Abstract
Importance
In atrial fibrillation (AF)–related acute ischemic stroke, the optimal oral anticoagulation strategy remains unclear.
Objective
To test whether rivaroxaban or warfarin sodium is safer and more effective for preventing early recurrent stroke in patients with AF-related acute ischemic stroke.
Design, Setting, and Participants
A randomized, multicenter, open-label, blinded end point evaluation, comparative phase 2 trial was conducted from April 28, 2014, to December 7, 2015, at 14 academic medical centers in South Korea among patients with mild AF-related stroke within the previous 5 days who were deemed suitable for early anticoagulation. Analysis was performed on a modified intent-to-treat basis.
Interventions
Participants were randomized 1:1 to receive rivaroxaban, 10 mg/d for 5 days followed by 15 or 20 mg/d, or warfarin with a target international normalized ratio of 2.0-3.0, for 4 weeks.
Main Outcomes and Measures
The primary end point was the composite of new ischemic lesion or new intracranial hemorrhage seen on results of magnetic resonance imaging at 4 weeks. Primary analysis was performed in patients who received at least 1 dose of study medications and completed follow-up magnetic resonance imaging. Key secondary end points were individual components of the primary end point and hospitalization length.
Results
Of 195 patients randomized, 183 individuals (76 women and 107 men; mean [SD] age, 70.4 [10.4] years) completed magnetic resonance imaging follow-up and were included in the primary end point analysis. The rivaroxaban group (n = 95) and warfarin group (n = 88) showed no differences in the primary end point (47 [49.5%] vs 48 [54.5%]; relative risk, 0.91; 95% CI, 0.69-1.20; P = .49) or its individual components (new ischemic lesion: 28 [29.5%] vs 31 of 87 [35.6%]; relative risk, 0.83; 95% CI, 0.54-1.26; P = .38; new intracranial hemorrhage: 30 [31.6%] vs 25 of 87 [28.7%]; relative risk, 1.10; 95% CI, 0.70-1.71; P = .68). Each group had 1 clinical ischemic stroke, and all new intracranial hemorrhages were asymptomatic hemorrhagic transformations. Hospitalization length was reduced with rivaroxaban compared with warfarin (median, 4.0 days [interquartile range, 2.0-6.0 days] vs 6.0 days [interquartile range, 4.0-8.0]; P < .001).
Conclusions and Relevance
In mild AF-related acute ischemic stroke, rivaroxaban and warfarin had comparable safety and efficacy.
Trial Registration
clinicaltrials.gov Identifier: NCT02042534
This randomized clinical trial examines whether rivaroxaban or warfarin sodium is safer and more effective for preventing early recurrent stroke in patients with atrial fibrillation–related acute ischemic stroke.
Key Points
Question
Is rivaroxaban safer and more effective compared with warfarin sodium for early anticoagulation in atrial fibrillation–related acute ischemic stroke?
Findings
In this randomized clinical trial of 195 patients with mild acute ischemic stroke and atrial fibrillation, new ischemic lesions or new intracranial hemorrhage on results of magnetic resonance imaging after 4 weeks occurred in 49.5% of patients receiving rivaroxaban and 54.5% receiving warfarin, a nonsignificant difference. Each group had 1 recurrence of clinical ischemic stroke, and no symptomatic intracranial hemorrhage occurred.
Meaning
Rivaroxaban and warfarin had comparable safety and efficacy for early anticoagulation in mild atrial fibrillation–related acute ischemic stroke.
Introduction
Patients with atrial fibrillation (AF)–related acute ischemic stroke are at high risk of recurrent ischemic stroke and intracranial hemorrhage, including hemorrhagic transformation, during the early period after stroke. Parenteral heparin is not recommended because it increases the risk of symptomatic intracranial hemorrhage. However, in patients with AF and recent ischemic stroke who are treated with aspirin, the risk of recurrent ischemic stroke within 2 weeks is 5%. The most widely used antithrombotic management strategy in clinical practice is to start aspirin and then to initiate oral anticoagulation after several days or 1 to 2 weeks when the risk of intracranial hemorrhage is likely to have subsided. Nonetheless, the optimal oral anticoagulation strategy for acute ischemic stroke, regarding when, in whom, and which drug, remains unclear.
To our knowledge, no trial has specifically tested oral anticoagulation in patients with AF-related acute ischemic stroke. In the European Atrial Fibrillation Trial, approximately 294 patients with AF were randomized to receive warfarin sodium, aspirin, or placebo within 2 weeks of symptom onset, but the efficacy and safety of warfarin vs aspirin or placebo in patients with acute cerebral ischemia was not reported. The pivotal non–vitamin K antagonist oral anticoagulant (NOAC) trials excluded patients who had ischemic stroke within 7-30 days.
For early oral anticoagulation after acute ischemic stroke, NOACs might have several advantages compared with warfarin: rapid onset of action, absence of transient hypercoagulability, stable and predictable anticoagulation effects, and expected lower risk of intracranial hemorrhage. Accordingly, this proof-of-concept trial (Acute Stroke With Xarelto to Reduce Intracranial Hemorrhage, Recurrent Embolic Stroke, and Hospital Stay [Triple AXEL]) compared the efficacy and safety between rivaroxaban and dose-adjusted warfarin using magnetic resonance imaging (MRI) surrogate markers and hospitalization length in patients who had mild AF-related acute ischemic stroke who were considered suitable for early anticoagulation.
