This study investigates the clinical spectrum associated with KCNB1 variants and the genotype-phenotype correlations.
Key Points
Question
How does the phenotype of patients with a KCNB1 mutation correlate with the type of mutation?
Findings
This study found that patients with a de novo mutation in KCNB1 present a variable phenotype of neurodevelopmental disorder.
Meaning
De novo mutations in KCNB1 are associated with a variable neurodevelopmental disorder.
Abstract
Importance
Knowing the range of symptoms seen in patients with a missense or loss-of-function variant in KCNB1 and how these symptoms correlate with the type of variant will help clinicians with diagnosis and prognosis when treating new patients.
Objectives
To investigate the clinical spectrum associated with KCNB1 variants and the genotype-phenotype correlations.
Design, Setting, and Participants
This study summarized the clinical and genetic information of patients with a presumed pathogenic variant in KCNB1.
Patients were identified in research projects or during clinical testing. Information on patients from previously published articles was collected and authors contacted if feasible.
All patients were seen at a clinic at one of the participating institutes because of presumed genetic disorder. They were tested in a clinical setting or included in a research project.
Main Outcomes and Measures
The genetic variant and its inheritance and information on the patient's symptoms and characteristics in a predefined format.
All variants were identified with massive parallel sequencing and confirmed with Sanger sequencing in the patient. Absence of the variant in the parents could be confirmed with Sanger sequencing in all families except one.
Results
Of 26 patients (10 female, 15 male, 1 unknown; mean age at inclusion, 9.8 years; age range, 2-32 years) with developmental delay, 20 (77%) carried a missense variant in the ion channel domain of KCNB1, with a concentration of variants in region S5 to S6. Three variants that led to premature stops were located in the C-terminal and 3 in the ion channel domain. Twenty-one of 25 patients (84%) had seizures, with 9 patients (36%) starting with epileptic spasms between 3 and 18 months of age. All patients had developmental delay, with 17 (65%) experiencing severe developmental delay; 14 (82%) with severe delay had behavioral problems. The developmental delay was milder in 4 of 6 patients with stop variants and in a patient with a variant in the S2 transmembrane element rather than the S4 to S6 region.
Conclusions and Relevance
De novo KCNB1 missense variants in the ion channel domain and loss-of-function variants in this domain and the C-terminal likely cause neurodevelopmental disorders with or without seizures. Patients with presumed pathogenic variants in KCNB1 have a variable phenotype. However, the type and position of the variants in the protein are (imperfectly) correlated with the severity of the disorder.
Introduction
Early infantile epileptic encephalopathies (EEs) form a group of disorders that are characterized by epileptic seizures starting during the first year of life. Patients have developmental delay (DD) or even regression to which the epileptic discharges are presumed to contribute. The seizures are often unresponsive to treatment. Many of those epilepsies have a genetic disposition.
In the past few years, several new genes have been identified that can cause EE when mutated. One of the more recently discovered genes is KCNB1 (potassium voltage-gated channel subfamily B member 1 or delayed rectifier potassium channel 1 [DRK1]) (OMIM 616056), which acts in a dominant manner. Variants are described by Torkamani et al, who identified a de novo KCNB1 variant when exome sequencing a family with a child with EE. They subsequently found 2 other independent patients with EE and KCNB1 variants. One of those patients had earlier been described in the Epi4K effort as having Lennox-Gastaut syndrome. Subsequently, more patients with variants in KCNB1 were described.
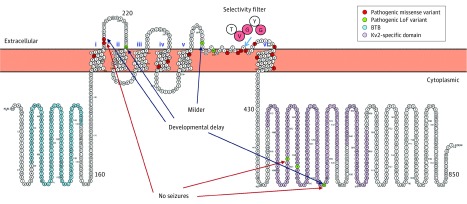
The KCNB1 gene encodes the Kv2.1 pore-forming, voltage-sensing α-subunit of a delayed rectifier potassium channel. It is expressed in various neuronal cells in the brain. In Ensembl (GRCh38.p3), only 1 transcript is known (ENST00000371741). It consists of only 2 exons and contains 4 known domains (Figure): the T1 domain, which is involved in multimerization; the ion_trans domain, which contains the transmembrane elements; and 2 intracellular Kv2 channel–specific domains (Pfam; http://smart.embl-heidelberg.de/). The protein has 6 transmembrane elements in the ion_trans domain; the first 4 form the voltage-sensor domain, with S4 being the actual voltage sensor, whereas S5 and S6 form the pore domain of the protein. The extracellular loop between S5 and S6 contains the selectivity filter (TVGYG amino acids; UNIPROT.org). This structure is similar to other voltage-gated potassium channels, such as KCNQ2 (OMIM 602235) and KCNQ3 (OMIM 602232). The protein forms homotetramers and heterotetramers with various other voltage-gated potassium channel proteins, such as KCNB2 (OMIM 607738), and proteins from the KCNG (Kv6), KCNE, and KCNS (Kv9) families.
