healthy controls (HCs) were excluded for a lifetime history of psychiatric disorders or substance dependency. Patients were excluded for substance dependency within 1 year prior to participation or a lifetime diagnosis of schizophrenia or bipolar I disorder. Participants were included as having current BPD (cBPD) according to DSM-IV criteria and as having remitted BPD (rBPD) if a previous lifetime diagnosis of BPD was reported but 3 or fewer DSM-IV criteria for BPD were met at the time of participation and within the 2 preceding years. Current use of selective serotonin reuptake inhibitors was tolerated (5 participants; all cBPD).
Eighty HCs were randomly assigned to a second participant, forming dyadic pairs, either with another HC (HC-HC; 20 pairs), a patient with cBPD (cBPD-HC; 23 pairs), or a patient with rBPD (rBPD-HC; 17 pairs). All participants were unknown to their partner but met briefly before the experiment exclusive of verbal communication.
One-way analysis of variance (ANOVA) revealed a significant difference of group age (F2,117 = 5.7; P = .004), but not education (F2,117 = 1.1; P = .32), owing to older patients with rBPD (independent samples 2-tailed t tests, rBPD-HC/cBPD-HC T78 = 1.8; P = .07; rBPD-HC/HC-HC T56.5 = 3.5; P = .001; cBPD-HC/HC-HC T79.5 = 1.6; P = .11). Subsequently, age was controlled for in all analyses.
Task
The joint attention (JA) task probes an ontogenetically early, basic form of cooperation that is an important prerequisite for successful social-cognitive development by demanding mutual eye gaze performance (eAppendix and eFigure in the Supplement). It includes task phases of interaction (INT) (performed for 5 seconds) that required the transfer of information between participants via eye movement and thus cooperation for successful trial completion. The INT phase was followed by individual trial completion (NoINT) (performed for 5 seconds), and feedback (performed for 3 seconds). Details of fMRI data acquisition are in the eAppendix and eTable 1 in the Supplement.
As expected from the simplicity of the assignment, the participants’ task performance was at ceiling (ie, a very high success rate) (successful trial completion: overall mean [SD], 93.6% [6.6%]; cBPD-HC mean [SD], 93.4% [7.8%]; rBPD-HC mean [SD], 93.4% [6.1%]; and HC-HC mean [SD], 94.0% [5.8%]; reaction time: cBPD-HC mean [SD], 2.18 [0.3] seconds; rBPD-HC mean [SD], 2.33 [0.3] seconds; and HC-HC mean [SD], 2.25 [0.3] seconds; eAppendix in the Supplement), and we found no group differences in the rate of successful trial completion and the response time.
Statistical Analysis
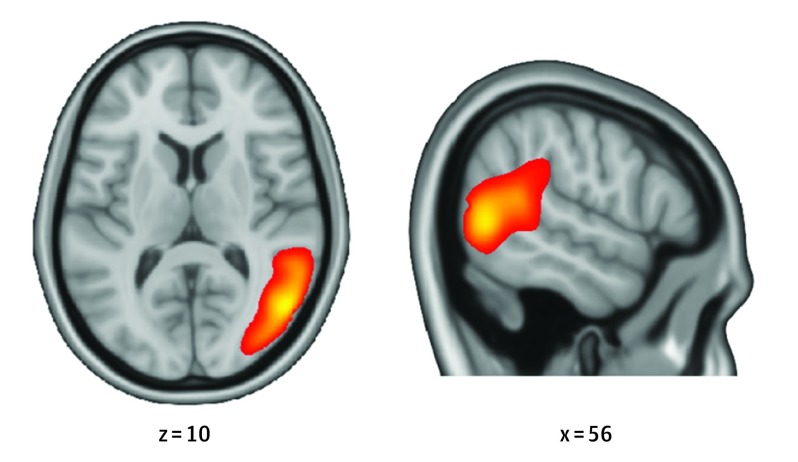
The analysis routine for 2-person hyperscanning data sets followed the advancements developed by Bilek et al. To examine neural coupling within interacting pairs, we performed a group analysis of independent components using GIFT toolbox for MATLAB, version 3.0a (http://mialab.mrn.org/software/gift/index.html), resulting in 16 components for further evaluation. Our focus on an rTPJ component is derived from previous work that found rTPJ as the core region driving cross-brain neural coupling, which was replicated in the current data set by identifying a component associated with social interaction that mapped on this region. We selected the component representing this brain region as the component of interest (COI) (Figure 2) to undergo further analysis (eAppendix and eTable 2 in the Supplement). Estimaton of group analysis of independent components is not limited to a specific region and may represent a neural network in which the rTPJ in particular participates.