Methods
Study Design and Participants
Triple AXEL was a phase 2, multicenter, randomized, parallel-group, open-label, blinded end point evaluation trial that enrolled patients from 14 academic hospitals in South Korea from April 28, 2014, to October 30, 2015; the patients’ last day of the study was December 7, 2015. Details of the study design have been published previously. The trial protocol and statistical analysis plan are provided in Supplement 1. Briefly, patients were eligible if they had an MRI-confirmed acute ischemic stroke within 5 days due to presumed cardioembolism, had nonvalvular AF (including paroxysmal AF) documented by electrocardiogram, and were considered suitable for early anticoagulation after taking into account the severity of the ischemic lesion as seen in results of diffusion-weighted imaging (DWI): acute ischemic lesion less than one-third of the middle cerebral artery territory, half of the anterior cerebral artery territory, half of the posterior cerebral artery territory, and half of 1 cerebellar hemisphere. Key exclusion criteria included significant hemorrhagic transformation, mechanical heart valve, stroke caused by presumed small vessel occlusion, severe renal impairment (creatinine clearance <30 mL/min/1.73 m2 [to convert to milliliters per second per meter squared, multiply by 0.0167]), and high risk of intracranial or systemic bleeding (eTable 1 in Supplement 2). This study was designed and conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines and was approved by the institutional review boards of Asan Medical Center, University of Ulsan College of Medicine; Ilsan Paik Hospital, Inje University; Korea University Ansan Hospital, Korea University College of Medicine; Ewha Women’s University School of Medicine; Yonsei University College of Medicine; Chonnam National University Medical School; Busan Paik Hospital, Inje University; Dong-A University Hospital; Pusan National University Hospital; Seoul National University Hospital; Samsung Medical Center, Sungkyunkwan University School of Medicine; Kyungpook National University School of Medicine and Hospital; Chosun University School of Medicine; and Hallym University Sacred Heart Hospital as well as the Korean Ministry of Food and Drug Administration (tracking number: 30078). Written informed consent from eligible patients or their legally authorized representatives was obtained. The trial is registered with ClinicalTrials.gov (Identifier: NCT02042534).
Randomization and Masking
We randomly allocated eligible patients in a 1:1 ratio to receive rivaroxaban or dose-adjusted warfarin (target international normalized ratio [INR], 2-3) using an interactive web response system. The randomization sequence was computer generated and stratified by sites with a block size of 4. Patients and responsible physicians were aware of the assigned treatment. However, an independent imaging review core laboratory, blinded to the treatment allocation and clinical data, assessed the MRI findings. Clinical events were reported by each investigator and were judged and categorized by the steering committee.
Procedures
Prior to randomization, all patients underwent magnetic resonance angiography and DWI, fluid-attenuated inversion recovery, gradient-recalled echo, or susceptibility-weighted imaging and received aspirin after the confirmation of an acute ischemic lesion on MRI results. Low-dose subcutaneous heparin or low-molecular-weight heparin for the prevention of deep venous thrombosis or pulmonary embolism was used at the discretion of the responsible physicians.
After randomization, the rivaroxaban group received rivaroxaban, 10 mg once daily, for the first 5 days followed by 20 mg once daily (patients with creatinine clearance ≥50 mL/min) or 15 mg once daily (patients with creatinine clearance of 30-49 mL/min). Aspirin or heparin for prophylaxis of deep venous thrombosis or pulmonary embolism was stopped 24 hours before initiation of rivaroxaban. In the warfarin group, aspirin or heparin for prophylaxis of deep venous thrombosis or pulmonary embolism was continued until an INR of 1.7 was reached after initiation of warfarin. To reduce inter-investigator variation in warfarin dosing, a web-based Bayesian algorithm (http://www.warfarindosing.org/) was recommended for warfarin dosing. For the anticoagulation quality in the warfarin arm, we assessed the mean INR values and the proportions of patients with an INR of 2 to 3 at day 5, 2 weeks, and 4 weeks because the 4-week trial period was too short to analyze the time in therapeutic range.
At 4 weeks, we performed MRI, including fluid-attenuated inversion recovery and gradient-recalled echo or susceptibility-weighted imaging to assess for new ischemic lesions and new intracranial hemorrhage. Patients who had experienced clinical ischemic stroke or symptomatic intracranial hemorrhage before the end of the trial underwent MRI at the time of the clinical event. We allowed computed tomographic evaluation for patients who were unstable and unable to undergo MRI. However, all patients included in the modified intent-to-treat (ITT) population completed the follow-up MRI.
During most of the trial period, the Korean insurance system did not cover the cost of NOACs for most patients with AF. Therefore, patients randomized to receive rivaroxaban had to make the transition from rivaroxaban to warfarin with a 5-day overlap to ensure adequate anticoagulation during the transition. Therefore, clinical ischemic and major bleeding events were followed up for an additional 7 days after the end of the trial and were included in the clinical event end points. However, patients who were willing to pay for rivaroxaban at their own expense continued taking rivaroxaban. Detailed follow-up procedures are summarized in eTable 2 in Supplement 2).
Outcomes
The primary end point was the composite of new ischemic lesion or new intracranial hemorrhage on results of follow-up MRI at 4 weeks. The definition of new ischemic lesion included symptomatic or asymptomatic new ischemic lesions on results of follow-up fluid-attenuated inversion recovery imaging. Symptomatic ischemic lesions were defined as clinical ischemic stroke recurrence associated with a relevant new ischemic lesion. New intracranial hemorrhage (hemorrhagic transformation, intracerebral hemorrhage, subarachnoid hemorrhage, subdural hematoma, or epidural hematoma) included symptomatic or asymptomatic hemorrhage on results of follow-up gradient-recalled echo or susceptibility-weighted imaging. Symptomatic intracranial hemorrhage was defined as any intracranial hemorrhage associated with clinical deterioration as judged by the responsible investigators.
Prespecified secondary efficacy end points were new ischemic lesion; new intracranial hemorrhage; length of hospitalization; major bleeding as defined by the International Society on Thrombosis and Hemostasis; acute coronary syndrome; composite of major vascular events including stroke, myocardial infarction, and vascular death; composite of a major vascular event plus major bleeding; composite of clinical ischemic events; and 4-week modified Rankin Scale score.
Statistical Analysis
A detailed description of the analytic approach is provided in the statistical analysis plan (Supplement 1). We designed the trial to show that rivaroxaban would be superior to warfarin for the prevention of new ischemic lesions or new intracranial hemorrhage on results of follow-up MRI. The primary and secondary end points were assessed in the modified ITT population, which included patients who were randomized, received at least 1 dose of study drugs, and completed follow-up MRI. Per-protocol analysis was additionally performed in patients who had no major protocol violation and took 80% or more of the assigned study medications. Adverse events were assessed in the safety population, which included patients who were randomized and received at least 1 dose of study drugs.
We performed the primary end point analysis using χ2 test for unadjusted analysis and Poisson regression analysis for adjusted analysis. In the adjusted analysis, we included variables of age, sex, center, prior use of a vitamin K antagonist (VKA), concomitant use of an antiplatelet agent, and variables showing difference between the 2 groups. Secondary and safety end point analyses were conducted using χ2 test, Fisher exact test, Wilcoxon rank sum test, or analysis of covariance, as indicated.