Figure. Distribution of Pathogenic Variants in KCNB1.
Schematic view of the Kv2.1 (KCNB1) protein in the membrane, showing pathogenic missense and loss-of-function (LoF) variants. BTB indicates broad-complex, tramtrack, and bric-brac domain.
Methods
In a recent screen of 358 children with EEs, we identified 3 novel KCNB1 variants (approximately 1%), all of which were found to be de novo. We subsequently collected data from 13 additional patients from research and diagnostic sources who were reported to carry a de novo novel KCNB1 variant not present in public databases. In this article, we describe the phenotypic and genetic characteristics of these patients. In addition, we collected more information about 10 patients who were described previously. For 3 of the 10 patients in previously published articles, the authors have provided updated information for the current article. We present the available information on 26 patients for a broad overview of the phenotypic and genetic variability and similarities. Statistical tests were performed using χ2 2 × 2 contingency tables; the significance of the enrichment of de novo mutation was calculated using a χ2 goodness-of-fit test. A 2-sided χ2 test with P < .05 determined statistical significance. Collection of data procedures agreed with the local ethics guidelines at the different institutes.
Results
Description of the Variants
We identified 16 patients with KCNB1 variants who were previously not described and collected information about 10 previously described patients (10 female, 15 male, 1 unknown; mean age at inclusion, 9.8 years; age range, 2-32 years). In these 26 patients, we identified 23 different variants; 3 variants were seen twice each. Twenty patients (77%) carried missense variants, and 6 (23%) carried nonsense or frameshift variants leading to a premature termination codon (Table 1). All the missense variants are located within the Pfam ion_trans domain (ie, the 6 transmembrane regions and their extracellular and intracellular connecting loops) (Figure). Two variants in 4 patients are gating charge residues in voltage sensor S4. Three variants are in the selectivity filter between S5 and S6. Three of the loss-of-function (LoF) variants are in the first Kv2 channel–specific domain in the intracellular C-terminus of the protein. The other 3 LoF variants are in the S1 to S2 linker and in the S5 to S6 P-loop. The gene has 2 exons, and all variants are in the second (last) exon. All nonsense or frameshift variants are expected to result in a truncated protein rather than nonsense-mediated decay because they are located in the terminal part of the gene. No variants were detected in the cytoplasmic N-terminal part of the gene. All missense variants alter strongly conserved amino acids and are predicted to be highly deleterious by SIFT (Sorting Intolerant From Tolerant), Polyphen2, and Combined Annotation Dependent Depletion (CADD) scores. The lowest CADD score was 23.3. In all individuals except one (patient 18), for whom parental samples were not available for segregation analysis, the variant was confirmed to be de novo. The variant not confirmed to be de novo, R312H, was seen as de novo in another patient and was absent from the Exome Aggregation Consortium ExAC Browser (http://exac.broadinstitute.org/); thus, we interpreted it to be pathogenic and included patient 18 in our series. An overview of characteristics of the patients and their variants is given in Table 1.
Table 1. Clinical Phenotypes of Previously Described and New Patients With Heterozygous De Novo and Putative De Novo KCNB1 Mutations.