Figure 2. Neural Coupling Across Interacting Brains.
Displayed is the full component of interest, spatially maximally associated with the posterior right temporoparietal junction, with a threshold of 0.19% signal change. Coordinates are given in Montreal Neurological Institute space.
Participant-specific COI time courses were derived by back-reconstruction and correlated for each pair, resulting in a pair measure quantifying neural coupling. Uniqueness of neural coupling to immediate social interaction was tested by permutation: pair assignments (eg, pair 1 = participants 1.1 and 1.2; pair 7 = participants 7.1 and 7.2) were randomized, forming samples of 60 randomly assigned nonpairs (nonpair 1 = participants 1.1 and 7.2). These nonpairs were scanned sequentially and were not interacting with each other; however, they underwent the same treatment, performing the JA task during interaction with a different partner (their actual partner). Neural coupling was estimated for the nonpairs and permutation was repeated 1000 times (referencing general neural synchrony). A comparison of frequencies for coupling nonpairs > coupling real pairs with the total event frequency delivered an empirical 1-sided P value, with P < .05 indicating uniqueness of neural coupling to true immediate interaction within real pairs (corrected for multiple comparisons by false discovery rate).
Group Comparisons of Neural Coupling
Neural Coupling
The group comparison of neural coupling was modeled within IBM SPSS, version 22.0 (IBM Corp) as follows: parameters were entered for pairs and task block as within-pair data (ie, 2 data points [A and B] per pair) and group as between-pair factor (cBPD-HC, rBPD-HC, or HC-HC) in a full factorial repeated-measures ANOVA model controlling for pairwise age (sum, difference). Following that step, direct group comparisons were performed via independent samples unpaired 2-tailed t tests.
Childhood Trauma and Risk of BPD
Adverse childhood experiences are assumed to lead to disorder-specific brain alterations, which create a risk for development of psychological disorders. Accordingly, we examined a Childhood Trauma Questionnaire–coupling association using a multiple regression analysis (cBPD and rBPD) while controlling for current symptom severity to account for bias induced by the current level of interpersonal functioning (International Personality Disorder Examination scores, Global Assessment of Functioning). Childhood Trauma Questionnaire data were available for 21 patients with cBPD and 15 with rBPD.
Post Hoc Tests
Additional analyses were conducted to investigate possible contributors to the observed aberrant neural coupling in cBPD-HC pairs. Groups were compared regarding task-associated and compensatory brain activity, spatial and temporal distribution of the COI, and gray matter volumes of the rTPJ.
Task-Related Brain Activity
Analysis of task-related brain activation was performed on the participant level within a random-effects model using Statistical Parametric Mapping software (SPM8; Wellcome Trust Centre for Neuroimaging, University College London; http://www.fil.ion.ucl.ac.uk/spm), modeling 4 regressors of interest, resulting from the combination of roles and task phase (ie, INT-sender, INT-receiver, NoINT-sender, and NoINT-receiver) and using realignment parameters as regressors of no interest. Data were high pass filtered (128-second cutoff) to account for low-frequency drifts. Brain activation relating to social interaction was computed by contrasting INT with individual performance ([INT-sender + INT-receiver] > [NoINT-sender + NoINT-receiver]). Resulting contrast images were entered in a 1-way between-participant ANOVA for statistical inference on the group level, controlled for age. Relevant contrasts were defined by comparing HC-HC pairs with combined BPD subsamples and with separate subsamples in both directions (BPD-HC [INT>NoINT], cBPD-HC [INT>NoINT], and rBPD-HC [INT>NoINT] vs HC-HC [INT>NoINT]). Results were obtained for the whole brain, as well as within the rTPJ.