On the basis of a review of earlier studies, we calculated the sample size by assuming that the primary end point rate would be 25% in the warfarin group and that the absolute risk reduction with rivaroxaban would be 15%. Using a 1-sided superiority test, 178 patients (89 in each group) assessable for the primary end point would give 80% power with a significance level of P < .05. Assuming a 10% dropout rate, we planned to enroll 196 patients. An independent statistician conducted the statistical analysis. At the inception, interim analysis was not planned because this was a phase 2, proof-of-concept, open-label trial. However, because there had been no trial of NOACs in patients with acute ischemic stroke, the steering committee and investigators agreed to conduct an interim analysis for safety after enrolling 100 patients. No safety concern was raised, and we continued to enroll the planned patients.
Consistency of treatment effect for the primary end point and individual components was assessed for 8 post hoc subgroups regarding age, sex, prior use of a VKA within 30 days before randomization, prestroke CHA2DS2-VASc (congestive heart failure, hypertension, age ≥75 years [doubled], diabetes, stroke [doubled], vascular disease, age 65-74 years, sex category [female]) score, prestroke HAS-BLED (hypertension, abnormal renal/liver function, stroke, bleeding history or predisposition, labile INR, elderly, drugs/alcohol concomitantly) score, baseline DWI volume, concomitant use of an antiplatelet agent, and creatinine clearance level.
Results
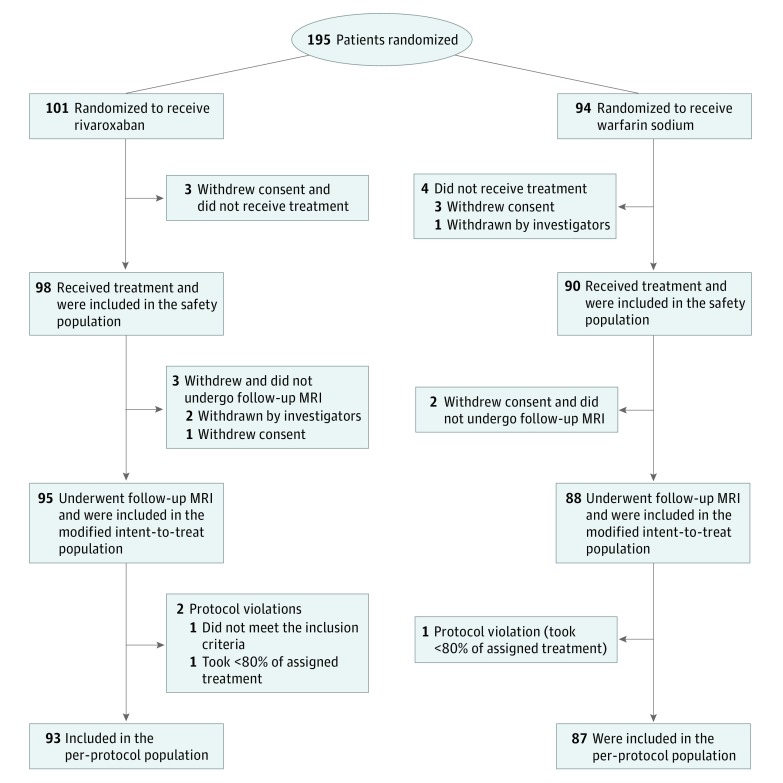
We randomized 195 patients to 1 of 2 treatment groups: 101 to rivaroxaban and 94 to warfarin. All patients had an acute ischemic lesion confirmed by MRI results before randomization. Of the 195 patients randomized, 7 did not receive study treatments, and 5 withdrew and did not undergo follow-up MRI. Therefore, 183 patients (95 in the rivaroxaban group and 88 in the warfarin group) were included in the modified ITT population for the primary end point analysis, and 188 patients (98 in the rivaroxaban group and 90 in the warfarin group) were included in the safety population. Comparisons of patients included in the modified ITT population and those excluded from the modified ITT population are provided in eTable 3 in Supplement 2. Three patients had major protocol violations or had less than 80% adherence to the study treatment (2 in the rivaroxaban group and 1 in the warfarin group), leaving 180 patients (93 in the rivaroxaban group and 87 in the warfarin group) in the per-protocol population (Figure).
Figure. Study Flowchart.
MRI indicates magnetic resonance imaging.
Baseline demographic and clinical characteristics were well balanced except that there were more patients in the rivaroxaban group than the warfarin group with a history of type 2 diabetes (24 [25.3%] vs 10 [11.4%]), and patients in the rivaroxaban group had a smaller median initial ischemic lesion volume on DWI than did patients in the warfarin group (2.6 cm3 [interquartile range, 0.3-10.8] vs 5.5 cm3 [interquartile range, 1.1-14.5]) (Table 1). The median interval from stroke onset to randomization was 2 days (interquartile range, 2.0-3.0) (eFigure 1 in Supplement 2), the mean (SD) prestroke CHA2DS2-VASc score was 2.5 (1.6) (Table 1), the mean (SD) prestroke HAS-BLED score was 1.3 (0.9), and the median National Institutes of Health Stroke Scale score at the time of randomization was 2.0 (interquartile range, 0.0-4.0). There was a history of VKA use within 30 days before randomization in 75 patients (41.0%), and 57 patients (31.1%) received concomitant antiplatelet therapy during the trial.
Table 1. Baseline Characteristics of the Patients Included in the Modified Intent-to-Treat Population.