| Patient No. | Mutation (hg19; NM_004975.2) |
Channel Domain | Age, y/Sex/Age at Seizure Onset | Epilepsy | DD | Behavior | MRI Findings | EEG Findings | Neurologic Examination Findings | Other |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | chr20:47990350G>Ap.Arg583* | C-terminal | 11/F/NA | No seizures | Moderate-severe (able to speak and walk) | Severe behavioral problems, ADHD, and ASD | Normal at age 10 y | Not performed | NA | NA |
| 2 | chr20:47990498G>Tp.Y533* | C-terminal | 32/M/5 mo | Infantile spasms | Unspecified, walking at 2 y | NA | cCT normal | Hypsarrhythmia | NA | NA |
| 3 | chr20:47990593T>TAp.K502fs | C-terminal | 10/M/NA | No seizures | Severe | Severe behavioral problems, ADHD, and ASD | Chiari 1 malformation at 2 y | Not done | NA | NA |
| 4 | chr20:47990849G>Cp.F416L | S6 | 18/F/42 mo | Tonic, myoclonic, and atypical absences | Severe | NA | Periventricular white matter abnormalities | Slow background activity and sharp-slow-wave discharges at 2-2.5 Hz alternating with rapid discharges | Hypertonia, dystonia | Gastrotube feeding |
| 5 | chr20:47990849G>Cp.F416L | S6 | 15/F/14 mo | Tonic-clonic, status epilepticus, and stimulus evoked | Severe | Stereotypies and hand-wringing | NA | At 14 y, multifocal and generalized ETP | Ataxia | Scoliosis |
| 6 | chr20:47990896C>Tp.G401R | S6 | 4/M/17 mo | Clonic and focal clonic | Severe: no words and unable to sit by 4 y | NA | Normal at 9 mo and progressive brain atrophy at 1 y 6 mo and 2 y 2 mo | Diffuse polyspikes and waves with intermittent multifocal spikes at 1 y 6 mo | Choreic movements, myoclonia | NA |
| 7 | chr20:47990904C>Ap.C397F | S6 | 22/M/10 mo | Tonic-clonic and multifocal | Severe | NA | At 2 y, periventricular leukomalacia and lateral ventricles | NA | Spastic tetraparesis | NA |
| 8 | chr20:47990924T>Gp.K391N | S5-S6 linker (P-loop) | NA | NA | NA | NA | NA | NA | Abnormal | NA |
| 9 | chr20:47990944C>Ap.P385T | S5-S6 linker (P-loop) | 17/M/13 mo | Focal dyscognitive, drop attacks, tonic, nocturnal tonic, clonic, and myoclonus | Severe: walks only with assistance, ataxic gait, and nonverbal | ASD, stereotypies, and hand-wringing | Normal at 6 y and mild cerebellar volume loss at 11 y | Multifocal sharps and slowing and disorganization of the background activity at 13 y and slow disorganized background with near continuous epileptiform activity at 16 y | Hypertonia, ataxia, tremor, and spasticity | Scoliosis and gastrotube feeding |
| 10 | chr20:47990956C>Tp.G381R | S5-S6 linker (selectivity filter) | 8/M/3 mo | Startle episodes at 3 mo evolving to IS (40/d), stimulus-induced tonic seizures, focal dyscognitive, and focal motor seizures | Severe | NA | NA | At 7 y: multifocal, photosensitive, and slow | Hypertonia, spasticity | Progressive microcephaly |
| 11 | chr20:47990962C>Tp.G379R | S5-S6 linker (selectivity filter) | 9/M/8 mo | IS and later multiple seizure types, including focal dyscognitive, atonic, and generalized tonic-clonic | Severe: motor and language | ASD, stereotypies, and hand-wringing | Normal at 9 mo | At 15 mo, modified hypsarrhythmia | Truncal hypotonia and brisk reflexes | Strabismus |
| 12 | chr20:47990964T>Cp.V378A | S5-S6 linker (selectivity filter) | 4/F/13 mo | Myoclonic seizure followed by a tonic component, generalized tonic-clonic seizure, and IS | Severe: no speech, limited vocalization; at 3 y, functioning as 5-8 mo; by 3 y, able to sit independently but no standing or walking | NA | Normal | Lack of a posterior-dominant rhythm, generalized slowing, generalized spikes, polyspikes and sharp waves, frequent bursts of abortive frontal-dominant generalized spikes, and polyspikes and sharp wave discharges | Hypotonia | Cortical visual impairment |
| 13 | chr20:47990976G>Ap.T374I | S5-S6 linker (P-loop) | 5/F/6 mo | Tonic-clonic, atonic, focal dyscognitive atypical absence, and IS | Yes, unspecified | NA | Normal | Unspecified | Spasticity | NA |
| 14 | chr20:47990976G>Ap.T374I | S5-S6 linker (P-loop) | 11/F/13 mo | IS that progressed to Lennox-Gastaut syndrome; currently, tonic and absence seizures | Severe: at 7 y 6 mo, could sit independently, walk with 2 hands assisted, and communicate with 20 signs | ASD, stereotypies, and hand-wringing | T2-hyperintensity of periventricular white matter at 9 mo and normal at 4 y | At 5 y, bilateral CSWS with spike and wave complexes with shifting asymmetries; at 10 y, nearly continuous epileptiform activity characterized by independent left, right, or bilateral spike and wave activity | Choreic movements, truncal hypotonia, and spasticity | Recurrent bacterial infections, low immunoglobulin levels, and incomplete vaccination response |