COI Structure
Group independent component analysis provided participant-specific spatial maps for each component. To test for group-specific differences in the estimation of the rTPJ component and compensatory enclosure of other brain regions to the COI, images were entered in 1-way between-participant ANOVAs controlling for age. Group-level contrasts compared HC-HC pairs with combined BPD subsamples and with separate subsamples in both directions (BPD-HC, cBPD-HC, and rBPD-HC vs HC-HC).
Voxel-Based Morphometry
For analysis of brain structure, T1-weighted, anatomical whole-brain images were acquired and analyzed within the Voxel-Based Morphometry toolbox (VBM8; http://www.neuro.uni-jena.de/vbm/) applying default settings (eAppendix in the Supplement). Gray matter volumes of rTPJ were extracted and compared between groups within a univariate ANOVA. These data were available for 108 participants: all rBPD-HC pairs, 20 patients with cBPD, 20 HC-cBPD pairs, and 34 HC-HC pairs (all missing data were owing to failure to acquire the data).
Results
Permutation Tests
Permutation was computed for the full sample and for each subsample individually. Consistent with prior results, neural coupling occurred during direct social interaction and was unique to truly interacting pairs (coupling real pairs mean = 0.18; nonpairs mean = 0.12; P < .001). Correspondingly, all subsamples showed significantly higher neural coupling within real pairs than nonpairs (cBPD-HC mean, 0.14, P = .03; rBPD-HC mean, 0.20, P = .005; and HC-HC mean, 0.21, P < .001).
Group Tests
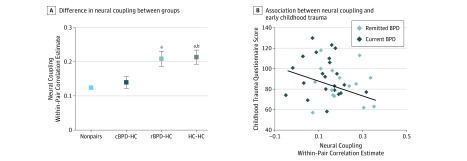
Groups were compared regarding pairwise neural coupling parameters. As hypothesized, groups differed as a function of current symptoms, with lower neural coupling in pairs involving BPD (Figure 3A; cBPD-HC mean, 0.14; rBPD-HC mean, 0.20; and HC-HC mean, 0.21). Correspondingly, direct comparison revealed a main effect of group (F2,55 = 5.5; P = .007; after exclusion of patients with cBPD who were receiving medication: F2,50 = 3.6; P = .04), indicating a significant difference of neural coupling between cBPD-HC, rBPD-HC, and HC-HC pairs.
Figure 3. Neural Coupling in Borderline Personality Disorder (BPD).
A, Difference in neural coupling between groups, driven by significantly lower indices within current BPD–healthy control (cBPD-HC) pairs compared with remitted BPD–HC (rBPD-HC) pairs and HC-HC pairs. Bars represent mean values and whiskers represent SEM. B, Association between neural coupling and early childhood trauma in patients with BPD. A multiple regression analysis was used to generate the curve and the statistic that summarizes the association between the dependent and independent variables.
aP < .05 compared with cBPD-HC pairs.
bP = .88 compared with rBPD-HC pairs.
Comparisons of groups via independent-samples t tests indicated lower neural coupling for cBPD-HC pairs than for both other subsamples (cBPD-HC/HC-HC T41 = 2.8; P = .009; cBPD-HC/rBPD-HC T38 = 2.85; P = .02); however, there were no statistically significant differences in group means between remission and health (rBPD-HC/HC-HC T35 = 0.2; P = .88). This finding suggested a state dependency of neural coupling. The quantification of childhood maltreatment of patients was significantly associated with the neural parameters in a regression model (BPD T28 = 2.3; P = .03; Figure 3B).
Post Hoc Tests
Further analyses investigating brainwide structural and functional group differences failed to provide a plausible cause for the observed aberrations in neural coupling. The comparison of interaction-related brain activity showed no difference between investigated groups, which held for both directions of group contrasts (BPD [JA>NoJA] vs HC [JA>NoJA]), 2 group definitions (full BPD-HC or BPD samples [JA>NoJA] vs the HC-HC [JA>NoJA] sample), and on a whole-brain level as well as within rTPJ only. Participant-level COI spatial maps were compared and yielded no significant difference for any contrast or group definition. Similarly, we found no significant whole-brain and rTPJ gray matter volumes between the groups. Post hoc tests showed that impaired cross-brain information flow was not explained by group differences in the task-related activation or structure of the brain systems involved.