| Characteristic | Rivaroxaban Group (n = 95) |
Warfarin Sodium Group (n = 88) |
Total (n = 183) |
|---|---|---|---|
| Age, mean (SD), y | 70.2 (10.1) | 70.6 (10.9) | 70.4 (10.4) |
| Female sex, No. (%) | 40 (42.1) | 36 (40.9) | 76 (41.5) |
| Time from onset to randomization, median (IQR), d | 2.0 (2.0-3.0) | 2.0 (2.0-3.0) | 2.0 (2.0-3.0) |
| NIHSS score at randomization, median (IQR) | 2.0 (1.0-4.0) | 2.0 (0.0-4.0) | 2.0 (0.0-4.0) |
| Initial DWI volume, median (IQR),a cm3 | 2.6 (0.3-10.8) | 5.5 (1.1-14.5) | 3.5 (0.5-12.3) |
| Prestroke CHA2DS2-VASc scoreb | |||
| 0 | 11 (11.6) | 10 (11.4) | 21 (11.5) |
| 1 | 16 (16.8) | 15 (17.0) | 31 (16.9) |
| 2 | 14 (14.7) | 26 (29.5) | 40 (21.9) |
| 3 | 20 (21.1) | 17 (19.3) | 37 (20.2) |
| 4 | 22 (23.2) | 14 (15.9) | 36 (19.7) |
| 5 | 8 (8.4) | 6 (6.8) | 14 (7.7) |
| 6 | 3 (3.2) | 0 | 3 (1.6) |
| 7 | 1 (1.1) | 0 | 1 (0.5) |
| Mean (SD) | 2.7 (1.7) | 2.3 (1.4) | 2.5 (1.6) |
| Prestroke HAS-BLED scorec | |||
| 0 | 13 (13.7) | 15 (17.0) | 28 (15.3) |
| 1 | 46 (48.4) | 44 (50.0) | 90 (49.2) |
| 2 | 25 (26.3) | 25 (28.4) | 50 (27.3) |
| 3 | 9 (9.5) | 4 (4.5) | 13 (7.1) |
| 4 | 2 (2.1) | 0 | 2 (1.1) |
| Mean (SD) | 1.4 (0.9) | 1.2 (0.8) | 1.3 (0.9) |
| Paroxysmal atrial fibrillation, No. (%) | 25 (26.3) | 24 (27.3) | 49 (26.8) |
| Prior VKA use, No. (%)d | 39 (41.1) | 36 (40.9) | 75 (41.0) |
| Risk factor, No. (%) | |||
| Hypertension | 65 (68.4) | 52 (59.1) | 117 (63.9) |
| Diabetes mellituse | 24 (25.3) | 10 (11.4) | 34 (18.6) |
| Hyperlipidemia | 16 (16.8) | 18 (20.5) | 34 (18.6) |
| Coronary artery disease | 13 (13.7) | 12 (13.6) | 25 (13.7) |
| Baseline neuroimaging performed, No. (%) | |||
| CT | 67 (70.5) | 63 (71.6) | 130 (71.0) |
| MRI | 95 (100.0) | 88 (100.0) | 183 (100.0) |
| DWI | 95 (100.0) | 87 (98.9) | 182 (99.5) |
| FLAIR | 95 (100.0) | 87 (98.9) | 182 (99.5) |
| GRE | 87 (91.6) | 79 (89.8) | 166 (90.7) |
| SWI | 9 (9.5) | 9 (10.2) | 18 (9.8) |
| Other | 18 (18.9) | 20 (22.7) | 38 (20.8) |
| Blood pressure at study entry, mean (SD), mm Hg | |||
| Systolic | 131.8 (19.1) | 128.0 (17.0) | 130.0 (18.2) |
| Diastolic | 76.8 (11.7) | 77.4 (10.9) | 77.1 (11.3) |
| Heart rate, mean (SD), beats per minute | 84.5 (22.7) | 83.3 (23.3) | 83.9 (22.9) |
| Serum creatinine, mean (SD), mg/dL | 0.89 (0.24) | 0.89 (0.25) | 0.89 (0.24) |
| Concomitant antiplatelet agent use, No. (%) | 34 (35.8) | 23 (26.1) | 57 (31.1) |
| Single antiplatelet agent | 28 (29.5) | 20 (22.7) | 48 (26.2) |
| Aspirin | 25 (26.3) | 11 (12.5) | 36 (19.7) |
| Clopidogrel bisulfate | 2 (2.1) | 7 (8.0) | 9 (4.9) |
| Other | 1 (1.1) | 2 (2.3) | 3 (1.6) |
| Dual antiplatelet agents | 6 (6.3) | 3 (3.4) | 9 (4.9) |
Abbreviations: CHA2DS2-VASc, congestive heart failure, hypertension, age ≥75 years (doubled), diabetes, stroke (doubled), vascular disease, age 65-74 years, sex category (female); CT, computed tomography; DWI, diffusion-weighted imaging; FLAIR, fluid-attenuated inversion recovery; GRE, gradient-recalled echo; HAS-BLED, hypertension, abnormal renal/liver function, stroke, bleeding history or predisposition, labile international normalized ratio, elderly, drugs/alcohol concomitantly; IQR, interquartile range; MRI, magnetic resonance imaging; NIHSS, National Institutes of Health Stroke Scale; SWI, susceptibility-weighted imaging; VKA, vitamin K antagonist.
SI conversion factor: To convert creatinine to micromoles per liter, multiply by 88.4.
P = .04.
The CHA2DS2-VASc scores range from 0 to 9, with higher scores indicating a greater risk of stroke: congestive heart failure, hypertension, diabetes, 65 to 74 years of age, female sex, and vascular disease are each assigned 1 point, and prior stroke or transient ischemic attack and being 75 years of age or older are assigned 2 points.
The HAS-BLED scores range from 0 to 9, with higher scores indicating a greater bleeding risk: hypertension, abnormal renal function, abnormal liver function, prior stroke history, bleeding, labile international normalized ratios, elderly (>65 years of age), prior alcohol or drug use, and medication use predisposing to bleeding are each assigned 1 point.
Use of VKA within 30 days before randomization.
P = .02.
Of the modified ITT population, the proportion of patients taking more than 80% of the study medications was 98.9% in the rivaroxaban group (n = 94) and 97.7% in the warfarin group (n = 86) at a mean (SD) of 5 (2) days and 98.9% in the rivaroxaban group (n = 94) and 98.9% in the warfarin group (n = 87) at 4 weeks. After the end of the trial, 73.7% of patients (n = 70) in the rivaroxaban group continued rivaroxaban treatment, and 26.3% (n = 25) transitioned from rivaroxaban to warfarin. In the warfarin group, the proportion of patients who achieved the target INR of 2.0-3.0 was 40.9% (n = 36) at 5 days, 53.4% (n = 47) at 2 weeks, and 46.6% (n = 41) at 4 weeks. The mean (SD) INR value in the warfarin group was 2.04 (0.62) at 5 days, 2.67 (0.92) at 2 weeks, and 2.39 (0.83) at 4 weeks (eFigure 2 in Supplement 2).