| 15 | chr20:47990990C>Tp.W369* | S5-S6 linker (P-loop) | 5/M/18 mo | Head drop, brief symmetric axial, muscle contraction, evocative of IS, later head drop, myoclonic, astatic, tonic, and tonic-clonic | Severe; development before epilepsy onset: psychomotor developmental delay; development after epilepsy onset: increased after seizures started | ADHD and ASD | Normal | Generalized spikes and waves, focal sharp waves, and spikes | Truncal hypotonia and spasticity | Neonatal respiratory distress |
| 16 | chr20:47991009delG,p.S363fs*13 | S5-S6 linker (P-loop) | 2/M/14 mo | Focal seizures in which patient becomes distant and pale, sometimes with cyanosis of the lips, eye rolling, and lip smacking of 20 s to 2 min duration | Moderate: no speech at 2 y | None | Normal | Sharp waves and sharp low waves in left fronto-temporo-central region | Normal | Normal |
| 17 | chr20:47991056G>Tp.S347R | S5 (pore) | 9/F/48 mo | Tonic-clonic; tonic, atonic, focal, and focal with secondary generalization | Severe: motor and language | NA | Subtle volume loss in left hippocampus | Mild, diffuse slowing and abundant bihemispheric, multifocal epileptiform discharges | Hypotonia | Strabismus and migraine |
| 18 | chr20:47991162C>Tp.R312H | S4 (VSD) | 11/M/14 mo | Focal seizure with eye and mouth deviation to the right, usually with secondary generalization | Severe/regression: stopped walking at 5 y and never developed language | ASD and temper tantrums | Normal | CSWS pattern and generalized discharges of diphasic spikes and slow waves and polyspikes | Ataxia and spasticity | Suspected retinopathy |
| 19 | chr20:47991162C>Tp.R312H | S4 (VSD) | 9/M/10 mo | IS, myoclonic, atypical absences, and tonic | Severe: developmental delay since first year of life, no active speech at age 8 y, and walking on tiptoe | ASD and temper tantrums | Normal at 8 y | CSWS pattern, bilateral slow waves, and multiregional sharp waves | Ataxia and spasticity | Sleep disturbance |
| 20 | chr20:47991181G>Ap.R306C | S4 (VSD) | 7/M/12 mo | IS at 1 y and tonic-clonic, myoclonic, and focal with head deviation at 2 y | Severe, but able to speak some words and walk | ASD and temper tantrums | Normal at 2 y 3 mo | Generalized discharges of high-amplitude spikes, waves, and polyspikes at 2 y 11 mo | NA | Macrocephaly |
| 21 | chr20:47991181G>Ap.R306C | S4 (VSD) | 9/M/1 y | Few: febrile convulsions (absences/atonic) | Severe | ADHD (severe hyperactivity) and aggressive outbursts | Retrocerebellar arachnoidal cyst | ESES pattern, multifocal spikes, and diffuse polyspikes | Hypotonia (as infant), ataxia, and extrapyramidal movement disorder | NA |
| 22 | chr20:47991415G>Ap.Q228* | S1-S2 linker | 3.5/F/10 mo | Dialeptic seizure, automotor seizure, and generalized tonic-clonic | Moderate, global developmental delay: speech and motor | ADHD | Normal | Focal spikes, ictal pattern temporal left, and ictal generalized seizure pattern | Normal | NA |
| 23 | chr20:47991465A>Gp.L211P | S1-S2 linker | 7/F/11 mo | FS, GTCS, and focal epilepsy | Moderate delay and dyspraxia | None | Normal | Paroxysmal activity with centroparital spikes and polyspikes, generalized and accentuated during sleep | Hypotonia | NA |
| 24 | chr20:47991468G>Ap.T210M | S1-S2 linker | 4/F/NA | No seizures | Unspecified | NA | Normal | Not applicable | Normal | Gastrotube feeding |
| 25 | chr20:47991468G>Tp.T210K | S1-S2 linker | 7/M/4 mo | Myoclonic: generalized tonic-clonic, focal dyscognitive with motor signs, atonic, subclinical seizures during sleep, and history of ESES | Global delay, speaks sentences, and walking | Hyperactivity and inattention | Normal | Right central sharps with a broad field, increase in sleep, and bilateral central spike wave discharges | Hypotonia | NA |
| 26 | chr20:47991492G>Ap.S202F | S1-S2 linker | 7/M/2 y | Onset: complex partial seizures, fever associated; recent: subclinical seizures during sleep | Global delay, speaks some words and short phrases, and able to walk | Hyperactivity, inattention, impulsivity, self-injurious behavior, and autism | Normal | Multifocal epileptiform discharges, generalized bursts of spike and waves during sleep not consistent with ESES, and 2 generalized seizures at awakening | Normal | Short stature, wide-spaced teeth, increased drooling, inguinal hernia, and sleep dysfunction |
Abbreviations: ADHD, attention-deficit/hyperactivity disorder; ASD, autism spectral disorder; cCT, cardiovascular computed tomography; CSWS, continuous spike and wave during slow-wave sleep; DD, developmental delay; EEG, electroencephalography; ESES, electrical status epilepticus during slow-wave sleep; ETP, epilepsy typical potential; F, female; FS, febrile seizure; GTCS, generalized tonic-clonic seizure; IS, infantile spasm; M, male; MRI, magnetic resonance imaging; NA, not applicable; VSD, ventricular septal defect.