Discussion
Our aim was the investigation, using hyperscanning, of neural correlates of disturbed social behavior in patients with BPD, the association with early trauma, and their state dependency. As hypothesized, we observed significantly lower cross-brain neural coupling during cooperation within pairs involving cBPD. This finding highlights a neural mechanism that may underpin patients’ difficulties in initiating and maintaining social interactions and relationships in everyday life.
More important, this finding was not associated with any between-group differences in individual brain structure or function, which suggests that neither a deviant volume of rTPJ nor a decreased brain activation nor TPJ embedding in a social brain network was accountable for observed aberrations. Moreover, the finding emerged only in the study of information flow between dyads, indicating a necessity of 2-person approaches. As hypothesized, the disturbance in neural interaction during a JA task, a function that emerges early in development, was linked to early traumatization, which is assumed to cause other functional and structural developmental changes in later developed BPD. This finding suggests neural coupling deficits as 1 additional neural mechanism through which early trauma and altered brain development may translate to impairments in social function. Our findings are associated with a core domain of BPD, since the symptoms of BPD are closely associated with unstable and unsuccessful relationships, and the coupling we measured is a quantitative neurobiological measure associated with human-to-human interaction that estimates social expertise.
The ability to synchronize brain activity with a conspecific was a process we observed during interaction. Given the simplicity of the performed interaction, our study provided few data on unsuccessful social contact. However, we expect neural coupling and similar parameters to prove as highly valuable for a biology-based understanding of the formation of social bonds and relationship-defining variables such as interpersonal sympathy.
Against our a priori hypothesis, between-brain coupling during interaction with patients with rBPD did not differ from that in HCs. Personality disorders are by definition stable across the lifespan and believed to remain largely inflexible. In a cross-sectional approach, we detected no differences in our interaction-related neural coupling indices between rBPD-HC pairs and HC-HC pairs. This finding is in line with the high remission rate of BPD of 75% to 85% after 6 to 10 years and joins few available findings on neural function in rBPD that suggest higher plasticity and therapeutic accessibility than often assumed, providing neuroscientific support for an active therapeutic stance in these common, severe, and often disabling disorders.
We believe that our work illustrates the usefulness and necessity of a shift toward 2-person approaches in clinical neuroscience, as suggested elsewhere. Novel approaches will be valuable for treatment and, following our results, might consider rTPJ function: for example, neural synchronization might be nudged through brain stimulation or through intake of “social drugs,” such as oxytocin.
Limitations
Our study has some limitations. We exclusively studied women owing to the low admission rate of men with BPD in psychiatric care. Although preserved neural coupling has been shown for male HCs, generalizability of the reported deficits to men with BPD cannot be assumed. Similarly, the examination of cross-brain coupling during patient-patient interaction is advised.
Second, patients with rBPD were naturally older than those in the other subsamples owing to treatment duration, and we controlled for age in all analyses. Moreover, directionality of age effects did not correspond to directionality of neural coupling differences and therefore did not affect interpretation of results.
Third, the JA task was designed to probe a fundamental neural deficit and, thus, core aspects of social interaction during cooperation. We therefore aimed for a high success rate to avoid behavioral abnormalities during task performance per se, which would have confounded the interpretation of neural coupling group differences. The advancement of hyperscanning (alongside analysis methods) toward complex social behavior is desirable given the reports on disturbed trust and relationship development in BPD-HC pairs.
Also, JA has not been known to be disturbed in BPD (to our knowledge, this is the first study investigating JA in patients with BPD). Therefore, disorder specificity should be tested for conditions in which JA deficits have been reported. Finally, our cross-sectional design provides valuable insight into the neurobiological mechanism of BPD remission, although we support further validation through longitudinal approaches.