The primary end point occurred in 47 patients (49.5%) in the rivaroxaban group and 48 patients (54.5%) in the warfarin group in the modified ITT population (relative risk, 0.91; 95% CI, 0.69-1.20, P = .49) (Table 2). After adjusting for age, sex, initial ischemic lesion volume on DWI, diabetes, prior VKA, concomitant antiplatelet use, and center, the difference was not significant (relative risk, 0.97; 95% CI, 0.79-1.18; P = .73). Sensitivity analyses assuming worst-case and best-case scenarios for patients who received study drug but did not undergo follow-up MRI showed similar findings (eTable 4 in Supplement 2). In the per-protocol population, the 2 groups showed no difference in the primary end point rate (rivaroxaban, 46 of 93 [49.5%] vs warfarin, 47 of 87 [54.0%]; relative risk, 0.92; 95% CI, 0.69-1.21; P = .54) (eTable 5 in Supplement 2).
Table 2. End Points in the Modified Intent-to-Treat Population.
| End Point | Rivaroxaban Group, No. (%) (n = 95) |
Warfarin Sodium Group, No. (%) (n = 88) |
Risk Difference (95% CI) |
Relative Risk (95% CI) |
P Value | Adjusted Relative Risk (95% CI)a |
P Value |
|---|---|---|---|---|---|---|---|
| Intracranial hemorrhage or recurrent ischemic lesion on results of 4-wk MRI (primary end point) | 47 (49.5) | 48 (54.5) | –5.07 (–19.52 to 9.49) |
0.91 (0.69 to 1.20) |
.49 | 0.97 (0.79 to 1.18) |
.73 |
| Recurrent ischemic lesion on results of 4-wk MRIb | 28 (29.5) | 31 (35.6) | –6.16 (–20.48 to 8.45) |
0.83 (0.54 to 1.26) |
.38 | 0.85 (0.56 to 1.30) |
.45 |
| Intracranial hemorrhage on results of 4-wk MRIb | 30 (31.6) | 25 (28.7) | 2.84 (–11.68 to 17.29) |
1.10 (0.70 to 1.71) |
.68 | 1.17 (0.74 to 1.85) |
.50 |
| Clinical recurrent ischemic stroke | 1 (1.1) | 1 (1.1) | –0.08 (–14.54 to 14.42) |
0.93 (0.06 to 14.59) |
>.99 | NA | NA |
| Symptomatic hemorrhagic conversion or hemorrhagic stroke | 0 | 0 | NA | NA | >.99 | NA | NA |
| Major bleeding | 1 (1.1) | 0 | 1.05 (–13.44 to 15.53) |
NA | >.99 | NA | NA |
| Systemic embolism | 0 | 0 | NA | NA | >.99 | NA | NA |
| Acute coronary syndrome | 0 | 0 | NA | NA | >.99 | NA | NA |
| Composite of stroke, MI, or vascular death | 1 (1.1) | 1 (1.1) | –0.08 (–14.54 to 14.42) |
0.93 (0.06 to 14.59) |
>.99 | NA | NA |
| Composite of stroke, MI, vascular death, or major bleeding | 2 (2.1) | 1 (1.1) | 0.97 (–13.50 to 15.46) |
1.85 (0.17 to 20.08) |
>.99 | NA | NA |
| Composite of clinical ischemic events | 1 (1.1) | 1 (1.1) | –0.08 (–14.54 to 14.42) |
0.93 (0.06 to 14.59) |
>.99 | NA | NA |
| Duration of hospitalization, median (IQR), d | 4.0 (2.0-6.0) | 6.0 (4.0-8.0) | NA | NA | <.001 | NA | .002 |
| mRS score 0–1 at 4 wkc | 79 (84.0) | 64 (74.4) | 9.62 (–5.06 to 23.95) |
1.13 (0.97 to 1.31) |
.11 | 1.04 (0.83 to 1.29) |
.73 |
Abbreviations: IQR, interquartile range; MI, myocardial infarction; MRI, magnetic resonance imaging; mRS, modified Rankin Scale; NA, not applicable.
Adjusted for age, sex, initial ischemic lesion volume on diffusion-weighted imaging, diabetes, prior use of vitamin K antagonist, concomitant use of antiplatelet agent, and center.
Of the patients in the warfarin group who were included in the intent-to-treat population and were evaluated for the primary end point, recurrent ischemic lesion on results of 4-week MRI was not evaluated in 1 patient, and new intracranial hemorrhage on 4-week MRI was not evaluated in 1 patient (detailed description in eTable 4 in Supplement 2). Sensitivity analyses assuming worst-case and best-case scenarios for these patients are provided in eTable 4 in Supplement 2.
Of the intent-to-treat population, the mRS score at 4 weeks was not available in 1 patient in the rivaroxaban group and in 2 patients in the warfarin group.
A new ischemic lesion was seen in 28 patients (29.5%) in the rivaroxaban group and 31 of 87 patients (35.6%) in the warfarin group (relative risk, 0.83; 95% CI, 0.54-1.26; P = .38) (Table 2). Each group had 1 clinical ischemic stroke recurrence, which was presumed to be cardioembolism. The proportions of new ischemic lesions greater than 10 mm and multiple lesions did not differ between the 2 groups (eTable 6 in Supplement 2).
New intracranial hemorrhage was seen in 30 patients (31.6%) in the rivaroxaban group and 25 of 87 patients (28.7%) in the warfarin group (relative risk, 1.10; 95% CI, 0.70-1.71; P = .68) (Table 2). There was no symptomatic intracranial hemorrhage in the 2 groups; all intracranial hemorrhages were asymptomatic hemorrhagic transformations within or adjacent to the qualifying ischemic lesion. Of the hemorrhagic transformations, 49 of 55 (89.1%) were minimal or mild hemorrhagic transformation without mass effect. Although there was no statistically significant difference, parenchymal hematoma with mass effect was observed more frequently in the warfarin group than in the rivaroxaban group, while type I hemorrhagic infarction was more frequent in the rivaroxaban group than in the warfarin group (eTable 7 in Supplement 2).
The treatment effects were generally consistent across post hoc subgroups for the primary end point and individual components. However, there was a significant interaction between prior VKA use and treatment effect for the recurrent ischemic lesion end point, suggesting that rivaroxaban was associated with fewer recurrent ischemic lesions in patients with no prior VKA use than was warfarin (eTables 8-10 in Supplement 2).