Description of the Patients
The observed phenotypes in patients are summarized in Table 2 and described in detail in this section. All 26 patients presented with primary DD in the first year of life, preceding onset of epilepsy. First signs of DD were unspecific, with muscular hypotonia or hypertonia being recurrently reported (11 of 23 individuals [48%] with available neurologic examination findings). Twenty-one of 25 patients (84%) had intractable epilepsy, with seizure onset in infancy or early childhood (median age at onset, 12 months; range, 3 months to 4 years). Epileptic spasms alone or as part of West syndrome were observed in 9 patients (36%), with a median onset of seizures of 12 months (range, 3-18 months). The only patient who developed spasms before 6 months of age was patient 10, who carried the prominent variant G381R within the selectivity filter of the P-loop. In most patients with epilepsy, the semiologic features developed over time into multiple seizure types, including tonic, focal-clonic, myoclonic, and atypical absences. Three patients did not have epileptic seizures (patients 1, 3, and 24), 1 reported only infantile spasms (patient 2), and no data were available for 1 (patient 8). The patients with LoF variants in the C-terminal region had no seizures or had infantile spasms only; in addition, 1 patient with a variant in the S1 to S2 linker reported no seizures. However, one patient (patient 3) with LoF variant in the C-terminus reported severe DD. Of the patients with LoF variants, 2 were reported to have severe DD and 4 had moderate or moderate-severe DD (1 unspecified). In contrast, the only patient with a missense variant for whom moderate DD was reported was patient 23, who had a missense variant in the S1 to S2 linker. In contrast, all patients with severe epilepsy harbored variants within the voltage sensor (S4) and the pore-forming domains (S5, P-loop, and S6) of KCNB1. These observations suggest an association between location of mutation and phenotype and severity of disease.
Table 2. Major Clinical Characteristics of the Patients Carrying a Presumed Pathogenic KCNB1 Mutation.
| Phenotype | No. of Patients/Patients With Data Available (%) |
|---|---|
| Developmental delay | 26/26 (100) |
| Intractable epilepsy | 21/25 (84) |
| Epileptic spasms | 9/25 (36) |
| EE-EEG | 20/21 (95) |
| Focal-generalized discharges | 9/21 (43) |
| CSWS or ESES | 5/21 (24) |
| Poor motor function | 10/13 (77) |
| Hypotonia | 11/23 (48) |
| Ataxia | 4/23 (17) |
| Abnormal behavior | 14/17 (82) |
| ADHD | 7/20 (35) |
| ASD | 10/20 (50) |
| Hand-wringing | 4/20 (20) |
| Temper tantrums | 3/20 (15) |
| Abnormal brain imaging | 4/23 (17) |
Abbreviations: ADHD, attention-deficit/hyperactivity disorder; ASD, autism spectrum disorder; CSWS, continuous spike and wave during slow-wave sleep; EE, epileptic encephalopathy; EEG, electroencephalography; ESES, electrical status epilepticus during slow-wave sleep.
In general, the epilepsy in most patients was highly refractory to diverse and multiple antiepileptic drugs. Anecdotally, partial efficacy has been reported for ketogenic diet, which resulted in a significant decrease in seizure burden in 2 patients (patients 14 and 18) but seemed ineffective in 2 others (patients 11 and 20). Improvement was also observed in 1 patient who received steroid pulses (patient 17), whereas intramuscular adrenocorticotropic hormone therapy for epileptic spasms was reported as not being of value in 3 patients (patients 2, 11, and 14). Vagus nerve stimulation and corpus callosotomy surgery were performed in 1 patient (patient 9) without substantial improvement of epilepsy. Brain biopsy in this patient revealed minimal cortical dysplasia consisting of excessive but not dysplastic neurons, a finding that argues against a role of KCNB1 in disorders of neuronal migration.
Electroencephalographic (EEG) data were reported for 21 patients. The EEG displayed features of EE in 20 of 21 patients (95%), including hypsarrhythmia and general slowing of background activity together with multifocal spikes and polyspikes, and 9 of 21 patients (43%) with abnormal EEG results had a combination of focal and generalized epileptic discharges, with 1 being mainly focal. Five patients (24%) had continuous spike and wave during slow-wave sleep (CSWS) or electrical status epilepticus during slow-wave sleep symptoms. Patients 14, 18, and 19 had CSWS on EEG, and patients 21 and 25 had a history of electrical status epilepticus during slow-wave sleep.
For 13 patients, more detailed information on motor function was available. Motor performance was poor, with limited ability to walk freely and significant signs of upper motor neuron disease (brisk reflexes, contractures, clonus, and positive plantar response) or ataxia in 10 patients (77%), all with missense variants within vital channel domains (S4-S6). The remaining 3 patients had normal neurologic examination findings and carried variants in S2 or the C-terminal.