Conclusions
This study exemplifies the application of a 2-person neuroimaging approach for the investigation of the neurobiology of severe psychiatric conditions during immediate social cooperation. Our findings suggest a disruption of a cross-brain marker of social interaction only during acute BPD states, not after remission of diagnostic criteria. Although longitudinal verification is necessary, this study supports neural coupling as a state-dependent neuroimaging marker of human social interaction and challenges the current understanding of BPD as an inflexible cognitive and neurobiological style.
eAppendix. Recruitment and Screening of Subjects
eTable 1. Quality Control Report for Subject-Specific fMRI Data
eTable 2. COI Definition Based on Spatial Consistency
eFigure. Joint Attention Task
References
- 1.Krause-Utz A, Winter D, Niedtfeld I, Schmahl C. The latest neuroimaging findings in borderline personality disorder. Curr Psychiatry Rep. 2014;16(3):438. [DOI] [PubMed] [Google Scholar]
- 2.Leichsenring F, Leibing E, Kruse J, New AS, Leweke F. Borderline personality disorder. Lancet. 2011;377(9759):74-84. [DOI] [PubMed] [Google Scholar]
- 3.Lazarus SA, Cheavens JS, Festa F, Zachary Rosenthal M. Interpersonal functioning in borderline personality disorder: a systematic review of behavioral and laboratory-based assessments. Clin Psychol Rev. 2014;34(3):193-205. [DOI] [PubMed] [Google Scholar]
- 4.King-Casas B, Sharp C, Lomax-Bream L, Lohrenz T, Fonagy P, Montague PR. The rupture and repair of cooperation in borderline personality disorder. Science. 2008;321(5890):806-810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bungert M, Liebke L, Thome J, Haeussler K, Bohus M, Lis S. Rejection sensitivity and symptom severity in patients with borderline personality disorder: effects of childhood maltreatment and self-esteem. Borderline Personal Disord Emot Dysregul. 2015;2:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Winter D, Herbert C, Koplin K, Schmahl C, Bohus M, Lis S. Negative evaluation bias for positive self-referential information in borderline personality disorder. PLoS One. 2015;10(1):e0117083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rüsch N, Lieb K, Göttler I, et al. . Shame and implicit self-concept in women with borderline personality disorder. Am J Psychiatry. 2007;164(3):500-508. [DOI] [PubMed] [Google Scholar]
- 8.Gutz L, Renneberg B, Roepke S, Niedeggen M. Neural processing of social participation in borderline personality disorder and social anxiety disorder. J Abnorm Psychol. 2015;124(2):421-431. [DOI] [PubMed] [Google Scholar]
- 9.Matthies SD, Philipsen A. Common ground in attention deficit hyperactivity disorder (ADHD) and borderline personality disorder (BPD)—review of recent findings. Borderline Personal Disord Emot Dysregul. 2014;1(1):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Philipsen A. Differential diagnosis and comorbidity of attention-deficit/hyperactivity disorder (ADHD) and borderline personality disorder (BPD) in adults. Eur Arch Psychiatry Clin Neurosci. 2006;256(suppl 1):i42-i46. [DOI] [PubMed] [Google Scholar]
- 11.Philipsen A, Feige B, Hesslinger B, et al. . Borderline typical symptoms in adult patients with attention deficit/hyperactivity disorder. Atten Defic Hyperact Disord. 2009;1(1):11-18. [DOI] [PubMed] [Google Scholar]
- 12.Philipsen A, Limberger MF, Lieb K, et al. . Attention-deficit hyperactivity disorder as a potentially aggravating factor in borderline personality disorder. Br J Psychiatry. 2008;192(2):118-123. [DOI] [PubMed] [Google Scholar]
- 13.Lieb K, Völlm B, Rücker G, Timmer A, Stoffers JM. Pharmacotherapy for borderline personality disorder: Cochrane systematic review of randomised trials. Br J Psychiatry. 2010;196(1):4-12. [DOI] [PubMed] [Google Scholar]
- 14.American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders. 5th ed Arlington, VA: American Psychiatric Association; 2013. [Google Scholar]
- 15.World Health Organization The ICD-10 Classification of Mental and Behavioural Disorders: Clinical Descriptions and Diagnostic Guidelines. Geneva, Switzerland: World Health Organization; 1992. [Google Scholar]