One patient in the rivaroxaban group developed intraocular bleeding leading to transient visual disturbance, which was categorized as major bleeding. However, it did not prolong hospitalization, and the patient recovered without sequelae. There was no acute coronary syndrome during the trial. The proportions of modified Rankin Scale scores of 0 to 1 at 4 weeks did not differ between the rivaroxaban and warfarin groups (79 of 94 [84.0%] vs 64 of 86 [74.4%]; relative risk, 1.13; 95% CI, 0.97-1.31; P = .11) (Table 2). However, median hospitalization length was significantly shorter in the rivaroxaban group than in the warfarin group (4.0 days [interquartile range, 2.0-6.0 days] vs 6.0 days [interquartile range, 4.0-8.0 days]; P < .001) (Table 2). In the per-protocol population analysis, the secondary end point results were similar to those of the modified ITT population analysis (eTable 5 in Supplement 2).
The rates of adverse events, adverse drug reactions, and serious adverse events were similar between the 2 groups (Table 3). There were no deaths during the trial.
Table 3. Adverse Events in the Safety Population.
| Characteristic | No. (%) | Risk Difference, % (95% CI) |
P Value | |
|---|---|---|---|---|
| Rivaroxaban Group (n = 98) |
Warfarin Sodium Group (n = 90) |
|||
| ≥1 Adverse events | 46 (46.9) | 51 (56.7) | −9.73 (−23.84 to 4.66) |
.18 |
| ≥1 Adverse drug reactions | 9 (9.2) | 13 (14.4) | −5.26 (−19.41 to 9.11) |
.26 |
| ≥1 Serious adverse events | 5 (5.1) | 5 (5.6) | −0.45 (−14.76 to 13.82) |
>.99 |
| Withdrawal owing to adverse events | 1 (1.0) | 0 | 1.02 (−13.32 to 15.32) |
>.99 |
| Death | 0 | 0 | NA | >.99 |
Abbreviation: NA, not applicable.
Discussion
In this first trial to compare NOAC and VKA in patients with AF-related acute ischemic stroke, there was no difference between rivaroxaban and warfarin in the combined surrogate end point of new ischemic lesion or new intracranial hemorrhage on results of follow-up MRI at 4 weeks. In addition, there were no differences between the 2 groups in the individual components of new ischemic lesion and new intracranial hemorrhage. No patient had symptomatic intracranial hemorrhage, and 1 patient (1.1%) in each group had clinical recurrence of ischemic stroke. Therefore, either rivaroxaban or warfarin initiated within 5 days of stroke onset was comparably safe and effective for preventing clinical recurrence of ischemic stroke in patients with AF and mild acute ischemic stroke. However, initiation of rivaroxaban instead of warfarin reduced hospitalization length by almost 2 days.
A recent European practical guide recommends the “1-3-6-12 day rule” for the initiation of NOACs after transient ischemic attack or acute ischemic stroke. However, the recommendations are based on expert opinion and are not supported by clinical trial data. An analysis of the Virtual International Stroke Trials Archive (VISTA) database found that the early initiation of anticoagulants (2-3 days after stroke) was associated with substantially fewer recurrent events during the following weeks without an increased risk of symptomatic intracerebral hemorrhages. However, the finding is subject to substantial confounding by indication. In a prospective observational study that enrolled 1029 consecutive patients with acute ischemic stroke and known or newly diagnosed AF without contraindications to anticoagulation, compared with initiation of treatment before 4 days or more than 14 days after stroke onset, initiation of anticoagulants within 4 to 14 days of stroke onset was associated with a significant reduction in the composite of stroke, transient ischemic attack, symptomatic systemic embolism, symptomatic cerebral bleeding, and major extracranial bleeding within 90 days from acute stroke. In a recent small, prospective observational study of 60 patients initiating rivaroxaban at a median time of 3 days after mild to moderate cardioembolic stroke or transient ischemic attack, no patient developed symptomatic hemorrhagic transformation, and 8 patients had asymptomatic new hemorrhagic transformation or worsening of hemorrhagic transformation on results of follow-up MRI obtained 7 days after initiation of rivaroxaban, suggesting the safety of rivaroxaban treatment in the acute stage. Our findings support the recommendations and are generally in accordance with the findings of the earlier observational studies.
Given the lower risk of intracranial hemorrhage with NOACs vs warfarin for long-term prevention therapy, patients with AF-related acute ischemic stroke who were treated with rivaroxaban vs those treated with warfarin were expected to have a lower risk of new intracranial hemorrhage, including hemorrhagic transformation. However, the risk was not different between the 2 treatment groups. Although, of intracranial hemorrhage types, minimal or mild hemorrhagic transformation was more frequent with rivaroxaban whereas parenchymal hematoma was more frequent with warfarin, our study was not adequately powered to detect the difference in the types of intracranial hemorrhage.
Although there were no statistically significant differences, the rivaroxaban group had fewer recurrent ischemic lesions and more new intracranial hemorrhages compared with the warfarin group. Full anticoagulation would be immediately achieved with rivaroxaban and delayed with warfarin, which might contribute to fewer ischemic lesions and more hemorrhagic lesions with rivaroxaban in the acute stage when the risks of both recurrent ischemia and intracranial hemorrhage would be greatest. The post hoc analysis finding of a greater difference in the recurrent ischemic lesion end point in patients with no recent VKA use than in those with recent VKA use might support our speculation. However, the results from the post hoc analysis and multiple comparisons would limit the significance of the finding.
Although there were no differences in the imaging and clinical end points between the 2 groups, initiation of oral anticoagulation with rivaroxaban vs warfarin significantly reduced the length of hospitalization for acute stroke. More rapid achievement of full anticoagulation with rivaroxaban likely enables patients to be discharged earlier. Previous studies showed that, for long-term therapy, use of NOACs vs warfarin was cost-effective. Our trial showed that rivaroxaban would reduce the cost of hospitalization for acute stroke in patients who are deemed suitable for early anticoagulation, but the cost-saving effect might not be applicable to different health care settings.