For 14 of 17 patients (82%) with available data, behavioral problems were reported. Autism spectrum disorder was reported in 10 individuals, with different variants located through S4 to S6 and the C-terminus. In 4 patients, caregivers noted specifically nonpurposeful hand-wringing stereotypies. In addition, attention-deficit/hyperactivity disorder or hyperactivity with inattention in 7 patients and temper tantrums in 3 patients were mentioned.
Information on brain imaging was available from 23 of 26 patients (22 with magnetic resonance imaging and 1 with computed tomography). Results were normal in 19 patients (83%) or revealed unspecific features after infancy, such as Arnold-Chiari malformation type 1 (patient 3), progressive supratentorial brain atrophy (patient 6, G401R, with infantile epilepsy), cerebellar volume loss (patient 8, P385T, infantile epilepsy proceeding to Lennox-Gastaut syndrome), and white matter abnormalities (patient 14, T374I, West syndrome progressing to Lennox-Gastaut syndrome), corresponding to seizure burden in these patients.
Discussion
All missense variants clustered within the ion_trans domain, in which few natural segregating variants are found. On the exome variants server with data from more than 6500 individuals (http://evs.gs.washington.edu/EVS/), no coding variants were found in the ion_trans domain. In the ExAC database with more than 60 000 individuals, 9 coding variants were present in this domain.
On the basis of the work of Samocha et al, we explored the frequency of missense variants in the ExAC database in the different regions of the gene relative to the neutral expectation to identify gene regions intolerant to missense variation. The ratio of missense to synonymous variants is 0.26 in the ion_trans domain; 0.33 in the upstream region, including the T1 domain; and 2.14 in the downstream region, including the Kv2 channel–specific domains. This ratio is expected to be 2.3 for the whole gene after neutral expectation according to Samocha et al; therefore, both the ion_trans domain and the upstream region of the gene are intolerant to missense variants (P < 1 × 10−5). Missense variants in those regions thus have a high probability of being pathogenic. We assume that all missense variants discussed in the present study are causal for the neurodevelopmental disorder in the patient.
We also consider the LoF variants described in the present study to be pathogenic. In the ExAC database, only 2 rare LoF variants were found: p.R854* (flagged as dubious) and p.K776Qfs*23. These variants are located in the C-terminal part of the gene, closer to the terminal part than were the LoF variants found in the patients and beyond the Kv2 channel–specific domains (Figure). Therefore, it can be expected that these variants, if they exist, do not affect normal function of Kv2.1. In contrast, the 3 C-terminal mutations observed in the patients were in the Kv2.1-specific domain located more upstream and therefore may be expected to have a functional effect, for example, lead to differences in protein folding or affect formation of tetramers. All LoF variants in the patients were in the last exon of the gene; thus, we expect that they will lead to truncation rather than nonsense-mediated decay. Because the protein forms (hetero)tetramers, we expect that the effect of the missense and truncating variants is dominant negative unless the mutated protein is not incorporated in the membrane.
Because we consider all variants described in this article as causal for the phenotype, the phenotypic range described is likely to be representative of variants in this gene. There may be a bias, however, toward patients with epilepsy because our initial screen was conducted in a panel of patients with EE and many colleagues in our network have a special interest in this phenotype. Torkamani et al may also have searched only for patients with seizures when following up their initial finding. The patients without seizures in this article were from exome-wide diagnostic screening of patients with (neuro)developmental problems and from the Deciphering Developmental Disorders program in the United Kingdom, which also was a general screening of children with developmental disorders. As described in the Results section and as seen in Table 1, the range of symptoms in patients with variants in KCNB1 was diverse in many aspects.
Genotype-Phenotype Associations
Most missense variants were in the S5 to S6 linker, which forms the pore region, particularly in or near the selectivity filter. Three patients had missense variants in one of the positively charged R-residues in voltage sensor S4. As described in the Results section, the distribution of variants in patients over the domains is different from that in the general population. The variants that we found in the less constrained C-terminus were LoF variants.
Missense variants within the voltage sensor domain or the pore region of the channel were found in patients with severe epilepsy and global DD, including intellectual disability and often spasticity. Therefore, missense variants in the S4 to S6 region seem to be correlated with a more severe prognosis. An LoF variant close to S2 and one in the C-terminus were found in patients with milder DD, who acquired walking and were able to communicate. Two of the 3 LoF variants in the C-terminus were in patients without seizures (at 11 and 32 years of age), whereas a patient with a missense variant in the S1 to S2 linker, close to S1, had no seizures at 4 years of age. The degree of intellectual disability in this patient was unspecified. The LoF variant 15 (p.W369*) in the pore region was seen in a patient with severe DD and epilepsy at the age of 5 years, whereas the LoF variant near that in LoF variant 16 (p.S363fs*) was found in a patient at 2 years of age with moderate DD and focal seizures but no additional reported problems.