- 16.Donegan NH, Sanislow CA, Blumberg HP, et al. . Amygdala hyperreactivity in borderline personality disorder: implications for emotional dysregulation. Biol Psychiatry. 2003;54(11):1284-1293. [DOI] [PubMed] [Google Scholar]
- 17.Ruocco AC, Amirthavasagam S, Choi-Kain LW, McMain SF. Neural correlates of negative emotionality in borderline personality disorder: an activation-likelihood-estimation meta-analysis. Biol Psychiatry. 2013;73(2):153-160. [DOI] [PubMed] [Google Scholar]
- 18.Hazlett EA, Zhang J, New AS, et al. . Potentiated amygdala response to repeated emotional pictures in borderline personality disorder. Biol Psychiatry. 2012;72(6):448-456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koenigsberg HW, Denny BT, Fan J, et al. . The neural correlates of anomalous habituation to negative emotional pictures in borderline and avoidant personality disorder patients. Am J Psychiatry. 2014;171(1):82-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Minzenberg MJ, Fan J, New AS, Tang CY, Siever LJ. Fronto-limbic dysfunction in response to facial emotion in borderline personality disorder: an event-related fMRI study. Psychiatry Res. 2007;155(3):231-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koenigsberg HW, Fan J, Ochsner KN, et al. . Neural correlates of the use of psychological distancing to regulate responses to negative social cues: a study of patients with borderline personality disorder. Biol Psychiatry. 2009;66(9):854-863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ruocco AC, Medaglia JD, Tinker JR, et al. . Medial prefrontal cortex hyperactivation during social exclusion in borderline personality disorder. Psychiatry Res. 2010;181(3):233-236. [DOI] [PubMed] [Google Scholar]
- 23.Mier D, Lis S, Esslinger C, et al. . Neuronal correlates of social cognition in borderline personality disorder. Soc Cogn Affect Neurosci. 2013;8(5):531-537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Domsalla M, Koppe G, Niedtfeld I, et al. . Cerebral processing of social rejection in patients with borderline personality disorder. Soc Cogn Affect Neurosci. 2014;9(11):1789-1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bilek E, Ruf M, Schäfer A, et al. . Information flow between interacting human brains: identification, validation, and relationship to social expertise. Proc Natl Acad Sci U S A. 2015;112(16):5207-5212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Babiloni F, Astolfi L. Social neuroscience and hyperscanning techniques: past, present and future. Neurosci Biobehav Rev. 2014;44:76-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Montague PR, Berns GS, Cohen JD, et al. . Hyperscanning: simultaneous fMRI during linked social interactions. Neuroimage. 2002;16(4):1159-1164. [DOI] [PubMed] [Google Scholar]
- 28.Corbetta M, Patel G, Shulman GL. The reorienting system of the human brain: from environment to theory of mind. Neuron. 2008;58(3):306-324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bzdok D, Langner R, Schilbach L, et al. . Characterization of the temporo-parietal junction by combining data-driven parcellation, complementary connectivity analyses, and functional decoding. Neuroimage. 2013;81:381-392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krall SC, Rottschy C, Oberwelland E, et al. . The role of the right temporoparietal junction in attention and social interaction as revealed by ALE meta-analysis. Brain Struct Funct. 2015;220(2):587-604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Van Overwalle F. Social cognition and the brain: a meta-analysis. Hum Brain Mapp. 2009;30(3):829-858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Decety J, Lamm C. The role of the right temporoparietal junction in social interaction: how low-level computational processes contribute to meta-cognition. Neuroscientist. 2007;13(6):580-593. [DOI] [PubMed] [Google Scholar]
- 33.Haas BW, Miller JD. Borderline personality traits and brain activity during emotional perspective taking. Personal Disord. 2015;6(4):315-320. [DOI] [PubMed] [Google Scholar]
- 34.Frick C, Lang S, Kotchoubey B, et al. . Hypersensitivity in borderline personality disorder during mindreading. PLoS One. 2012;7(8):e41650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mauchnik J, Schmahl C. The latest neuroimaging findings in borderline personality disorder. Curr Psychiatry Rep. 2010;12(1):46-55. [DOI] [PubMed] [Google Scholar]