In the current study, we found that about 50% of patients had asymptomatic new ischemic lesions or intracranial hemorrhage on results of MRI within 4 weeks after stroke, which was greater than expected. Despite the early use of anticoagulation, about 30% of the patients had a new ischemic lesion. In a prior study, 40% of patients who had a cardioembolic stroke within 6 hours and were treated with antiplatelet drugs or anticoagulation had a recurrent ischemic lesion on MRI results within the first week. Our findings, along with those of the earlier study, indicate that the risk of early recurrent cerebral ischemia is high in patients with AF-related acute ischemic stroke. However, intracranial hemorrhage was also observed in about 30% of patients in our study. All of the intracranial hemorrhages were asymptomatic and most were minor hemorrhagic transformations, which might spontaneously develop rather than be caused by anticoagulation. Because we had no placebo group, we were unable to assess whether rivaroxaban or warfarin increased the risk of asymptomatic hemorrhagic transformation. Although the ischemic and hemorrhagic lesions were clinically silent in most patients, their effect on cognitive function was not explored in this study and would be of interest as part of a future investigation. In addition, the high frequency of both new ischemic and hemorrhagic lesions emphasizes the importance of a proper balance between the efficacy and safety of an antithrombotic strategy in patients with AF and acute cerebral ischemia.
We selected a low dose of 10 mg of rivaroxaban for the first 5 days because no safety data on rivaroxaban in patients with acute ischemic stroke were available, and all patients received aspirin before randomization and the residual effect of aspirin would remain for 5 to 7 days after rivaroxaban initiation. In a pharmacodynamics study in healthy Chinese individuals, the inhibition of factor Xa activity, as measured by the median percentage change from baseline, was about 38% with a single dose of rivaroxaban, 10 mg, and about 46% with a single dose of rivaroxaban, 20 mg. Therefore, a single dose of rivaroxaban, 10 mg, might achieve about 83% of the factor Xa activity inhibition as a single dose of rivaroxaban, 20 mg.
When NOAC is compared with warfarin, the anticoagulation quality of warfarin therapy must be adequately maintained. Because of the short trial duration, rather than time in therapeutic range, we assessed the mean INR and the proportion of INR values ranging from 2.0 to 3.0. Although the mean INR values in the warfarin arm were within 2.0 to 3.0 at each time point, the proportion of INR values from 2.0 to 3.0 ranged between 40.9% (n = 36 of 88) and 53.4% (n = 47 of 88), even though we followed a recommended warfarin dosing program. In the pivotal NOAC trials, the time in therapeutic range of Korean patients ranged between 48% and 56%. Our findings, along with those of the large trials, indicate that Korean patients have difficulty in achieving good-quality anticoagulation with warfarin therapy.
Limitations
This was an open-label study, which was at risk of a reporting bias for clinical events. However, the primary end point was an imaging surrogate marker evaluated in a blinded fashion. The quality of anticoagulation with warfarin in our study was less than optimal. Therefore, our findings might not be applicable to populations with high-quality anticoagulation with warfarin therapy. Because this study selected patients with mild stroke severity who were considered at low risk of intracranial hemorrhage with early oral anticoagulation, the findings would not be applicable to those with moderate to severe stroke.
Conclusions
Both rivaroxaban and warfarin initiated within 5 days of stroke onset in patients with mild AF-related acute ischemic stroke were safe and effective for preventing early clinical stroke recurrence.
Trial Protocol
eTable 1. Inclusion and Exclusion Criteria
eTable 2. Study Procedures
eTable 3. Characteristics of Patients Included in Modified Intent-To-Treat Population (ITT) and Those Excluded From the ITT Population
eTable 4. Sensitivity Analyses
eTable 5. Endpoints in the Per-Protocol Population
eTable 6. Characteristics of New Ischemic Lesion in the Modified ITT Population
eTable 7. Characteristics of New Intracranial Hemorrhage in the Modified ITT Population
eTable 8. Subgroup Analysis for Primary Endpoint in the Modified ITT Population
eTable 9. Subgroup Analysis for Recurrent Ischemic Lesion at Week 4 in the Modified ITT Population
eTable 10. Subgroup Analysis for Intracranial Hemorrhage at Week 4 in the Modified ITT Population
eFigure 1. Onset to Randomization
eFigure 2. INR Values in the Warfarin Group
References
- 1.Paciaroni M, Agnelli G, Micheli S, Caso V. Efficacy and safety of anticoagulant treatment in acute cardioembolic stroke: a meta-analysis of randomized controlled trials. Stroke. 2007;38(2):423-430. [DOI] [PubMed] [Google Scholar]
- 2.Sandercock P, Collins R, Counsell C, et al. ; International Stroke Trial Collaborative Group . The International Stroke Trial (IST): a randomised trial of aspirin, subcutaneous heparin, both, or neither among 19435 patients with acute ischaemic stroke. Lancet. 1997;349(9065):1569-1581. [PubMed] [Google Scholar]
- 3.CAST (Chinese Acute Stroke Trial) Collaborative Group CAST: randomised placebo-controlled trial of early aspirin use in 20,000 patients with acute ischaemic stroke. Lancet. 1997;349(9066):1641-1649. [PubMed] [Google Scholar]
- 4.Berge E, Abdelnoor M, Nakstad PH, Sandset PM. Low molecular-weight heparin versus aspirin in patients with acute ischaemic stroke and atrial fibrillation: a double-blind randomised study: HAEST Study Group: Heparin in Acute Embolic Stroke Trial. Lancet. 2000;355(9211):1205-1210. [DOI] [PubMed] [Google Scholar]
- 5.Hart RG, Palacio S, Pearce LA. Atrial fibrillation, stroke, and acute antithrombotic therapy: analysis of randomized clinical trials. Stroke. 2002;33(11):2722-2727. [DOI] [PubMed] [Google Scholar]
- 6.EAFT (European Atrial Fibrillation Trial) Study Group Secondary prevention in non-rheumatic atrial fibrillation after transient ischaemic attack or minor stroke. Lancet. 1993;342(8882):1255-1262. [PubMed] [Google Scholar]