No pathogenic variants have been reported thus far in the highly constrained N-terminal region. A handful of rare missense variants were described for this region in the ExAC control population. It remains to be determined whether missense or LoF variants in this region also lead to neurodevelopmental disorders. The strong constraints on variation in this region suggest that many of the possible variants are deleterious or even fatal. Finally, patients carrying missense mutations more often had abnormal neurologic examination results (17 of 19 available [89%]) compared with 1 of 3 LoF carriers (33%) (P = .02, χ2 test for independence). Similarly, normal magnetic resonance imaging results were reported for all 6 patients carrying an LoF mutation. Overall, patients with KCNB1 mutations have a variable phenotype, a finding highlighted by the observation that even patients carrying the same mutation have differences in phenotype.
Functional studies have been performed for several of the described variants. In general, the electrophysiologic characteristics of the different variants have a variety of functional aberrations, such as loss of voltage sensing (p.R306C), reduced current (p.G410R), and suppression of repetitive firing (p.R306C, p.G410R). Of interest, all 3 missense mutations in the ion-selectivity filter domain have been studied and render Kv2.1, a nonselective cation channel that is not voltage activated. However, the general loss of channel function and dominant-negative effects seen in most studied variants are in support of the presumed pathogenicity and link to the EE phenotype. Finally, a Kv2.1−/− mouse model was not prone to spontaneous seizures but exhibited hypocampal hyperexcitability and epileptiform activity on stimulation. Together, these observations further support our conclusion that the KCNB1 variants described here are likely to be pathogenic.
Comparison to KCNQ2
KCNB1 forms a gene family with KCNB2, with which it can form heterotetramers. To date, KCNB2 has not been implicated in monogenic disorders. KCNB1 has a similar structure as other potassium channels involved in severe epilepsy, such as KCNQ2. Pathogenic mutations leading to EE in the other potassium channels are found in the transmembrane regions. This finding is similar to the mutations in KCNB1 described in this study. Furthermore, 2 of the pathogenic variants found in KCNQ2 were homologous to presumed pathogenic variants described in this article: the charged residue in the voltage sensor (p.R306C; variant 20) and missense variants 13 and 14 (p.T374I) in the pore region. This finding underlines the importance of these residues for the functioning of the proteins.
Limitations
Our study also has some limitations. Our patients may have been selected for certain phenotypes, in particular for epilepsy. Our focus on EE may have excluded cases with much milder phenotypes. Furthermore, the number of observations remain small, and description of additional cases will further elucidate the full clinical spectrum associated with mutations in KCNB1.
Conclusions
Missense and LoF variants in the ion_trans domain of KCNB1 and LoF variants in the C-terminal region can cause neurodevelopmental disorder with or without epileptic seizures. Missense variants in the C-terminus are less likely to cause developmental disorders, whereas the consequences of variants in the N-terminus is not clear. The more severely affected patients consistently harbored missense variants within vital channel domains of KCNB1 and experienced late-onset epileptic spasms that evolved to treatment-refractive epilepsy and severe global DD. The effect of LoF variants was less clear, although a tendency existed toward less severe phenotypes. However, more outcome data on patients with LoF variants are needed to support clinical predictions and counseling of such patients. There was a marked tendency to develop spastic paraparesis or tetraparesis and ataxia as well as autistic spectrum disorder. Finally, patients carrying missense mutations were more likely to have neurologic and magnetic resonance imaging abnormalities.