- 36.Afifi TO, Mather A, Boman J, et al. . Childhood adversity and personality disorders: results from a nationally representative population-based study. J Psychiatr Res. 2011;45(6):814-822. [DOI] [PubMed] [Google Scholar]
- 37.Waxman R, Fenton MC, Skodol AE, Grant BF, Hasin D. Childhood maltreatment and personality disorders in the USA: specificity of effects and the impact of gender. Personal Ment Health. 2014;8(1):30-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Skodol AE, Pagano ME, Bender DS, et al. . Stability of functional impairment in patients with schizotypal, borderline, avoidant, or obsessive-compulsive personality disorder over two years. Psychol Med. 2005;35(3):443-451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weber K, Miller GA, Schupp HT, et al. . Early life stress and psychiatric disorder modulate cortical responses to affective stimuli. Psychophysiology. 2009;46(6):1234-1243. [DOI] [PubMed] [Google Scholar]
- 40.Gilbert R, Widom CS, Browne K, Fergusson D, Webb E, Janson S. Burden and consequences of child maltreatment in high-income countries. Lancet. 2009;373(9657):68-81. [DOI] [PubMed] [Google Scholar]
- 41.Pietrek C, Popov T, Steffen A, Miller GA, Rockstroh B. Neuromagnetic indication of dysfunctional emotion regulation in affective disorders. Depress Res Treat. 2012;2012:156529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thomsen MS, Ruocco AC, Carcone D, Mathiesen BB, Simonsen E. Neurocognitive deficits in borderline personality disorder: associations with childhood trauma and dimensions of personality psychopathology [published online September 12, 2016]. J Pers Disord. [DOI] [PubMed] [Google Scholar]
- 43.Weber K, Rockstroh B, Borgelt J, et al. . Stress load during childhood affects psychopathology in psychiatric patients. BMC Psychiatry. 2008;8(1):63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.First M, Spitzer R, Gibbon M, Williams J. Structured Clinical Interview for DSM-IV Axis I Disorders. New York, NY: New York State Psychiatric Institute, Biometrics Research; 1995. [Google Scholar]
- 45.Loranger AW, Sartorius N, Andreoli A, et al. . The International Personality Disorder Examination. The World Health Organization/Alcohol, Drug Abuse, and Mental Health Administration international pilot study of personality disorders. Arch Gen Psychiatry. 1994;51(3):215-224. [DOI] [PubMed] [Google Scholar]
- 46.Gunderson JG, Stout RL, McGlashan TH, et al. . Ten-year course of borderline personality disorder: psychopathology and function from the Collaborative Longitudinal Personality Disorders study. Arch Gen Psychiatry. 2011;68(8):827-837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lieb K, Zanarini MC, Schmahl C, Linehan MM, Bohus M. Borderline personality disorder. Lancet. 2004;364(9432):453-461. [DOI] [PubMed] [Google Scholar]
- 48.Zanarini MC, Frankenburg FR, Reich DB, Silk KR, Hudson JI, McSweeney LB. The subsyndromal phenomenology of borderline personality disorder: a 10-year follow-up study. Am J Psychiatry. 2007;164(6):929-935. [DOI] [PubMed] [Google Scholar]
- 49.Bertsch K, Rausch J, Nagy K, Kleindienst N, Herpertz S. Increased testosterone levels and cortisol awakening responses in patients with borderline personality disorder: sex and trait aggressiveness matter. Psychoneuroendocrinology. 2015;61:55. [DOI] [PubMed] [Google Scholar]
- 50.Schilbach L. Towards a second-person neuropsychiatry. Philos Trans R Soc Lond B Biol Sci. 2016;371(1686):20150081. doi: 10.1098/rstb.2015.0081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Meyer-Lindenberg A, Domes G, Kirsch P, Heinrichs M. Oxytocin and vasopressin in the human brain: social neuropeptides for translational medicine. Nat Rev Neurosci. 2011;12(9):524-538. [DOI] [PubMed] [Google Scholar]
- 52.Mundy P, Gwaltney M, Henderson H. Self-referenced processing, neurodevelopment and joint attention in autism. Autism. 2010;14(5):408-429. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix. Recruitment and Screening of Subjects
eTable 1. Quality Control Report for Subject-Specific fMRI Data
eTable 2. COI Definition Based on Spatial Consistency
eFigure. Joint Attention Task