- 7.Connolly SJ, Ezekowitz MD, Yusuf S, et al. ; RE-LY Steering Committee and Investigators . Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361(12):1139-1151. [DOI] [PubMed] [Google Scholar]
- 8.Patel MR, Mahaffey KW, Garg J, et al. ; ROCKET AF Investigators . Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365(10):883-891. [DOI] [PubMed] [Google Scholar]
- 9.Granger CB, Alexander JH, McMurray JJ, et al. ; ARISTOTLE Committees and Investigators . Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365(11):981-992. [DOI] [PubMed] [Google Scholar]
- 10.Giugliano RP, Ruff CT, Braunwald E, et al. ; ENGAGE AF-TIMI 48 Investigators . Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369(22):2093-2104. [DOI] [PubMed] [Google Scholar]
- 11.Hong KS, Choi YJ, Kwon SU; Triple AXEL Investigators . Rationale and design of Triple AXEL: trial for early anticoagulation in acute ischemic stroke patients with nonvalvular atrial fibrillation. Int J Stroke. 2015;10(1):128-133. [DOI] [PubMed] [Google Scholar]
- 12.World Medical Association World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. [DOI] [PubMed] [Google Scholar]
- 13.Bang OY, Hong KS, Heo JH, et al. . New oral anticoagulants may be particularly useful for Asian stroke patients. J Stroke. 2014;16(2):73-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schulman S, Kearon C; Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis . Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost. 2005;3(4):692-694. [DOI] [PubMed] [Google Scholar]
- 15.Kang DW, Latour LL, Chalela JA, Dambrosia J, Warach S. Early ischemic lesion recurrence within a week after acute ischemic stroke. Ann Neurol. 2003;54(1):66-74. [DOI] [PubMed] [Google Scholar]
- 16.Heidbuchel H, Verhamme P, Alings M, et al. . Updated European Heart Rhythm Association practical guide on the use of non–vitamin K antagonist anticoagulants in patients with non-valvular atrial fibrillation. Europace. 2015;17(10):1467-1507. [DOI] [PubMed] [Google Scholar]
- 17.Abdul-Rahim AH, Fulton RL, Frank B, et al. ; VISTA Collaborators . Association of improved outcome in acute ischaemic stroke patients with atrial fibrillation who receive early antithrombotic therapy: analysis from VISTA. Eur J Neurol. 2015;22(7):1048-1055. [DOI] [PubMed] [Google Scholar]
- 18.Paciaroni M, Agnelli G, Falocci N, et al. . Early recurrence and cerebral bleeding in patients with acute ischemic stroke and atrial fibrillation: effect of anticoagulation and its timing: the RAF Study. Stroke. 2015;46(8):2175-2182. [DOI] [PubMed] [Google Scholar]
- 19.Gioia LC, Kate M, Sivakumar L, et al. . Early rivaroxaban use after cardioembolic stroke may not result in hemorrhagic transformation: a prospective magnetic resonance imaging study. Stroke. 2016;47(7):1917-1919. [DOI] [PubMed] [Google Scholar]
- 20.Lee S, Anglade MW, Pham D, Pisacane R, Kluger J, Coleman CI. Cost-effectiveness of rivaroxaban compared to warfarin for stroke prevention in atrial fibrillation. Am J Cardiol. 2012;110(6):845-851. [DOI] [PubMed] [Google Scholar]
- 21.Dorian P, Kongnakorn T, Phatak H, et al. . Cost-effectiveness of apixaban vs current standard of care for stroke prevention in patients with atrial fibrillation. Eur Heart J. 2014;35(28):1897-1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harrington AR, Armstrong EP, Nolan PE Jr, Malone DC. Cost-effectiveness of apixaban, dabigatran, rivaroxaban, and warfarin for stroke prevention in atrial fibrillation. Stroke. 2013;44(6):1676-1681. [DOI] [PubMed] [Google Scholar]
- 23.Shah SV, Gage BF. Cost-effectiveness of dabigatran for stroke prophylaxis in atrial fibrillation. Circulation. 2011;123(22):2562-2570. [DOI] [PubMed] [Google Scholar]
- 24.Magnuson EA, Vilain K, Wang K, et al. ; ENGAGE AF–TIMI 48 Trial Investigators . Cost-effectiveness of edoxaban vs warfarin in patients with atrial fibrillation based on results of the ENGAGE AF-TIMI 48 trial. Am Heart J. 2015;170(6):1140-1150. [DOI] [PubMed] [Google Scholar]
- 25.Zhao X, Sun P, Zhou Y, et al. . Safety, pharmacokinetics and pharmacodynamics of single/multiple doses of the oral, direct factor Xa inhibitor rivaroxaban in healthy Chinese subjects. Br J Clin Pharmacol. 2009;68(1):77-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wallentin L, Yusuf S, Ezekowitz MD, et al. ; RE-LY investigators . Efficacy and safety of dabigatran compared with warfarin at different levels of international normalised ratio control for stroke prevention in atrial fibrillation: an analysis of the RE-LY trial. Lancet. 2010;376(9745):975-983. [DOI] [PubMed] [Google Scholar]
- 27.Singer DE, Hellkamp AS, Piccini JP, et al. ; ROCKET AF Investigators . Impact of global geographic region on time in therapeutic range on warfarin anticoagulant therapy: data from the ROCKET AF clinical trial. J Am Heart Assoc. 2013;2(1):e000067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wallentin L, Lopes RD, Hanna M, et al. ; Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation (ARISTOTLE) Investigators . Efficacy and safety of apixaban compared with warfarin at different levels of predicted international normalized ratio control for stroke prevention in atrial fibrillation. Circulation. 2013;127(22):2166-2176. [DOI] [PubMed] [Google Scholar]
- 29.Shimada YJ, Yamashita T, Koretsune Y, et al. . Effects of regional differences in Asia on efficacy and safety of edoxaban compared with warfarin—insights from the ENGAGE AF-TIMI 48 Trial. Circ J. 2015;79(12):2560-2567. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eTable 1. Inclusion and Exclusion Criteria
eTable 2. Study Procedures
eTable 3. Characteristics of Patients Included in Modified Intent-To-Treat Population (ITT) and Those Excluded From the ITT Population
eTable 4. Sensitivity Analyses
eTable 5. Endpoints in the Per-Protocol Population
eTable 6. Characteristics of New Ischemic Lesion in the Modified ITT Population
eTable 7. Characteristics of New Intracranial Hemorrhage in the Modified ITT Population
eTable 8. Subgroup Analysis for Primary Endpoint in the Modified ITT Population
eTable 9. Subgroup Analysis for Recurrent Ischemic Lesion at Week 4 in the Modified ITT Population
eTable 10. Subgroup Analysis for Intracranial Hemorrhage at Week 4 in the Modified ITT Population
eFigure 1. Onset to Randomization
eFigure 2. INR Values in the Warfarin Group