References
- 1.Deprez L, Jansen A, De Jonghe P. Genetics of epilepsy syndromes starting in the first year of life. Neurology. 2009;72(3):273-281. [DOI] [PubMed] [Google Scholar]
- 2.Torkamani A, Bersell K, Jorge BS, et al. . De novo KCNB1 mutations in epileptic encephalopathy. Ann Neurol. 2014;76(4):529-540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allen AS, Berkovic SF, Cossette P, et al. ; Epi4K Consortium; Epilepsy Phenome/Genome Project . De novo mutations in epileptic encephalopathies. Nature. 2013;501(7466):217-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Soden SE, Saunders CJ, Willig LK, et al. . Effectiveness of exome and genome sequencing guided by acuity of illness for diagnosis of neurodevelopmental disorders. Sci Transl Med. 2014;6(265):265ra168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Srivastava S, Cohen JS, Vernon H, et al. . Clinical whole exome sequencing in child neurology practice. Ann Neurol. 2014;76(4):473-483. [DOI] [PubMed] [Google Scholar]
- 6.Deciphering Developmental Disorders Study Large-scale discovery of novel genetic causes of developmental disorders. Nature. 2015;519(7542):223-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Allen NM, Conroy J, Shahwan A, et al. . Unexplained early onset epileptic encephalopathy: exome screening and phenotype expansion. Epilepsia. 2016;57(1):e12-e17. [DOI] [PubMed] [Google Scholar]
- 8.Thiffault I, Speca DJ, Austin DC, et al. . A novel epileptic encephalopathy mutation in KCNB1 disrupts Kv2.1 ion selectivity, expression, and localization. J Gen Physiol. 2015;146(5):399-410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saitsu H, Akita T, Tohyama J, et al. . De novo KCNB1 mutations in infantile epilepsy inhibit repetitive neuronal firing. Sci Rep. 2015;5:15199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Latypova X, Matsumoto N, Vinceslas-Muller C, Bézieau S, Isidor B, Miyake N. Novel KCNB1 mutation associated with non-syndromic intellectual disability. J Hum Genet. 2017;62(5):569-573. [DOI] [PubMed] [Google Scholar]
- 11.Bishop HI, Guan D, Bocksteins E, et al. . Distinct cell- and layer-specific expression patterns and independent regulation of Kv2 channel subtypes in cortical pyramidal neurons. J Neurosci. 2015;35(44):14922-14942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Letunic I, Doerks T, Bork P. SMART: recent updates, new developments and status in 2015. Nucleic Acids Res. 2015;43(database issue):D257-D260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Finn RD, Bateman A, Clements J, et al. . Pfam: the protein families database. Nucleic Acids Res. 2014;42(database issue):D222-D230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuang Q, Purhonen P, Hebert H. Structure of potassium channels. Cell Mol Life Sci. 2015;72(19):3677-3693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ruppersberg JP, Schröter KH, Sakmann B, Stocker M, Sewing S, Pongs O. Heteromultimeric channels formed by rat brain potassium-channel proteins. Nature. 1990;345(6275):535-537. [DOI] [PubMed] [Google Scholar]
- 16.Ottschytsch N, Raes A, Van Hoorick D, Snyders DJ. Obligatory heterotetramerization of three previously uncharacterized Kv channel α-subunits identified in the human genome. Proc Natl Acad Sci U S A. 2002;99(12):7986-7991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bocksteins E, Mayeur E, Van Tilborg A, Regnier G, Timmermans JP, Snyders DJ. The subfamily-specific interaction between Kv2.1 and Kv6.4 subunits is determined by interactions between the N- and C-termini. PLoS One. 2014;9(6):e98960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Kovel CG, Brilstra EH, van Kempen MJ, et al. ; EuroEPINOMICS RES Consortium . Targeted sequencing of 351 candidate genes for epileptic encephalopathy in a large cohort of patients. Mol Genet Genomic Med. 2016;4(5):568-580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Islas LD, Sigworth FJ. Voltage sensitivity and gating charge in Shaker and Shab family potassium channels. J Gen Physiol. 1999;114(5):723-742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ng PC, Henikoff S. Predicting the effects of amino acid substitutions on protein function. Annu Rev Genomics Hum Genet. 2006;7:61-80. [DOI] [PubMed] [Google Scholar]
- 21.Adzhubei IA, Schmidt S, Peshkin L, et al. . A method and server for predicting damaging missense mutations. Nat Methods. 2010;7(4):248-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kircher M, Witten DM, Jain P, O’Roak BJ, Cooper GM, Shendure J. A general framework for estimating the relative pathogenicity of human genetic variants. Nat Genet. 2014;46(3):310-315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lek M, Karczewski KJ, Minikel EV, et al. ; Exome Aggregation Consortium . Analysis of protein-coding genetic variation in 60,706 humans. Nature. 2016;536(7616):285-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Samocha KE, Robinson EB, Sanders SJ, et al. . A framework for the interpretation of de novo mutation in human disease. Nat Genet. 2014;46(9):944-950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baker KE, Parker R. Nonsense-mediated mRNA decay: terminating erroneous gene expression. Curr Opin Cell Biol. 2004;16(3):293-299. [DOI] [PubMed] [Google Scholar]
- 26.Speca DJ, Ogata G, Mandikian D, et al. . Deletion of the Kv2.1 delayed rectifier potassium channel leads to neuronal and behavioral hyperexcitability. Genes Brain Behav. 2014;13(4):394-408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weckhuysen S, Mandelstam S, Suls A, et al. . KCNQ2 encephalopathy: emerging phenotype of a neonatal epileptic encephalopathy. Ann Neurol. 2012;71(1):15-25. [DOI] [PubMed] [Google Scholar]